Abstract
Pulmonary fibrosis (PF) is a progressive, irreversible disease with limited effective treatments. Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) have recently shown promise in PF therapy. This study evaluated the therapeutic effects and mechanisms of UC-MSCs combined with pirfenidone (PFD) in a bleomycin-induced PF model. C57BL/6 mice were divided into nine groups: control, model, PFD (100 mg/kg), UC-MSCs (0.5 × 106, 1.0 × 106, and 2.0 × 106 cells per animal), and PFD (100 mg/kg) + UC-MSCs (0.5 × 106, 1.0 × 106, and 2.0 × 106 cells per animal). The model was established by intratracheal bleomycin instillation. PFD was administered intraperitoneally twice daily from day 4 to 21 in the PFD and PFD + UC-MSCs groups, and UC-MSCs were injected via the tail vein on day 4 in the UC-MSCs and PFD + UC-MSCs groups. Outcomes included pulmonary function, histopathology, inflammatory factors (TGF-β1, INF-γ, IL-6) in serum, bronchoalveolar lavage fluid (BALF) and lung tissues, and markers of fibrosis (α-SMA, Collagen I) and collagen deposition. The results showed that the high-dose UC-MSCs group and the PFD + high-dose UC-MSCs group achieved the most significant improvements across all parameters compared to other groups (P < 0.05). The PFD + high-dose UC-MSCs group showed the most reduction in p-SMAD 2/3 levels (P < 0.05) and greater inhibition of pro-fibrotic gene expression. This suggests that the combined treatment effectively mitigates bleomycin-induced PF by inhibiting the TGF-β/SMAD pathway. High-dose UC-MSCs combined with PFD offers superior therapeutic effects for PF treatment.
Similar content being viewed by others
Introduction
The most common type of interstitial lung disease (ILD) is idiopathic pulmonary fibrosis (IPF), accounting for one-third of ILD cases1. IPF is a chronic, progressive fibrosing interstitial lung disease of unknown cause. It results from chronic epithelial cell injury2,3 leading to increased pulmonary fibrosis. The median survival time for IPF patients is only 3–5 years4 with a prognosis worse than that of many types of cancer5,6. In Xinjiang Kashi, IPF is particularly prevalent due to local environmental factors and residents’ lifestyle habits. According to the IPF treatment guidelines (2015 edition), pirfenidone (PFD) is conditionally recommended as a therapeutic drug, but its efficacy as a monotherapy is limited due to the high effective doses and numerous adverse effects7. Therefore, finding an improved treatment approach is critically important and an urgent need for patients with pulmonary fibrosis in Kashi.
Human umbilical cord-derived mesenchymal stem cells (UC-MSCs), sourced from human umbilical tissue, are widely used in preclinical studies on pulmonary regenerative medicine due to their broad availability, safe collection process, lack of ethical restrictions, and low immunogenicity8. Some studies have compared the efficacy of pirfenidone (PFD) or nintedanib with MSCs derived from various sources (bone marrow, adipose tissue, or umbilical cord) in treating bleomycin-induced fibrosis models7,9,10. However, research on the combination of traditional anti-fibrotic drugs with MSCs remains limited.
In the pathogenesis of IPF, persistent inflammatory responses stimulate excessive fibroblast proliferation, ultimately leading to fibrosis. Among these mechanisms, the TGF-β pathway plays a pivotal role in the initiation and maintenance of fibrosis11. PFD not only exhibits anti-inflammatory and antioxidant effects but also inhibits fibrosis by suppressing the TGF-β pathway, thereby reducing collagen synthesis12.
Thus, this study used bleomycin-induced pulmonary fibrosis mice model to evaluate the therapeutic efficacy and potential mechanisms of PFD, UC-MSCs, and their combination. This would advance a foundation for developing more effective and targeted therapeutic strategies for PF patients.
Results
Culture and identification of UC-MSCs
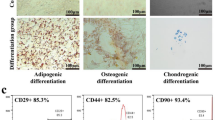
Primary UC-MSCs (P0) were cultured by adherence and passaged to the fourth generation (P4). After adherence culture and passaging, cell morphology gradually became fusiform (Fig. 1b). Flow cytometry was then performed on the P4 UC-MSCs to confirm their phenotype. The results showed negative expression for CD19, CD45, and CD34, while CD90 and CD105 were positively expressed (Fig. 1c), aligning with the identification criteria for MSCs derived from umbilical cord set by the International Society for Cellular Therapy.
