Abstract
Social and physical environment can largely influence behavioral phenotypes in fish. Additionally, developmental environment may not only influence the behavior of an individual, but also that of its offspring. The objective of this study was to investigate the effects of parental social experience (social housing or chronic isolation) on offspring feeding behavior, growth performance, and intestinal health when fed fishmeal-based or soybean meal-based diets. Offspring from zebrafish raised in chronic isolation (CIO) and offspring from zebrafish raised in social housing (SHO) were randomly assigned to 3.0 L tanks with 25 fish per tank. Each tank was randomly selected to be fed either a fishmeal-based (FM) or soybean meal-based (SBM) diet. There were three replicate tanks (n = 3) for each of the four treatment groups (SHOF, SHOS, CIOF, CIOS) with a total of 12 tanks. At the end of the experiment, the offspring of the chronically isolated parents had grown significantly more than the offspring of the socially housed parents regardless of diet type. However, the SHO fish consumed more as a percent of tank biomass during the feed intake tests when compared to the CIO fish of the same diet type. Fish fed with a SBM-based diet had higher numerical expression of inflammation-related genes in the gut in both offspring origin groups suggesting some inflammatory response to the diet. Overall, it appears that parental social experience had an influence on the growth performance of offspring, but the mechanisms remain unclear.
Similar content being viewed by others
Introduction
Environment can largely influence patterns of behavior in fish1,2,3,4,5,6. This is especially true in early development; an individual is particularly sensitive to external sensory input and the brain is most prone to changes induced by the environment during early life stages7,8,9. Both physical1,5,8,10,11 and social2,4,7,9,12,13,14,15 environment has been shown to cause variation in behavioral phenotypes across all life stages of fish.
Social experience impacts a variety of behaviors in fish as individuals learn from and are influenced by the actions of conspecifics2,14,15,16,17. While isolation is often thought to be stressful for social animals, several studies have found that social isolation can reduce stress18,19,20,21, anxiety-related behaviors4,22 and aggression2. Additionally, developmental environment may not only influence the behavior of the individual, but also that of its offspring2,3,5. Studies have found that fish may transfer information about their environment to offspring, resulting in transmission of behavioral adaptation to the next generation2,3,5. For example, a study of isolated versus socially housed zebrafish (Danio rerio) found that those raised in social isolation from fertilization to reproduction had reduced aggression-related behaviors. This behavioral trait was also observed in the next generation, even when the offspring were raised in social groups2. While there may be complex interactions between genetics and the environment on the expression of a given trait1, this suggests some role of parental social context on the behavioral phenotypes of their progeny2.
Studies surrounding the influence of social context on the epigenetic factors of behavior are increasing2,23, but little is known on how parental social experience affects nutrition-related behavior in the offspring. If physical and social environment can have generational effects on behavior2,3,5, there may also be a significant effect on foraging behavior and thus nutrient uptake24. Nutrition is a critical component in any animal study as there may be consequences related to growth, reproduction, and overall health25. Additionally, feed intake and utilization are highly prioritized in fish culture26, and zebrafish may serve as a model for further improvement of husbandry and dietary practices in aquaculture27. The heritability of feed intake has previously been reported in multiple aquaculture species including channel catfish (Ictalurus punctatus)28, sea bass (Dicentrarchus labrax)29, Nile tilapia (Oreochromis niloticus)30, and Atlantic salmon (Salmo salar)31. While heritability of feed intake was not explicitly examined, Oswald and Robinson24 found significant strain variations in feed intake and specific growth rate in zebrafish. The two genetic lines that were derived from wild populations consumed significantly less than the two genetic lines maintained in the laboratory for over 30 generations. This may be a result of adaptation to captivity leading to behavioral changes influenced by both environmental and genetic factors24.
The heritability of feed intake may be particularly important in improving utilization of alternative diets, specifically those with plant-based protein sources. Soybean meal (SBM), commonly used as a protein replacement for fishmeal (FM), has a high protein content and is more sustainable and cost effective than FM26,27,28,29,30,31,32,33,34,35,36. However, SBM has also been shown to negatively influence feed ingestion, growth rate, and intestinal health due to the presence of anti-nutritional factors37,38. Anti-nutritional factors have been regarded as a causative agent leading to gut dysfunction, poor feed utilization, and immune system modulation in fish39. Improving SBM utilization via methods of mitigating SBM-induced intestinal inflammation has been investigated extensively over time.
Evidence of transgenerational improvement in utilization of a plant-based diet can be seen in a study of zebrafish offspring from broodstock that were fed a SBM diet. The offspring showed a strong trend of improved growth performance when fed the same SBM diet40. This combined with the influence of parental social experience on the behavior of offspring2 and strain specific feed intake24 suggest that the housing conditions, diet, and feeding behavior of broodstock could have an additive effect and strongly influence feed intake and feed utilization in their offspring. Because social isolation has been found to reduce stress, anxiety, and aggression in zebrafish, it may be a useful tactic to select for broodstock, creating a genetic line of improved feed intake and utilization of a plant-based diet. The objective of this study was to evaluate the effects of parental social experience on offspring 1) feed intake and feed utilization, 2) appetite, and 3) intestinal health of zebrafish fed FM- and SBM-based diets.
Results
Growth performance and feed utilization
The initial weight of fish (n = 30) at 21 days-post-fertilization (dpf) was significantly higher in the offspring of parents raised in chronic isolation (CIO) compared to the offspring of parents raised in social housing (SHO; t58 = 3.83, p < 0.001; Fig. 1). A similar trend was observed in the initial total length with CIO individuals having a greater total length compared to SHO (t58 = 3.11, p = 0.003).
Representative initial (21 days-post-fertilization) weights of offspring from parents raised in chronic isolation (CIO) and offspring of parents raised in social housing (SHO; n = 30). Error bars represent 95% confidence interval. The bracket with asterisk (*) indicates significant difference between groups (p < 0.001).
Final growth performance and survival results are presented in Table 1. Statistical analyses were only performed on survival, final individual weight, specific growth rate, and feed conversion ratio to avoid redundant analyses. Statistical values for two-way ANOVA are detailed in Table 2. There were no significant differences in survival or feed conversion ratio (FCR) between treatment groups. Mean final (53 dpf) individual weight was significantly affected by offspring origin – the CIO fish were larger than the SHO fish. There was no effect of diet type on final weight. Numerically, the CIO fed fishmeal (CIOF) group had the greatest final weight and absolute weight gain followed by CIO fed soybean meal (CIOS), SHO fed fishmeal (SHOF), and then SHO fed soybean meal (SHOS).
Due to the differences in initial weight between CIO and SHO fish, specific growth rate (SGR) was also examined. There was a significant effect of offspring origin on SGR: the SHO fish had a higher SGR than the CIO fish. The same trend was seen in numerical values of overall percent weight gain.
