Abstract
Pre-eclampsia remains a leading contributor to maternal and perinatal mortality, particularly in resource-limited settings, prompting the urgent search for accessible early biomarkers. Capitalising on growing evidence that bile-acid dysregulation participates in hypertensive disorders of pregnancy, we conducted a case–control study in which fasting serum from 30 women with preeclampsia and 30 gestational-age-matched healthy pregnant controls was subjected to targeted LC–MS/MS quantification of 59 bile-acid subtypes after DMED derivatisation. 30 analytes differed significantly (unpaired t-test, FDR-adjusted q-value < 0.05; fold-change ≥ 2), with glycochenodeoxycholic acid (GCDCA) achieving an AUC of 0.879 (95% CI 0.782–0.946). A two-metabolite panel comprising GCDCA and glycodeoxycholic acid-3-O-β-glucuronide delivered AUCs of 0.856 under support-vector. These data reveal extensive disruption of bile-acid homeostasis in preeclampsia, implicate gut–liver axis perturbation in its pathophysiology, and identify a parsimonious serum signature that merits prospective multi-centre validation.
Similar content being viewed by others
Introduction
Globally, pre-eclampsia affects 2–8% of pregnancies1, causing more than 500 000 fetal and neonatal deaths, and 70 000 maternal deaths every year worldwide2, and 99% of these deaths occur in low- and middle-income countries1.
The prediction and diagnosis of pre-eclampsia represents a crucial aspect of antenatal maternity care. however, the accurate diagnosis of pre-eclampsia remains a clinical challenge. The presentation of pre-eclampsia is highly variable, and women with pre-eclampsia may be asymptomatic, even in the presence of severe disease. The clinical disease course can progress for weeks before a diagnosis is confirmed. The diagnostic criteria for pre-eclampsia, as defined by the American College of Obstetricians and Gynecologists, relies on the assessment of new-onset hypertension and one or more features of multi‐organ disease3. However, the diagnostic performance of these signs and symptoms do not meet the clinical needs of the field4,5.
Currently, metabolomics are widely applied in exploring the pathophysiological mechanisms of diseases6,7,8and in the identification of biomarkers9,10. Pre-eclampsia is a disease associated with multiple factors and has a complex pathophysiology11,12,13,14. Given that metabolomics can simultaneously identify multiple metabolites, it has become an essential method for investigating the pathogenesis of pre-eclampsia and for identifying potential biomarkers15,16,17,18. Although some biomarkers have been applied in clinical use, such as the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) the limitations still exist19.There remains a significant demand for reliable biomarkers for the prediction and diagnosis of pre-eclampsia.
More and more researchers are now interested in bile acid metabolomics. Qi et al.20 analyzed serum samples from different populations by non-targeted metabolomics techniques and found that the development of hypertension is associated with vascular inflammation caused by abnormal bile acid metabolism, and that bile acid metabolism plays an important role in the development of prehypertension by regulating vascular inflammatory responses. Louise et al.21 found that the differences of 12-ketocholestatic acid in patients with pre-eclampsia and healthy pregnant women were significant. significant differences. Several studies have established a correlation between bile acid metabolism and the pathophysiological mechanisms underlying pre-eclampsia22,23,24. So we accurately quantified various bile acids in serum by liquid chromatography tandem mass spectrometry (LC-MS/MS), explored the association between bile acids and the development of pre-eclampsia, searched for potential biomarkers that can realize the early diagnosis of pre-eclampsia, and provided new perspectives and directions for the clinical diagnosis of pre-eclampsia, which can help in the early treatment of pre-eclampsia.
