Abstract
The fibrosis mechanism in endometriosis is not elucidated. We investigated the role of eosinophil in fibrosis of endometriosis. The endometriotic stromal cells (ESCs) and endometrial epithelial cells (EECs) were subjected to in vitro experiments. Within endometriotic lesions, eosinophils were prominently observed by Hematoxylin-Eosin (HE) staining. Immunohistochemical staining for Major Basic Protein (MBP), a granule protein of eosinophils, revealed that MBP-positive eosinophils were predominantly found in the endometrial stromal region beneath the epithelial cell layer. Eosinophil clusters were found in 17 of 18 ovarian endometrioma (EMoma) cases, compared to 3 of 16 in non-endometriotic ovarian cyst/tumor (non-EMoma) (P < 0.0005). HE staining revealed eosinophils in fibrotic areas, and fields with ≥ 3 eosinophils per 100 × 100 μm area showed higher counts of nucleolus-positive fibroblasts (P < 0.05). Serial Masson Trichrome staining revealed blue-stained extracellular matrix around MBP-positive eosinophils, indicating fibrosis. GM-CSF levels, a stimulator of eosinophils, were higher in EMoma contents (93.6 ± 16.9 pg/ml) compared to non-EMoma (3.6 ± 2.1 pg/ml, P < 0.05). Coculture of eosinophils with ESCs or EECs upregulated PAI-1 mRNA and protein expression. Immunohistochemistry confirmed PAI-1 expression in eosinophil-rich lesions. These findings suggest that eosinophils promote fibrosis in endometriosis through PAI-1 induction, offering new therapeutic targets.
Similar content being viewed by others
Introduction
Endometriosis is a prevalent condition known for its significant morbidity and profound impact on women’s quality of life1. Characterized as an inflammatory disease, it manifests through an increased presence of inflammatory chemical mediators and immune cells such as macrophages within the peritoneal cavity1,2. Our prior studies have highlighted elevated levels of chemical mediators like IL-6, IL-8, IL-17, and sphingosine-1 phosphate (S1P) in endometriosis, underscoring the disease’s complex inflammatory profile3,4,5. Additionally, we have identified IL-33 as an alarmin released in response to cellular stress6, highlighting its critical role in mediating inflammatory and immune responses that contribute to the pathogenesis of endometriosis7.
A critical aspect of endometriosis is fibrosis, which contributes to severe symptoms including dyspareunia, painful bowel movements, and infertility8. Notably, fibrosis around the fallopian tubes impairs fertility by obstructing oocyte capture, and fibrotic replacement of ovarian tissue diminishes the ovarian reserve, as evidenced by reduced serum Anti-Mullerian Hormone (AMH) levels in affected individuals9,10. Although research has extensively focused on TGF-β as a fibrotic factor, translating these findings into safe, effective treatments remains a challenge due to issues with in vivo stability, tissue specificity, and potential side effects11.
Recent studies have shown that eosinophil-mediated activation of fibroblasts plays a role in the pathogenesis of adhesions in conditions such as asthma and radiation enteritis12,13. Therapeutic agents targeting eosinophils, such as anti-IL-5 monoclonal antibodies, have already been developed for these diseases14. In endometriosis, Blumenthal RD et al. reported the presence of eosinophils in endometriotic lesions for the first time15. However, little is known about the role of eosinophils in the formation of fibrotic lesions. In this study, we aim to investigate the involvement of eosinophils in the development of fibrotic lesions associated with endometriosis.
Results
Eosinophil localization in endometriotic tissue
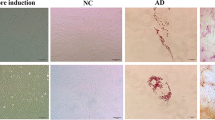
Eosinophils were prominently observed within endometriotic lesions, as demonstrated by Hematoxylin-Eosin (HE) staining (Fig. 1a, arrows). To further elucidate the distribution of eosinophils, immunostaining for Major Basic Protein (MBP), a granule protein in eosinophils, was performed. MBP-positive eosinophils were predominantly located in the endometriotic stromal region beneath the epithelial cell layer (Fig. 1b). In areas with preserved normal ovarian structures, such as regions surrounding primary and tertiary follicles, MBP immunostaining did not reveal eosinophil accumulation (Fig. 1c). We evaluated the presence of eosinophil clusters from ovarian endometrioma (EMoma) and non-endometriotic ovarian cyst/tumor (non-EMoma) by MBP staining. In the EMoma group, 17 of 18 cases showed clusters, while in the non-EMoma group, 3 of 16 cases (serous adenoma 1of 5, mucinous adenoma 1 of 5, and dermoid cyst 1 of 6) showed clusters and a significant accumulation of eosinophils in EMoma (P < 0.0005, Fig. 1d).