UC-MSCs with or without PFD improved lung function in bleomycin induced pulmonary fibrosis mice
To evaluate the efficacy of different treatment methods on bleomycin-induced pulmonary fibrosis mice, we first assessed the pulmonary function status in each group (Fig. 1d). Compared with the control group, mice in the bleomycin group (model group) exhibited significantly impaired lung function, as evidenced by increased respiratory rate (f) and enhanced pause (Penh) (P < 0.05), and significantly decreased dynamic lung compliance (Cdyn), minute volume (MV), forced ventilation capacity (FVC), tidal volume (TV), inspiratory time (Ti), expiratory time (Te), peak expiratory flow (PEF), peak inspiratory flow (PIF), and expiratory flow at 50% tidal volume (EF50) (P < 0.05). Compared with the model group, the PFD group, UC-MSCs groups, and PFD + UC-MSCs groups showed significant improvement in pulmonary function status (P < 0.05). Compared with the PFD group, the UC-MSCs groups and PFD + UC-MSCs groups had more noticeable recovery in pulmonary function (P < 0.05), and the medium- and high-dose UC-MSCs groups as well as the PFD combined with medium and high-dose UC-MSCs groups showed significantly better effects than the low-dose groups (P < 0.05). There was no statistically significant difference in pulmonary function between the UC-MSCs groups and the PFD + UC-MSCs groups (P > 0.05). Compared with the control group, there was no difference in Te, Penh, FVC, PIF, Cydn, and TV after treatment with high-dose UC-MSCs and PFD combined with high-dose UC-MSCs (P > 0.05).
It was evident that high-dose of UC-MSCs, with or without PFD, could significantly improve lung function in IPF mice. Additionally, we observed that compared to the PFD + UC-MSCs groups, mice in the PFD group had poorer appetite, and the combination with UC-MSCs mitigated, to some extent, the gastrointestinal adverse reactions caused by PFD.
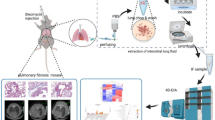
PFD combined with UC-MSCs improves lung function in IPF mice more effectively. (a) Illustration of the study design. Day 1: the control group received 50 µL of saline intratracheally, the remaining mice were administered intratracheally with the same volume of bleomycin (3 mg/kg) to induce pulmonary fibrosis. From day 4 to day 21, mice in the PFD group and the PFD combined cell therapy groups received PFD (100 mg/kg, two times/day) via intraperitoneal injection. Mice in the remaining groups received intraperitoneal injections of an equivalent volume of saline. Mice treated with UC-MSCs (200 µL per animal) received one time different concentrations (low UC-MSCs: 0.5 × 106 cells per animal, medium UC-MSCs: 1.0 × 106 cells per animal, high UC-MSCs: 2.0 × 106 cells per animal) via tail vein on day 4. Mice in the remaining groups received the same volume of saline once by tail vein on day 4. (b) The P4 UC-MSCs morphology (40 × ). (c) Flow cytometric analysis of UC-MSCs after the fourth passage of culture. (d) Lung function after UC-MSCs injection with or without PFD (n = 6). f: respiratory rate, Penh: enhanced pause, Cdyn: dynamic lung compliance, MV: minute volume, FVC: forced ventilation capacity, TV: tidal volume, Ti: inspiratory time, Te: expiratory time, PEF: peak expiratory flow, PIF: peak inspiratory flow, EF50: expiratory flow 50. Data were expressed as means ± SD. The significant difference was analyzed by the letter-based method. Groups that share at least one common letter were considered not significantly different from each other, while groups with completely different letters were considered significantly different. (P < 0.05). Abbreviations: PFD: pirfenidone. PFD + Low: PFD + Low UC-MSCs. PFD + Medium: PFD + Medium UC-MSCs. PFD + High: PFD + High UC-MSCs. Low: Low UC-MSCs. Medium: Medium UC-MSCs. High: High UC-MSCs.
UC-MSCs with or without PFD reduced inflammation and collagen deposition
To better understand the inflammatory infiltration before and after treatments, we measured the levels of TGF-β1, INF-γ, and IL-6 in serum, BALF, and lung tissues, and assessed the cell counts in BALF. Additionally, we evaluated the levels of hydroxyproline (HYP) in the lung tissues.
Compared to the controls, levels of TGF-β1, INF-γ, and IL-6 in serum, BALF, and lung tissue were significantly increased in the model group (P < 0.05). Compared to the model group, the levels of TGF-β1, INF-γ, and IL-6 in the serum (Fig. 2a), BALF (Fig. 2b) and tissues (Fig. 2c) were significantly decreased in the PFD group, UC-MSCs groups, and PFD + UC-MSCs groups (P < 0.05). Compared to the PFD group, the decrease in TGF-β1 in serum and BALF was more pronounced in the high-dose UC-MSCs group and the PFD + high-dose UC-MSCs group (P < 0.05), with no difference in tissues (P > 0.05). The levels of INF-γ and IL-6 in serum, BALF, and tissues significantly decreased after treatment with high-dose UC-MSCs and PFD combined with high-dose UC-MSCs (P < 0.05). There was no statistical difference in TGF-β1, INF-γ, and IL-6 between the UC-MSCs groups and the PFD + UC-MSCs groups (P > 0.05). Compared to the control group, there was no difference in the levels of TGF-β1, INF-γ, and IL-6 in serum, BALF, and tissues after treatments with high-dose UC-MSCs and PFD combined with high-dose UC-MSCs (P > 0.05).
