Abstract
Background and purpose
Taxane plus ramucirumab (RAM) is the standard second-line treatment for advanced gastric cancer (AGC). However, data on second-line therapy following first-line treatment with nivolumab remains limited. This study aimed to evaluate the efficacy, safety, and prognostic factors of taxane plus RAM in patients with advanced AGC who previously received chemotherapy plus nivolumab.
Materials and methods
We collected data from AGC patients at multiple institutions who received taxane plus RAM after failure of chemotherapy plus nivolumab. All patients received first-line chemotherapy with nivolumab before December 2022.
Results
In total, 51 patients received taxane plus RAM (41 received paclitaxel plus RAM, and ten received nab-paclitaxel plus RAM). The median age was 70 years (range 41–83), 30 (59%) patients were male, and 45 (89%) had PS 0/1. Fourteen patients (27%) had liver metastases, 30 (59%) had peritoneal dissemination, and 25 (49%) had ascites; 43 patients had measurable lesions, nine (21%) achieved a partial response, and 21 (49%) achieved stable disease. Median progression-free survival was 3.7 months (95% confidence interval (CI) 3.1–5.1) and median overall survival was 8.9 months (95% CI 6.3–11.6). Major adverse events of grade 3 or higher were neutropenia (41%), anemia (10%), febrile neutropenia (8%), proteinuria (6%), and infection (4%). Multivariate analysis showed that ascites was a poor prognostic factor for overall survival.
Conclusion
Taxane plus RAM was effective in patients with AGC who have received prior chemotherapy plus nivolumab. The presence of ascites may be a poor prognostic factor in patients receiving taxane plus RAM.
Similar content being viewed by others
Introduction:
As of 2022, gastric cancer ranked sixth globally in both incidence and mortality among cancers, causing approximately 660,000 deaths annually1. Chemotherapy is the standard treatment for unresectable advanced gastric cancer (AGC). For first-line chemotherapy, combination therapy with platinum-based agents and fluoropyrimidine drugs supplemented with trastuzumab is used in cases where human epidermal growth factor receptor 2 (HER2) amplification is positive2. HER2-negative AGC is treated with a combination of chemotherapy and zolbetuximab if claudin18.2 (CLDN18.2) is positive3,4. If both HER2 and CLDN18.2 expression are negative, chemotherapy alone or in combination with immune checkpoint inhibitors (ICIs) is considered5,6. In cases of treatment failure or intolerance, transitioning to second-line therapy that focuses on taxane-based agents is recommended.
Weekly paclitaxel (PTX) demonstrated efficacy equivalent to that of irinotecan in a randomized phase III trial (WJOG4007 trial), becoming one of the standard second-line treatments for patients with AGC7. Nab-PTX is a 130-nm nanoparticle formulation that links albumin to PTX, rendering it soluble. Weekly nab-PTX showed non-inferiority in overall survival compared with weekly PTX in a randomized phase III trial (ABSOLUTE trial) and reduced hypersensitivity reactions8, establishing it as a standard second-line treatment for patients with AGC. Ramucirumab (RAM) is a human IgG1 monoclonal antibody receptor antagonist designed to bind to the extracellular domain of the vascular endothelial growth factor receptor-2 (VEGFR-2). It binds to the extracellular domain of VEGFR-2, inhibits its binding to multiple VEGF ligands, and suppresses receptor activation9. PTX plus RAM has become the standard second-line treatment for AGC patients, demonstrating superiority over PTX in a randomized phase III trial (RAINBOW trial)10. Additionally, nab-PTX plus RAM showed promising results, with a response rate of 54.8% and a median progression-free survival of 7.6 months in a single-arm phase II trial11, establishing it as a second-line therapy option.
Despite their promising findings, these trials focused on patients with AGC who did not receive ICIs as part of their first-line treatment, and data on the efficacy and safety of taxane plus RAM for patients with AGC who received chemotherapy and ICIs as first-line treatment are limited. This retrospective study evaluated the efficacy and safety of taxane plus RAM as a second-line treatment for patients with AGC who previously received combination chemotherapy and ICIs.