Materials and methods
Study participants
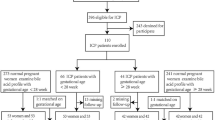
30 pregnant women diagnosed with pre-eclampsia at the Third Hospital of Peking University from September 2021 to October 2021(7 cases) and from July 2023(63 cases) to October 2023 were included as the pre-eclampsia group, while 30 healthy pregnant women were included as the control group. Informed consent was obtained from all participants. Inclusion criteria and exclusion criteria are described in the Supplementary Material. The general clinical data of the pregnant women were recorded, and the remaining serum samples were collected from routine laboratory examinations. The samples were collected in yellow vacuum blood collection tubes containing separation gel and centrifuged at 2793 g for 10 min to collect the serum. The pregnant women were fasting at the time of sampling and the samples were free from jaundice, hemolysis, and lipemia. Serum samples were dispensed into cryopreservation tubes and stored at -80 °C in a freezer until testing, avoiding repeated freezing and thawing. Sample preparation, derivatization, and LC-MS/MS analysis methods were used as developed by Xiong Xin et al.25. Details are provided in the Supplementary Material3. Pregnancy outcome and gestational week of delivery were followed and recorded. This study was approved and consented by the Medical Ethics Committee of the Third Hospital of Peking University (Ethics Approval No. IRB00006761-M2021032). All methods were performed in accordance with the relevant guidelines and regulations.
Data processing and statistical analysis
The general clinical data and routine biochemical indicators of the patients were statistically analyzed using GraphPad Prism 10.0. The normality of the quantitative indicators was detected using the D’Agostino & Pearson normality test. If both the pre-eclampsia group and the control group conformed to the normal distribution, the results were described as the mean ± standard deviation, and t-tests were performed to determine whether there was a significant difference. In the event that one of the pre-eclampsia and control groups did not conform to a normal distribution, the results were described as the median [25% quartile, 75% quartile], and the Mann-Whitney test was used to determine whether there was a significant difference between the two groups. The number of cases statistic was described as the number of cases (percentage), and the Yates corrected chi-square test was used to determine whether there was a statistical difference between the two groups. All statistical tests were two-tailed, and p < 0.05 was considered a statistically significant difference.
The acquired mass spectrometry data were processed by AB SCIEX MultiQuant2.1, and bile acid subtypes that were undetectable in more than 20% of the samples were excluded. Substances below 80% of the lower limit of quantification(LLOQ) were assigned a value of ½ of LLOQ. Substances below the LLOQ but above 80% of the LLOQ were assigned the original value (LLOQ ± 20% is considered valid). The acquired mass spectral data were statistically analyzed using MetaboAnalyst 6.0 (http://www.metaboanalyst.ca/). A 2-sample unpaired t test was performed with unequal group variance at a threshold of P < 0.05 to identify differential bile acid subtypes. The receiver operating characteristic curve (ROC) was then analyzed using MedCalc. Differences between ROC curves were compared using the DeLong test.
Machine learning models
Machine learning models were constructed using MetaboAnalyst 6.0 for multivariate ROC curve analysis to screen better combination of biomarkers. Logistic regression model, random forest model and support vector machine model were used.
Results
Subject characteristics
The clinical characteristics of the study participants are shown in Table 1. There was no statistically significant difference in age and gestational age between the pre-eclampsia and control groups. There was also no statistical difference in glycocholic acid and total bile acids (TBA) detected by routine biochemical tests.
Differences in bile acid profiles
We performed absolute quantification of 59 bile acid subtypes in 60 samples by LC-MS/MS. An unpaired t test with unequal group variance at a threshold of P < 0.05 and FDR < 0.05 showed 30 differential bile acid subtypes (12 up-regulated and 18 down-regulated). Details are provided in Table 2. The heatmap shows the differences in bile acid profiles between pre-eclamptic patients and healthy pregnant women (Fig. 1). In partial least squares discriminant analysis (PLS-DA), there was a clear trend toward separation between the pre-eclampsia group and the control group (Fig. 2). Next, we decided to idenitify biomarkers in differential bile acid subtypes with FC ≥ 2 and raw p-value < 0.05(Fig. 3).
Differences between pre-eclampsia and control groups in bile acid profile. The heatmap shows the differential expression of bile acid subtypes pre-eclampsia and control groups. The information for metabolites corresponding to the abbreviations is presented in Table 2. PE, pre-eclampsia.
2D(A) and 3D(B) view of partial least squares discriminant analysis. There was a clear trend toward separation between the pre-eclampsia group and the control. The biplot (C) and VIP score (D) are also presented. The bile acid subtypes that contribute significantly to the separation trend are shown.PE, pre-eclampsia.
Volcanic plot of potential biomarkers of pre-eclampsia diagnosis. A total of 26 bile acid subtypes exhibited significant differences, meeting the criteria of a fold change (FC) ≥ 2 and a raw p-value < 0.05. The information for metabolites corresponding to the abbreviations is presented in Table 2.