Localization of eosinophils in endometriotic lesions. (a) Hematoxylin-Eosin (HE) staining reveals the presence of eosinophils (arrows) within fibrotic sections of endometriosis lesions. (b) Immunostaining for Major Basic Protein (MBP) identifies eosinophils in the stromal regions beneath the epithelial layers. Rabbit IgG serves as the negative control. (c) Immunostaining for MBP and corresponding HE staining around a normal tertiary follicle. (d) Distribution of eosinophil clusters in sections of ovarian tumors (1. endometrioma, 2. serous adenoma, 3. mucinous adenoma, 4. Dermoid cyst).
Eosinophils’ effect on fibroblasts
Utilizing HE-stained sections, we quantified nucleolus-positive fibroblasts adjacent to eosinophils across a defined area (100 μm×100 μm). The eosinophil count was assessed in 10 randomly selected areas of each HE stained section for EMoma across 36 patients (Fig. 2a). Among these patients, 20 had received hormonal therapy, including GnRH analogs (n = 3) and progestin (n = 17). Findings indicated that an increased presence of eosinophils correlates with a higher activation of surrounding fibroblasts. Specifically, regions containing three or more eosinophils showcased a significantly greater number of nucleolus-positive fibroblasts compared to areas devoid of eosinophils (Fig. 2b). Furthermore, the eosinophil count within EMoma per field of view remained unchanged regardless of hormonal therapy (HT: n = 20, non-HT: n = 16, Fig. 2c).
The extent of fibrosis in areas with eosinophil accumulation was evaluated using serial sections from the same samples stained with Masson Trichrome. In regions surrounding MBP-positive eosinophils, marked blue staining was observed, indicating collagen deposition. These areas corresponded to pale pink regions in the HE-stained sections, suggesting an increase in extracellular matrix (Fig. 3).
Interaction between eosinophils and fibroblasts in endometriotic lesions. (a) Representative HE staining showing the spatial relationship between eosinophils and fibroblast cells. Arrow: nucleoli-positive fibroblast cell; white arrow heads: eosinophil. (b) Analysis of the relationship between eosinophil count and the presence of nucleolus-positive (activated) fibroblasts around eosinophils per field of view in lesion areas (n = 36, 360 fields of view). (c) Examination of eosinophil counts in endometriosis lesions per field of view, comparing cases with hormonal therapy (HT) including GnRH analogs and progestin therapy (n = 20), and without HT (non-HT, n = 16).
Histological evaluation of eosinophil accumulation and associated fibrosis in endometriotic lesions. (a, b) Low-power (a) and high-power (b) views of the same region stained for major basic protein (MBP), with eosinophil-rich areas indicated by white circles. (c, d) Masson Trichrome staining of the same region shown at low power (c) and high power (d). The arrow in (d) indicates an area of blue-stained extracellular matrix accumulation. (e, f) Hematoxylin and eosin (HE) staining of the same region shown at low power (e) and high power (f).
The mechanism of eosinophil activation in endometriotic cell
To examine the mechanism of eosinophil activation, the expression of some eosinophil activators and migratory factors including GM-CSF, IL-5, eotaxin16,17 was examined through immunohistochemical analysis. Immunostaining showed the expression of GM-CSF within the endometriotic lesion, however, the expressions of IL-5 and eotaxin were scanty (Fig. 4a). Subsequently, the GM-CSF level in the contents of ovarian cysts/tumors was quantified. GM-CSF concentrations in the ovarian contents of EMoma were significantly higher at 93.6 ± 16.9 pg/ml (average ± SEM), compared to the non-EMoma groups (3.6 ± 2.1 pg/ml, P < 0.05, Fig. 4b).