The cell counts in BALF (Fig. 2d) showed that compared to the control group, the total cells, lymphocytes, neutrophils, and macrophages were significantly elevated (P < 0.05). Compared to the model group, the total cells, neutrophils, lymphocytes, and macrophages significantly decreased after treatments in the PFD group, UC-MSCs groups, and PFD + UC-MSCs groups (P < 0.05). Compared to the PFD group, the total cells were significantly reduced after treatments with UC-MSCs and PFD + UC-MSCs (P < 0.05), while the neutrophils were only different in the high-dose UC-MSCs group and the PFD + high-dose UC-MSCs group (P < 0.05), with no differences observed in lymphocytes and macrophages (P > 0.05). There were no statistically significant difference in the number of various types of cells between the UC-MSCs groups and the PFD + UC-MSCs groups (P > 0.05).
The HYP results (Fig. 2e) showed that compared to the controls, the levels of HYP were significantly elevated in the model group (P < 0.05). Compared to the model group, the PFD group, UC-MSCs groups, and PFD + UC-MSCs groups all significantly reduced HYP levels after treatments (P < 0.05). Although there were no significant differences in HYP levels among the PFD group, UC-MSCs groups, and PFD + UC-MSCs groups (P > 0.05), the high-dose UC-MSCs group and the PFD + high-dose UC-MSCs group showed a greater reduction in HYP levels. (P > 0.05). Compared to the control group, there were no differences in HYP levels after treatments with the high-dose UC-MSCs group and the PFD + high-dose UC-MSCs group (P > 0.05).
It was evident that high-dose of UC-MSCs, with or without PFD, could significantly reduce inflammation infiltration and collagen deposition in IPF mice. The combination with high-dose UC-MSCs showed a better effect.
Levels of inflammatory cytokines and HYP. (a) Levels of TGF-β1, INF-γ and IL-6 in serum (n = 6). (b) Levels of TGF-β1, INF-γ and IL-6 in BALF (n = 6). (c) Levels of TGF-β1, INF-γ and IL-6 in lung tissues (n = 6). (d) Cell counts in BALF (n = 6). (e) HYP levels in lung tissues (n = 6). Data were expressed as means ± SD. The significant difference was analyzed by the letter-based method. Groups that share at least one common letter were considered not significantly different from each other, while groups with completely different letters were considered significantly different. (P < 0.05). Abbreviations: PFD: pirfenidone. PFD + Low: PFD + Low UC-MSCs. PFD + Medium: PFD + Medium UC-MSCs. PFD + High: PFD + High UC-MSCs. Low: Low UC-MSCs. Medium: Medium UC-MSCs. High: High UC-MSCs.
UC-MSCs with PFD reverse histopathological damage
The ultimate goal of this study was to evaluate the therapeutic potential of UC-MSCs, with or without PFD, for treating bleomycin-induced pulmonary fibrosis model.
H&E staining (Fig. 3a) showed that prominent infiltration of neutrophils, lymphocytes, and macrophages around bronchioles in the model group. Structural damage such as thinning or rupture of alveolar walls and enlarged alveolar spaces was observed in H&E staining. Inflammation scores were significantly higher (P < 0.05). Compared to the model group, treatments with PFD, UC-MSCs, and PFD + UC-MSCs could alleviate the infiltration of inflammatory cells around the bronchioles in lung tissues. The degree of inflammatory cell infiltration was further reduced when UC-MSCs were used in combination. Additionally, the effect of UC-MSCs alone was similar to PFD + UC-MSCs groups. The inflammation scores (Fig. 3c) indicated that high-dose UC-MSCs and PFD combined with high-dose UC-MSCs were the most effective in reducing the inflammation scores (P < 0.05), which was consistent with histology findings.
