Abstract
The purpose of this study was to investigate the relationship. between serum osmolality levels and 28-day mortality in patients with DKA. Data for this observational cohort study were obtained from the MIMIC-IV3.0 database. The participants were divided into five groups based on the serum osmolality quintiles. The primary outcome was 28-day mortality. We employed Cox proportional hazards regression analysis and threshold effect analysis to assess. the relationship between serum osmolality levels and 28-day mortality in patients with DKA. The study included 1026 patients; the mean age was 52 years, 55.0% were male. Our findings indicate that serum osmolality is associated with an increased risk of 28-day mortality, exhibiting a U-shaped relationship. Altered serum osmolality levels, whether lower or higher, are linked to a heightened risk of mortality. When osmolality < 306.8 mOsm/kg, the 28-day mortality risk decreased by 6.2% (HR 0.938, 95% CI 0.912–0.964) for every 1 mOsm/kg increase. At osmolality ≥ 306.8 mOsm/kg, there was a 3.7% (HR 1.037, 95% CI 1.030–1.045) increase in the 28-day mortality risk for every 1 mOsm/kg increase in osmolality. The risk of mortality was lower at osmolality of 284–299 mOsm/kg. A U-shaped correlation between initial serum osmolality and 28-. days all-cause mortality in patients with DKA was identified. These results underscore. serum osmolality’s critical role in early mortality among patients with DKA.
Similar content being viewed by others
Introduction
Diabetic ketoacidosis (DKA) is a common diabetic emergency characterized by hyperglycemia, ketosis, and acidosis. This severe metabolic disorder results from the combined effects of insulin deficiency, insulin antagonism, and counterregulatory hormones. While type 1 diabetes is prone to spontaneous DKA, type 2 diabetes can also develop DKA1. DKA is linked to considerable morbidity and a high demand for health care resources, representing 4 ~ 9% of all hospital discharges among patients whose primary cause for acute hospitalization is diabetes mellitus2. The treatment of DKA remains a costly endeavor. In the United States, the estimated expense for a single DKA episode is around US$26,5663. Conversely, in the United Kingdom, the cost of treating one episode of DKA is approximated at £2,064 for adults and £1,387 for adolescents aged 11 to 18 years4,5. Elevated blood glucose levels and high blood ketone concentrations increase serum osmolality, leading to the movement of intracellular fluid outside the cells. This process results in cellular dehydration and osmotic diuresis. The severe depletion of water can lead to inadequate blood volume, decreased blood pressure, and potentially culminate in circulatory failure6,7. Serum osmolality refers to the total osmolality of ions and particles dissolved in body fluid, which is influenced by the concentrations of sodium (Na), potassium (K), glucose, and urea8. Elevated serum osmolality have been linked to negative outcomes in various clinical conditions, including heart failure9, acute myocardial infarction10, cerebral ischemia11 or sepsis12.
However, there is a scarcity of research investigating the relationship between serum osmolality levels and the prognosis in patients with DKA. In order to address this gap, an observational cohort study was conducted to investigate the potential of serum osmolality levels in predicting mortality in patients with DKA.
Methods
Study population
The researchers conducted an observational study using data from the publicly accessible Medical Information Mart for Intensive Care IV (MIMIC-IV 3.0) database. MIMIC-IV 3.0 (released 2022) is a publicly available critical care database containing de-identified ICU data from 2008 to 2022 at Beth Israel Deaconess Medical Center. It is an important database in the field of critical care. The database includes information such as demographic data, vital signs, laboratory parameters, comorbidities, and more, which can be found at https://mimic.mit.edu13. The patient cohort for this research comprised individuals with a confirmed diagnosis of DKA, following the codes outlined in the International Classification of Diseases, 9th and 10th Revision (25013, 25012, 25011, 24911, 25010, E1010, E1110, E1310, E0910, E0810, E1011, E1311). Ethical review and approval were waived for this study, due to reason: The use of the MIMIC-IV database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center and patient’s data were anonymized prior to publication.
