Abstract
Cardamonin (CDN) has been shown to have promising anticancer effects against osteosarcoma (OS). Nevertheless, the molecular processes of it are still not well understood. Our investigation revealed that CDN has significant cytotoxic effects and diminishes cell viability in OS. The self-renewal ability was assessed using a sphere formation test, and the presence of marker proteins associated with cancer stem cells (CD133, SOX2, Nanog, and Oct4) was determined using Western blotting. These findings suggest that CDN significantly inhibits cancer stem cells (CSCs). Autophagy-related proteins (LC3, SQSTM1, Beclin1, and Atg5) were quantified via Western blotting, and the number of endogenous LC3 puncta was quantified via mRFP-GFP-LC3B adenovirus transfection. These outcomes manifest that CDN raises autophagy. The inhibition of autophagy with chloroquine (CQ) worsened the adverse effect of CDN on cancer stem cell stemness. This shows that CDN-induced autophagy inhibited the growth of osteosarcoma stem cells. The weakening of CSCs’ ability to maintain their stemness in OS is achieved by blocking the Wnt/β-catenin (WBC) signaling pathway via the action of CDN. Moreover, CDN induces autophagy, which has a negative regulatory effect on the WBC signaling pathway. Conversely, the WBC signaling pathway can also regulate autophagy induced by CDN. Ultimately, the antitumor activity of CDN was verified via in vivo experiments in an OS xenograft model. Immunohistochemical analysis revealed that CDN upregulated LC3 expression and decreased CD133 and β-catenin expression, aligned with the findings of the in vitro experiments. Overall, this study establishes an empirical basis for using CDN as a therapeutic medication that specifically targets CSCs in treating OS.
Similar content being viewed by others
Introduction
Tumor stem cells have been found in several malignant tumors in recent years1,2. These cells possess the capacity to induce tumors, to renew themselves, and to differentiate in multiple directions. Furthermore, they play an instrumental role in tumor formation, development, and metastasis3. Tumor stem cells are also highly resistant to drugs. These tumors can recur locally and metastasize remotely despite radiotherapy treatment4. Osteosarcoma (OS) is a heterogeneous malignant tumor that comprises several different types of cells, including a small number of CSCs5. The presence of tumor stem cells is often observed in OS patients with a poor prognosis. Drug toxicity and resistance pose significant challenges during osteosarcoma treatment. Currently, there are no ideal drugs for treating this disease, and finding safe and effective treatments remains a top priority for both domestic and international researchers.
Cardamonin (CDN) is a well-defined natural flavonoid compound with multiple biological activities. Its chemical structure is 2,4-dihydroxy-6-methoxy-chalcone (Fig. 1A), and it is found mainly in the seeds of the ginger plant herb CDN6. Research has confirmed that CDN possesses antitumor, anti-inflammatory, antidiabetic, antiviral, hypoglycemic, and anti-infection properties7. CDN has demonstrated anticancer effects in breast cancer8multiple myeloma9lung10colon11and ovarian cancers12. Its antitumor properties are effective, and it has nontoxic side effects on normal cells and organs, which make it a promising antitumor drug. In particular, the effect of CDN on osteosarcoma stem cells has not been reported either domestically or internationally.
Impact of CDN on Cell Viability in Various Osteosarcoma Cells: Cell survival was evaluated using the CCK8 assay 24 and 48 h post-CDN treatment. (A) Molecular Composition of CDN. Various concentrations of CDN (0,0.625, 1.25, 2.5, 5, 10, 20, 40 microns) were used to treat MG63 (B), 143 B (C), U2OS (D), HOS (E), and SAOS2 (F) osteosarcoma cell lines. Cell viability ratio was assessed at 24 and 48 h after treatment. The control group (0 μM) did not receive CDN treatment, and cell viability remained at 100%. * p < 0.05; *** p < 0.001 vs. the un-treated control.
Autophagy is a catabolic process that is widely preserved. Autophagy entails the creation of autophagosomes, which are double-membrane vesicles that enclose various cellular cargo, such as organelles, protein aggregates, and intracellular pathogens. Subsequently, the autophagosomes merge with lysosomes, leading to the obliteration of the contents13. Autophagy is a cellular process that promotes survival and is triggered by cancer therapy and many forms of stress. It may enhance the ability of cancer cells to survive in a dormant state for extended periods, ultimately resulting in the development of metastatic illness14. Autophagy breaks down large cellular structures and promotes cell survival by recycling their constituent metabolites15.