Eosinophil activators in endometriotic lesions. (a) Immunostaining for expression of eosinophil activators in endometriotic lesions. 1. major basic protein (MBP), 2 IL-5, 3. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), 4. Eotaxin (Chemokine C-C Motif Ligand 11; CCL11). (b) GM-CSF concentrations in the fluid contents of non-endometriomas (n = 3) and endometriomas (n = 9). Horizontal bars represent median values. * indicates a statistically significant difference (P < 0.05).
Eosinophils and fibrosis
Using a transwell coculture system to explore the interaction between eosinophils and endometriotic stromal cells (ESCs) or endometriotic epithelial cells (EECs), we examined the impact on mRNA expression of the fibrosis-associated factor plasminogen activator inhibitor-1 (PAI-1). PAI-1 mRNA expression in ESCs and EECs increased approximately 3-fold and 2-fold in the presence of eosinophils compared to controls (P < 0.05, Fig. 5a,b). And, PAI-1 concentrations in cell culture supernatants increased 2- and 1.3-fold in ESCs and EECs, respectively (P < 0.05, Fig. 5c,d). Furthermore, immunohistochemical analysis revealed PAI-1 expression proximal to MBP-positive eosinophils, highlighting their role in fibrosis within endometriosis lesions (Fig. 5e).
Influence of co-culture of eosinophils and endometriotic cells on Plasminogen Activator Inhibitor-1 (PAI-1) expression. (a) PAI-1 mRNA expression following eosinophil co-culture in endometriotic stromal cells (ESCs). mRNA expression data was normalized to GAPDH mRNA levels. Results from three independent experiments shown as mean ± SEM (*P < 0.05). (b) PAI-1 mRNA expression following eosinophil co-culture in endometriotic epithelial cells (EECs). mRNA expression data was normalized to GAPDH mRNA levels. Results from three independent three experiments shown as mean ± SEM (*P < 0.05). (c) Culture supernatant concentration of PAI-1 following eosinophil co-culture in ESCs. Results from three independent three experiments shown as mean ± SEM (*P < 0.05). (d) Culture supernatant concentration of PAI-1 following eosinophil co-culture in EECs. Results from three independent three experiments shown as mean ± SEM (*P < 0.05). (e) Immunohistochemical staining for PAI-1 and major basic protein (MBP) in endometriotic lesions.
Discussion
This study is the first to investigate the mechanism by which eosinophils are involved in the formation of fibrotic lesions in endometriosis. In the present study, we demonstrated that eosinophils play a role in promoting the fibrosis in endometriotic lesions via PAI-1 production (Fig. 6).
Hypothetical framework for fibrosis mechanism in endometriotic lesions. Hypothesis: Hypoxic environment in ovarian endometrioma induces the production of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) from endometriotic stroma cells (ESCs) followed by the migration and activation of eosinophils. Activated eosinophils increases Plasminogen Activator Inhibitor-1 (PAI-1) expression from ESCs or endometriotic epithelial cells (EECs) and promotes the formation of fibrosis in endometriotic lesions.