Materials and methods
This was a multicenter retrospective study. We collected data from patients with AGC who received taxane plus RAM after the failure of chemotherapy plus nivolumab at the Himeji Red Cross Hospital, Kobe City Medical Center General Hospital, Kansai Medical University Hospital, and Ichinomiyanishi Hospital. All patients received chemotherapy plus nivolumab as a first-line treatment before December 2022. The eligibility criteria for this study were unresectable or advanced recurrent gastric cancer with histologically confirmed adenocarcinoma that was refractory to chemotherapy plus nivolumab as a first-line treatment, and patients receiving PTX or nab-PTX plus RAM as second-line therapy. The exclusion criteria were a history of treatment with PTX-, nab-PTX-, or RAM-containing regimens, or patients with other advanced cancers requiring systemic or surgical treatment. Under Japan’s clinical research regulations, the opt-out approach is permitted for retrospective observational studies and the need for informed consent to participate was waived by the Ethics Committee of Kansai Medical University. Informed consent was not applicable to this study, and an opt-out approach was adopted to ensure the opportunity for patients to refuse participation. This study was approved by the Ethics Committee of Kansai Medical University (approval no. 2023033) and was conducted in accordance with the principles of the Declaration of Helsinki.
The PTX plus RAM regimen consisted of intravenous administration of 80 mg/m2 PTX over 60 min on days 1, 8, and 15, along with 8 mg/kg RAM intravenously on days 1 and 15 of each 28-day cycle. The nab-PTX plus RAM regimen consisted of the intravenous administration of 100 mg/m2 of nab-PTX over 30 min on days 1, 8, and 15, along with 8 mg/kg of RAM intravenously on days 1 and 15 of each 28-day cycle. We defined cases with ascites observed on CT scan as those with ascites.Based on the study by Nakajima et al10, we classified ascites using CT scans as follows; massive, extending throughout the abdominal cavity; moderate, neither mild nor massive; mild, located only in the upper or lower abdominal cavity; no ascites, ascites not detected by CT scan. Cases with massive or moderate ascites were defined as large amount ascites.An immunohistochemical PD-L1 antibody assay (Dako 28–8) was used to assess PD-L1 expression in formalin-fixed paraffin-embedded tissue samples. Microsatellite instability (MSI) was determined using an MSI-IVD Kit (Falco). In patients with measurable lesions, tumor assessment was performed using imaging according to the Response Evaluation Criteria in Solid Tumors (version 1.1). Adverse events were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Overall survival (OS) was defined as the time from the date of initiation of PTX or nab-PTX plus RAM to the date of death. Living patients were censored at their last follow-up visit. Progression-free survival (PFS) was defined as the duration from the date of initiation of PTX or nab-PTX plus RAM to the date of disease progression or death from any cause. PFS was estimated using the Kaplan–Meier method. Comparisons of clinicopathological backgrounds were conducted using Student’s t-tests for continuous variables and Fisher’s exact tests for categorical variables. Univariate and multivariate analyses were performed using Cox regression models. Age was included as variables in the multivariate analysis. Additionally, taxane type11,12, ECOG PS13,14, the presence of ascites15,16,17 and the effect of chemotherapy plus nivolumab18, which were previously reported as prognostic factors, were incorporated as variables. All statistical analyses were performed using JMP version 17.2.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
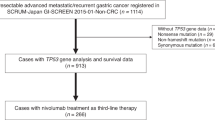
Between July 2017 and December 2022, 98 patients with AGC received chemotherapy plus nivolumab as a first-line treatment at the participating institutions; 83 discontinued first-line treatment, and 55 (66%) received second-line treatment. Among them, four were excluded due to other chemotherapies, and 51 patients who received taxane plus RAM were analyzed (Fig. 1). Patient background information is shown in Table 1. Forty-one patients (80%) received PTX plus RAM and ten patients (20%) received nab-PTX plus RAM. The median patient age was 70 years (range: 41–83); 12% had an ECOG PS of 2 or 3, and 27% had undergone prior gastrectomy. Of all patients, 63% had diffuse-type histology, 27% had liver metastases, 59% had peritoneal dissemination, and 49% had ascites. PD-L1 expression was ≥ 5 in 35%, ≥ l in 57%, unmeasured in 29%, and MSI-high in 4% of patients. The progression-free survival after first-line treatment was 7.1 months, with 45% of patients responding; 96% of patients discontinued treatment due to disease progression, and 4% discontinued treatment due to adverse events. No significant differences were observed in patient backgrounds between the nab-PTX plus RAM and PTX plus RAM groups. Twenty-four patients (47%) began taxane with a reduced dose and no cases began with a reduced dose of ramucirumab. Of these patients, 18 (44%) were on PTX and six (60%) were on nab-PTX (p = 0.4853).
