Abstract
In Oriental medicine, silkworms and their derivatives have been used for anti-inflammatory and diabetic purposes. Bombycis Feces exhibit anti-atopic effects and anti-proliferative activity; however, the role of BF extract (BFE) in adipogenesis and obesity and management remains underexplored. This study examined the effects of BFE on amino acid oxidation during adipocyte differentiation. An adipogenesis model was established using 3T3-L1 cells. Intracellular lipid accumulation was assessed via Oil Red O staining, while cytotoxicity was evaluated using a cell viability assay. The effects of BFE on gene and protein expression, lipolysis, real-time oxygen consumption rate, and substrate oxidation during adipocyte differentiation were analyzed. The involvement of the branched-chain amino acid (BCAA) catabolic pathway and leptin gene expression was also evaluated. BFE treatment significantly downregulated the expression level of key adipogenic transcription factors, including PPARγ and C/EBPα, as well as aP2 gene expression. Lipid accumulation was significantly reduced, accompanied by increased expression of Sirt1 and Sirt6, but not Sirt3, and a concurrent reduction in Parp1 expression. In addition to adipogenesis, the oxygen consumption rate and extracellular acidification rate were downregulated by BFE treatment in adipocytes. Conversely, BFE enhanced amino acid-dependent oxidation during adipogenesis. These findings suggest that BFE supports energy production via BCAA catabolism rather than fatty acid or glucose oxidation. BFE inhibits adipogenesis by modulating the expression of Sirt1, Sirt6, and Parp1, reducing lipid metabolism, and enhancing BCAA catabolism in 3T3-L1 cells. These results highlight BFE’s potential as a therapeutic agent for obesity management.
Similar content being viewed by others
Introduction
Adipose tissue is a major organ found in both humans and mice, which acts as an endocrine organ by producing hormones and adipokines that influence systemic inflammation, metabolism, and energy storage1,2. It is composed of adipocytes embedded within a matrix containing blood vessels, collagen, lymphatic vessels, and the stromal vascular fraction, which includes immune cells, smooth muscle cells, endothelial cells, mesenchymal stem cells, and adipocyte precursor cells (preadipocytes)3. Adipocytes play a central role in regulating energy metabolism and storage. Specifically, triglycerides, which are synthesized from excess dietary fats and carbohydrates, are stored within adipocytes as lipid droplets4. During energy-demanding states such as fasting or physical activity, adipocytes mobilize stored lipids via lipolysis, providing a primary energy source for various physiological processes5. The transcriptional regulation of adipogenesis in adipocytes has been well characterized, with the process controlled by master transcriptional regulators such as C/EBPs and PPARγ6,7.
Mitochondria, often referred to as the cellular powerhouses, are essential to adipocyte metabolism, contributing to ATP biosynthesis, fatty acid metabolism, and triglyceride homeostasis8. Although adipocytes typically contain fewer mitochondria than other cell types, mitochondrial function is vital for maintaining energy balance9. Mitochondrial dysfunction in adipocytes is associated with obesity-related metabolic complications, including type 2 diabetes, insulin resistance, atherosclerosis, and non-alcoholic fatty liver disease. Compared to brown or beige adipocytes, white adipocytes contain fewer mitochondria; however, their functional mitochondria are crucial for regulating adipocyte biology and systemic metabolic functions9. Mitochondria are involved in regulating cellular survival, adipocyte differentiation, glucose and lipid homeostasis, and branched-chain amino acids (BCAAs) metabolism at the homeostatic level10. The efficiency of mitochondrial processes such as lipogenesis and lipolysis depends on ATP production11. While the impact of glucose and dietary fats on lipid metabolism is well-documented, the role of amino acids in adipocyte function remains underexplored.