Performance of machine learning models
The univariate analysis of the eligible bile acid subtypes (13 up-regulated and 13 down-regulated), as illustrated in Fig. 3, revealed 11 bile acid subtypes with area under the curve (AUC) > 0.8, with the AUC of Glycochenodeoxycholic acid(GCDCA) reaching 0.879(95%CI[0.782,0.946]) (Fig. 4). To investigate the efficacy of various biomarker panels, we generated ROC curves using support vector machine, each validated through 4-fold cross-validation. The results for the 3 biomarker combinations with the highest AUC are presented in Table 3. Consequently, it is the best combination to include two biomarkers, glycochenodeoxycholic acid and glycodeoxycholic acid-3-O-β-glucuronide. In the PE cohort, glycochenodeoxycholic acid was up-regulated, whereas glycodeoxycholic acid-3-O-β-glucuronide was markedly down-regulated (Fig. 5).
ROC of 11 bile acid subtypes: GCDCA(A),3β12βOH-5βBA(B), GDCA-3-G(C), Glyco-LCA-S(D), IsoLCA(E), LCA-3-S(F), TCDCA(G), LagoDCA(H), 3β-GCA(I), DCA-3-S(J) and TCA(K). The information for metabolites corresponding to the abbreviations is presented in Table 2. There are 11 bile acid subtypes with area under the curve (AUC) > 0.8, with the AUC of Glycochenodeoxycholic acid(GCDCA) reaching 0.879(95%CI[0.782,0.946])
Discussion
This study employed the LC-MS/MS method developed by Xiong Xin et al.25, which has been previously validated for specificity, sensitivity, accuracy, and precision. The DMED labeling technique used in this method, which tags the carboxyl groups of bile acids, significantly enhanced the separation of bile acid subtypes, thereby greatly improving the sensitivity and specificity of bile acid profiling.
Previous research has indicated that dysbiosis of the gut microbiota can induce symptoms of pre-eclampsia through the gut-placenta axis26,27, closely linking gut microbiota with bile acid metabolism28,29,30,31. Pre-eclampsia is a hypertensive disorder of pregnancy and several previous studies have found that TBA is significantly associated with pre-eclampsia32,33,34. In addition, bile acid metabolism is a potential mediator of blood pressure regulation, and some bile acid subtypes are negatively associated with blood pressure in humans35,36. Our study found severe abnormalities in bile acid metabolism in patients with pre-eclampsia, corroborating these earlier findings. Furthermore, we identified 30 distinct bile acid subtypes that exhibited significant differences in patients with pre-eclampsia compared to the control group. 5β-cholanic acid-3,7β-diol (UDCA) are significantly elevated in the serum of patients with pre-eclampsia.
Univariate ROC curve analysis indicated that GCDCA had the highest AUC, reaching 0.879 (95% CI [0.782, 0.946]). Previous studies have shown that GCDCA can be used for early prediction of gestational diabetes37, a known risk factor for pre-eclampsia38,39, corroborating our findings. This discovery also suggests the potential for simultaneous clinical diagnosis of both pregnancy complications. The study found that the combination of GCDCA and GDCA-3-G consistently achieved an test AUC of over 0.856. Further increasing the number of biomarkers did not significantly enhance the efficacy of the biomarker combination. Considering the cost of clinical testing and the economic burden on patients, the combination of GCDCA and GDCA-3-G is identified as the optimal panel. Although our sampling window limits the findings to late-gestation triage, the analytical platform is readily adaptable to early-pregnancy specimens; longitudinal studies are planned to explore this translational pathway.
This study, focusing on bile acid metabolism and utilizing metabolomics technology, analyzed over 90 bile acid subtypes to comprehensively reflect the differences in bile acid metabolism between patients with pre-eclampsia and healthy pregnant women. While traditional biochemical markers such as TBA and glycocholic acid showed no significant differences between the groups, metabolomics studies revealed distinct differences in bile acid metabolism, demonstrating the technology’s ability to more comprehensively and accurately reflect metabolic characteristics and its unique advantages in biomarker discovery.