A key observation is the location relationship of eosinophils and fibroblasts in endometriosis lesions. Eosinophils, recently spotlighted for their role in fibrotic diseases12,13, were predominantly located in epithelial and stromal regions of EMoma, implicating the role in fibroblast activation. In endometriotic section, we actually observed a mix of sparse and dense fibroblast populations in fibrotic areas, with a significant presence of eosinophils among the denser fibroblast areas, indicating a potential role of eosinophils in fibroblast activation. Major basic protein (MBP) of eosinophils is a potent basic protein abundant in eosinophil granules, and its release is known to not only induce inflammation and tissue damage, but also activate surrounding fibroblasts, suggesting the direct effect of eosinophil for promoting fibrosis18. Interestingly, PAI-1 production from ESCs and EECs, linked to fibrosis, was significantly up-regulated in the presence of eosinophils. The immunohistochemical findings showing PAI-1 expression in fibrotic areas of endometriotic lesions further support the hypothesis that eosinophil-derived factors play a role in fibrosis. In addition, fibrosis in areas of eosinophil accumulation was histologically evaluated using Masson Trichrome staining, which revealed strong blue staining—indicative of increased extracellular matrix—at the sites where eosinophils had accumulated. These histological findings suggest that fibrosis is progressing in the regions surrounding eosinophil clusters and further support the role of eosinophils in fibrotic remodeling of endometriotic lesions. Previous studies have shown that stromal cells derived from endometriotic tissue produce higher levels of PAI-1 compared to those from normal endometrium, and that this expression is selectively upregulated by TGF-β1. In our study, increased PAI-1 expression was observed in fibrotic areas with high eosinophil density, suggesting that eosinophil-derived factors such as TGF-β may contribute to PAI-1 induction in both epithelial and stromal cells, thereby promoting fibrosis deevelopment. Although we did not directly demonstrate co-localization of PAI-1 expression with eosinophils or fibrotic regions in tissue sections, our in vitro coculture experiments showed that eosinophils induced PAI-1 expression in both endometriotic epithelial and stromal cells. This finding suggests a potential indirect mechanism by which eosinophils may promote fibrosis. Furthermore, previous reports19 support that TGF-β1 selectively enhances PAI-1 production in endometriosis-derived stromal cells, which may help explain our observed upregulation of PAI-1 in eosinophil-rich fibrotic areas.
Factors known to activate or attract eosinophils include GM-CSF, eotaxin, and IL-5, however, based on the results of immunostaining of endometriotic tissue, we focused in particular on GM-CSF as an essential for eosinophil activation. Hypoxia and alarmins are reported to promote the production of GM-CSF20,21. Hypoxia within EMoma stimulates ESCs to secrete GM-CSF, thereby promoting fibrosis in endometriosis lesions22. We found that the GM-CSF mRNA in ESCs was increased 3.0-fold by hypoxic stimulation (P = 0.019, Supplemental Fig. 1), whereas the expression of GM-CSF in EECs was very weak and difficult to evaluate. Previous report shows that adenosine Triphosphate (ATP), an alarmin, mediates activation of eosinophils via GM-CSF in the pathogenesis of fibrosis in radiation enteritis13. Consistent with this, we observed that ATP significantly increased the expression of GM-CSF mRNA in ESC (Supplemental Fig. 2). These multiple factors may be involved in the GM-CSF production.
In the present study, hormonal treatments, including progestin therapy or GnRH analogs, did not affect eosinophil counts within the lesions, highlighting the need for alternative approaches to address fibrosis beyond hormonal therapy. Targeting fibrosis in endometriotic lesions is crucial for mitigating disease progression23. TGF-β is a key driver of fibrosis, but systemic TGF-β inhibition carries risks, including side effects that may be unacceptable for patients attempting conception24 Our findings suggest eosinophils as novel therapeutic targets for endometriosis fibrosis treatment.
In the present study, although we demonstrated that eosinophils contribute to fibrosis formation, the observation of both dense and sparse eosinophil distributions within endometriotic lesions suggests that more complex mechanisms regulate eosinophil activity. It is also crucial to accumulate clinical data on endometriosis patients using eosinophil inhibitors, which are already in practical use for asthma and other conditions. Further investigation into eosinophil-mediated fibrosis mechanisms may open new avenues for innovative endometriosis therapies.
One of the limitations of this study is the absence of healthy control tissue. However, for ethical reasons, it is not feasible to obtain ovarian or peritoneal tissue from healthy women solely for research purposes. Therefore, the inclusion of control samples from healthy individuals was not possible within the current study design. As an alternative approach, we analyzed regions within the resected specimens that retained normal ovarian architecture. In these areas, no eosinophil accumulation was observed, suggesting that eosinophil infiltration may be specific to endometriotic lesions. While this internal comparison provided some insight, further studies incorporating ethically feasible control groups are warranted. Another limitation is the lack of direct histological evidence showing spatial co-localization between eosinophils, PAI-1 expression, and fibrotic areas. While immunostaining demonstrated the presence of PAI-1 and eosinophils in adjacent regions, further studies are needed to clarify their spatial and functional relationships at the tissue level.
In the present study, we demonstrated that eosinophils may contribute to the progression of fibrosis in endometriosis by promoting PAI-1 expression in both epithelial and stromal cells. These findings reveal a previously overlooked mechanism of fibrotic remodeling in endometriotic lesions and suggest that targeting eosinophil-mediated pathways could lead to the development of novel therapeutic strategies beyond conventional hormonal treatments.