Feed intake
Initial and final feed intake measured as a percent of biomass are presented in Fig. 2. Three-way mixed ANOVA was used to evaluate the effects of offspring origin, diet type, and time on satiation feed intake tests. The three-way interaction between offspring origin, diet type, and time was not significant (F1,8 = 0.04, p = 0.84, η2 = 0.005). Two-way ANOVA was used to evaluate the Offspring x Time and Diet x Time interactions. There was a significant effect of offspring origin on the initial feed intake test (F1,10 = 19.8, p = 0.001, η2 = 0.665) with the SHO fish consuming more as a percent of biomass than the CIO fish. Offspring origin did not have a significant effect on the final feed intake test (p = 0.15). Diet type did not significantly affect initial feed intake (p = 0.27) but it did significantly affect the final feed intake test (F1,10 = 8.33, p = 0.016, η2 = 0.455). Full statistical details can be found in the Supplementary Data (S1).
Gene expression
Statistical details for relative gene expression in the intestinal tissue are detailed in Table 3.
Growth-related genes growth hormone receptor a (ghra), growth hormone receptor b (ghrb), and insulin-like growth factor 1 (igf1a) expressed in the gut were analyzed (Table 3, Fig. 3). The offspring origin by diet interaction was not significant for any of the growth-related genes. There was a significant effect of offspring origin on the expression of ghra (p < 0.005). The SHO fish had a higher expression of ghra relative to the CIO fish. No significant effects were observed in the relative expression of ghrb or igf1a between treatment groups.
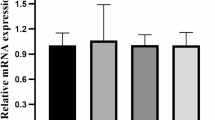
Appetite-related genes cholecystokinin (cck), ghrelin, and leptin were also examined (Table 3, Fig. 4). There were no significant differences in the relative expression of these genes.
Inflammation-related genes interleukin 1 beta (il-1b), matrix metallopeptidase 9 (mmp9), and tumor necrosis factor a (tnfa) were examined (Table 3, Fig. 5). There were no significant differences in the relative expression of any of these genes (p > 0.05).
Histological analysis
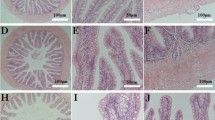
Intestinal villi length, villi width, and villi length-to-width ratio were examined to investigate intestinal morphology (Table 4). Multivariate analysis of variance revealed no significant effect of offspring origin, diet type, or treatment interaction on intestinal morphology (Table 5).
Discussion
Historically, selective breeding has been used to improve livestock production and in recent decades, it has also become an area of interest in the aquaculture industry. Improving feeding efficiency through selective breeding could help to improve production while also reducing waste and production costs in the process41. It has previously been shown that broodstock social and physical environment can influence the behavioral phenotypes of offspring2,3,5 and heritability of feed intake and feed utilization have been found in several aquaculture species28,29,30,31. Increasing feed intake by altering the social environment may be a useful tactic in selecting for broodstock that produces similarly efficient offspring.
Growth performance
Although conditions were kept consistent between the separate common gardens, the offspring in the CIO group weighed significantly more than the offspring in the SHO group at the start of the experiment. Broodstock nutrition and dietary intake can significantly affect breeding success and offspring development42,43,44. Reproduction is energetically costly; nutrition is critical in reproductive performance and nutrient availability can affect gonadal development and the production of gametes45,46. The parental CI zebrafish had significantly higher feed intake than the SH fish on average47, potentially leading CI parents to be better nutritionally equipped for breeding success. A study of zebrafish that were fed either 60 mg or 5 mg of food per day, found those with increased food availability were more likely to breed successfully and produce higher quality eggs44. Additionally, the progeny of the 60 mg group had a significantly higher survival rate by 18 dpf compared to those in the 5 mg group44. Similarly, studies of female plaice (Pleuronectes platessa)48 and yellowtail (Seriola quinqueradiata)49 found that those fed a low ration or high restriction feeding rate had delayed ovarian development and reduced fecundity when compared to those fed a high ration or satiation feeding rate.
Stress may have influenced offspring size as it has been shown to negatively affect broodstock feeding efficiency, growth performance, and reproduction50,51. In a study of zebrafish that were exposed to increasing levels of stress in the form of net chasing, females that experienced no net chasing had higher final weights, lower FCR, improved reproductive performance, and better offspring survival51. Social stress and crowding may also negatively affect reproduction. A study of damselfish (Pomacentrus amboinensis) found that females housed at low densities had the lowest cortisol levels and produced the largest larvae50. Group housing of the SH parents in the present study may have altered reproductive performance due to social stress, however, stress levels of the broodstock were not specifically measured47.
Growth performance was evaluated in several ways to account for the difference in average initial weights between treatment groups. The CIO fish had a higher final weight and absolute weight gain compared to the SHO fish. When measured as percent weight gain and SGR, the SHO fish grew more in terms of daily and overall percent weight gain compared to the CIO fish. It is important to note, however, that percent gain and SGR have limited application in fish feeding experiments where the fish have differing initial weights. This is due to the influence of fish life stage and size on percent gain and SGR52,53,54. There is a gradual decrease in percent gain and SGR with increasing body size53. The larger initial weight of the CIO fish may explain the lower percent gain and SGR in comparison to the SHO fish.
Body size is a critical trait affecting many physiological aspects of fish performance including survival and reproduction55,56,57. Past studies have found that increased fish body size is positively correlated with egg size and yolk sac volume of the eggs they produce, as well as offspring survival55,57,58. While there were no significant differences in the growth performance of SH and CI parents, the production of larger offspring by chronically isolated parents may aid in developing a lineage of fish with increased body size and improved fitness.
Growth is regulated through complex endocrine pathways involving multiple hormones and organs59. Additionally, physiological processes like growth and energy mobilization that are mediated by the growth hormone (GH) axis are highly responsive to external stimuli59,60. Genes involved in the GH axis were examined, namely GH receptors ghra and ghrb, and insulin-like growth factor igf1a. There was a significant effect of offspring origin on the expression of ghra – the SHO fish had an elevated relative expression of ghra compared to the CIO fish. Interestingly, a study of juvenile rainbow trout found differential expression of ghr subtypes dependent on social status61. Dominant trout showed higher relative transcript abundance of ghra in the liver and muscle whereas subordinate trout displayed increased ghrb expression61. The increased expression of ghra in SHO fish may therefore be a result of differing social status between treatment groups.
Nutritional state, feed intake, and diet composition can also affect expression of growth-related hormones59,60. In a study investigating overexpression of GH through the use of transgenic zebrafish, GH-transgenic fish exhibited significantly higher feed intake and a higher expression of ghrb and igf1a whereas ghra was not differentially expressed between the transgenic and non-transgenic individuals59. Fasting and re-feeding has also been shown to influence ghr expression62. A study of juvenile zebrafish found ghra expression significantly increased throughout the fasting experiment while ghrb decreased in 3–6 h after feeding and increased again 12 h after feeding62.