The research excluded individuals below the age of 18 during their initial admission, individuals who experienced multiple admissions to the ICU due to DKA (only data from the first admission were considered). We excluded patients who met the following criteria: (1) under 18 years; (2) length of ICU stay < 24 h (we excluded these patients due to the substantial amount of missing laboratory test data for these patients); (3) patients diagnosed with Hyperosmolar Hyperglycemic State; (4) patients with missing baseline sodium, potassium, urea nitrogen, and glucose at admission; (5) data for which the calculated serum osmolality value is anomalous.
Data collection
To conduct the data extraction, we utilized PostgresSQL software and pgAdmin 4 tool by employing Structured Query Language (SQL). The extraction process prioritized four distinct categories of potential variables: demographic factors, vital signs, laboratory parameters, severity of illness score, comorbidities and treatment during ICU stay. All laboratory tests and vital signs data were measured for the first time within 24 h of ICU admission. Serum osmolality was calculated using the equation [2 × Na++ (glucose/18)]. Hyperglycemia in DKA can cause osmotic shifts of water into the extracellular space, diluting serum sodium levels and potentially masking true sodium concentrations, To address this, we have calculated corrected serum sodium using the established formula: Measured Na⁺ + 1.6 × [(Glucose – 100)/100].Variables with a missingness rate exceeding 20% were excluded from the model to avoid bias that might result from directly filling in missing values.
Outcomes
The main outcome of this study was 28-day mortality after ICU admission. Secondary outcomes focused on hospital length of stay and ICU length of stay.
Statistical analysis
The patients were categorized into five groups based on their admission serum osmolality levels: Quintile 1 (< 297 mOsm/kg), Quintile 2 (297–305 mOsm/kg), Quintile 3 (306–314 mOsm/kg), Quintile 4 (315–326 mOsm/kg) and Quintile 5 (> 326 mOsm/kg). Continuous variables were presented as the mean ± SD or median and interquartile range (IQR). Categorical variables were expressed as numbers or percentages (%). ANOVA analysis, the Kruskal-Wallis test for continuous variables, or the chi-square test for categorical data, as applicable.
Kaplan-Meier survival analysis was used to assess the incidence rate of primary outcome events in different stratified groups based on the serum osmolality. The log-rank test was employed to examine any observed disparities. Binary logistic regression analysis was conducted to evaluate factors influencing the risk of all-cause death.
Cox regression analysis was conducted to investigate the relationship between serum osmolality and 28-day mortality. We included all variables in the survival analysis. Model 1: unadjusted; Model 2 was adjusted for age, gender, temperature, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and saturation of peripheral oxygen (SPO2); Model 3 was further adjusted for white blood cell (WBC), platelet, hemoglobin, sodium, potassium, glucose, urea nitrogen, creatinine, lactate, Sequential Organ Failure Assessment (SOFA), Acute Physiology Score III (APS III), Simplified Acute Physiology Score II (SAPSII), Charlson Comorbidity Index (Charlson), type 2 diabetes, type 1 diabetes, sepsis, acute myocardial infarction (AMI), chronic kidney disease (CKD), acute kidney injury (AKI), the use of continuous renal replacement therapy (CRRT) and ventilation. HRs were counted and the findings were presented with 95% confidence intervals (CI).
Furthermore, an analysis was conducted to examine the association between serum osmolality and 28-day mortality among patients diagnosed with DKA, utilizing a multivariate-adjusted restricted cubic spline method. To identify the inflection point, a recursive algorithm was applied, and a two-piecewise Cox proportional hazards regression model was established. This model incorporated the values from both sides of the inflection point to assess the threshold impact of serum osmolality on 28-day mortality. The data analyses were conducted using R software (version 4.2.2). For all analyses, a 2-side P < 0.05 was considered statistically significant.
Results
Baseline characteristics
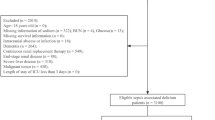
The study enrolled a total of 1026 critically ill patients with DKA from the MIMIC-IV database, as shown in Fig. 1.