Research has shown that multiple signal transduction pathways can influence the activity and function of osteosarcoma tumor stem cells16. One of these pathways is the Wnt/β-catenin (WBC) signal transduction pathway, which can control cell growth, apoptosis, and differentiation. It plays a crucial role in embryo and organ development as well as maintaining stem cell self-renewal. This pathway also has significant effects on osteosarcoma tumor stem cells17. A faulty WBC signal transduction pathway can alter the biological traits of osteosarcoma stem cells, causing uncontrolled self-renewal, aberrant proliferation, and disrupted differentiation18,19. The degradation of β-catenin is a pivotal occurrence in the WBC signaling pathway20. β-catenin that has built up over time moves into the nucleus and attaches itself to TCF transcription factors in order to initiate the transcription of downstream genes, encompassing CD4421, MYC22and LGR523. These genes may improve stem cells and contribute to the progression of OS. Consequently, there is significant interest among cancer researchers in developing WBC pathway inhibitors as new anticancer treatments. Nevertheless, despite significant efforts, the lack of known kinases and other drug-capable targets in the Wnt pathway means that anti-Wnt-based therapies are currently very challenging.
This study revealed that CDN effectively suppressed cell viability, cell proliferation, and cancer stemness in OS. Autophagy was significantly increased by CDN, and autophagy inhibited the formation of spheres in OS. Furthermore, our study demonstrated that CDN attenuates cancer stemness by suppressing the WBC signaling pathway. Furthermore, autophagy triggered by CDN overlaps with the WBC pathway. Ultimately, the effectiveness of CDN in inhibiting tumor growth was verified in vivo by employing an OS xenograft model. This study offers experimental evidence for OS treatment using CDN as antitumor agents targeted against CSCs.
Materials and methods
Cell culture, treatments, and SiRNA transfection
Five OS cell lines, MG63, 143B, U2OS, HOS, and SAOS2, were obtained from The World Cell Factory (iCell Bioscience, Inc., Shanghai), Osteoblast cell line hFOB1.19 was obtained from the American Type Culture Collection (ATCC). The five cell lines were cultured in medium (DMEM) supplemented with fetal bovine serum (10%, Gibco) and penicillin/streptomycin (10 U/ml, HyClone) in an incubator (5% CO2, 37 °C). Prior to cell collection and subsequent cell detection, the cells were treated with a specified CDN concentration. The cells went through treatment with 5 µl of siAtg5 (Shanghai GenePharma Co.,Ltd), using Lipofectamine 2000 to dilute the solution in Opti-MEM for 5 min. The solution was thereafter mixed and allowed to incubate at ambient temperature for a duration of 20 min, followed by introducing the composite into the cell culture plate. After a 48 h period of transfection, the cells were gathered for additional assessments. The sequences were recorded in Table S1 (Supporting Information).
Reagents and antibodies
All reagents and antibodies used in this study were purchased from suppliers in China (in brackets): CDN (MCE, HY-N0279), Chloroquine (Sigma, C6628), dimethyl sulfoxide (DMSO, Sigma, 67-68-5), Cell Counting Kit-8 (CCK-8, MCE, HY-K0301), AD-mRFP-GFP-LC3 (Hanbio, HB-AP210 000), hESC-Qualified Matrix, LDEV-free (Corning, 354277), BML284 (MCE, HY-19987), CD133 (Abcam, ab19898), SOX2 (Abcam, ab97959), Nanog (Abcam, ab21624 ), Oct4 (Abcam, ab19857), Beclin1 (Abcam, ab62557), Atg5 (Abcam, ab108327), Wnt1 (Abcam, ab15251), β-catenin (Abcam, ab32572), and SQSTM1 (Origene, UM570012), LC3 (Proteintech, 14600-1-AP) and Ki67(Proteintech, 27309-1-AP).
Cell viability assay
Typically, 3,000 cells were distributed evenly in a 96-well plate and treated with the specified concentration of CDN. After 24–48 h of treatment, the cells were subjected to treatment with Cell Counting Kit-8 (CCK-8, 10 µL/well) and placed in an incubator at 37 °C for 2 h. The absorbance at 450 nm was then quantified employing the OD value of the medium lacking cells as a blank control. Cell viability was quantified by calculating the proportion of CDN-treated cells that took up compared to DMSO-treated cells (i.e., control).
Spheroid formation analysis
Typically, 2,000 cells were seeded and treated for approximately 14 days following instructions in a 24-well ultralow cluster plate (Corning). The spheres were cultivated in DMEM/F12 serum-free media (HyClone) enriched with EGF, FGF, B-27, and N-2. Ultimately, the colonies were scrutinized and captured in images using a microscope.
Western blot (WB) analysis
The proteins were extracted employing RIPA buffer, and their concentration was quantified by deploying a BCA kit. The protein lysates were separated utilizing a 10% SDS-PAGE method and then deposited onto PVDF membranes. Following the sealing process, the PVDF membrane was subjected to incubation with both primary and secondary antibodies. The target proteins were seen employing an improved chemiluminescence reagent (Merck Millipore, Billerica, MA).
Immunofluorescence
The cells were treated with a solution containing 4% paraformaldehyde, 0.5% Triton X-100, and 5% bovine serum albumin to fix, infilter, and block them. Following PBS washing, the cells were subjected to incubation with an LC3 antibody and goat anti-rabbit IgG H&L. DAPI was utilized to stain the nuclei, and laser scanning confocal microscopy (Olympus FV1000, Tokyo, Japan) was employed to observe the cells.