However, the study has limitations. It was a case-control study conducted after the diagnosis of pre-eclampsia. Additionally, matching was based only on gestational age and maternal age, not considering other maternal health factors such as BMI40. Moreover, conducted at a single center with a small cohort, the study did not design an independent validation set due to sample size limitations. Therefore, our findings need to be validated in a larger, more diverse population.
Conclusions
We identified 30 bile acid subtypes that were significantly upregulated or downregulated in patients with pre-eclampsia. The highest AUC was 0.879(95%CI[0.782,0.946]) when glycochenodeoxycholic acid was used as a single biomarker. In the multi-factor models, the best combination was the modle including two biomarkers: glycochenodeoxycholic acid and glycodeoxycholic acid-3-O-β-glucuronide.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Duley, L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 33 (3), 130–137 (2009).
Brown, M. A. et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72 (1), 24–43 (2018).
Gestational Hypertension and Preeclampsia. ACOG practice bulletin, number 222. Obstet. Gynecol. 135 (6), e237–e260 (2020).
Moutquin, J. M. et al. A prospective study of blood pressure in pregnancy: prediction of preeclampsia. Am. J. Obstet. Gynecol. 151 (2), 191–196 (1985).
Shi, H., Wang, W., Li, F., Guo, A. & Liu, T. Model construction and application for predicting pre-eclampsia by sonoclot coagulation analyzer. Noncoding RNA Res. 9 (2), 288–293 (2024).
Da Cunha, P. A. et al. Metabolomic Analysis of Plasma from Breast Cancer Patients Using Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry: An Untargeted Study, Metabolites 12(5) (2022).
Rath, S., Hawsawi, Y. M., Alzahrani, F. & Khan, M. I. Epigenetic regulation of inflammation: the metabolomics connection. Semin Cell. Dev. Biol. 154(Pt C), 355–363 (2024).
Selvaraj, S. et al. Metabolomic profiling of the effects of Dapagliflozin in heart failure with reduced ejection fraction: DEFINE-HF. Circulation 146 (11), 808–818 (2022).
Amidfar, M., Askari, G. & Kim, Y. K. Association of metabolic dysfunction with cognitive decline and alzheimer’s disease: A review of metabolomic evidence. Prog Neuropsychopharmacol. Biol. Psychiatry. 128, 110848 (2024).
Masoodi, M. et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 18 (12), 835–856 (2021).
Ali, M. et al. Preeclampsia: A comprehensive review. Clin. Chim. Acta. 563, 119922 (2024).
Ma, Y. et al. Impact of the Immunomodulatory factor soluble B7-H4 in the progress of preeclampsia by inhibiting essential functions of extravillous trophoblast cells. Cells 13(16), 1372 (2024).
Najeeb, M. N. et al. Interleukin-6 (-174G/C), interleukin-1β (-511 C/T), and apolipoprotein B-100 (2488 C/T) gene polymorphism in pre-eclampsia. Medicine (Kaunas) 60(8) (2024).
Staff, A. C. The two-stage placental model of preeclampsia: an update. J. Reprod. Immunol. 134–135, 1–10 (2019).
Bahado-Singh, R. et al. Integrated proteomic and metabolomic prediction of term preeclampsia. Sci. Rep. 7 (1), 16189 (2017).
Chen, T., He, P., Tan, Y. & Xu, D. Biomarker identification and pathway analysis of preeclampsia based on serum metabolomics. Biochem. Biophys. Res. Commun. 485 (1), 119–125 (2017).
Jääskeläinen, T. et al. A non-targeted LC-MS metabolic profiling of pregnancy: longitudinal evidence from healthy and pre-eclamptic pregnancies. Metabolomics 17 (2), 20 (2021).
Sander, K. N. et al. Untargeted analysis of plasma samples from pre-eclamptic women reveals Polar and apolar changes in the metabolome. Metabolomics 15 (12), 157 (2019).
Biomarker Prediction of Preeclampsia With Severe Features, Obstetrics and Gynecology. (2024).
Ql, Y. C., Zou, L. Z., Han, X. Y., Jiang, H. Q. & Li, Y. L. Underlying mechanisms of bile acids in progression of hypertension based on metabolomics and network analysis. Chin. J. Experimental Traditional Med. Formulae. 28 (5), 178–186 (2022).
Kenny, L. C. et al. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension 56 (4), 741–749 (2010).