Table 1 presents the baseline characteristics of all patients. The mean age of the study participants was 52 ± 16 years, with males comprising 55.0% of the sample. The patients in the group with higher serum osmolality were older, and had higher levels of WBC, sodium, glucose, urea nitrogen, creatinine, lactate, anion gap. These patients had higher disease severity scores, and comorbidities such as sepsis and acute AKI were relatively more common. There was no statistical difference in vital signs among the five groups (all p > 0.05). There was a higher utilization of CRRT and ventilation among patients with a higher serum osmolality. Overall, the all-cause mortality rate at 28 days of admission was 44.6%, and the difference in mortality rates between the five groups was statistically significant (47.8% vs. 38.0% vs. 34.8% vs. 48.0 vs. 54.3%, p < 0.001), indicating that lower or higher serum osmolality is associated with a higher risk of 28-day mortality.
Primary outcomes
Survival analysis
The 28-day risk of mortality among groups was analyzed using Kaplan-Meier survival analysis curves, based on the serum osmolality quintile as presented. Patients exhibited a higher short-term survival rate when serum osmolality was between 292 and 299 mOsm/kg and a lower survival rate when serum osmolality was below 284 mOsm/kg (Fig. 2).
Multivariable Cox regression analysis
Table 2 presents the results of the Cox regression conducted to assess the risk of all-cause death in patients with DKA. In the unadjusted model, the risk of mortality was increased by 54% (HR 1.54, 95%CI 1.10–2.05) in Q1 when compared with serum osmolality in Q3, and the risk of mortality was elevated by 83% (HR 1.83, 95%CI 1.43–2.31) in the highest quintile Q5. In model 2 (adjusted for age, gender, temperature, heart rate, SBP, DBP, SPO2), the results remained significant. In model 3 (adjusted for age, gender, temperature, heart rate, SBP, DBP, SPO2 and potential confounders), the risk of mortality was elevated by 72% (HR 1.72, 95%CI 1.30–2.21) in Q1 and 52% (HR 1.52, 95%CI 1.21–2.01) in Q5.
A lower and similar risk of mortality in Q2 and Q3 of osmolality was observed. As a result, Q2 and Q3 were merged, and Q2 + Q3 served as a reference for a multivariable Cox regression analysis. After adjusting for the covariates in model 3, we found that there was a 67% (HR 1.67, 95%CI 1.28–2.12) higher risk of mortality at 28 days in Q1 compared to Q2 + Q3, and a 62% (HR 1.62, 95%CI 1.12–2.14)) higher in the Q5 (Table 3).
Threshold effect analysis for the relationship between serum osmolality levels and 28-day mortality
The study employed a restricted cubic splines regression model to investigate the relationship between serum osmolality and the risk of 28-day mortality. After adjusting for covariates in model 3, we found a U-shaped relationship between serum osmolality and 28-day mortality in curve ftting (P for non-linearity < 0.001). Additional examination employing a segmented regression model indicated a turning point at a serum osmolality level of 306.8 mOsm/kg in relation to the risk of 28-day mortality (Fig. 3). When osmolality < 306.8 mOsm/kg, the 28-day mortality risk decreased by 6.2% (HR 0.938, 95% CI 0.912–0.964) for every 1 mOsm/kg increase. At osmolality ≥ 306.8 mOsm/kg, there was a 3.7% (HR 1.037, 95% CI 1.030–1.045) increase in the 28-day mortality risk for every 1 mOsm/kg increase in osmolality (Table 4).
Sensitivity analysis
To confirm the stability of the U-shaped association between serum osmolality and mortality at 28 days, we conducted curve fitting stratified by diabetes, AKI, CKD, hypertension, and sepsis. The results demonstrated that this U-shaped association remained consistent across various subgroups (Fig. 4).
Discussion
To the best of our knowledge, this study represents the first investigation into the association between serum osmolality and the risk of 28-day mortality in patients with DKA. Our findings indicate that serum osmolality is associated with an increased risk of 28-day mortality, exhibiting a U-shaped relationship. This association remains significant even after adjusting for potential confounding factors.