Confocal microscopy
HOS cells and MG63 cells were placed in 24-well plates and transfected until they attained a confluence of 50%-70%. The mRFP-GFP-LC3 adenoviral vector was obtained from Han Biotechnology (Shanghai, China). This experiment is dependent on the consistency of green and red fluorescent proteins when exposed to different pH levels. The eGFP fluorescence signal is susceptible to quenching in acidic circumstances (pH below 5) inside the lysosome, but the mRFP fluorescent signal is very stable in acidic settings. The combined green and red pictures show autophagosomes as yellow dots (RFP + GFP+) and autolysosomes as red dots (RFP + GFP). The autophagic flux exhibited an increase when there was an elevation in both yellow and red dots inside the cell. Conversely, the autophagic flux was hindered when there was a drop in both yellow and red dots within the cell. The adenovirus infection was conducted in accordance with the instructions provided by the manufacturer. The OS cells were cultivated in a growth medium with adenovirus at a temperature of 37 °C for a duration of 2 h. Subsequently, they were incubated in a media supplemented with F. nucleatum and certain concentrations of miRNA at a temperature of 37 °C for a period ranging from 0 to 24 h. The presence of LC3 puncta was seen using a Zeiss LSM710 confocal microscope (Carl Zeiss) equipped with a 63X oil immersion objective.
Animal studies
BALB/C mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd and maintained at a constant temperature (23 ± 1 °C) with a 12 h light/dark cycle under specific pathogen-free conditions. All animal research procedures were carried on according to the detailed rules of the Animal Ethics Committee of Shaoxing People’s Hospital (approval number: 2020 − 118, date: 24 September 2020), and all methods were carried out in accordance with relevant guidelines and regulations and reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). Immunocompromised nude mice, aged 6–8 weeks, were subcutaneously injected with MG63 cells to create a model of osteosarcoma xenograft. Mice were randomly divided into 2 groups (5 mice per group), and DMSO and CDN (50 mg/kg) was injected via the tail vein once every 5 days until Day 20 when the tumor volume was measured every 5 days. One day after treatment ended, the animals were euthanized with an anesthetic overdose (thiopental, 100 mg/kg), and tumors were excised, weighed and processed for histological analysis.
Immunohistochemistry
Immunohistochemistry was performed on sections that were treated with 4% paraformaldehyde and then embedded in paraffin. Following the usual process of removing wax and adding water, the sections were immersed in a solution containing sodium citrate (0.01 M, pH 6.0) at a temperature of 60 °C for the whole night to retrieve the antigens. Following a 10-minute incubation in the dark with 3% hydrogen peroxide, the sections were treated with a solution containing 1% goat serum and 0.2% Triton X-100 (Beyotime, Shanghai, China) at room temperature for 30 min to enhance permeability and prevent non-specific-binding-. Following that, the principal antibodies employed were anti-LC3, CD133, and β-catenin. Next, the slides were treated with HRP (horse-radish peroxidase)-conjugated goat anti-rabbit IgG H&L (Proteintech Group, IL, United States) at a temperature of 37 °C for a duration of 60 min. This was followed by the use of 3, 3-diaminobenzidine (DAB) (ZSGB-Bio, Beijing, China) to visualize the results. The microscopic examination was conducted using an Olympus IX50 microscope (Olympus, Tokyo, Japan).
Apoptosis assay
The cells were cultivated on 6-well plates for one night. The cells were gathered and re-suspended in 195 µl solution f binding buffer for the apoptosis experiments. Next, 5 µl of Annexin V-FITC and 10 µl of PI (4 A Biotech) were introduced. Following a 30-minute incubation at room temperature, the sample was assessed using flow cytometry (Beckman Coulter, USA). For the purpose of the cell cycle study, the cells were rendered immobile by exposure to 70% ethanol overnight. Afterwards, the cells were stained with PI dye for a duration of 30 min. Finally, the cells were examined and evaluated using the technique of flow cytometry.
Statistical analysis
The statistical analysis was carried out using SPSS statistical software version 20. These values were compared using one-way ANOVA or an independent sample Student’s t-test. Statistical significance was ascertained at *p < 0.05, **p < 0.01, or ***p < 0.001. The values are expressed as the mean ± SEM. Error bars manifest the SEM unless otherwise stated.