Although not significant, the relative expression of ghra was higher in SBM-fed fish and the relative expression of ghrb was higher in FM-fed fish. This may suggest a potential effect of dietary protein on expression of the different growth hormone receptors. It is important to note that in the present study, samples for gene expression were taken after zebrafish were fasted overnight. While the SHOF fish had the highest final feed intake, higher ghra, and numerically highest relative expression of igf1a, they exhibited lower weight gain than both CIOF and CIOS fish. These results reiterate the complex interactions involved in endocrine growth regulation.
Feed utilization
The larger initial size and absolute weight gain of the CIO fish may have been a result of inherited behavioral phenotype. Past studies have found that feed intake exhibits genetic components and is responsive to selective breeding28. Broodstock diet can also have profound effects on offspring nutrition and growth63. Nutritional programming is a tactic that involves brief exposure of an individual to a dietary component to improve utilization of that ingredient when the individual encounters it later in life40,63. The effects of nutritional programming have been explored in several fish species40,63,64,65,66,67,68. Additionally, nutritional programming of broodstock with a plant-based diet has previously been shown to improve feed utilization and growth performance of offspring40,63,68.
While both broodstocks were exposed to the same diets for two weeks each, the CI parents consumed significantly more than the SH parents47. Perhaps increased CI consumption and exposure to these diets aided in production of offspring that were better able to utilize both FM- and SBM-based diets. While not significant, the FCR values possibly suggested the CIOF fish had the highest conversion efficiency and the CIOS fish had the lowest. However, it is important to note that the fish were not weighed weekly and with only one midpoint weighing the calculated daily feed portions may not have represented the true percentage of biomass in each tank as growth rates varied. The significant difference in growth performance suggests a reasonable ability to convert the food to biomass.
While there was a significant effect of parental social housing treatment on the initial feed intake test and numerically SHO fish consumed more than the CIO fish during both feed intake tests, this seemingly increased appetite and subsequent feed intake did not translate to improved growth performance. Relative expression of appetite-related genes was analyzed to further investigate the differences in feed intake observed; no significant differences in relative expression of these genes were detected. However, the SHOS fish had the numerically highest relative expression of leptin followed by CIOS, SHOF, and finally CIOF. Leptin has roles in both regulation of feed intake and control of body weight69,70. Increased leptin levels are typically associated with decreased feed intake and increased energy expenditure70. During the final feed intake test, the SBM groups (SHOS and CIOS) consumed significantly less than the FM groups. The numerically higher expression of leptin in these two groups could suggest rising satiety signaling in the SBM groups leading to lower feed intake. Interestingly, leptin has also been associated with innate immune response regulation in rodents71,72, and thus it is possible that the higher relative expression of leptin in the SBM fed groups is linked to a diet-induced inflammatory response.
An individual’s environment can cause alterations in gene activity via epigenetic mechanisms, resulting in altered phenotypic responses to environmental stimuli73,74. The increased feed intake by the CIO parents47 may have been a result of epigenetic changes; social environment may have altered behavior of the parental fish to increase feed intake17,75. It is possible that parents have a “programming” effect on offspring, influencing progeny to inhabit an environment similar to the one they experienced as juveniles. This can potentially aid in offspring behavioral adaptation to that environment6. While the CIO fish grew significantly more, they consumed less than the SHO fish when challenged with feed intake tests. The combination of improved growth performance and lower feed intake may indicate improved dietary utilization potentially as a result of parental adaptation and transgenerational effects. This may be particularly true for the CIOF group that exhibited the highest absolute weight gain and numerically lowest FCR.
Intestinal health
Interleukin-1beta (il-1b) and tumor necrosis factor alpha (tnfa) are pro-inflammatory cytokines that are important for modulating inflammatory reactions through innate and adaptive immune responses76,77. These cytokines induce matrix metalloproteinases (mmp9) that aid in extracellular breakdown and cellular remodeling76,78. Upregulation of these inflammatory genes is often observed in fish fed SBM-based diets76,77,78,79,80,81, however, the present study found no significant effects of treatment on the relative expression of these genes. Further, there were no significant differences in the morphometric measurements of intestinal villi. Slightly reduced villi length-to-width ratio in the SBM-fed groups compared to the FM-fed groups may indicate the onset of tissue swelling, and thus reduced surface area, in response to the SBM diet. The numerical trend of intestinal villi length-to-width ratio measurement was also observed in FCR results (CIOF < SHOF < SHOS < CIOS). Although not significant, the potential for reduced surface area within the intestines of the SHOS and CIOS could provide an explanation for the increased feed conversion ratio as intestinal inflammation interferes with digestion and nutrient absorptio 64,82.
A study investigating the use of plant-based proteins in fish diets found that inclusion of these proteins induced genome-wide epigenetic alterations in the mid-intestines of zebrafish after a 46-day feeding trial83. The zebrafish exhibited epigenetic changes involving neurogenesis, cell adhesion, and immune-related genes; Dhanasiri et al. 83 suggest potential for plant-based diets to promote regulation of genes involved in the innate and adaptive immune system to maintain intestinal integrity and prevent the development of severe enteritis. The zebrafish in our study were fed experimental diets for 31 days; it is possible that in addition to any parental exposure to SBM, offspring experienced further epigenetic changes in response to fishmeal-based or plant protein-based diets. Thus, parental social experience may not be the only factor influencing behavioral phenotype changes and diet may play a strong role in feeding behavior and feed utilization83,84.
There appears to be some effect of parental social experience on the growth performance of juvenile zebrafish. The CIO fish were significantly larger at the start of the experiment and had significantly higher final weight regardless of diet. This difference may be a result of the significantly higher feed intake observed in the CI parents leading to better nutritionally equipped offspring. Gene expression and histological analysis provided limited context for the differences observed. This may be a result of small sample size, a constraint of laboratory animal studies, and complex interactions of endocrine regulations on growth, appetite, and immune response. Further investigation into the epigenetic changes caused by social experience and feed intake in the first generation may help to explain the mechanisms behind the growth performance in the second generation.
Methods
Animal husbandry
The experiment was conducted in the Center for Fisheries, Aquaculture, and Aquatic Sciences at Southern Illinois University-Carbondale (SIUC), IL. All procedures were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of SIUC. The SIUC Institutional Animal Care and Use Committee approved all protocols utilized during experimentation (Protocol # 21-006). Involved researchers were trained in accordance with SIUC Institutional Animal Care and Use Committee requirements. The study was also in accordance with ARRIVE guidelines. All efforts were made to minimize pain, stress, and discomfort in the experimental animals. Zebrafish were observed daily for signs of distress and discomfort and no fish had to be euthanized prior to experimental termination.
Zebrafish were housed in 3.0 L polycarbonate aquaria on a recirculated aquaculture system (Pentair Aquatic Ecosystems, Cary, NC, USA) that was modified to be an open system with continuous flow-through of city water (Carbondale, IL, USA). The system was altered to prevent olfactory cues or hormonal signaling between tanks through shared system water4. All tanks were exposed to the same incoming water. City water was filtered through a carbon filter, aerated, and heated to 26.69 ± 0.47 °C. Water pH and conductivity were monitored daily through the system and averaged 7.66 ± 0.03 and 191 ± 6.48 µS, respectively. Automatic lights were used to create a 10:14 photoperiod with lights turning on at 08:00 and off at 18:00. The illumination provided was at 245 lx and the distance between the surface of the water and the light source was 10 cm.