Masson trichome staining (Fig. 3a) revealed that thickened alveolar walls and increased collagen deposition around bronchioles in the model group, with stronger blue staining and larger fibrotic areas. Ashcroft scores were significantly elevated (P < 0.05). Compared to the model group, treatments with PFD, UC-MSCs, and PFD + UC-MSCs resulted in a significant reduction in blue-stained collagen in lung tissues. Compared with the PFD group, blue-stained collagen was further reduced after combined treatment with UC-MSCs, with the high-dose UC-MSCs group showing the most pronounced reduction. The effect of UC-MSCs alone was similar to that of the combination with PFD in terms of blue staining. The Ashcroft scores (Fig. 3c) also showed a downward trend. Compared with the PFD group, the scores of the PFD + high-dose UC-MSCs, the medium- and high-dose UC-MSCs groups were significantly decreased (P < 0.05), further supporting the beneficial effects of UC-MSCs treatment, either alone or in combination with PFD. There was no significant difference in Ashcroft scores between the UC-MSCs groups and the PFD + UC-MSCs groups (P > 0.05).
Subsequently, the levels of fibrosis markers were assessed in each group (Fig. 3b, d). Western blot analysis revealed increased expression of α-SMA, Collagen I, and p-SMAD 2/3 in the model group (P < 0.05). Compared to the model group, treatment with PFD, UC-MSCs, and PFD + UC-MSCs significantly reduced the levels of α-SMA, Collagen I, and p-SMAD 2/3 (P < 0.05). There were no significant differences in α-SMA among the PFD group, UC-MSCs groups, and PFD + UC-MSCs groups (P > 0.05). Compared to the PFD group, the decrease in Collagen I was more pronounced in the high-dose UC-MSCs group and the PFD + high-dose UC-MSCs group (P < 0.05). Although the levels of p-SMAD 2/3 decreased in both UC-MSCs groups and PFD + UC-MSCs groups, only PFD + high-dose UC-MSCs group showed a statistically significant difference (P < 0.05). Compared to the PFD + UC-MSCs groups, the levels of α-SMA and Collagen I were slightly higher in UC-MSCs groups, but there were no statistical differences (P > 0.05). However, the decrease in p-SMAD 2/3 were significantly better in PFD + UC-MSCs groups than in UC-MSCs groups (P < 0.05). At the same time, although the levels of α-SMA, and p-SMAD 2/3 in PFD + UC-MSCs groups were higher than in control group, the differences were not statistically significant (P > 0.05).
It was evident that the combination of high-dose UC-MSCs with PFD had a more pronounced effect in reducing fibrosis markers and slowing the progression of fibrosis.
UC-MSCs with PFD reverse histopathological damage. (a) H&E and Masson trichome staining (n = 6). Scale bars are 1000 and 200 μm. (b, d) WB analysis of α-SMA, collagen I, SMAD2/3, and p-SMAD2/3 (n = 3). Original gels were presented in Supplementary Figs. 1 and 2. (c) Inflammatory scores and Ashcroft scores (n = 6). Data were expressed as means ± SD. The significant difference was analyzed by the letter-based method. Groups that share at least one common letter were considered not significantly different from each other, while groups with completely different letters were considered significantly different. (P < 0.05). Abbreviations: PFD: pirfenidone. PFD + Low: PFD + Low UC-MSCs. PFD + Medium: PFD + Medium UC-MSCs. PFD + High: PFD + High UC-MSCs. Low: Low UC-MSCs. Medium: Medium UC-MSCs. High: High UC-MSCs.
Discussion
In this study, we used PFD, UC-MSCs, and the combination of UC-MSCs with PFD to treat bleomycin-induced pulmonary fibrosis mice model. We found that UC-MSCs alone or in combination with PFD had comparable effects on improving lung function and reducing inflammatory infiltration damage. Regarding anti-fibrotic effects, the combination of PFD and UC-MSCs was superior to UC-MSCs alone. Moreover, the combination treatment reduced the adverse effects of PFD in mice, which could enhance the tolerance and compliance of IPF patients with PFD therapy in clinical settings.
IPF patients primarily exhibited progressive dyspnea and hypoxemia13 with severe cases leading to death from respiratory failure14. Pulmonary function is a key indicator of therapeutic efficacy in clinical trials and is also used to assess the extent of fibrosis and treatment effects in animal models15. Previous clinical studies had shown that PFD helped restore FVC and better improved the 6-minute walk distance (6MWD)12,16. However, PFD did not ameliorate respiratory symptoms or improve the acute exacerbation rate, and patients’ lung function continued to decline17. Therefore, new treatment approaches or combination therapies were urgently needed to improve lung function more effectively. Clinical studies on MSCs for pulmonary diseases had demonstrated significant improvements in FVC, 6MWD, and diffusing capacity of the lung for carbon monoxide (DLCO) after 12 months of treatment18,19. In this study, both high-dose UC-MSCs alone and combined with PFD significantly restored lung function in IPF mice. Metrics such as Te, Penh, FVC, PIF, Cydn, and TV showed marked improvement following UC-MSCs treatment, with values approaching those of the control group and lacking statistical differences. These findings align with human clinical trials’ results, indicating that UC-MSCs, either alone or in combination with PFD, effectively restore pulmonary function and reduce pulmonary edema in IPF mice. It was beneficial for improving the quality of life of clinical patients, enhancing their treatment adherence, and contributing to better clinical outcomes.