Efficacy
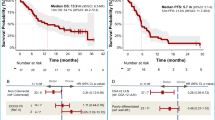
The median observational period was 6.1 months (range: 1.1–27.7). The median PFS and OS were 3.7 months (95% confidence interval [CI] 3.1–5.1 months) and 8.9 months (95% CI 6.3–11.6 months), respectively (Fig. 2). In patients with measurable disease, the overall response rate (ORR) was 21% and the disease control rate (DCR) was 70%. Comparing the PTX plus RAM and nab-PTX plus RAM groups, the median PFS was 3.3 vs. 4.5 months (p = 0.5303), respectively, and the median OS was 9.1 vs. 7.6 months (p = 0.9797) (Fig. 3); the ORR was 29% vs. 0% and DCR was 67% vs. 80% (Table 2). The ORR tended to be lower in the nab-PTX group; however, the DCR, PFS, and OS in this group were comparable to those in the PTX group. Univariate and multivariate analyses of PFS and OS according to Cox proportional hazards models are presented in Table 3. When compared between patients with PS of 0/1 or ≥ 2, PFS was 4.2 months vs. 1.8 months and OS was 9.1 months vs. 3.9 months, respectively (Fig. 4a, b); poor PS was significantly associated with poor PFS in multivariate analysis.
Safety
The proportion of patients with adverse events is shown in Table 4. The major adverse events ≥ grade 3 were neutropenia (41%), anemia (10%), febrile neutropenia (8%), proteinuria (6%), and infection (4%). The nab-PTX plus RAM group showed a trend toward increased neutropenia and febrile neutropenia; however, no other differences were observed. Of the 51 patients, treatment was discontinued in 45, of whom 20 (44%) received third-line therapy. Third-line therapy included irinotecan-containing regimens in eight cases, trifluridine/tipiracil-containing regimens in seven, and other regimens in five.
Relationship of efficacy/safety and ascites
Patient background, efficacy, and safety were compared based on the presence or absence of ascites.In patients with and without ascites,there was a significantly higher incidence of metastases involving two or more organs (81% versus 40%, p = 0.0042) and peritoneal dissemination (77% versus 40%, p = 0.0107). Additionally, cases with low PD-L1 expression were more prevalent (Supplementary Table 1). The frequency of cases starting with a reduced dose of taxane did not differ based on the presence or absence of ascites(54% vs. 40%, p = 0.4043). OS was significantly shorter in patients with ascites (6.9 vs. 10.0 months, p = 0.0284) (Fig. 4c, d), and ascites were significantly associated with poor OS in multivariate analysis. In our study, PFS (2.3 vs 3.2 months) and OS (6.2 vs 6.9 months) tended shorter in patients with large amount ascites than in patients with ascites. An exploratory analysis comparing adverse events based on the presence or absence of ascites revealed a tendency toward higher incidences of neutropenia, anemia, and febrile neutropenia in the group with ascites (Table 5).
Discussion
To the best of our knowledge, this is the first study using real-world data to evaluate the efficacy and safety of taxane plus RAM, including nab-PTX, in patients with AGC after receiving chemotherapy plus nivolumab. Taxane plus RAM after chemotherapy plus nivolumab showed a comparable response rate (21% vs 27%), PFS (3.7 vs 4.4 months), and OS (8.9 vs 9.6 months) to PTX plus RAM in the RAINBOW trial, though the response rate and PFS tended to be slightly lower in the present study19. This may be because 15% of patients in this study had PS ≥ 2 and 50% had ascites, suggesting more patients with worse prognoses were included than in the clinical trials. In addition, adverse events were not significantly different from those in the RAINBOW trial. Overall, taxane plus RAM may be considered a standard second-line therapy for gastric cancer patients who have received chemotherapy plus ICIs as first-line therapy.
Nab-PTX plus RAM has shown efficacy in phase II trials and has become the standard second-line treatment for patients in Japan20. Although no phase III trials have directly compared PTX plus RAM with nab-PTX plus RAM, two retrospective observational studies reported that they were equivalent or that nab-PTX plus RAM had better outcomes13,14. A randomized phase II trial was conducted to compare PTX plus RAM therapy with nab-PTX plus RAM as second-line therapy for gastric cancer with peritoneal dissemination. Patients previously treated with nivolumab were not included in the trial, and the superiority of nab-PTX plus RAM over PTX plus RAM was not demonstrated21.
Our study is the first observational study to evaluate the efficacy and safety of nab-PTX plus RAM in patients receiving chemotherapy plus nivolumab as first-line therapy. In our study, the objective ORR for nab-PTX plus RAM was 0%, which was lower than the 54.8% ORR previously reported in phase 2 trials20. However, the DCR was 80%, which is comparable to previous findings. In a randomised phase 2 trial in Japan focusing on cases with peritoneal metastasis, an ORR of 20% and a DCR of 56.7% were reported21. The lower ORR observed in our study, which had only 10 cases in the nab-PTX group, and the higher prevalence of peritoneal metastasis, likely influenced these results. Based on our study, there were no significant differences in efficacy and adverse events between PTX plus RAM and nab-PTX plus RAM, suggesting that both PTX plus RAM and nab-PTX plus RAM could be considered as treatment options as second-line therapies for AGC patients who received chemotherapy plus ICIs.