Results
CDN exhibits high toxicity to OS cell lines
To investigate the antitumor effects of CDN on OS cells and the osteoblast cell, MG63, 143B, U2OS, HOS, SAOS2 and HFOB1.19 cells were treated with the indicated CDN levels for 24 and 48 h. Cell viability was subsequently determined via a CCK8 assay. The results showed that the proliferation of MG63 (Fig. 1B), 143B (Fig. 1C), U2OS (Fig. 1D), HOS (Fig. 1E), and SAOS2 (Fig. 1F) cells were dose-dependent (0–40 µM) and time-dependent after exposure to CDN for 24 and 48 h, while the cell viability of the osteoblast cell line HFOB1.19 was not significantly affected (Supplementary Fig. 1). The IC50 values of CDN for all five osteosarcoma cell lines were less than 20 µM after 24 h (Table 1), with U2OS and MG63 cells showing the greatest and least sensitivity, respectively. After extending the incubation time to 48 h, the IC50 values of CDN decreased for all the cell lines, with the largest decrease for HOS cells and the smallest decrease for MG63 cells, at 10.99 µM and 1.87 µM, respectively. Therefore, we conducted further studies on HOS and MG63 cells. Subsequently, a flow cytometry study was conducted with Annexin V FITC/PI to assess the impact of CDN on apoptosis. The findings showed that tumors with CDN group did not emerge a significantly elevated percentage of apoptotic cells in comparison to tumors with control group (Supplementary Fig. 2) It indicates that CDN has little effect on the apoptosis of OS cells.
CDN attenuates cancer stem-like cells stemness in OS cells
To evaluate the effect of CDN on osteosarcoma, we cultured HOS and MG63 osteosarcoma cells in a serum-free medium for 7 days. We also concentrated the csc of these cells. We observed sphere formation and division morphology using electron microscopy (Fig. 2A). These morphologies are usually observed upon drying. We added graded concentrations of CDN to HOS and MG63 cell culture media. We monitored the in vitro sphere formation of HOS and MG63 cells daily. Cell sphere formation was observed on Day 3. With increasing CDN concentration in the experimental group, the formation time of the cell spheres gradually increased, and the diameter and formation density gradually decreased. These findings suggest that CDN inhibits the ball formation of HOS and MG63 cells in vitro (Fig. 2B). The expression levels of proteins labeled as CSCs, such as CD133, SOX2, Nanog, and Oct4, decreased in OS cells with increasing doses of CDN treatment (Fig. 2C). CD133 is a membrane-anchored cell surface protein24 and Oct4 are nuclear proteins25. By immunofluorescence staining and confocal laser microscopy, we found that CDN significantly reduced the expression of HOS stem cell markers in osteosarcoma (Fig. 2D). These findings suggest that CDN can effectively suppress the stem cell characteristics of osteosarcoma cells.
CDN treatment reduced the stemness of cancer stem cells in OS cells. (A)The formation and division patterns of spheroids were examined through electron microscopy. Scale bar: 5 μm. (B)Sphere formation assays revealed reduced self-renewal capacity in cells treated with CDN for a week. Scale bar: 200 μm. (C)Following the treatment of OS cells with CDN for 24 h, the levels of CSCs-labeled proteins CD133, SOX2, Nanog, and Oct4 were quantified using WB. (D)Following treatment, we detected the expression of marker proteins Oct3/4 and CD133 using immunofluorescence. Scale bar: 25 μm. *p < 0.05; **p < 0.01 and ***p < 0.001 vs. the untreated control.
CDN suppresses the OS cell’s migration and invasion
According to the CSC progression theory, this cell subpopulation plays a key role in driving neoplasm invasion in new tumor subclones. Therefore, we investigated the inhibitory effect of CDN on reducing CSCs in two OS cell lines to inhibit tumor cell metastasis. A certain concentration of CDN was added to the media of OS HOS and MG63 cells, and it was discovered that CDN could significantly inhibit the migration capacity of HOS cells in the scratch test at 12 and 24 h (Fig. 3A). The results from the transwell experiment indicated that CDN significantly suppressed the migration and invasion capacity of HOS and MG63 cells after 24 h in a dose-dependent manner (Fig. 3B).
Inhibition of migratory invasive ability of HOS and MG63 cells by CDN. (A)Scratch assay manifested that CDN significantly mitigated the migratory ability of HOS and MG63 cells. The migratory ability of HOS was significantly reduced with increasing CDN concentration. Scale bar: 50 μm. (B)Transwell showed that the migratory invasion ability of osteosarcoma HOS and MG63 was significantly decreased with increasing CDN concentration. Scale bar: 200 μm. Statistical analysis showed a statistically significant increase in inhibition over time with a significance level of ***p < 0.001.
Autophagy in OS cells induced by CDN
Considering the confirmed role of autophagy in chemotherapy described in several studies [1–5], we examined the capacity of CDN to stimulate autophagy in OS cells. To accomplish this goal, WB analysis was employed to identify the protein expression levels linked to autophagy. The results manifested that CDN enhances the LC3-II and Beclin1 expression levels in a way that depends on the dosage. However, the implication on the SQSTM1 protein was opposite in HOS and MG63 cells (Fig. 4A). Furthermore, contrasted to those in the control group, the number of endogenous LC3 puncta and lysosomes in the CDN group was considerably greater (Fig. 4B). Compared with the control treatment, CDN promoted autophagy formation. In order to examine the potential of CDN to enhance autophagic flux, OS cells were exposed to CDN in the existence or lack of the autophagy inhibitor chloroquine (CQ). Subsequently, we conducted a WB analysis to ascertain the LC3 and SQSTM1 expression levels. In OS cells treated with CDN mixed with CQ rather than CDN alone, the LC3-II and SQSTM1 expression levels elevated, demonstrating that CDN can promote complete autophagy in OS cells (Fig. 4C).