Feed preparation
The experimental diets were prepared at Southern Illinois University – Carbondale (SIUC; Table 6). Two experimental diets were investigated: a SBM-based diet with SBM fully replacing fishmeal (FM) inclusion, and a FM based diet. Both diets were formulated to be isonitrogenous (36% crude protein) and isolipidic (15% lipid) and to meet all essential nutrient requirements for zebrafish40,85,86. Dietary formulations were based on previous studies of zebrafish40,47,82,87 with some modifications to allow for higher SBM inclusion. The dry ingredients were uniformly mixed using a mixer (Farberware, Fairfield, CA). Water and oil components were added slowly to the mixture until all ingredients were homogenous. The mixture was then extruded (Caleva Extruder 20, Sturminster Newton Dorset, England), and freeze-dried (Labconco FreeZone 6, Kansas City, MO) to remove moisture. The diets were sieved to varying particle sizes and particle size of feed was adjusted based on fish gape size throughout the study. Particle sizes ranged between 150 and 355 µm.
Broodstock
The broodstock zebrafish were spawned from the SIU wild-type stock and raised in a common garden until 20 dpf. Larval fish were fed rotifers (Brachionus plicatilis) and Artemia nauplii before being trained on a commercial FM-based dry feed (Otohime, Japan) prior to separation into either chronic isolation (CI) or social housing (SH) treatments for two months. From 20 to 87 dpf, CI parental fish were housed 1 fish in 1.5 L tanks and SH parental fish were housed 6 fish in 9 L tanks to maintain a density of 1 fish per 1.5 L. To avoid any mortalities and for successful transition from live food to dry diet CI and SH fish were first fed commercial feed for 15 days (20–35 dpf). All fish were then transitioned to a SBM-based diet for the following 15 days (35–50 dpf) to investigate plant-based feed utilization under differing social treatments (CI and SH)32. Fish that were not sacrificed for analyses were raised for use as broodstock for the present study. After 50 dpf, broodstock fish were returned to the commercial FM-based diet regime to ensure proper gonadal growth and maturation. From experimental separation through spawning, fish were fed ad libitum three times per day (08:00–09:00, 12:00–13:00, and 16:00–17:00). For more details regarding the larval rearing, feeding regimes, and social treatment applied to the broodstock, please see Dissinger et al. 47.
Prior to spawning, CI fish were briefly combined for 48 h and then re-separated into isolation. SH males and females were separated into two tanks. This was done to prepare fish for breeding. Additionally, parental fish were fed Artemia nauplii along with commercial diet for a week before spawning for nutritional enrichment. From both treatment groups, three females and four males were used as broodstock; this sex ratio was a result of selection of broodstock prior to sexual maturation. Broodstock fish were combined into separate 9.0 L tanks (per social treatment) at 1700 and remained together overnight until approximately 0900 after spawning behavior was observed. A mesh insert was used to protect the fertilized eggs from the adults.
Larval fish
Offspring from socially housed parents (SHO) and offspring of chronically isolated parents (CIO) were raised in two separate 9.0 L common garden tanks from fertilization to 21dpf. Although the CIO and SHO fish were separated by offspring origin, fish were raised under the same conditions. Larval fish were fed in abundance with rotifers (Brachionus plicatilis) from 5 to 10 dpf, and then fed a combination of rotifers and Artemia nauplii from 10 to 14 dpf. Fish were transitioned to Artemia only at 14 dpf and trained on commercial dry feed (Otohime, Japan) from 18 dpf to experiment start (21 dpf).
Experimental design and feeding regime
The feeding trial was carried out over a month-long period, from 21 to 52 dpf, before zebrafish reach sexual maturity to avoid biases derived from sexual dimorphism. The treatment groups consisted of a two-factor design to investigate both offspring origin (socially housed parents or chronically isolated parents) and diet type (FM or SBM). Using a random number generator88, twelve 3.0 L tanks were randomly assigned to one of the four treatment groups in triplicate (n = 3): offspring fish that originated from socially housed parents (SHO) fed a FM-based diet (SHOF; control group), SHO fed a SBM-based diet (SHOS), offspring fish that originated from chronically isolated parents (CIO) fed a FM-based diet (CIOF), and CIO fed a SBM-based diet (CIOS; Fig. 6). Twenty-five fish from the respective common gardens were randomly stocked into each tank one fish at a time while cycling through tanks to ensure randomization; 300 fish in total were used in the experiment. Treatment tanks were treated as the experimental unit (N = 12).
Experimental design displaying the timeline for separation into treatment groups, feeding regime, and sampling schedule (dpf = days post-fertilization, CIOF = chronic isolation offspring fed fishmeal diet, CIOS = chronic isolation offspring fed soybean meal diet, SHOF = social housing offspring fed fishmeal diet, SHOS = social housing offspring fed soybean meal diet).
All tanks were fed at a restricted feeding rate throughout the feeding trial (21 to 52 dpf). The rate was calculated by performing two feed intake tests in the first week of the experiment. The first feed intake test took place approximately four hours after stocking fish into treatment tanks. The second feed intake test was performed on the third day of the study (23 dpf) to account for any stress-related anorexia from the initial stocking of tanks. Satiation feed intake tests were performed by offering an initial small amount of feed to each tank and closely observing feeding behavior. Tanks were provided additional small feed amounts if continued feeding behavior was observed. The test continued until only two fish remained eating or until a 30 min time period elapsed. Vials of feed were weighed before and after the feeding test to calculated the amount consumed. Satiation feed intake was calculated as the amount consumed by each tank as a percent of total biomass. The tank with the lowest intake as a percent of biomass was used to set the restricted feeding rate for all tanks. This was done to control for differences in palatability between diets and to separate dietary preference from feed utilization. Over the duration of the experiment, the restricted feeding rate was reduced to accommodate tanks with feed left uneaten. Thus, the feeding rate throughout the study ranged from 7 to 12.5% of daily estimated biomass. Estimated daily biomass was calculated based on the assumption that feed conversion ratio (FCR) was 1 and daily feed amounts were increased accordingly. One midpoint (37 dpf) weighing was performed to obtain updated biomasses for each tank. These values were used to adjust feed amounts based on the restricted feeding rate. At 52 dpf, another satiation feed intake test was performed to gauge how feed intake varied between treatment groups at the end of the feeding trial.
Sampling
At 21 dpf, 30 samples (n = 30) were randomly selected from both common gardens, euthanized using an ice bath89, patted dry, and measured to obtain initial lengths and weights. One midpoint weighing was performed (37 dpf) to obtain an updated biomass for daily feed calculations. Fish from each tank were netted out, patted dry, and weighed together in water using an analytical scale with a resolution of 0.0001 g (Mettler-Toledo, Columbus OH, USA).