In other cancers, the administration of cytotoxic anticancer drugs after anti-PD-1 antibody therapy enhances the therapeutic effect22,23,24,25,26. Preclinical studies have reported prolonged binding of nivolumab with T cells even after administration cessation, suggesting a potential reason why chemotherapy administered after ICI therapy may enhance efficacy27. RAM is an antibody–drug against VEGFR-2; inhibition of the VEGF pathway has been reported to reduce the number of immunosuppressive cells, including forkhead box P3 + CD25 + regulatory T cells and tumor-associated macrophages, as well as enhancing the antitumor activity of PD-1 inhibitors28,29,30. Sasaki et al. reported the potential effectiveness of a taxane plus RAM following the administration of ICIs31; in contrast, Jeon et al. reported insufficient efficacy compared with the RAINBOW trial32. Our study showed results similar to those of the RAINBOW trial, suggesting the need for further large-scale observational and prospective interventional studies.
In the RAINBOW trial, HER2 positivity was shown to be a favorable prognostic factor for PTX plus RAM33. However, in our study, all cases were HER2-negative. We performed univariate and multivariate analyses of PFS and OS with respect to age, PS, efficacy of chemotherapy plus nivolumab, presence of ascites, and taxane type. Ascites was identified as a significant adverse prognostic factor for OS in both univariate and multivariate analyses; additionally, its relationship with PFS also showed a detrimental trend, although not statistically significant. The presence of malignant ascites in GC is known to be associated with a poor prognosis15,16,17, however, in the RAINBOW trial, no data were available for patients with ascites. Data on its effect in the context of second-line treatment are extremely limited.Matsumoto et al. reported that large amounts of ascites was poor prognostic factor in patients who received PTX plus RAM34. The results of this study suggest that the presence of ascites may be a poor prognostic factor even in patients with advanced gastric cancer who have previously received chemotherapy and nivolumab.
Our study had some limitations, including the small number of enrolled patients which means that subgroup and multivariate analyses are also exploratory, potential inadequacy in analyzing OS for taxane plus RAM due to a relatively short observation period, its retrospective observational nature rather than an interventional study design, and lack of analysis of biomarkers. However, this study represents the most comprehensive research to date on taxane plus RAM in patients with AGC who received combination chemotherapy and ICIs. In conclusion, taxane plus RAM showed modest efficacy and safety in patients with AGC who received combination chemotherapy and ICIs as first-line treatment, irrespective of their response to primary therapy. There is the potential for reduced efficacy in cases of ascites, necessitating careful consideration and monitoring.
Data availability
The datasets generated and/or analyzed in the present study are available from the corresponding author upon reasonable request.
Abbreviations
- AGC:
-
Advanced gastric cancer
- CLDN:
-
18.2: Claudin18.2
- ECOG:
-
Eastern Cooperative Oncology Group
- HER2:
-
Human epidermal growth factor receptor 2
- ICIs:
-
Immune checkpoint inhibitors
- MSI:
-
Microsatellite instability
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PS:
-
Performance status
- PTX:
-
Paclitaxel
- RAM:
-
Ramucirumab
- VGFR-2:
-
Vascular endothelial growth factor receptor-2
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3), 209–249 (2021).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Shitara, K. et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet. 401(10389), 1655–68. https://doi.org/10.1016/S0140-6736(23)00620-7 (2023).
Shah, M. A. et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 29, 2133–2141. https://doi.org/10.1038/s41591-023-02465-7 (2023).
Janjigian, Y. Y. et al. First-line nivolumab pluschemotherapy versus chemotherapy alone for advanced gastric,gastro-oesophageal junction, and oesophageal adenocarcinoma(CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398(10294), 27–40 (2021).
Rha, S. Y. et al. KEYNOTE-859 investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 24(11), 1181–1195. https://doi.org/10.1016/S1470-2045(23)00515-6 (2023).
Hironaka, S. et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 31(35), 4438–4444 (2013).
Shitara, K. et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label,randomized, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 277–287 (2017).
Spratlin, J. L. et al. Phase I pharmacologic and biologic study of ramucirumab (IMC1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. 28, 780–787 (2010).
Eguchi Nakajima, T. et al. Randomized phase II/III study of 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer 23(4), 677–688 (2020).