Altered serum osmolality levels, whether lower or higher, are linked to a heightened risk of mortality. When osmolality < 306.8 mOsm/kg, the 28-day mortality risk decreased by 6.2% (HR 0.938, 95% CI 0.912–0.964) for every 1 mOsm/kg increase. At osmolality ≥ 306.8 mOsm/kg, there was a 3.7% (HR 1.037, 95% CI 1.030–1.045) increase in the 28-day mortality risk for every 1 mOsm/kg increase in osmolality. The risk of mortality was lower at osmolality of 284–299 mOsm/kg. Consequently, these results indicate that serum osmolality holds promise as a valuable decision-making tool for clinicians and may serve as an independent prognostic factor in patients with DKA. Our study found that lower serum osmolality was associated with a significantly higher risk of mortality compared to higher serum osmolality. Patients with DKA may experience a shift of water from intracellular to extracellular spaces due to hyperglycemia, resulting in dilutional hyponatremia14. In such cases, patients may suffer from severe dehydration, but the absence of a significant increase in osmolality may mask the true extent of dehydration, leading to inadequate treatment and an increased risk of mortality. In addition, rapid declines in serum glucose concentration without concurrent rise in serum sodium may precipitate cerebral edema by creating an osmotic gradient that drives water into brain cells15. The identification of 306.8 mOsm/kg as a threshold for serum osmolality carries clinical implications. This value serves as a practical marker for risk stratification in DKA management. For patients with osmolality ≥ 306.8 mOsm/kg, clinicians should prioritize interventions to mitigate hypertonicity, such as controlled insulin administration to avoid rapid glucose declines and judicious fluid replacement to address hypernatremia7,15.
The level of serum osmolality is determined by the number of solute particles present in a given volume of solution, independent of the type and size of the solute particles16. Serum osmolality plays a critical role in regulating water balance within and outside of cells and blood vessels, maintaining normal cell morphology, and ensuring proper water distribution in blood vessels, thereby sustaining blood volume17. Numerous studies have previously investigated the predictive value of serum osmolality across various clinical conditions. Nicholson conducted a retrospective analysis examining the correlation between the initial calculated blood serum osmolality and the risk of 30-day in-hospital mortality among 24,232 patients admitted to the emergency department. Their findings revealed that the first recorded serum osmolality in the emergency department was associated with an elevated risk of 30-day mortality18. Shen et al. conducted a large retrospective cohort study demonstrating that a hyperosmolar state at the time of admission was associated with increased mortality among critically ill patients suffering from cardiac, cerebral, vascular, and gastrointestinal diseases in the ICU, with a threshold of 300 mOsm/kg19. Similarly, the study by Holtfreter et al. reached a comparable conclusion, indicating that elevated serum osmolality is associated with an increased risk of mortality among critically ill individuals.
The mortality prediction value of serum osmolality falls between that of the APACHE II and SOFA scores. Notably, when the outcome prediction is restricted to long-term ICU patients, serum osmolality demonstrates superior performance compared to both the APACHE II and SOFA scores. Furthermore, serumosmolality is a more cost-effective and rapid measure than the clinical scoring systems currently employed in ICUs for predicting disease severity20. Increased serum osmolality plays a key role in activating intrarenal (polyol-fructokinase)21 and extrarenal (vasopressin)22 pathways, leading to renal injury. Elevated serum osmolality and reduced serum osmolality are viewed as independently linked to a greater likelihood of developing acute kidney injury (AKI)23.We found that as serum osmolality increased, the incidence of AKI also increased, reaching 68.4% in the Q5 (> 312 mOsm/kg) group, and the utilization rate of CRRT was the highest, reaching 7.3%.