CDN induces autophagy in OS cells. (A) WB detected the expression levels of autophagy marker proteins LC3, SQSTM1, Beclin1, and Atg5 after treating the cells for 24 h as directed. (B) As shown in (A), Immunofluorescence manifests the existence of endogenous LC3 puncta after cell treatment. Scale bar: 25 μm. (C) The WB technique was used to ascertain the LC3 and SQSTM1 expression levels after treating the cells with or without CQ. The significance levels are *p < 0.05 and ***p < 0.001.
CDN impairs cancer stemness by inducing autophagy in OS cells
As autophagy contributes to various physiological and pathological processes, we investigated the potential of CDN-induced autophagy to reduce CSC proliferation in OS cells. Figure 5A shows that blocking CDN-induced autophagy with the autophagy inhibitor CQ partially restored the sphere-forming ability of the cells compared to that of cells treated with CDN alone. Figure 5B demonstrates that combining CDN and CQ partially counteracted the decreases in the expression levels of CD133, SOX2, Nanog, and Oct4 in CSCs caused by CDN treatment. Atg5 siRNA was also adopted to block the CDN-induced autophagy. The inhibition efficacy siAtg5 was confirmed by western blotting (Supplementary Fig. 3A). Similar conclusions were obtained by using Atg5 siRNA rather than by an autophagy inhibitor (Supplementary Fig. 3B).
CDN damages CSCs stem cells by inducing autophagy in OS cells. (A) Spheroid-forming assays were conducted for two weeks on cells with and without a CDN, with or without CQ, to assess their self-renewal ability. Scale bar: 200 μm. (B) The protein expression levels of CD133, SOX2, Nanog, and Oct4, labeled as CSCs, were quantified via WB after treating cells with or without CQ for 24 h. The statistical significance was denoted as **p < 0.01 and ***p < 0.001.
CDN weakens CSCs through the WBC signaling pathway in OS cells
The WBC pathway is a conserved signaling pathway that is crucial for biological evolution. It possesses a significant role in regulating cell proliferation and differentiation, as well as in the development and maintenance of tissues, organs, and adult stem cells. Due to the excessive activation of the Wnt signaling pathway in many diseases, especially tumors and inflammatory diseases, regulating the Wnt signaling pathway is often a crucial focus of therapeutic development. Thus, we selected the WBC signaling pathway as the potential signaling pathway for CDN in OS cells. The WB analysis manifested that the Wnt1 and β-catenin expression levels were mitigated in a dose-dependent manner by CDN. Additionally, the expression of c-Myc was also lowered (Fig. 6A). The immunofluorescence results were also consistent (Fig. 6B). Collectively, our studies indicate that CDN inhibits the stem cell properties of cancer stem-like cells by inhibiting the WBC signaling pathway.
CDN impairs the Wnt/β-catenin pathway in OS cells. (A) Wnt1, β-catenin, and c-Myc expression levels were quantified via WB following 24 h of treatment of the cells as directed. (B) The β-catenin protein expression was quantified via immunofluorescence following cell treatment. The statistical significance was denoted as **p < 0.01 and ***p < 0.001.
CDN-stimulated autophagy intersects with the WBC signaling pathway
In order to evaluate the connection between CDN-stimulated autophagy and the WBC signaling pathway, we first examined the impact of autophagy on the WBC signaling pathway by obstructing CDN-stimulated autophagy using the autophagy inhibitor CQ. The WB analysis manifested that the addition of CQ to CDN led to a significant hindrance in the CDN hindrance on the Wnt1 and β-catenin expression (Fig. 7A). On the other hand, when the WBC pathway, which was blocked by CDN, was stimulated by the Wnt signaling pathway activator BML284, the WB analysis contrasted with POS alone, CDN plus BML284 significantly reduced LC3-II expression, suggesting that activation of the Wnt signaling pathway inhibited CDN-induced autophagy (Fig. 7B). In conclusion, CDN-induced autophagy and the WBC signaling pathway mutually inhibit one other.
CDN-stimulated autophagy intersects with the Wnt/β-catenin signaling pathway. (A) Wnt1 and β-catenin Expression levels were quantified via WB following the treatment of cells with or without CQ. (B) Expression levels of Wnt1 and LC3 were measured by WB after cells were treated with CDN with or without BML284. The statistical significance was denoted as **p < 0.01 and ***p < 0.001.