Materials and methods
Ethical approval and sample collection
The study was conducted with the approval of the institutional review boards at the University of Yamanashi (Approval number: 2493) and Kitasato University (Approval number: B18-265). Informed consent was obtained from each patient before collecting surgical specimens. Samples, including endometriotic stromal cells (ESCs), fluid contents from EMoma (n = 8) and non-endometriotic ovarian cyst/tumor (non-EMoma, n = 8), were sourced from patients undergoing laparoscopic surgery for benign gynecological conditions. For the experiments of in vitro study, all patients had regular menstrual cycles, and none had been taking hormonal medications for at least 3 months prior to laparoscopy. Surgery was performed in EMoma cases either because medical treatment failed to control dysmenorrhea or because the tumor was 6 cm or larger, raising concern for potential malignancy. In cases where surgery was performed due to suspected endometriotic cysts, endometriosis was confirmed by histopathological diagnosis. In the non-EMoma group, surgery was conducted due to suspected risks such as ovarian torsion or rupture. Histopathological examination in these cases revealed serous ovarian cystadenoma, mature teratoma, or mucinous cystadenoma.
In the in vivo experiments involving pathological sections, we analyzed cases of EMoma that were treated with hormonal therapy (HT, n = 20) as well as those without hormonal therapy (non-HT, n = 16). The average age of the participants was 37.1 years. For non-EMoma, pathological sections of 5 serous cystadenomas, 6 mature teratomas, and 5 mucinous cystadenomas were used.
Immunohistochemical analysis
The sections of ovarian cyst/tumor, fixed in formalin and embedded in paraffin, were stained for Major Basic Protein (MBP; Bio-RAD MCA5751, Hercules, California, USA, 1:50 dilution), GM-CSF (Proteintech 17762-1-AP, Rosemont, Illinois, 1:400 dilution), Chemokine C-C Motif Ligand 11 (CCL11[eotaxin]: Proteintech 11786-1-AP, Rosemont, Illinois, 50 dilution), IL-5 (: Proteintech 26677-1-AP, Rosemont, Illinois, 1: 400 dilution), and PAI-1(Abcam 66705, Cambridge, UK, 1:800 dilution), utilizing an Envision + System/HRP rabbit (DAB+) kit (Dako, Tokyo, Japan). Antigen retrieval was facilitated by microwaving in 10 mM sodium citrate buffer (pH 6.0) for 10 min. Control IgGs served as negative controls in each Immunohistochemical experiments. Masson Trichrome staining was performed for the evaluation of fibrosis. Immunohistochemical images were acquired using a ZEISS Axiolab 5 microscope (Carl Zeiss AG, Oberkochen, Germany).
Collection of endometriotic tissues
Human samples were obtained from patients undergoing laparoscopic surgery for benign gynecological diseases. Endometriotic tissue was meticulously collected from EMoma wall under sterile conditions and preserved in DMEM/F12. Fluid contents from EMoma (n = 9) and non-EMoma (serous or mucinous cystadenoma, n = 3) patients, without hormonal treatments for at least three months prior to surgery, were aspirated, centrifuged at 400×g for 10 min, and supernatant aliquots stored at -80 °C for subsequent analysis.