Additionally, significant inflammatory infiltration in the lung tissue of IPF patients is one of the primary drivers of disease progression13. Previous studies had shown that neutrophils20 macrophages21 lymphocytes22 and their derivatives participated in IPF progression at various stages. Research indicated that MSCs could reduce abnormal neutrophil accumulation in lung tissues, thereby alleviating inflammation23,24. In this study, high-dose UC-MSCs, whether combined with PFD or not, significantly alleviated inflammatory damage in the serum, BALF, and lung tissues of IPF mice, as well as reduced the number of inflammation-related cells in BALF. Notably, the combination with high-dose UC-MSCs led to a more pronounced reduction in inflammatory markers, although the differences were not statistically significant. These findings were consistent with previous research.
Another critical pathological mechanism of IPF is the excessive collagen deposition in lung tissues, which leads to distortion of the normal lung structure25. Therefore, quantifying the collagen content in lung tissues is crucial. Currently, the HYP content in total lung tissues is used as a surrogate marker for collagen content (1 mg HYP = 6.94 mg collagen) and serves as the gold standard for assessing the severity of pulmonary fibrosis25. In this study, after treatment with high-dose UC-MSCs combined with PFD, a significant reduction in HYP levels, Ashcroft scores, and fibrosis markers was observed in the lung tissues of IPF mice. This suggested that the combination of UC-MSCs and PFD indeed slowed the collagen deposition and fibrosis in lung tissues. Furthermore, PFD is a TGF-β inhibitor26 and TGF-β is a key factor in promoting the differentiation of fibroblasts into myofibroblasts11,27. Therefore, PFD can inhibit TGF-β-mediated fibroblast proliferation, differentiation into myofibroblasts, and excessive extracellular matrix (ECM) deposition by intervening in the TGF-β/SMAD pathway, thus slowing the progression of fibrosis28,29,30. In this study, although PFD, UC-MSCs, and PFD + UC-MSCs all significantly reduced the levels of α-SMA, Collagen I, and p-SMAD 2/3, the combined treatment of PFD and UC-MSCs showed markedly better results, with a more pronounced reduction in these markers (though the differences were not statistically significant). This suggested that the combination of UC-MSCs and PFD enhanced the anti-fibrotic effects. This may be because UC-MSCs can secrete pro-fibrotic factors such as TGF-β31 but when combined with PFD, PFD partially inhibited UC-MSCs’ secretion of these pro-fibrotic factors, making the combination treatment more effective than UC-MSCs alone7.
However, the study has some limitations. The model mice used in this study were primarily in the early stages of fibrosis progression. Further research would explore the therapeutic efficacy of UC-MSCs combined with PFD in chronic fibrosis stages of IPF models, as well as to investigate the underlying mechanisms of this combination treatment in more depth.
Conclusion
In conclusion, the present study demonstrated that UC-MSCs and PFD + UC-MSCs treatment had superior therapeutic effects (restoring lung function and alleviating inflammatory infiltration) compared to PFD treatment alone in the bleomycin-induced pulmonary fibrosis model. The combination of PFD and UC-MSCs showed a more pronounced inhibitory effect on pro-fibrotic genes and also suppressed the activation of the TGF-β/SMAD pathway, partially reversing the progression of fibrosis. This might provide a promising new perspective for the clinical treatment of IPF.
Methods
Animal ethics approval
All animal experiments were approved by the Ethics Committee of Xinjiang Medical University (IACUC-20231010-06), and adhered to ARRIVE guidelines and other relevant protocols. This study was conducted in accordance with relevant guidelines and regulations.
In this study, 8- to 9-week-old C57BL/6 male mice were provided by the Experimental Animal Center of Hangzhou Medical College [production license number SCXK (Zhejiang) 2019-0002]. The experiments were conducted in the SPF grade animal laboratory of Xinjiang Medical University Laboratory Animal Center. All experimental mice housed under standard condition with free access to food, water, temperature 22 ± 2 °C, and humidity of 60%-80%.
Experimental grouping and induction of pulmonary fibrosis in mice
Fifty-four C57BL/6 mice were randomized to the control group (n = 6), model group (n = 6), PFD group (n = 6), PFD + low UC-MSCs group (0.5 × 106 cells per animal, n = 6), PFD + medium UC-MSCs group (1.0 × 106 cells per animal, n = 6), PFD + high UC-MSCs group (2.0 × 106 cells per animal, n = 6), low UC-MSCs group (0.5 × 106 cells per animal, n = 6), medium UC-MSCs group (1.0 × 106 cells per animal, n = 6), and high UC-MSCs group (2.0 × 106 cells per animal, n = 6).