CDN reduces OS growth in vivo
Considering the suppressive impact of CDN on OS cells and CSCs, we conducted an investigation to determine whether CDN exhibits anticancer effects in vivo. In order to achieve this objective, we inserted MG63 cells subcutaneously into immunodeficient nude mice. The mice were given DMSO or CDN a total of 4 times, with each administration occurring at 5-day intervals. The tumor volume was measured at the same 5-day intervals. According to the data shown in Fig. 8A, the CDN group had a significantly hindrance number of tumors compared to the DMSO control group. Subsequently, Ki67 immunohistochemistry (Fig. 8B) on tumor tissues was performed. Consistent with the in vitro findings, the expression level of Ki67, as an important marker of cell proliferation, was significantly reduced compared with the control group (supplementary Fig. 4). In addition, the immunohistochemical examination manifested that CDN boosted the LC3 expression and mitigated the CD133 and β-catenin expression, which is aligned with the outcomes obtained from the in vitro tests (Fig. 8B). Overall, our findings unequivocally demonstrate that CDN has anticancer effects against osteosarcoma in vivo.
CDN inhibits OS tumor growth in vivo. (A) Immunodeficient nude mice were subcutaneously implanted with MG63 cells. Mice were randomly divided into 2 groups, and DMSO or CDN was administered every 5 days for 4 times. Tumor volumes were detected every 5 days. Left, tumor images taken on day 20. Right, tumor volumes were measured at different time points (n = 5), and tumor weight was measured at 20 days. (B) Immunohistochemical analysis of Ki67, CD133, LC3, and β-catenin expression in MG63 xenografts from DMSO or CDN-treated mice. Scale bar:100μM.
Discussion
The main options for OS therapy include surgery, radiotherapy, and adjuvant chemotherapy. Patients with OS benefitted from conventional treatments that improved their quality of life. Nevertheless, attaining full recovery is seldom accomplished. Treatment failure is strongly linked to CSCs. CSCs are a subset of cancer cells that possess the capacity for self-renewal and differentiation. They possess an essential involvement in the initiation and perpetuation of tumors, which is why they are sometimes referred to as tumor-initiating cells or tumor-spreading cells. CSCs initiate the tumors formation and contribute to the diversity of cancer by generating cancer cells that undergo differentiation while in a dormant state. In general, the survival of CSCs is critical to the effectiveness of cancer treatment regimens. In this study, by examining the transmission electron microscopy, self-renewal ability, and expression levels of classical CSC-labeled proteins (CD133, SOX2, Nanog, and Oct4), we showed that CDN could significantly weaken CSCs from OS cells, demonstrating that CDN is a potential CSC-targeted antitumor agent for OS treatment.
Autophagy is an essential step for the survival of CSCs. Autophagy is a cellular mechanism that involves the digestion of cytoplasmic components, such as proteins, damaged organelles, and lipids. These components are broken down and recycled inside a structure called the autophagosome. Autophagy is crucial for maintaining cell homeostasis under normal circumstances. Autophagy may have contrasting effects, either suppressing or promoting tumors, depending on several factors such as the kind and stage of the tumor, the local microenvironment of the tumor, and the particular therapy being used for cancer. Likewise, the contrasting impact of autophagy has been perceived as both beneficial and detrimental in CSCs. Autophagy is crucial for the preservation and viability of CSCs. For example, the pluripotency of CSCs requires autophagy26and the absence or inhibition of autophagy results in the inhibition of dry features and the CSC phenotype27,28. Co-administering autophagy inhibitors may enhance the effectiveness of pharmaceutical medicines29. On the other hand, autophagy induction can promote autophagy death or apoptosis in CSCs30. In the present study, multiple methods for detecting autophagy, including WB analysis of autophagy-labeled proteins, mRFP-GFP-LC3B adenovirus transfection, and observation of the number of autophagosomes and lysosomes, were used to determine whether CDN can induce autophagy in OS cells. When autophagy was blocked, the inhibitory effect of CDN on CSC characteristics was partially offset. Therefore, CDN-induced autophagy has an inhibitory effect on cancer stem-like cell stemness.
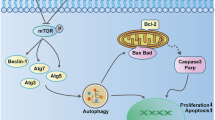
Accumulating data indicates that CSCs are controlled by crucial signaling pathways inside tumors, including the Wnt, Hedgehog, and Notch pathways31,32,33. For instance, the activation of Wnt in OS induces dryness and chemical resistance in circulating tumor cells (CSCs). The interaction between autophagy and the WBC pathway has been seen in several biological activities14,34. The presence of motifs for LC3 interactions was detected in β-catenin. Activation of autophagy leads to the inhibition of the Wnt signaling pathway by the binding of LC3 to β-catenin, followed by the destruction of β-catenin by autophagy35. Conversely, blocking the WBC pathway may trigger autophagy36. Autophagy and the Wnt/beta-catenin pathway exhibit reciprocal inhibition. The reciprocal regulatory connection between autophagy produced by CDN and the WBC signaling pathway was verified by employing WB analysis (Fig. 7).