Nakasya, A. et al. Nanoparticle albumin-bound paclitaxel and ramucirumab versus paclitaxel and ramucirumab as second-line chemotherapy for unresectable advanced or recurrent gastric cancer: A multicenter, propensity score-matched analysis (CROSS SELL study). Int. J. Clin. Oncol. 27(4), 684–694 (2022).
Chau, I. et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J. Clin. Oncol. 22(12), 2395–2403 (2004).
Takahari, D. et al. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer 20(5), 757–763 (2017).
Fang, N. et al. Clinicopathological characteristics and prognosis of gastric cancer with malignant ascites. Tumor Biol. 35(4), 3261–3268 (2014).
Honda, M. et al. An ascites grading system for predicting the prognosis of gastric cancer with peritoneum dissemination. Ann. Gastroenterol Surg. 4(6), 660–666 (2020).
Iwasa, S. et al. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer 15(1), 21–26 (2012).
Okunaka, M. et al. Retrospective cohort study of nanoparticle albumin-bound paclitaxel plus ramucirumab versus paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. BMC Cancer 20(1), 1111 (2020).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (rainbow): A double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–1235 (2014).
Bando, H. et al. A phase II study of nab-paclitaxel in combination with ramucirmub in patients with previously treated advanced gastric cancer. Eur. J. Cancer. 91, 86–91 (2018).
Hirata K, Hamamoto Y, Shoji H, et al. A randomized phase II trial of paclitaxel plus ramucirumab versus nab-paclitaxel plus ramucirumab for gastric cancer with peritoneal dissemination refractory to first-line therapy (WJOG10617G/P-SELECT). J Clin Oncol 2022;40:suppl #280
Shiono, A. et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 10, 775–781 (2019).
Park, S. E. et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol 13, 106–111 (2018).
Schvartsman, G. et al. Response rates to singleagent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 112, 90–95 (2017).
Harada, D. et al. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res 39, 4987–4993 (2019).
Reck, M. et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 37, 537–546 (2019).
Osa, A. et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight 3, e59125. https://doi.org/10.1172/jci.insight.59125 (2018).
Sachdev, J. C. et al. Safety, pharmacodynamic, and pharmacokinetic profile of TSR-042, an anti–PD–1 monoclonal antibody, in patients (PTS) with advanced solid tumors. Ann. Oncol. 28, v420–v427 (2017).
Chen, C.-W. et al. FRI-471-Regorafenib may enhance efficacy of anti-program cell death-1 therapy in hepatocellular carcinoma through modulation of macrophage polarization. J. Hepatol. 70, e605–e606 (2019).
Kato, Y. et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 14, e0212513 (2019).
Sasaki, A. et al. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open 4, e000775 (2020).
Jeon, Y. et al. The role of ramucirumab plus paclitaxel as second-line therapy after failure of nivolumab plus doublet chemotherapy in patients with advanced gastric cancer. J. Gastrointest Oncol. 14(6), 2346–2353 (2023).
De Vita, F. et al. Ramucirumab and paclitaxel in patients with gastric cancer and prior trastuzumab: Subgroup analysis from RAINBOW study. Future Oncol. 15(23), 2723–2731 (2019).
Matsumoto, H. et al. A retrospective study of the safety and efficacy of paclitaxel plus ramucirumab in patients with advanced or recurrent gastric cancer with ascites. BMC Cancer 18, 120. https://doi.org/10.1186/s12885-018-4057-7 (2018).
Acknowledgements
We gratefully thank the participants of this study and their families as well as the study investigators and their teams for their contributions to the publication of this study.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception or design. TM, TI and TT were involved in data acquisition. TM wrote the original draft of the manuscript. All the authors reviewed and approved the final version of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interests
Toshihiko Matsumoto received research funding from Ono Pharmaceutical Co, Ltd and Sanofi Co, Ltd; honoraria from Bayer Co, Ltd, Bristol-Myers Squibb Co, Ltd, Chugai Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Eli Lilly Japan Co, Ltd, Merck Bio Pharma Co, Ltd, MSD Co, Ltd, Ono Pharmaceutical Co, Ltd, Sanofi Co, Ltd, Taiho Pharmaceutical Co, Ltd, Takeda Co, Ltd, Teijin Pharmaceutical Co, Ltd and Yakult Honsha Co, Ltd. Hironaga Satake received lecture fees from Chugai, Takeda, and Merck Biopharma. All other authors have declared no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matsumoto, T., Ikoma, T., Makiyama, C. et al. Efficacy and safety of taxane plus ramucirumab for advanced gastric cancer after chemotherapy plus nivolumab. Sci Rep 15, 28841 (2025). https://doi.org/10.1038/s41598-025-13714-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13714-9