Adipogenesis is associated with mitochondrial biogenesis and reactive oxygen species (ROS) production, with a metabolic shift from glycolysis to oxidative phosphorylation being essential for mature adipocyte function12. Loss of mitochondrial function disrupts adipogenesis and leads to inflammation, adipose tissue dysfunction, and cell death. The NAD+ /NADH redox balance is fundamental to mitochondrial metabolism, with imbalances linked to obesity and metabolic diseases. NAD+ -dependent enzymes, including sirtuins (SIRTs) and poly (ADP-ribose) polymerases (PARPs), play opposing roles in regulating mitochondrial function13. Sirtuins, particularly SIRT1, promote mitochondrial biogenesis and enhance mitochondrial function14,15, whereas PARP1 reduces mitochondrial activity; its inhibition has been shown to improve mitochondrial function in animal models. Since SIRTs and PARPs rely on the same NAD+ pool, PARP1 may inhibit SIRT1 through PARylation, a mechanism that warrants further study in adipose tissue13. SIRT1 influences adipogenesis by downregulating CACUL1 and reducing the adipogenic activity of PPARγ, while SIRT6 inhibits lipid accumulation through AMPK activation14. In contrast, SIRT3 deficiency impairs FOXO3a activity during adipogenesis, although it does not significantly affect overall differentiation in 3T3-L1 cells14. Inhibiting PARP1 has been shown to reduce the expression of C/EBPα and PPARγ, both key regulators of adipocyte differentiation13.
In the small intestine, both adipocytes and enterocytes produce the hormone leptin, which regulates energy balance by suppressing appetite, increasing energy expenditure, and reducing fat storage. Leptin expression is influenced by glucose, amino acids, and lipid intake. Diets enriched in BCAAs can activate mTOR signaling, thereby promoting leptin production in white adipose tissue16. Adipose triglyceride lipase (ATGL) is the main enzyme responsible for triglyceride lipolysis and lipid β-oxidation. In white adipose tissue exposed to BCAAs, ATGL protein levels are reduced, which correlates with a significant decrease in adipocyte size17,18.
Bombycis excrementum (BE), also known as silkworm excrement or droppings, is a traditional medicine made from the silkworm (Bombyx mori L.), which serves as a source of chlorophyll, sodium copper chlorophyllin, carotenoids, pectin, and phytol19. Previous studies have reported that bioactive constituents of Bombycis Feces (BF) suppress migraine pain, reduce blood glucose levels, and mitigate obesity 20,21. Megastigmane sesquiterpenes and flavonoids isolated from BE have been shown to increase SIRT1 expression and enhance the activity of the antioxidant enzyme heme oxygenase-1, which suppresses inflammatory mediators22,23.
However, despite extensive research on lipid and glucose metabolism during adipogenesis, the role of Bombycis Feces Extract (BFE) in this process remains unclear. This study aims to investigate the effects of BFE on mitochondrial BCAA metabolism during adipocyte differentiation.
Results
Cytotoxicity
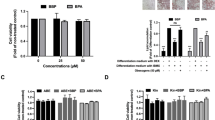
The potential cytotoxic effects of BFE on 3T3-L1 adipocytes were assessed both prior to adipogenesis (day -1) and during differentiation (day 10) by evaluating cell viability at concentrations of 50, 100, 200, and 400 µg/mL. A slight decrease in cell viability was observed with increasing concentrations of BFE. However, at 50 and 100 µg/mL, no significant cytotoxicity was detected in either undifferentiated or fully differentiated adipocytes. Therefore, these concentrations were deemed suitable for further experiments (Fig. 1A, B).
Adipogenesis and intracellular lipid accumulation
To assess the impact of BFE on adipogenesis and lipid accumulation, Oil Red O staining was used to visualize lipid content in 3T3-L1 cells on day 8 post-differentiation. Intracellular lipid levels were significantly reduced in cells treated with 50 and 100 µg/mL BFE compared to controls (Fig. 2A, B). The expression of key adipogenic transcription factors, including PPARγ and C/EBPα, was also noticeably reduced at 50 µg/mL BFE on days 3, 6, and 9 of differentiation, with a more pronounced reduction observed at 100 µg/mL (Fig. 2C). Similarly, the expression of aP2, a PPARγ target gene, was reduced in BFE-treated cells (Fig. 2C).
Suppression of adipogenesis in 3T3-L1 adipocytes by Bombycis Feces extract (BFE) treatment. (A) Schematic representation of experimental set-up. (B) Effects of BFE on adipogenesis and lipid accumulation assessed by Oil Red O staining and quantification using Image J software (40x: left, 100 × right, scale bar: 100 μm). (C) qPCR analysis of adipogenesis-associated gene expression in 3T3-L1 adipocytes differentiated for 9 days in the presence and absence of BFE. All data are representative of 2–3 independent experiments. Data are presented as the mean ± SD. *p < 0.05; *** p < 0.001 indicate significant differences between the indicated groups.