Isolation and culture of human endometriotic stromal cells
Primary cultures of endometriotic stromal cells (ESCs) were established following the protocol detailed in reference3. In brief, endometriotic tissue was carefully dissected to remove underlying parenchyma, then minced into small fragments, and incubated in DMEM/F12 medium containing type I collagenase (2.5 mg/ml) and DNase I (15 U/ml) at 37 °C for 1–2 h. Following enzymatic digestion, the cell suspension was subjected to serial filtration. A 100 μm nylon cell strainer (Becton Dickinson, Lincoln Park, NJ) was used to remove debris, while a 70 μm nylon cell strainer was employed to eliminate dispersed epithelial glands. The stromal cells remaining in the filtrate were pelleted by centrifugation, resuspended in DMEM/F12 supplemented with 10% charcoal-stripped FBS, penicillin (100 U/ml), streptomycin (100 ng/ml), and amphotericin B (250 ng/ml), and subsequently plated onto 100 mm culture dishes (Iwaki Co, Chiba, Japan). Upon reaching confluence after 2 days, cells were detached using 0.25% trypsin, collected by centrifugation, and replated in 6-well plates at a density of 2 × 10⁵ cells/well. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air until confluence was achieved. For endometriotic epithelial cells (EECs), it was not possible to isolate a sufficient number of primary cells from endometriotic lesions; therefore, immortalized EECs (Applied Biological Materials, Cat. No. T0764-C, Lot: C24H27NC, Richmond, Canada) were used. DMEM/F12 medium and fetal bovine serum (FBS) was obtained from Life Technologies (Tokyo, Japan). Antibiotics (a mixture of penicillin, streptomycin, and amphotericin B) were obtained from Wako Pure Chemical Industries (Osaka, Japan). AnaeroPackTM-Anaero was purchased from MITSUBISHI GAS CHEMICAL (Tokyo, Japan), and used for hypoxia stimulation of cultured cells. Adenosine 5′-triphosphate disodium salt (ATP; CAS No. 987-65-5; A2383, Sigma-Aldrich, St. Louis, MO, USA) for stimulation experiments.
Quantitative PCR
Total RNA was isolated from ESCs and EECs using the RNeasy Mini Kit (QIAGEN, Venlo, Netherlands). Reverse transcription utilized the ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Tokyo, Japan). Quantitative PCR, performed on the Mx3000P system (Agilent Technologies, CA, USA), quantified mRNA levels, normalizing against GAPDH. Specific PCR primers targeted different exons to avoid chromosomal DNA contamination, with conditions set for 40 cycles of denaturing, annealing, and extension. The following conventional PCR primers were used: glyceraldehyde 3-phosphate dehydrogenase (GAPDH; NM_002046-7; 602–621 and 1053 − 1034), GM-CSF (NM_000758.4; 125–146 and 236 − 213), IL-5 (XM_054352521.1; 289–310 and 415 − 393), and plasminogen activator inhibitor-1 (PAI-1; NM_001386456.1; 310–333 and 501 − 478).
Transwell barrier cultures
Eosinophils, isolated from Peripheral blood using Microbeads (Eosinophil isolation kit; Miltenyi Biotec, Bergisch Gladbach, Germany), were cocultured with ESCs or EECs in transwell plates featuring 0.4-mm pores (Corning Inc., New York, USA). The ratio of the number of eosinophils to ESCs or EECs per well was at 1:50. Cultures were maintained in DMEM media and incubated at 37 °C for 24 h. Post-culture, RNA was extracted from ESCs or EECs for cDNA synthesis and quantitative PCR analysis. and RPMI-1640 was obtained from Life Technologies (Tokyo, Japan).
Cytokine measurement
ELISA kits of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and Plasminogen Activator Inhibitor-1 (PAI-1) were purchased from R&D Systems, Inc (Minneapolis, MN, USA). Concentrations of PAI-1 in cell culture supernatants and GM-CSF in fluid contents of ovarian cyst/ tumor were measured in duplicate using an enzyme-linked immunosorbent assay. The sensitivity limit of these kits was 10 pg/mL. The intra-assay and inter-assay coefficients of variation were < 5% in the assays.
Statistical analysis
Experiments were conducted in triplicate, with data subjected to nonparametric statistical analysis (Mann–Whitney test, Fisher’s exact test, chi-square test) using GraphPad Prism software (version 10.5.0; GraphPad Software, San Diego, CA, USA). Significance was set at a P-value of < 0.05. Schematic illustrations were created using Microsoft PowerPoint 2019 MSO (Version 2506, Build 16.0.18925.20076, 64-bit; Microsoft Corporation, Redmond, WA, USA).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zondervan, K. T. et al. Nat. Rev. Dis. Prim. 4, 9 (2018).
Sampson, J. A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14, 422–469 (1927).
Yoshino, O. et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 52, 306–311 (2004).
Yoshino, O. et al. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J. Reprod. Immunol. 72, 85–93 (2006).