At experimental termination (53 dpf), all zebrafish were euthanized using ice slurry with equal proportion water and ice89. All fish were measured for total length using calipers (General Tools & Instruments, Secaucus, NJ, USA), patted dry, and weighed using an analytical scale with a resolution of 0.0001 g (Mettler-Toledo, Columbus OH, USA). Three fish per treatment tank were randomly selected for gene expression analysis; the digestive tracts of these fish were dissected and stored in RNAlater (Sigma-Aldrich, St. Louis, MO, USA). Five fish per treatment tank were randomly selected for histological analysis. Bodies were dissected at an angle slightly posterior to the anal vent and the anterior portion of the body was stored in formalin.
Survival was calculated by dividing the number of fish remaining by the initial number of fish (25) and multiplying that by 100. Growth performance was measured as individual absolute weight gain, individual percent weight gain, and individual specific growth rate (SGR) using the following equations:
Feed intake was recorded daily and two satiation feed intake tests were performed. Feed intake amounts were calculated based on dry matter (feed intake = amount of feed * (1-moisture content)). Cumulative feed intake was determined by totaling daily feed intake for each tank. Satiation feed intake was calculated as a percent of biomass determined the day of testing. Feed conversion ratio (FCR) was calculated by dividing the cumulative feed intake by total biomass gain (FCR = total feed intake/biomass gained).
Experimenters were not blinded to treatment groups, however analysts were. Analytical samples were labeled as tank numbers rather than treatment and thus gene expression and histological analyses were performed blind.
Gene expression analysis
Three dissected zebrafish digestive tracts per tank (n = 3 tanks per treatment group) were pooled and stored in RNAlater at −20 °C after sampling. Samples were homogenized (PowerGen 1000 Homogenizer, Fisher Scientific, Hampton, NH, USA) and processed using TRIzol Reagent (Ambion, Foster City, CA, USA). RNA was extracted using the On-Column PureLink™ DNase Treatment (PureLink™ RNA Mini Kit and PureLink™ DNase, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Once the RNA was extracted and purified, the nanograms/μl of each RNA sample was obtained using a Qubit Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) and RNA broad range assay kit (Qubit™ RNA Broad Range Assay Kit, Invitrogen™, Waltham, MA, USA). These values were used to reverse transcribe RNA from each sample using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) to obtain 2 µg cDNA from intestinal samples. The RT-reactions were then diluted (1:10) and 4 μl of each diluted cDNA were used in 10 μl quantitative PCR (qPCR) reaction for a reaction concentration of 8 ng.
The qPCR protocol consisted of an initial denaturation step of 95 °C for 2 min, followed by 40 cycles of denaturation for 20 s at 95 °C and annealing/extension for 35 s at 60 °C. Gene expression of each cDNA sample was measured using CFX384 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Genes related to growth, appetite, and inflammation were selected based on previous zebrafish nutrition studies59,64,76,77,78,79,80,81. Primers (Table 7) were obtained from Integrated DNA Technologies (Coralville, IA, USA). Each qPCR reaction mixture (10 µl) consisted of 5 µl of PowerUp™ SYBR™ Green, 0.5 µl each of forward and reverse primers, and 4 µl of sample cDNA. Each reaction was run in technical triplicates and negative controls without sample templates were performed for each primer set. Reaction efficiencies ranged from 97.49 to 108.18%. The specificity of PCR reactions was verified by analysis of melting curves (ramping rates of 0.5 °C/5 s over a temperature range of 65–95 °C). Intra-assay variation ranged from 0.0 to 3.72%. Fluorescence raw data was analyzed by Bio-Rad CFX Manager 2.1 software. The 2ΔΔCt method was used to obtain relative gene expression by normalizing the expression of the target gene to the expression of the reference gene ef1a.
Histological analysis
Slides for histological analysis were prepared at Saffron Scientific Histology Services (Carbondale, IL). Zebrafish samples dissected at an angle slightly posterior to the anal vent to open the body cavity and allow for intestinal fixation in 10% neutral buffered formalin. Samples were moved through increasing concentrations of ethanol until all water was removed from the tissue. Ethanol was then replaced with paraffin wax, infiltrating the tissues. Samples were embedded in a paraffin wax block, sectioned, and stained on the slide. Slides were viewed at 100 × magnification using a microscope (Nikon SMZ1500) and pictures were taken using Nikon Digital Sight. Intestinal villus length and width were measured using NIS Elements software (Nikon Instruments Inc., Melville, NY). Only two fish per tank were used for villi measurements due to the loss of some samples through the fixation process.
Statistical analysis
Sample size calculation was based on a power analysis of final weight measurements from the parental study47 the present study was based on. With a significance criterion of α = 0.05 and power = 0.80, the minimum sample size needed with this effect size is N = 2.31, indicating the need for 3 replicate tanks per treatment group. Aside from initial fish samples for weight, all growth performance and feed utilization metrics represent treatment tank means (n = 3 tanks per group) rather than individual fish. Relative gene expression values are representative of pooled cDNA from three fish per tank (n = 3 tanks per group). Statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria, Version 4.3.1 “Eye Holes”). There were no experimental units excluded from statistical analyses.
An independent samples t-test was used to compare initial weights and total lengths of SHO and CIO samples (n = 30) from the common gardens. Correlation coefficients were evaluated to examine correlation between dependent variables measured (S1). Weak to moderate associations among growth performance metrics and gene expression values were detected. Thus, two-way ANOVA was used to compare survival, final individual weight, specific growth rate (SGR), feed conversion ratio (FCR), and relative gene expression levels (dCt) among treatment groups. Offspring origin by diet treatment interaction was evaluated first. If non-significant, the interaction was dropped from the model and main effects (offspring origin and diet type) were analyzed. A Bonferroni correction factor was applied to grouped datasets: growth performance and gene expression analysis. Effects were considered significant at p < 0.0125 and 0.005, respectively. All data met normality and homogeneity of variance assumptions.
Three-way ANOVA was used to evaluate offspring origin, diet, and time on initial and final satiation feed intake tests. The three-way interaction was not significant, so two-way interactions were investigated. A Bonferroni correction factor was used to evaluate significance (significant at p < 0.025).
Due to the correlations between histological measurements (r = 0.238–0.888), a two-way MANOVA was used. MANOVA results were considered significant at p < 0.05. The data met multivariate and univariate normality assumptions.
Data availability
The datasets generated during and/or analyzed during the current study are available within the article and its supplementary materials
References
Marks, M., West, T. N., Bagatto, B. & Moore, F. B. G. Developmental environment alters conditional aggression in zebrafish. Copeia 4, 901–908. https://doi.org/10.1643/0045-8511(2005)005[0901:DEACAI]2.0.CO;2 (2005).
Tamilselvan, P. & Sloman, K. A. Developmental social experience of parents affects behaviour of offspring in zebrafish. Anim. Behav. 133, 153–160. https://doi.org/10.1016/j.anbehav.2017.09.009 (2017).