Regulation of sirtuins and PARP during adipocyte differentiation
To investigate whether the effects of BFE on adipogenesis were mediated through sirtuins, gene expression levels of SIRT1, SIRT3, and SIRT6 were analyzed during differentiation. As shown in Fig. 3A, Sirt1 and Sirt6 showed significantly increased expression following BFE treatment during adipogenesis, especially on day 9, when considered mature adipocytes, but there was no significant increase in Sirt3. In addition to sirtuins, PARylated Parp1 is well known for its role in adipogenesis as a counteractant to sirtuins. PARylated Parp1 expression tended to be downregulated by BFE treatment on day 9 of adipogenesis (Fig. 3B).
Administration of Bombycis Feces extract (BFE) activates sirtuin gene expression in fully differentiated 3T3-L1 adipocytes. (A) Gene expression of sirtuins during adipogenesis in 3T3-L1 adipocytes. (B) Protein expression of PARP1 with BFE treatment in mature 3T3-L1 adipocytes, with quantification by Image J software. Data are presented as the mean ± SD. **p < 0.01 indicates significant differences between the indicated groups.
Lipolysis and mitochondrial activity
The effects of BFE on lipolysis were assessed by measuring glycerol release in fully differentiated adipocytes. As shown in Fig. 4A, isoproterenol-induced lipolysis was markedly reduced in BFE-treated cells compared to controls, while basal glycerol levels remained unchanged (Fig. 4A). Similarly, expression of ATGL, a key enzyme involved in triglyceride hydrolysis, was decreased in BFE-treated cells (Fig. 4A). Mitochondrial respiration was evaluated by measuring oxygen consumption rates (OCRs). BFE-treated cells exhibited a significant reduction in both basal and ATP-linked OCRs, although maximal respiratory capacity remained unaffected (Fig. 4B). Additionally, expression levels of fatty acid oxidation-related genes such as Cox5b, Cox8b, and MCAD were significantly downregulated in BFE-treated adipocytes (Fig. 4C, D).
Effects of Bombycis Feces extract (BFE) on lipolysis, mitochondrial Oxygen Consumption Rate (OCR), and extracellular acidification rate (ECAR) of 3T3-L1 adipocytes. (A) Effects of BFE on lipolysis and expression of lipolysis-associated genes in mature 3T3-L1 cells. (B) OCR in mature 3T3-L1 adipocytes treated with BFE. (C) ECAR in BFE-treated 3T3-L1 cells. (D) Mitochondrial fatty acid oxidation-linked gene expression in 3T3-L1 adipocytes under BFE treatment. Data are presented as the mean ± SD. **p < 0.01 indicates significant differences between the indicated groups.
Mitochondrial fuel dependency and the BCAAs catabolic pathway
Given the observed alterations in mitochondrial function, the dependency of adipocytes on different fuel substrates was assessed. Fatty acid dependency was reduced in BFE-treated cells, while metabolic flexibility toward alternative fuels was enhanced relative to controls (Fig. 5A). Furthermore, both glutamine dependency and flexibility were increased, whereas glucose utilization showed no significant change (Fig. 5A). Analysis of mitochondrial branched-chain amino acid (BCAA) metabolism revealed upregulated expression of genes involved in BCAA catabolism, including BCAT1, BCAT2, BCKDHα, and BCKDHβ, in BFE-treated cells. These findings indicate a metabolic shift toward increased amino acid oxidation and reduced fatty acid utilization during differentiation (Fig. 5B). Correspondingly, enhanced BCAA catabolism was associated with increased leptin expression in BFE-treated adipocytes, suggesting an influence on energy metabolism and adipocyte function (Fig. 5C).
Effects of Bombycis Feces extract (BFE) on mitochondrial fuel oxidation in 3T3-L1 adipocytes. (A) Mitochondrial fuel oxidation in fully differentiated 3T3-L1 adipocytes treated with BFE. (B) Expression of genes related to branched-chain amino acid (BCAA) catabolism in 3T3-L1 adipocytes with or without BFE treatment. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 indicate significant differences between the indicated groups.