Finally, this project has certain limitations. This investigation suggested that CDN-stimulated autophagy is involved in inhibiting OS stemness via CDN. However, the molecular mechanism of CDN-induced autophagy crosstalk and CSC remodeling remains unclear. Furthermore, the findings of the current research indicate that CDN has the potential to attenuate the activity of CSCs derived from OS cells. Given the role of CSCs in initiating and maintaining the tumor, it is necessary to explore the chemotherapy resistance of CDN for OS treatment. Therefore, considerable effort should be made to solve these questions in future research work to reveal a complete story.
The current investigation revealed that CDN successfully suppressed the viability, proliferation, and stemness of OS cells. Notably, CDN significantly induced autophagy, which prevented the stemness of OS. Our study also revealed that CDN attenuates the stemness of OS by inhibiting the WBC signaling pathway. In addition, the induction of autophagy by CDN had an impact on the WBC pathway. Ultimately, the effectiveness of CDN in inhibiting tumor activity was verified by in vivo investigations conducted on an OS xenograft model. This investigation provides an experimental basis for the use of CDN as antitumor drugs for targeting CSCs for the treatment of OS (Fig. 9).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Wang, S. et al. Bruceine D inhibits tumor growth and stem cell-like traits of osteosarcoma through Inhibition of STAT3 signaling pathway. Cancer Med. 8 (17), 7345–7358. https://doi.org/10.1002/cam4.2612 (2019).
Filbeck, S., Cerullo, F., Pfeffer, S. & Joazeiro, C. A. P. Ribosome-associated quality-control mechanisms from bacteria to humans. Mol. Cell. 82 (8), 1451–1466. https://doi.org/10.1016/j.molcel.2022.03.038 (2022).
Wang, W. et al. Targeting CSC-related transcription factors by E3 ubiquitin ligases for cancer therapy. Semin Cancer Biol. 87, 84–97. https://doi.org/10.1016/j.semcancer.2022.11.002 (2022).
Köseer, A. S. et al. Immunotargeting of cancer stem cells. Cancers (Basel). 15 (5). https://doi.org/10.3390/cancers15051608 (2023).
Zhang, H. et al. A novel molecular classification method for osteosarcoma based on tumor cell differentiation trajectories. Bone Res. 11 (1), 1. https://doi.org/10.1038/s41413-022-00233-w (2023).
Bheemasankara Rao, C., Namosiva Rao, T. & Suryaprakasam, S. Cardamonin and alpinetin from the seeds of amomum subulatum. Planta Med. 29 (4), 391–392. https://doi.org/10.1055/s-0028-1097682 (1976).
Yadav, V. R., Prasad, S. & Aggarwal, B. B. Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins. Br. J. Pharmacol. 165 (3), 741–753. https://doi.org/10.1111/j.1476-5381.2011.01603.x (2012).
Basit, A. et al. Evaluation of the anti-inflammatory, antioxidant, and cytotoxic potential of cardamine Amara L. (Brassicaceae): A comprehensive biochemical, toxicological, and in Silico computational study. Front. Chem. 10, 1077581. https://doi.org/10.3389/fchem.2022.1077581 (2022).
Qin, Y. et al. Cardamonin exerts potent activity against multiple myeloma through Blockade of NF-κB pathway in vitro. Leuk. Res. 36 (4), 514–520. https://doi.org/10.1016/j.leukres.2011.11.014 (2012).
Zhou, X. et al. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anticancer Drugs. 30 (3), 241–250. https://doi.org/10.1097/cad.0000000000000709 (2019).
Hou, S. et al. Cardamonin attenuates chronic inflammation and tumorigenesis in colon. Cell. Cycle. 18 (23), 3275–3287. https://doi.org/10.1080/15384101.2019.1673620 (2019).
Chen, H. et al. Anti-inflammatory effects of Cardamonin in ovarian cancer cells are mediated via mTOR suppression. Planta Med. 84 (16), 1183–1190. https://doi.org/10.1055/a-0626-7426 (2018).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147 (4), 728–741. https://doi.org/10.1016/j.cell.2011.10.026 (2011).
Debnath, J., Gammoh, N. & Ryan, K. M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell. Biol. 24 (8), 560–575. https://doi.org/10.1038/s41580-023-00585-z (2023).
Piletic, K., Alsaleh, G. & Simon, A. K. Autophagy orchestrates the crosstalk between cells and organs. EMBO Rep. 24 (9), e57289. https://doi.org/10.15252/embr.202357289 (2023).
Martins-Neves, S. R., Sampaio-Ribeiro, G. & Gomes, C. M. F. Self-renewal and pluripotency in osteosarcoma stem cells’ chemoresistance: Notch, hedgehog, and Wnt/β-catenin interplay with embryonic markers. Int. J. Mol. Sci. 24 (9). https://doi.org/10.3390/ijms24098401 (2023).
Najafi, M., Farhood, B. & Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 234 (6), 8381–8395. https://doi.org/10.1002/jcp.27740 (2019).