Induction of fibrosis in mice and the different treatment protocols were as follows (Fig. 1a). The control group received 50 µL of saline intratracheally at day 1. The remaining mice were anesthetized with 5% isoflurane and bleomycin was administered intratracheally at a dose of 3 mg/kg in 50 µL of saline on day 1 to induce pulmonary fibrosis.
From day 4 to day 21, mice in the PFD group and the PFD combined cell therapy groups received PFD via intraperitoneal injection (100 mg/kg, twice a day). Mice in the remaining groups received intraperitoneal injections of an equivalent volume of saline, twice a day. Mice treated with UC-MSCs (200 µL per animal) received one time different concentrations via tail vein on day 4. Mice in the remaining groups received the same volume of saline once by tail vein on day 4. All mice were sacrificed by carbon dioxide overdose on day 22 for relevant tests.
Culture and identification of UC-MSCs
In this study, UC-MSCs at passage 0 (P0) (CP-CL11, Punosai, China) were purchased from Wuhan Punosai Life Science and Technology Co. P0 was cultivated (37℃, 5% CO2) using specialised medium for UC-MSCs, replacing the cell medium every 48 h and observing the cell status under a microscope. The cells could be passaged when the cells reach 80–90% confluence. The UC-MSCs used in our experiments were the passage 4 cells. Cells were washed with saline after trypsinization, and the cell suspension was passed through a 100 μm filter to eliminate cell clumps. Then, cells were counted and resuspended in saline before injection and placed on ice for use.
Flow cytometry analysis
To identify the UC-MSCs, flow cytometry analysis was performed according to the guidelines of the International Society for Cell Therapy (ISCT). The ISCT defined UC-MSCs as expressing surface markers CD90 and CD105, while lacking expression of CD19, CD34, and CD45. UC-MSCs at the fifth passage were trypsinized, washed twice with PBS, and 1 × 105 cells were stained separately with CD19-FITC, CD34-FITC, CD45-APC, CD90-PE-Cy7, and CD105-APC (Thermo, USA). The cells were incubated with the antibodies at 4 °C in the dark for 15 min. After washing, the cells were resuspended in 400 µL of pre-cooled PBS and analyzed by flow cytometry (BF 710, URIT, China).
Pulmonary function assessment
Whole Body Plethysmography (WBP, Buxco Research Systems, Wilmington, NC) was used to perform pulmonary function tests. The small animal pulmonary function tester used in this study was non-invasive. The mice were placed into the tester, and after the mice were in a stable state with stable respiration, the basal values of respiration were recorded. Changes in respiratory rate (f), enhanced pause (Penh), dynamic lung compliance (Cdyn), minute volume (MV), forced ventilation capacity (FVC), tidal volume (TV), inspiratory time (Ti), expiratory time (Te), peak expiratory flow (PEF), peak inspiratory flow (PIF), and expiratory flow 50 (EF50) were expressed as an average of 3 min recordings.
Analysis of cells in BALF
BALF was collected as previously32. Then BALF was centrifuged (500 g, 10 min), the supernatant was used for cytokine analysis, the cell sediments were re-selected with PBS (1 mL). Hemocytometer was used to count total cells in BALF, and neutrophils, lymphocytes, macrophages count were performed using Wright and Giemsa staining (BASO, Zhuhai, China).
ELISA
The levels of TGF-β, INF-γ,and IL-6 were determined using ELISA kits (Multi Sciences, China) following the recommended protocols. HYP was assessed by the absorbance at 560 nm with a HYP assay kit (Elabsciecne, China).
Histology
Part of the left lung was used for histopathologic evaluation. Paraffin-embedded lung tissue blocks were serially cut into 4 μm thick Sect. (3 sections per mouse, three mice for each group) according to a previous study33. The sections were then stained with hematoxylin and eosin (H&E) and Masson trichome staining to evaluate the lung inflammatory and fibrosis level. H&E staining lung sections were observed under a light microscope (E200, Nikon, Japan) and scored for inflammation based on the infiltration degree of inflammatory cells around bronchioles, bronchi, blood vessels and pulmonary interstitium34. Each lung specimen was blindly scored by three independent pathologists.
Inflammation scores34 was as follows: 0 (no inflammatory cells infiltration), 1 (occasional inflammatory cells), 2 (1–2 layers of inflammatory cells around bronchioles or vessels), 3 (3–4 s layer of inflammatory cells), 4 (more than 5 layers of inflammatory cells around most bronchioles or vessels).