Discussion
The catabolism of BCAAs in adipocytes is important for maintaining metabolic homeostasis, as it affects glycolytic flux, mitochondrial respiration, and fatty acid synthesis12,18. Although mitochondria are less abundant in white adipocytes, impaired adipogenesis, fatty acid synthesis, and BCAA catabolism can lead to metabolic diseases such as diabetes, obesity, and lipoatrophy18. Elevated blood BCAAs are often associated with insulin resistance and diabetes, possibly due to reduced cellular BCAA oxidation24. Moreover, BCAA catabolism promotes adipogenesis via key adipogenic regulators in obesity and diabetes25,26. However, elevated BCAA levels have been observed in both metabolically healthy and obese individuals27. The positive effects of BCAAs on body weight, composition, aerobic capacity, and insulin sensitivity suggest a physiological demand for amino acids and/or BCAA intake during catabolic states12. By contrast, BCAA-mediated suppression of lipogenesis and lipolysis could compromise insulin sensitivity18. Notably, variations in metabolic phenotypes are not always reflected in circulating BCAA levels. Mitochondrial BCAA catabolism is also known to play an adaptive role (e.g., during fever) where the mitochondrial BCAA carrier (MBC), SLC25A44, facilitates BCAA import for oxidation and thermogenesis28. Adipogenesis is also linked to BCAA catabolism in white adipocyte mitochondria16. Thus, this study provides evidence that, beyond glucose and fatty acids, BFE enhances mitochondrial BCAA metabolism in 3T3-L1 cells, leading to suppressed adipogenesis and lipolysis.
BF has primarily been studied for its effects on blood glucose regulation, particularly via α-glucosidase inhibition, which helps lower postprandial blood glucose in rats challenged with disaccharides. In this study, fagomine, cis-5-hydroxy-L-pipecolic acid, and 1-deoxynojirimycin were identified as active constituents of BF through bioassay-guided fractionation23. This regulation of glucose levels is essential for maintaining mitochondrial function, as the mitochondria rely on glucose metabolism for ATP production. Another study found that petroleum ether fractions of BF contain seven compounds, including phytol, which exhibits anti-migraine properties22. Whether BF directly serves as a metabolic fuel for mitochondrial BCAA catabolism remains unclear due to complex metabolic interactions. Thus, no direct evidence currently supports the notion that BFE directly impacts mitochondrial respiration.
Nevertheless, our findings do suggest that suppression of adipogenesis and lipolysis through the reduction of mitochondrial fatty acid oxidation may complement enhanced BCAA catabolism in BFE-treated adipocytes.
In addition to its effects on adipogenesis, BFE significantly altered mitochondrial respiratory dynamics and fuel dependency. By enhancing glutamine flexibility and promoting BCAA catabolism, BFE-treated cells demonstrated a metabolic preference for amino acid oxidation. This metabolic reprogramming could reduce reliance on fatty acids and glucose, with potential therapeutic relevance for metabolic disorders involving dysregulated adipocyte function.
The regulatory roles of sirtuins and PARP1 in mitochondrial function and adipogenesis are well-established. Sirtuins, particularly SIRT1 and SIRT6, support mitochondrial activity, lipid metabolism, and energy balance, whereas PARP1 negatively regulates these processes through NAD + competition13,14. This study demonstrates that BFE treatment upregulates SIRT1 and SIRT6 expression while suppressing PARP1 activity, as reflected by reduced PARylation. These findings align with previous research indicating that sirtuins promote mitochondrial integrity and inhibit adipogenesis29. By modulating the NAD + pool and enhancing sirtuin activity, BFE may contribute to the observed metabolic changes in 3T3-L1 adipocytes.
The ability of BFE to reduce lipid accumulation, alter mitochondrial respiration, and promote BCAA catabolism underscores its potential as a therapeutic candidate for metabolic disorders. BFE may be beneficial in treating metabolic dysfunctions such as type 2 diabetes, obesity, and insulin resistance by redirecting adipocyte metabolism toward amino acid oxidation and away from fatty acid and glucose reliance. Furthermore, increased leptin expression in BFE-treated adipocytes suggests potential roles in energy balance and appetite regulation.
Although this work offers new insights into BFE’s role in adipocyte metabolism, several questions remain. Future studies are needed to identify the bioactive compounds responsible for these effects and elucidate their mechanisms of action. In vivo investigations will also be critical to confirm the in vitro findings and evaluate the systemic metabolic effects of BFE. Additionally, exploring the interaction of BFE with other metabolic pathways, such as glucose homeostasis and thermogenesis, could offer more comprehensive insights into its therapeutic potential.