Serum osmolality is maintained in a narrow range both by neuroendocrine functions through hypothalamic-pituitary axis and the kidney on controlling thirst and regulating water and electrolyte balance24. Serum osmolality is often elevated in patients with DKA. Under normal circumstances, the serum osmolality ranges from 280 to 310 mOsm/kg. The average effective serum osmolality among patients with DKA is (299.73 ± 13.99) mOsm/kg25. It has been reported that high osmolality is independently associated with mortality in DKA25,26. Hyperosmolality primarily arises from hyperglycemia in DKA, leading to significant impacts on CNS function and consciousness. The most critical complication of DKA is brain edema, and untreated DKA can result in cardiac arrest and death. Among the various factors contributing to altered mental status, such as hyperglycemia, ketonemia, or metabolic acidosis, serum osmolality serves as the key determinant of the level of awareness in these patients27,28. Therefore, abnormal serum osmolality should be promptly diagnosed and treated clinically.
Subgroup analyses stratified by AKI, CKD, sepsis, hypertension, and diabetes revealed persistent U-shaped associations between serum osmolality and mortality. Post hoc analyses revealed no statistically significant interactions between serum osmolality and these comorbidities in modifying 28-day mortality (all P for interaction > 0.05). However, clinical nuances merit discussion: For instance, AKI patients demonstrated heightened mortality risk at hyperosmolar levels (≥ 306.8 mOsm/kg), likely reflecting reduced renal capacity to buffer osmotic fluctuations, thereby amplifying hypertonicity-induced organ injury21,23. Conversely, CKD patients exhibited blunted risk gradients, possibly due to chronic adaptations to osmolality dysregulation (e.g., altered vasopressin signaling)24. In diabetic subgroups, the association remained robust regardless of diabetes type, though delayed ketosis resolution in type 2 diabetes may prolong exposure to hyperosmolar stress25,26. These observations, while exploratory, underscore the need for tailored management in high-risk subgroups.
The observed U-shaped relationship between serum osmolality and mortality underscores the complexity of osmotic regulation in DKA. While hyperosmolality-driven risks are well-documented7,25, the association between hypo-osmolality (< 306.8 mOsm/kg) and increased mortality merits mechanistic exploration. We hypothesize that hypo-osmolality may reflect iatrogenic dilution (e.g., overzealous hypotonic fluid resuscitation) or delayed resuscitation, leading to “masked” intracellular dehydration and persistent tissue hypoperfusion14,27. Rapid glucose reduction without concurrent sodium correction could exacerbate cerebral edema risk, even in adults15. Additionally, hypo-osmolality might indicate underlying comorbidities (e.g., CKD, heart failure) that impair osmotic adaptation, rendering patients more vulnerable to DKA complications23,24. For example, CKD patients often exhibit chronic hyponatremia due to impaired free water excretion, which may worsen during acute illness. Future studies should investigate whether targeted sodium-glucose co-correction or slower fluid administration rates mitigate risks in hypo-osmolar patients.
Overall, this study used a large and publicly available critical-care database to first assess the relationship between serum osmolality and the risk of 28-day mortality in patients with DKA. However, our study has several limitations. (1) As this study was observational in nature, we were unable to definitively establish causality. Despite the use of multivariate adjustment and subgroup analyses, there is still a possibility of residual confounding factors influencing the clinical outcomes. (2) Serum osmolality obtained from the initial measurements of blood glucose, sodium, potassium, and urea nitrogen might not completely reflect the overall change in the body. It is important to consider this limitation when interpreting the results. (3) The observational design limits causal inference, and residual confounding from unmeasured variables must be acknowledged. For example, fluid resuscitation rates and insulin therapy specifics (e.g., dosing, timing) were not fully captured in our dataset. Fluid administration may transiently dilute serum sodium, lowering osmolality without addressing intracellular dehydration14, while insulin protocols influence glucose and ketone clearance rates, indirectly modulating osmolality15. These unmeasured factors could bias the association between osmolality thresholds and mortality. (4) While our cohort excluded patients with primary HHS diagnoses, the biochemical profile in Q5 (severe hyperosmolality with moderate acidosis) suggests some patients may represented mixed DKA-HHS