Zajitschek, S., Herbert-Read, J. E., Abbasi, N. M., Zajitschek, F. & Immler, S. Paternal personality and social status influence offspring activity in zebrafish. BMC Evol. Biol. 17, 157. https://doi.org/10.1186/s12862-017-1005-0 (2017).
Shams, S., Amlani, S., Buske, C., Chatterjee, D. & Gerlai, R. Developmental Social Isolation affects adult social behavior, social interaction, and dopamine metabolites in zebrafish. Dev. Psychobiol. 60(1), 43–56. https://doi.org/10.1002/dev.21581 (2018).
Green, M. R. & Swaney, W. T. Interacting effects of environmental enrichment across multiple generations on early life phenotypes in zebrafish. Front. Cell Dev. Biol. 9, 657591. https://doi.org/10.3389/fcell.2021.657591 (2021).
Jonsson, B. & Jonsson, N. Early environment influences later performance in fishes. J. Fish Biol. 85, 151–188. https://doi.org/10.1111/jfb.12432 (2014).
Groneberg, A. H. et al. Early-life social experience shapes social avoidance reactions in larval zebrafish. Curr. Biol. 30, 4009–4021. https://doi.org/10.1016/j.cub.2020.07.088 (2020).
Prentice, P. M., Alcalde, M. C., Císař, P. & Planella, S. R. Early-life environmental enrichment promotes positive animal welfare for juvenile Atlantic salmon (Salmo salar) in aquaculture research. Sci Rep. 15, 5828. https://doi.org/10.1038/s41598-025-88780-0 (2025).
Eachus, H., Choi, M. K. & Ryu, S. The effects of early life stress on the brain and behaviour: Insights from zebrafish models. Front. Cell Dev. Biol. 9, 657591. https://doi.org/10.3389/fcell.2021.657591 (2021).
Fontana, B. D., Gibbon, A. J., Cleal, M., Norton, W. H. J. & Parker, M. O. Chronic unpredictable early-life stress (CUELS) protocol: Early-life stress changes anxiety levels of adult zebrafish. Prog. Neuro-Psychoph. 108, 110087. https://doi.org/10.1016/j.pnpbp.2020.110087 (2021).
Zhang, Z. et al. Physical enrichment for improving welfare in fish aquaculture and fitness of stocking fish: A review of fundamentals, mechanisms and applications. Aquaculture 574(15), 739651. https://doi.org/10.1016/j.aquaculture.2023.739651 (2023).
Parker, M. O., Millington, M. E., Combe, F. J. & Brennan, C. H. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytics. PLoS ONE 7(4), e34992. https://doi.org/10.1371/journal.pone.0034992 (2012).
Otsuka, A., Inahata, M., Shimomura, Y. & Kagawa, N. Physiological changes in response to social isolation in male medaka fish. Fish. Sci. 86, 775–781. https://doi.org/10.1007/s12562-020-01441-1 (2020).
Hesse, S. & Thünken, T. Growth and social behavior in a cichlid fish are affected by social rearing environment and kinship. Sci. Nat. 101(4), 273–283. https://doi.org/10.1007/s00114-014-1154-6 (2014).
Herbert-Read, J. E. et al. How predation shapes the social interaction rules of shoaling fish. Proc. R. Soc. B. 284, 1861. https://doi.org/10.1098/rspb.2017.1126 (2017).
Moretz, J. A., Martins, E. P. & Robison, B. D. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ. Biol. Fish 80, 91–101. https://doi.org/10.1007/s10641-006-9122-4 (2007).
Herczeg, G., Gonda, A. & Merilä, J. The social cost of shoaling covaries with predation risk in nine-spined stickleback, Pungitius pungitius, populations. Anim. Behav. 77(3), 575–580. https://doi.org/10.1016/j.anbehav.2008.10.023 (2009).
Giacomini, A. C. V. V. et al. My stress, our stress: Blunted cortisol response to stress in isolated housed zebrafish. Physiol. Behav. 139, 182–187. https://doi.org/10.1016/j.physbeh.2014.11.035 (2015).
Forsatkar, M. N., Safari, O. & Boiti, C. Effects of social isolation on growth, stress response, and immunity of zebrafish. Acta. Ethol. 20, 255–261. https://doi.org/10.1007/s10211-017-0270-7 (2017).
Shams, S., Khan, A. & Gerlai, R. Early social deprivation does not affect cortisol response to acute and chronic stress in zebrafish. Stress 24, 273–281. https://doi.org/10.1080/10253890.2020.1807511 (2021).
Onarheim, T., Janczak, A. M. & Nordgreen, J. The effects of social vs. individual housing of zebrafish on whole-body cortisol and behavior in two tests of anxiety. Front. Vet. Sci. 9, 859848. https://doi.org/10.3389/fvets.2022.859848 (2022).
Shams, S., Chatterjee, D. & Gerlai, R. Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav. Brain Res. 292, 283–287. https://doi.org/10.1016/j.bbr.2015.05.061 (2015).
Jeffrey, J. D. & Gilmour, K. M. Programming of the hypothalamic-pituitary–interrenal axis by maternal social status in zebrafish (Danio rerio). J. Exp. Biol. 219(11), 1734–1743. https://doi.org/10.1242/jeb.138826 (2016).
Oswald, M. & Robison, B. D. Strain-specific alteration of zebrafish feeding behavior in response to aversive stimuli. Can. J. Zool. 86(10), 1085–1094. https://doi.org/10.1139/Z08-085 (2008).
Fowler, L. A., Williams, M. B., D’Abramo, L. R. & Watts, S. A. Zebrafish nutrition: Moving forward. Zebrafish Biomed Res 33, 379–401. https://doi.org/10.1016/B978-0-12-812431-4.00033-6 (2020).
Eriegha, O. J. & Ekokotu, P. A. Factors affecting feed intake in cultured fish species: a review. Anim. Res. Int. 14(2), 2697–2709 (2017).
Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 64, 82–92. https://doi.org/10.1016/j.dci.2016.02.020 (2016).
Silverstein, J. T., Bosworth, B. G., Waldbieser, G. C. & Wolters, W. R. Feed intake in channel catfish: Is there a genetic component?. Aquac. Res. 32, 199–205. https://doi.org/10.1046/j.1355-557x.2001.00015.x (2001).
Besson, M. et al. Feed intake with genomic data to improve feed efficiency in sea bass. Front. Genet 10, 219. https://doi.org/10.3389/fgene.2019.00219 (2019).
de Verdal, H., Vandeputte, M., Mekkawy, W., Chatain, B. & Benzie, J. A. H. Quantifying the genetic parameters of feed efficiency in juvenile Nile tilapia Oreochromis niloticus. BMC Genet. https://doi.org/10.1186/s12863-018-0691-y (2018).
Thodesen, J., Gjerde, B., Grisdale-Helland, B. & Storebakken, T. Genetic variation in feed intake, growth and feed utilization in Atlantic salmon (Salmo salar). Aquaculture 194(3–4), 273–281. https://doi.org/10.1016/S0044-8486(00)00527-5 (2001).