Ono, Y. et al. Sphingosine 1-phosphate (S1P) in the peritoneal fluid skews M2 macrophage and contributes to the development of endometriosis. Biomedicines 9, 1519 (2021).
Yang, D., Han, Z. & Oppenheim, J. J. Alarmins and immunity. Immunol. Rev. 280, 41–56 (2017).
Ono, Y. et al. IL-33 exacerbates endometriotic lesions via polarizing peritoneal macrophages to M2 subtype. Reprod. Sci. 27, 869–876 (2020).
Vigano, P. et al. Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim. Invasive Gynecol. 27, 287–295 (2020).
Michio, K. et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 96, 685–691 (2011).
Arisa, T. et al. Endometriosis triggers excessive activation of primordial follicles via PI3K-PTEN-Akt-Foxo3 pathway. J. Clin. Endocrinol. Metab. 104, 5547–5554 (2019).
Walton, K. L., Johnson, K. E. & Harrison, C. A. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 8, 461 (2017).
Thiam, F. et al. Understanding fibroblast-immune cell interactions via co-culture models and their role in asthma pathogenesis. Front. Immunol. 14, 1128023 (2023).
Naoki, T. et al. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci. Transl Med. 10, eaan0333 (2018).
Walsh, G. M. Anti-IL-5 monoclonal antibodies for the treatment of asthma: an update. Expert Opin. Biol. Ther. 20, 1237–1244 (2020).
Blumenthal, R. D. et al. Degranulating eosinophils in human endometriosis. Am. J. Pathol. 156, 1581–1588 (2000).
Dougan, M., Dranoff, G., Dougan, S. K. & GM-CSF IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity 50, 796–807 (2019).
Kampen, G. T. et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood 95, 1911–1917 (2000).
Rochester, C. L. et al. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J. Immunol. 156, 4449–4456 (1996).
Guan, Y. M. et al. Impact of epidermal growth factor and transforming growth factor beta-1 on the release of fibrinolytic factors from cultured endometrial and ovarian endometriotic stromal cells. Gynecol. Obstet. Invest. 55, 7–13 (2003).
Bui, B. P. et al. Hypoxia-inducible factor-1: A novel therapeutic target for the management of cancer, drug resistance, and cancer-related pain. Cancers (Basel). 14, 6054 (2022).
Montanari, E. et al. Interleukin-33 stimulates GM-CSF and M-CSF production by human endothelial cells. Thromb. Haemost. 116, 317–327 (2016).
Li, W. N., Wu, M. H. & Tsai, S. J. Hypoxia and reproductive health: the role of hypoxia in the development and progression of endometriosis. Reproduction 161, F19–F31 (2021).
Garcia, J. M. G. et al. Endometriosis: cellular and molecular mechanisms leading to fibrosis. Reprod. Sci. 30, 1453–1461 (2023).
Akhurst, R. J. & Hata, A. Targeting the TGF-β signaling pathway in disease. Nat. Rev. Drug Discov. 11, 790–801 (2012).
Acknowledgements
The authors thank Shoko Yamamoto, Ayano Oka, Yuki Kyushima, Souma Kumano, and Atsushi Hosokawa for their excellent supports.
Funding
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (Grant Number19K09823 and 22K09616).
Author information
Authors and Affiliations
Contributions
O.Y., Y.O.: conception and design; O.Y., Y.O., and H.T.: acquiring and processing samples; Y.O., E.S., K.T., T.O, M.H and M.I.: execution of the experiment; O.Y., Y.O: analysis of data; O.Y., Y.S, S.K., K.K and Y.O.: interpretation of data; Y.O., O.Y., S.K.: drafting the manuscript; O.Y., Y.O: revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study was carried out in accordance with the recommendations of the Ethical Guidelines for Medical and Health Research Involving Human Subjects, Animal Subjects, the Ministry of Health, Labor and Welfare, Japan. The experiment was approved by the committee of the University of Kitasato (Approval number: B18-265) and the University of Yamanashi (Approval number: 2493). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ono, Y., Tanaka, K., Sato, E. et al. Activated eosinophil plays a role in promoting fibrosis in endometriotic lesion. Sci Rep 15, 28015 (2025). https://doi.org/10.1038/s41598-025-13855-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13855-x