Pulmonary fibrosis severity was analyzed by Ashcroft scores (score range, 0–8)35. 0 (no fibrosis); 1–3 (mild fibrosis, localized to small areas with mostly preserved alveolar architecture); 4–5 (moderate fibrosis, distinct fibrotic regions with some alveolar structural disruption); 6–7 (severe fibrosis, majority of alveolar structures replaced by fibrotic tissue); 8 (very severe fibrosis, almost entire lung parenchyma replaced by fibrotic tissue).
RNA isolation and RT-qPCR
The left lung tissues of mice were washed by cold phosphate-buffered saline and lysed in TRIzol Reagent. DNase I (RNase-free) (M0303S, NEB, UK) was used to remove DNA. In the 50 µL reaction mixture, 2.5 µg RNA, 5 µL DNase I Reaction Buffer (10×), and 0.5 µL DNase I (RNase-free) were included, and the volume was adjusted to 50 µL with nuclease-free H2O. The reaction was incubated at 37 °C for 10 min, followed by heat inactivation at 75 °C for 10 min. RNA was changed into complimentary-DNA (AG11706, Accurate Biology, China). Expression of relative mRNA were analyzed by LightCycler® 480 SYBR Green I Master (Roche, Basel, Switzerland). The 2−△△Ct method was used to measure expression in reference to that of GAPDH.
Primers’ name | Primers’ sequences (5’→ 3’) |
|---|---|
TGF-β1-Forward | CTCCCGTGGCTTCTAGTGC |
TGF-β1-Reverse | GCCTTAGTTTGGACAGGATCTG |
INF-γ-Forward | ATGAACGCTACACACTGCATC |
INF-γ-Reverse | CCATCCTTTTGCCAGTTCCTC |
IL-6-Forward | CCAAGAGGTGAGTGCTTCCC |
IL-6-Reverse | CTGTTGTTCAGACTCTCTCCCT |
GAPDH-Forward | AGGTCGGTGTGAACGGATTTG |
GAPDH-Reverse | TGTAGACCATGTAGTTGAGGTCA |
Western blotting analysis
The left lung tissues were lysed in RIPA lysate containing protease inhibitor. Protein concentration was determined using bicinchoninic acid (BCA) reagent (TransGen Biotech, China). Equal amounts of proteins were added to SDS polyacrylamide gels for electrophoresis, and then transferred to PVDF membranes (Millipore, USA). After incubation with appropriate primary and secondary antibodies as followed, the protein bands were visualized with the Chemi scope imaging system.
Antibodies’ name | Company | Catalog number |
|---|---|---|
Anti-alpha smooth muscle actin | Abcam | ab124964 |
Anti-Collagen I | Abcam | ab270993 |
Anti-SMAD2/3 | Boster | BA1395 |
phospho-SMAD2/SMAD3 (Thr8) | Bioss | bs8853R |
Anti-mouse IgA + IgG + IgH (H + L) | Abcam | ab205719 |
Anti-rabbit IgG (H + L) | Abcam | ab205718 |
Statistical analysis
The statistical analysis and graphing were performed using GraphPad Prism 10 software (GraphPad Software, USA). Data were expressed as means ± SD. Differences among the groups were analyzed by one-way ANOVA followed by Dunnett’s T3 multiple comparisons test or Tukey’s multiple comparisons test. The differences between groups were indicated using letter labeling, groups with the same letter label indicate no significant difference, while groups with different letter labels indicate significant differences. P < 0.05 was considered statistically significant.
Data availability
The original data presented in the study are included in the article/ supplementary material, further inquiries can be directed to the corresponding author/s.
References
Maher, T. M. Interstitial lung disease: A review. JAMA 331, 1655–1665 (2024).
Wang, J. et al. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin B. 12, 18–32 (2022).
Cottin, V. et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 27, 180076 (2018).
Spagnolo, P. et al. Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol. Ther. 222, 107798 (2021).
Hutchinson, J. et al. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur. Respir J. 46, 795–806 (2015).
Richeldi, L. et al. Idiopathic pulmonary fibrosis. Lancet 389, 1941–1952 (2017).
Wu, X. et al. Human umbilical cord mesenchymal stem cells combined with Pirfenidone upregulates the expression of RGS2 in the pulmonary fibrosis in mice. Respir Res. 23, 270 (2022).
Wang, G. et al. Pre-clinical study of human umbilical cord mesenchymal stem cell transplantation for the treatment of traumatic brain injury: safety evaluation from Immunogenic and oncogenic perspectives. Neural Regen Res. 17, 354–361 (2022).
Fikry, H. et al. Therapeutic effect of adipose-derived mesenchymal stem cells (AD-MSCs) compared to Pirfenidone on corticosteroid resistance in a mouse model of acute exacerbation of idiopathic pulmonary fibrosis. Histol. Histopathol. 37, 1065–1083 (2022).