phenotypes. This aligns with current understanding of hyperglycemic crises as a spectrum7,15. Despite potential phenotypic heterogeneity, serum osmolality maintained a U-shaped mortality relationship across all subgroups (Fig. 4), including those with sepsis, CKD, and diabetes. This suggests osmolality’s prognostic role transcends strict diagnostic boundaries. Clinically, osmolality ≥ 306.8 mOsm/kg should heighten vigilance for complications like cerebral edema or renal injury7,15,27, irrespective of absolute pH or glucose values. (5) Previous studies have shown that anion gap and ketone are important factors affecting patient prognosis. MIMIC-IV lacks ketone the data of ketone, so we were unable to perform relevant statistical analyses. (6) The individuals involved in the study were patients in the ICU, and additional validation is necessary to assess the relevance of serum osmolality for patients who are admitted to general wards. (7) As the study data came from a single center (the MIMIC-IV database), our findings may not generalise to different healthcare settings, particularly those with different ICU resources or patient demographics. (8) Our study excluded patients with ICU stays < 24 h or incomplete laboratory data to ensure robust calculation of serum osmolality and outcome assessment. While this strengthens internal validity, it may results in selection bias by excluding individuals who died rapidly or had milder presentations requiring shorter ICU stays. For example, early deaths (e.g., due to refractory shock or cerebral edema) might exhibit extreme osmolality values not captured in our cohort, potentially skewing the observed U-shaped mortality relationship. Conversely, patients discharged quickly may represent less severe cases, limiting the generalizability of our risk thresholds to all DKA populations. Similar exclusion criteria are common in critical care studies using the MIMIC-IV database to balance data quality and representativeness13,19. Despite this, our findings remain relevant to the majority of hospitalized DKA patients requiring sustained critical care. Future studies incorporating pre-ICU and non-ICU settings are essential to confirm the broader applicability of serum osmolality as a prognostic marker. (9) With electronic health records data, there may be potential inaccuracies or inconsistencies in data entry, missing data, or variable measurements that may affect study validity. Therefore, further research is essential to comprehensively explore how this bias impacts clinical outcomes.
Conclusion
Our research demonstrates a U-shaped relationship between serum osmolality levels and 28-day all-cause mortality in patients with DKA following ICU admission, while accounting for confounding variables. Due to its simplicity, accessibility, and cost-effectiveness, measuring serum osmolality highlights the importance of managing osmolality, assisting physicians in recognizing high-risk patients. However, further studies are crucial to determine if interventions targeting serum osmolality levels can positively impact clinical outcomes within this group.
Data availability
Data is provided within the manuscript or supplementary information files(MIMIC IV 3.0: https://mimic.mit.edu).
References
Dhatariya, K. K. et al. Diabetic ketoacidosis. Nat. Rev. Dis. Primers. 6 (1), 40 (2020).
Centers for Disease Control and Prevention. Age-adjusted hospital discharge rates for diabetic ketoacidosis as first-listed diagnosis per 10,000 population, United States, 1988–2009. CDC (2013). https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html
Desai, D., Mehta, D., Mathias, P., Menon, G. & Schubart, U. K. Health care utilization and burden of diabetic ketoacidosis in the U.S. Over the past decade: a nationwide analysis. Diabetes Care. 41, 1631–1638 (2018).
Dhatariya, K. K., Skedgel, C. & Fordham, R. The cost of treating diabetic ketoacidosis in the UK: a National survey of hospital resource use. Diabet. Med. 34, 1361–1366 (2017).
Dhatariya, K. K. et al. The cost of treating diabetic ketoacidosis in an adolescent population in the UK: a National survey of hospital resource use. Diabet. Med. 36, 982–987 (2019).
Vellanki, P. & Umpierrez, G. E. Increasing hospitalizations for DKA: A need for prevention programs. Diabetes Care. 41, 1839–1841 (2018).
Kitabchi, A. E., Umpierrez, G. E., Miles, J. M. & Fisher, J. N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 32, 1335–1343 (2009).
Büyükkaragöz, B. & Bakkaloğlu, S. A. Serum osmolality and hyperosmolar States. Pediatr. Nephrol. 38, 1013–1025 (2023).
Zou, Q. et al. Association between serum osmolality and 28-day all-cause mortality in patients with heart failure and reduced ejection fraction:a retrospective cohort study from the MIMIC-IV database. Front. Endocrinol. (Lausanne). 15, 1397329 (2024).