Park, H. et al. Towards the development of a sustainable soya bean-based feedstock for aquaculture. Plant Biotechnol. J. 15, 227–236. https://doi.org/10.1111/pbi.12608 (2017).
Lin, S. & Luo, L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. AFST 168(1–2), 80–87. https://doi.org/10.1016/j.anifeedsci.2011.03.012 (2011).
Zhou, Q. C., Mai, K. S., Tan, B. P. & Liu, Y. J. Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentron canadum). Aquac. Nutr. 11(3), 17–182. https://doi.org/10.1111/j.1365-2095.2005.00335.x (2005).
Macusi, E. D. et al. Protein fishmeal replacement in aquaculture: A systematic review and implications on growth and adoption viability. Sustainability 15(16), 12500. https://doi.org/10.3390/su151612500 (2023).
Blaufuss, P. & Trushenski, J. Exploring soy-derived alternatives to fish meal: Using soy protein concentrate and soy protein isolate in hybrid striped bass feeds. N. Am. J. Aquac. 74(1), 9–19. https://doi.org/10.1080/15222055.2011.635782 (2012).
Hua, K. et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 1(3), 316–329. https://doi.org/10.1016/j.oneear.2019.10.018 (2019).
Francis, G., Makkar, H. P. S. & Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199, 197–227. https://doi.org/10.1016/S0044-8486(01)00526-9 (2001).
Krogdahl, Å., Bakke-McKellep, A. M. & Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 9, 361–371. https://doi.org/10.1046/j.1365-2095.2003.00264.x (2003).
Kwasek, K. et al. Does exposure of broodstock to dietary soybean meal affect its utilization in the offspring of zebrafish (Danio rerio)?. Animals 12(12), 1475. https://doi.org/10.3390/ani12121475 (2022).
de Verdal, H. et al. Improving feed efficiency in fish using selective breeding: A review. Rev. Aquacult. 10, 833–851. https://doi.org/10.1111/raq.12202 (2018).
Izquierdo, M. S., Fernández-Palacios, H. & Tacon, A. G. J. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197, 25–42. https://doi.org/10.1016/S0044-8486(01)00581-6 (2001).
Markovich, M. L., Rizzuto, N. V. & Brown, P. B. Diet affects spawning in zebrafish. Zebrafish 4(1), 69–74. https://doi.org/10.1089/zeb.2006.9993 (2007).
Newman, T., Jhinku, N., Meier, M. & Horsfield, J. Dietary intake influences adult fertility and offspring fitness in zebrafish. PLoS ONE 11(11), e0166394. https://doi.org/10.1371/journal.pone.0166394 (2016).
Muchlisin, Z. A. A general overview on some aspects of fish reproduction. Aceh Int. J. Sci. Technol. 3(1), 43–52. https://doi.org/10.13170/AIJST.0301.05 (2014).
Volkoff, H. & London, S. Nutrition and reproduction in fish. Encycl Reprod 6, 743–748. https://doi.org/10.1016/B978-0-12-809633-8.20624-9 (2018).
Dissinger, A., Rimoldi, S., Terova, G. & Kwasek, K. Chronic social isolation affects feeding behavior of juvenile zebrafish (Danio rerio). PLoS ONE 19(7), e0307967. https://doi.org/10.1371/journal.pone.0307967 (2024).
Horwood, J. W., Greer Walker, M. & Witthames, P. The effect of feeding levels on the fecundity of plaice (Pleuronectes platessa). J. Mar. Biol. Assoc 69(1), 81–92. https://doi.org/10.1017/s0025315400049122 (1989).
Higuchi, K. et al. Effect of long-term food restriction on reproductive performances in female yellowtail Seriola quinqueradiata. Aquaculture 486(3), 223–231. https://doi.org/10.1016/j.aquaculture.2017.12.032 (2018).
McCormick, M. I. Mothers matter: Crowding leads to stressed mothers and smaller offspring in marine fish. Ecology 87(5), 1104–1109. https://doi.org/10.1890/0012-9658(2006)87[1104:mmclts]2.0.co;2 (2006).
Abdollahpour, H., Falahatkar, B., Jafari, N. & Lawrence, C. Effect of stress severity on zebrafish (Danio rerio) growth, gonadal development and reproductive performance: Do females and males respond differently?. Aquaculture 522, 735099. https://doi.org/10.1016/j.aquaculture.2020.735099 (2020).
Lovell, R. T. Nutrition and feeding of fish (Van Nostrand Reinhold, New York, 1989).
Jobling, M. Growth studies with fish: Overcoming the problems of size variation. J. Fish Biol. 22, 153–157. https://doi.org/10.1111/j.1095-8649.1983.tb02946.x (1983).
Halver, J. E. & Hardy, R. W. Fish nutrition 3rd edn. (Academic Press, Cambridge, 2002).
Uusi-Heikillä, S., Wolter, C., Meinelt, T. & Arlinghaus, R. Size-dependent reproductive success of wild zebrafish Danio rerio in the laboratory. J. Fish Biol. 77, 552–569. https://doi.org/10.1111/j.1095-8649.2010.02698.x (2010).
Breck, J. E. Body composition in fishes: Body size matters. Aquaculture 433, 40–49. https://doi.org/10.1016/j.aquaculture.2014.05.049 (2014).
Ferosekhan, S. et al. Maternal size on reproductive performance, egg and larval quality in the endangered Asian catfish. Clarias magur. Aquac. Res. 52(11), 5168–5179. https://doi.org/10.1111/are.15385 (2021).
Amaral, I. P. G. & Johnston, I. A. Experimental selection for body size at age modifies early life-history traits and muscle gene expression in adult zebrafish. J. Exp. Biol. 215(22), 3895–2904. https://doi.org/10.1242/jeb.068908 (2012).
Meirelles, M. G. et al. Growth hormone overexpression induces hyperphagia and intestinal morphophysiological adaptations to improve nutrient uptake in zebrafish. Front. Physiol. 12, 723853. https://doi.org/10.3389/fphys.2021.723853 (2021).
Bertucci, J. I. et al. Nutrient regulation of endocrine factors influencing feeding and growth in fish. Front. Endocrinol. 10, 83. https://doi.org/10.3389/fendo.2019.00083 (2019).
Mennigen, J. A. et al. Social status-dependent regulation and function of the somatotropic axis in juvenile rainbow trout. Mol. Cell. Endocrinol. 554, 111709. https://doi.org/10.1016/j.mce.2022.111709 (2022).
Tian, J. T., Mai, K. & Liu, C. Effects of postprandial starvation on mRNA expression of endocrine-, amino acid and peptide transporter-, and metabolic enzyme-related genes in zebrafish (Danio rerio). Fish Physiol. Biochem. 41, 773–787. https://doi.org/10.1007/s10695-015-0045-x (2015).
Izquierdo, M. S. et al. Nutritional programming through broodstock diets to improve utilization of very low fishmeal and fish oil diets in gilthead sea bream. Aquaculture 449, 18–26. https://doi.org/10.1016/j.aquaculture.2015.03.032 (2015).