Gad, E. S. et al. The Anti-fibrotic and Anti-inflammatory potential of bone Marrow-Derived mesenchymal stem cells and nintedanib in Bleomycin-Induced lung fibrosis in rats. Inflammation 43, 123–134 (2020).
Bellaye, P. S. et al. Macitentan reduces progression of TGF-beta1-induced pulmonary fibrosis and pulmonary hypertension. Eur Respir J 52, 1701857 (2018).
King, T. E. Jr. et al. A phase 3 trial of Pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl. J. Med. 370, 2083–2092 (2014).
George, P. M. et al. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 8, 807–815 (2020).
Yang, Y. et al. Mesenchymal stem cells and pulmonary fibrosis: a bibliometric and visualization analysis of literature published between 2002 and 2021. Front. Pharmacol. 14, 1136761 (2023).
Aggarwal, S. et al. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L433–452 (2016).
Maher, T. M. et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 8, 147–157 (2020).
Srour, N. & Thebaud, B. Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: A systematic review. Stem Cells Transl Med. 4, 1500–1510 (2015).
Averyanov, A. et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med. 9, 6–16 (2020).
Glassberg, M. K. et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): A phase I safety clinical trial. Chest 151, 971–981 (2017).
Le Saux, C. J. & Chapman, H. A. Idiopathic pulmonary fibrosis: cell death and inflammation revisited. Am. J. Respir Cell. Mol. Biol. 59, 137–138 (2018).
Rao, L. Z. et al. IL-24 deficiency protects mice against bleomycin-induced pulmonary fibrosis by repressing IL-4-induced M2 program in macrophages. Cell. Death Differ. 28, 1270–1283 (2021).
Senoo, S. et al. Essential role of IL-23 in the development of acute exacerbation of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 321, L925–L940 (2021).
Silva, J. D. et al. Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue-derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell. Res. Ther. 10, 264 (2019).
Navas, A. et al. Anti-Inflammatory and Anti-Fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cells Transl Med. 7, 906–917 (2018).
Heukels, P. et al. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med. 147, 79–91 (2019).
Reddy, M. et al. Human Adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: comparison with Pirfenidone. Int. J. Stem Cells. 9, 192–206 (2016).
Huang, Y. et al. TGF-beta1-induced miR-424 promotes pulmonary myofibroblast differentiation by targeting Slit2 protein expression. Biochem. Pharmacol. 180, 114172 (2020).
Molina-Molina, M. et al. Anti-fibrotic effects of Pirfenidone and Rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm Med. 18, 63 (2018).
Mei, Q. et al. Idiopathic pulmonary fibrosis: an update on pathogenesis. Front. Pharmacol. 12, 797292 (2021).
Lacy, S. H. et al. Activated human lung fibroblasts produce extracellular vesicles with antifibrotic prostaglandins. Am. J. Respir Cell. Mol. Biol. 60, 269–278 (2019).
Alvites, R. et al. Mesenchymal stem/stromal cells and their paracrine Activity-Immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics 14, 381 (2022).
Zhang, W. et al. Schistosoma Japonicum peptide SJMHE1 suppresses airway inflammation of allergic asthma in mice. J. Cell. Mol. Med. 23, 7819–7829 (2019).
Moroncini, G. et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS One. 13, e0196048 (2018).
Dong, L. et al. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell. Res. Ther. 12, 4 (2021).
Akter, T. et al. IQGAP1 regulates actin polymerization and contributes to Bleomycin-Induced lung fibrosis. Int J. Mol. Sci 25, 5244 (2024).
Acknowledgements
We thanks all the participants in this study.
Funding
This study was supported by the Kashi Regional Science and Technology Program Projects (grants and KS2022033 and KS2023012), the Hospital Scientific research project of the First People’s Hospital of Kashi (grants KDYY202312), and Dedicated funding for stem cell research project of the First People’s Hospital of Kashi (grants 2023-13). The funder had no role in study design, data collection/analysis/interpretation or manuscript preparation.
Author information
Authors and Affiliations
Contributions
L.L. conceived and designed the study. J.X., Z.A., H.G., and L.X. performed the experiments. J.X., X.M.Z., and X.G.Z conducted the analysis. J.X wrote the first version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were approved by the Ethics Committee of Xinjiang Medical University (IACUC-20231010-06). This study was conducted in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, J., Abudureheman, Z., Gong, H. et al. Pirfenidone combined with UC-MSCs reversed bleomycin-induced pulmonary fibrosis. Sci Rep 15, 28339 (2025). https://doi.org/10.1038/s41598-025-14286-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14286-4