Luo, X. et al. Association between serum osmolality and deteriorating renal function in patients with acute myocardial infarction: analysis of thenMIMIC-IV database. BMC Cardiovasc. Disord. 24 (1), 490 (2024).
Brathwaite, S. & Macdonald, R. L. Current management of delayed cerebral ischemia: update from results of recent clinical trials. Transl Stroke Res. 5 (2), 207–226 (2014).
Liang, M. et al. The U-shaped association between serum osmolality and 28-day mortality in patients with sepsis: a retrospective cohort study. Infection 52(5), 1931–1939 (2024).
Johnson, A. et al. MIMIC-IV (version 3.0). PhysioNet https://doi.org/10.13026/hxp0-hg59 (2024).
Abbas, E., Kitabchi, G. E., Umpierrez, J. M. & Miles, Joseph, N. Fisher; hyperglycemic crises in adult patients with diabetes. Diabetes Care. 32 (7), 1335–1343 (2009).
Wolfsdorf, J. I. et al. ISPAD Clinical Practice Consensus Guidelines 2018:Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes 19(Suppl.27), 155–177 (2018).
Star, R. A. Southwestern internal medicine conference: hyperosmolar States. Am. J. Med. Sci. 300, 402–412 (1990).
Argyropoulos, C. et al. Hypertonicity: pathophysiologic concept and experimental studies. Cureus 8, e596 (2016).
Nicholson, T., Bennett, K. & Silke, B. Serum osmolality as an outcome predictor in hospital emergency medical admissions. Eur. J. Intern. Med. 23 (2), e39–43 (2012).
Shen, Y. et al. Association between serum osmolality and mortality in patients who are critically ill:a retrospective cohort study.
Holtfreter, B. et al. Serum osmolality and outcome in intensive care unit patients. Acta Anaesthesiol. Scand. 50 (8), 970–977 (2006).
Roncal Jimenez, C. A. et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 86 (2), 294–302 (2014).
García-Arroyo, F. E. et al. Vasopressin mediates the renal damage induced by limited Fructose rehydration in recurrently dehydrated rats. Int. J. Biol. Sci. 13 (8), 961–975 (2017).
Yang, J., Cheng, Y., Wang, R. & Wang, B. Association between serum osmolality and acute kidney injury in critically ill patients: a retrospective cohort study. Front. Med. 8, 745803 (2021).
Seay, N. W., Lehrich, R. W. & Greenberg, A. Diagnosis and management of disorders of body tonicity-hyponatremia and hypernatremia: core curriculum. Am. J. Kidney Dis. 75, 272–286 (2020).
Wu, X. Y. et al. Clinical profiles, outcomes and risk factors among type 2 diabetic inpatients with diabetic ketoacidosis and hyperglycemic hyperosmolar state: a hospital-based analysis over a 6-year period. BMC Endocr. Disord. 20 (1), 182 (2020).
Blank, S. P., Blank, R. M. & Ziegenfuss, M. D. The importance of hyperosmolality in diabetic ketoacidosis. Diabet. Med. 37 (12), 2001–2008 (2020).
Rondon-Berrios, H. et al. Hypertonicity: clinical entities, manifestations and treatment. World J. Nephrol. 6, 1–13 (2017).
Agrawal, S. et al. Pediatric diabetic ketoacidosis with hyperosmolality: clinical characteristics and outcomes. Endocr. Pract. 24, 26–732 (2018).
Acknowledgements
We are particularly grateful to all the people who have given us help on our article.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, Jian Liao; Methodology, Dingyu Lu; Validation, Zhi Liang; Formal Analysis, Jian Liao; Data Curation, Hongwei Tan; Writing-Original Draft Preparation, Jian Liao; Writing-Review & Editing, Dingyu Lu; Supervision, Maojuan Wang. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liao, J., Lu, D., Liang, Z. et al. Association of serum osmolality levels with all-cause mortality risk in patients with DKA. Sci Rep 15, 31874 (2025). https://doi.org/10.1038/s41598-025-14405-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14405-1