Deng, W. et al. The association of serum total bile acid with new-onset hypertension during pregnancy. BMC Pregnancy Childbirth. 22 (1), 879 (2022).
Hou, Y., Yun, L., Zhang, L., Lin, J. & Xu, R. A risk factor-based predictive model for new-onset hypertension during pregnancy in Chinese Han women. BMC Cardiovasc. Disord. 20 (1), 155 (2020).
Ramirez Zamudio, A. F., Monrose, E., Pan, S. & Ferrara, L. From pruritus to cholestasis: Building a statistical model and online application to predict a diagnosis prior to bile acid determination. Am. J. Perinatol. 38 (9), 889–996 (2021).
Xiong, X. et al. A chemical derivatization-based pseudotargeted liquid chromatography-tandem mass spectrometry method for sensitive and high coverage determination of bile acids in human serum. Anal. Chim. Acta. 1287, 342119 (2024).
Chen, X. et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 69 (3), 513–522 (2020).
Jin, J. et al. Gut dysbiosis promotes preeclampsia by regulating macrophages and trophoblasts. Circul. Res. 131 (6), 492–506 (2022).
Ridlon, J. M., Kang, D. J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47 (2), 241–259 (2006).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell. Metab. 17 (2), 225–235 (2013).
Staley, C., Weingarden, A. R., Khoruts, A. & Sadowsky, M. J. Interaction of gut microbiota with bile acid metabolism and its influence on disease States. Appl. Microbiol. Biotechnol. 101 (1), 47–64 (2017).
Wahlström, A., Sayin, S. I., Marschall, H. U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell. Metab. 24 (1), 41–50 (2016).
Deng, W. et al. The association of serum total bile acid with new-onset hypertension during pregnancy. BMC Pregnancy Childbirth 22(1) (2022).
Hou, Y., Yun, L., Zhang, L., Lin, J. & Xu, R. A risk factor-based predictive model for new-onset hypertension during pregnancy in Chinese Han women. BMC Cardiovasc. Disord. 20(1) (2020).
Ramirez Zamudio, A. F., Monrose, E., Pan, S. & Ferrara, L. From pruritus to cholestasis: Building a statistical model and online application to predict a diagnosis prior to bile acid determination. Am. J. Perinatol. 38 (9), 889–896 (2021).
Chakraborty, S. et al. Conjugated bile acids are nutritionally re-programmable antihypertensive metabolites. J. Hypertens. 41 (6), 979–994 (2023).
Shi, H. et al. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Circul. Res. 128 (9), 1240–1254 (2021).
Diboun, I. et al. Metabolic profiling of pre-gestational and gestational diabetes mellitus identifies novel predictors of pre-term delivery. J. Transl Med. 18 (1), 366 (2020).
Garcia-Vargas, L., Addison, S. S., Nistala, R., Kurukulasuriya, D. & Sowers, J. R. Gestational diabetes and the offspring: implications in the development of the cardiorenal metabolic syndrome in offspring. Cardiorenal Med. 2 (2), 134–142 (2012).
Hung, S. C. et al. Lysophosphatidylcholine impairs the mitochondria homeostasis leading to trophoblast dysfunction in gestational diabetes mellitus. Antioxidant (Basel) 13(8) (2024).
Wang, Y. et al. The roles of obesity and ASB4 in preeclampsia pathogenesis. Int. J. Mol. Sci. 25(16) (2024).
Poon, L. C. et al. The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 145 (Suppl 1) (2019).
Funding
This work were supported by grants from National Natural Science Foundation of China (No.62071007 and No.21405007) .
Author information
Authors and Affiliations
Contributions
Su Jiaqi wrote the main manuscript text and prepared all figures. Ma Lijuan was responsible for the supervision and the revision of the manuscript. Xiong Xin was responsible for formal analysis, methodology, and validation. Gu Xunke was responsible for data curation and supervision. Wang Yongqing was responsible for funding acquisition and supervision. Jia Keke reviewed and edited the manuscript and applied for the fund.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, J., Ma, L., Xiong, X. et al. Metabonomics of serum bile acids in patients with pre-eclampsia. Sci Rep 15, 34028 (2025). https://doi.org/10.1038/s41598-025-13611-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13611-1