Kwasek, K. et al. Nutritional programming improves dietary plant protein utilization in zebrafish Danio rerio. PLoS ONE 15(3), 1–19. https://doi.org/10.1371/journal.pone.0225917 (2020).
Geurden, I. et al. The positive impact of the early-feeding of a plant-based diet on its future acceptance and utilisation in rainbow trout. PLoS ONE 8(12), e83162. https://doi.org/10.1371/journal.pone.0083162 (2013).
Clarkson, M. et al. Early nutritional intervention can improve utilisation of vegetable-based diets in diploid and triploid Atlantic salmon (Salmo salar L.). Br. J. Nutr. 118(1), 17–29. https://doi.org/10.1017/S0007114517001842 (2017).
Kemski, M., Wick, M. & Dabrowski, K. Nutritional programming effects on growth and reproduction of broodstock and embryonic development of progeny in yellow perch (Perca flavescens) fed soybean meal-based diets. Aquaculture 497, 452–461. https://doi.org/10.1016/j.aquaculture.2018.07.001 (2018).
Kumar, S. et al. Does nutritional history impact on future performance and utilization of plant based diet in common carp?. Aquaculture 551, 737935. https://doi.org/10.1016/j.aquaculture.2022.737935 (2022).
Mania, M. et al. Expression and distribution of leptin and its receptors in the digestive tract of DIO (diet-induced obese) zebrafish. Anzeiger 212, 37–47. https://doi.org/10.1016/j.aanat.2017.03.005 (2017).
Volkoff, H. et al. Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 142, 3–19. https://doi.org/10.1016/j.ygcen.2004.11.001 (2005).
Lord, G. M. et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901. https://doi.org/10.1038/29795 (1998).
Audira, G. et al. Zebrafish mutants carrying leptin a (lepa) gene deficiency display obesity, anxiety, less aggression and fear, circadian rhythm and color preference dysregulation. Int. J. Mol. Sci. 19(12), 4038. https://doi.org/10.3390/ijms19124038 (2018).
Szyf, M., McGowan, P. & Meaney, M. J. The social environment and the epigenome. Environ. Mol. Mutagen. 49(1), 46–60. https://doi.org/10.1002/em.20357 (2008).
Gavery, M. R. & Roberts, S. B. Epigenetic considerations in aquaculture. PeerJ 5, e4147. https://doi.org/10.7717/peerj.4147 (2017).
Soengas, J. L., Cerdá-Reverter, J. M. & Delgado, M. J. Central regulation of food intake in fish: An evolutionary perspective. J. Mol. Endocrinol. 60(4), R171–R199. https://doi.org/10.1530/JME-17-0320 (2018).
Perera, E. & Yúfera, M. Soybean meal and soy protein concentrate in early diet elicit different nutritional programming effects on juvenile zebrafish. Zebrafish 13(1), 61–69. https://doi.org/10.1089/zeb.2015.1131 (2016).
Carnovali, M., Valli, R., Banfi, G., Porta, G. & Mariotti, M. Soybean meal-dependent intestinal inflammation induces different patterns of bone-loss in adult zebrafish scale. Biomedicines 9(4), 393. https://doi.org/10.3390/biomedicines9040393 (2021).
Fuentes-Appelgren, P. et al. Effect of the dietary inclusion of soybean components on the innate immune system in zebrafish. Zebrafish 14(1), 41–49. https://doi.org/10.1089/zeb.2013.0934 (2014).
Hedrera, M. I. et al. Soybean meal induces intestinal inflammation in zebrafish larvae. PLoS ONE 8(7), e69983. https://doi.org/10.1371/journal.pone.0069983 (2013).
Fehrman-Cartes, K. et al. Anti-inflammatory effects of aloe vera on soy meal-induced intestinal inflammation in zebrafish. Fish Shellfish Immun. 95, 564. https://doi.org/10.1016/j.fsi.2019.10.075 (2019).
Micheloni, G. et al. Soy diet induces intestinal inflammation in adult zebrafish: Role of OTX and P53 family. Exp. Pathol-Jena. 103(1), 13–22. https://doi.org/10.1111/iep.12420 (2021).
Molinari, G. S. et al. Can intestinal absorption of dietary protein be improved through early exposure to plant-based diet?. PLoS ONE 15(6), e0228758. https://doi.org/10.1371/journal.pone.0228758 (2020).
Dhanasiri, A. et al. Dietary inclusion of plant ingredients induces epigenetic changes in the intestine of zebrafish. Epigenetics 15(10), 1035–1051. https://doi.org/10.1080/15592294.2020.1747777 (2020).
Volkoff, H. & Peter, R. E. Feeding behavior of fish and its control. Zebrafish 3(2), 131–140. https://doi.org/10.1089/zeb.2006.3.131 (2006).
Jobling, M. National research council (NRC): Nutrient requirements of fish and shrimp. Aquacult. Int. 20, 601–602. https://doi.org/10.1007/s10499-011-9480-6 (2012).
Fernandes, H., Coronado, M., Hernández, A. J., Allende, M. L. & Feijoo, C. G. Dietary protein requirement during juvenile growth of zebrafish (Danio rerio). Zebrafish 13(6), 548–555. https://doi.org/10.1089/zeb.2016.1303 (2016).
Patula, S. et al. Nutritional programming with dietary soybean meal and its effect on gut microbiota in zebrafish (Danio rerio). Zebrafish 18(2), 125–138. https://doi.org/10.1089/zeb.2020.1952 (2021).
Haahr, M. RANDOM.ORG: True random number service. Retrieved from https://www.random.org (2025).
Wallace, C. K. et al. Effectiveness of rapid cooling as a method of euthanasia for young zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 57(1), 58–63 (2018).
Stockhammer, O. W., Zakrzewska, A., Hegedûs, Z., Spaink, H. P. & Meijer, A. H. Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 182(9), 5641–5643. https://doi.org/10.4049/jimmunol.0900082 (2009).
Galindo-Villegas, J., García-Moreno, D., de Oliveira, S., Meseguer, J. & Mulero, V. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. PNAS 109(39), E2605–E2614. https://doi.org/10.1073/pnas.1209920109 (2012).
Yossa, R., Sarker, P. K., Karanth, S., Ekker, M. & Vandenberg, G. W. Effects of dietary biotin and avidin on growth, survival, feed conversion, biotin status and gene expression of zebrafish Danio rerio. Comp. Biochem. Phys. B. 160(4), 150–158. https://doi.org/10.1016/j.cbpb.2011.07.005 (2011).
Acknowledgments
We thank Saffron Scientific Histology Services (Carbondale, IL) for providing the histological services for this study.
Funding
There was no specific funding associated with this research.
Author information
Authors and Affiliations
Contributions
Aubrey Dissinger—Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft; Karolina Kwasek—Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
No competing financial interests exist.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dissinger, A., Kwasek, K. Parental social environment has transgenerational effects on zebrafish (Danio rerio) growth. Sci Rep 15, 28848 (2025). https://doi.org/10.1038/s41598-025-13802-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13802-w