Wang, J. et al. N6-Methyladenosine-Mediated Up-Regulation of FZD10 regulates liver cancer stem cells’ properties and lenvatinib resistance through WNT/β-Catenin and Hippo signaling pathways. Gastroenterology 164 (6), 990–1005. https://doi.org/10.1053/j.gastro.2023.01.041 (2023).
Nusse, R. & Clevers, H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell 169 (6), 985–999. https://doi.org/10.1016/j.cell.2017.05.016 (2017).
Liu, X. et al. Section 62 promotes stemness and chemoresistance of human colorectal cancer through activating Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res. 40 (1), 132. https://doi.org/10.1186/s13046-021-01934-6 (2021).
Huang, Q. et al. CD44(+) lung cancer stem cell-derived pericyte-like cells cause brain metastases through GPR124-enhanced trans-endothelial migration. Cancer Cell. 41 (9), 1621–1636. https://doi.org/10.1016/j.ccell.2023.07.012 (2023). .e1628.
Zhou, M. et al. N(6)-methyladenosine modification of REG1α facilitates colorectal cancer progression via β-catenin/MYC/LDHA axis mediated glycolytic reprogramming. Cell. Death Dis. 14 (8), 557. https://doi.org/10.1038/s41419-023-06067-6 (2023).
Mehdawi, L. M. et al. LGR5 expression predicting poor prognosis is negatively correlated with WNT5A in colon cancer. Cells 12 (22). https://doi.org/10.3390/cells12222658 (2023).
Liu, K. et al. Hypoxia-induced GLT8D1 promotes glioma stem cell maintenance by inhibiting CD133 degradation through N-linked glycosylation. Cell. Death Differ. 29 (9), 1834–1849. https://doi.org/10.1038/s41418-022-00969-2 (2022).
Yuan, L. et al. Identification and gene expression profiling of human gonadotrophic pituitary adenoma stem cells. Acta Neuropathol. Commun. 11 (1), 24. https://doi.org/10.1186/s40478-023-01517-w (2023).
Sharif, T. et al. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy 13 (2), 264–284. https://doi.org/10.1080/15548627.2016.1260808 (2017).
Zhang, D. et al. Defective autophagy leads to the suppression of stem-like features of CD271(+) osteosarcoma cells. J. Biomed. Sci. 23 (1), 82. https://doi.org/10.1186/s12929-016-0297-5 (2016).
Zhang, L., Xu, L., Zhang, F. & Vlashi, E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell. Cycle. 16 (8), 737–745. https://doi.org/10.1080/15384101.2016.1241929 (2017).
Pagotto, A. et al. Autophagy Inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell. Death Dis. 8 (7), e2943. https://doi.org/10.1038/cddis.2017.327 (2017).
Kumar, D., Shankar, S. & Srivastava, R. K. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol. Cancer. 12 (1), 171. https://doi.org/10.1186/1476-4598-12-171 (2013).
Zhao, H. et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol. Cancer. 21 (1), 144. https://doi.org/10.1186/s12943-022-01616-7 (2022).
Fan, X. et al. NOTCH pathway Blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 28 (1), 5–16. https://doi.org/10.1002/stem.254 (2010).
Liu, T. et al. Circulating glioma cells exhibit stem Cell-like properties. Cancer Res. 78 (23), 6632–6642. https://doi.org/10.1158/0008-5472.Can-18-0650 (2018).
Lorzadeh, S., Kohan, L., Ghavami, S. & Azarpira, N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. Biophys. Acta Mol. Cell. Res. 1868 (3), 118926. https://doi.org/10.1016/j.bbamcr.2020.118926 (2021).
Petherick, K. J. et al. Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. Embo J. 32 (13), 1903–1916. https://doi.org/10.1038/emboj.2013.123 (2013).
Nàger, M. et al. Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers. Autophagy 14 (4), 619–636. https://doi.org/10.1080/15548627.2017.1423439 (2018).
Funding
This work was funded by Zhejiang Province Traditional Chinese Medicine Science and Technology Project to X.L. (Grant# 2025ZL588), Shaoxing Health Science and Technology Project to L.Z. (Grant#2022KY004) and to M.L.( Grant# 2022KY001).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.Z. and Y.L.; methodology, X.L.; software, Y.J. and K.W.; validation, Z.L., M.Y. and X.L.; formal analysis, Z.L. and Y.J.; investigation, L.Z. and M.L.; resources, Y.L.; data curation, L.Y. and Y.J.; writing—original draft preparation, L.Z.; writing—review and editing, Q.D. and X.L.; visualization, M.Y.; supervision, Z.L.; project administration, L.Z.; funding acquisition, X.L., Z.L. and M.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Jin, Y., Ding, Q. et al. Cardamonin inhibits the growth and stemness of osteosarcoma stem cells by inducing autophagy and inhibiting the Wnt/β-catenin signaling pathway. Sci Rep 15, 29484 (2025). https://doi.org/10.1038/s41598-025-14209-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14209-3