In conclusion, this study demonstrates that BFE inhibits adipogenesis in 3T3-L1 cells by modulating mitochondrial BCAA metabolism, reducing lipogenesis, and suppressing fatty acid and glucose oxidation. These effects are partially mediated by sirtuin activation and PARP1 suppression. By promoting BCAA catabolism and altering mitochondrial fuel flexibility, BFE represents a promising strategy for treating metabolic disorders. Future research should focus on elucidating the specific molecular mechanisms underlying these effects and evaluating their clinical relevance.
Materials and methods
Bombycis Feces extraction (BFE)
BF were collected from Andong-si, Gyeongsangbuk-do, South Korea. After drying, the material was finely powdered, and 1 kg of this powdered BF then underwent ethanol extraction via reflux at a controlled temperature of 100 ± 3 °C for 3 h. The extract was then filtered using a 53 μm sieve, concentrated under vacuum, and freeze-dried to yield a powdered extract30,31.
In vitro cell culture model
Mouse 3T3-L1 preadipocytes, obtained from the American Type Culture Collection (ATCC, USA), were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% bovine calf serum and antibiotics (penicillin–streptomycin; Gibco, USA). Upon reaching confluence, differentiation into adipocytes was induced using DMEM containing 10% fetal bovine serum (FBS), 5 μM dexamethasone, 0.5 μg/mL insulin, and 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, USA) for two days. This was followed by a two-day incubation in DMEM with 10% FBS and 0.5 μg/mL insulin32,33. Differentiation continued for eight days, after which mature adipocytes were treated with DMSO (control) or BFE at concentrations specified in the experimental figures.
Oil Red O staining
On the final day of differentiation, 3T3-L1 cells were washed with PBS and fixed with 4% formaldehyde in PBS for 15 min at room temperature. The fixed cells were stained with Oil Red O working solution (Sigma-Aldrich, USA) for 1 h at room temperature, and excess stain was removed by rinsing with distilled water. Lipid droplet formation was visualized using an EVOS microscope (Invitrogen, USA).
Cell viability assay
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, USA). Cells (3 × 103 per well) were seeded into 96-well plates and treated with BFE at concentrations of 50 and 100 μg/mL for 48 h at 37 °C in a 5% CO₂ atmosphere. After incubation, each well was supplemented with the CCK-8 reagent for 2 h at 37 °C, and the absorbance was measured at 450 nm using a SpectraMax iD3 microplate reader (Molecular Devices, USA).
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Total RNA was extracted from 3T3-L1 cells using TRIzol reagent (Ambion, USA). Reverse transcription of 1 μg RNA was carried out using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, USA). Quantitative real-time PCR was performed using SYBR Green PCR Master Mix (Enzynomics, Korea) and gene-specific primers (listed in Table 1). Reactions were conducted using a Bio-Rad real-time PCR system (Bio-Rad, Hercules, CA, USA). mRNA levels were normalized to cyclophilin expression and presented as fold changes relative to controls.
Western blot analysis
Western blotting was performed as described previously34,35. Briefly, proteins were separated by SDS-PAGE, transferred onto nitrocellulose membrane (Millipore, USA), and incubated overnight at 4 °C with specific antibodies: anti-PARP-1 (MA5-15031; Invitrogen, USA), anti-PAR (4334-MC-100; R&D Systems, USA), and anti-β-actin (A5441; Sigma-Aldrich, USA). After washing, the membranes were incubated with horseradish peroxide-conjugated goat anti-mouse IgG secondary antibody (SC-2031; Santa Cruz Biotechnology, USA) for 1 h at room temperature. Detection was performed using a chemiluminescent substrate, and protein expression was quantified.
Lipolysis assay
Lipolytic activity was measured by quantifying glycerol release using a Free Glycerol Reagent (Sigma, USA), following previously published protocols with minor modifications. Briefly, differentiated adipocytes were treated with or without 1 μmol/L isoproterenol in DMEM supplemented with 2% fatty acid-free BSA. Absorbance at 540 nm was measured and normalized to total protein content determined using a BCA Protein Assay Kit (Thermo Fisher Scientific, USA).
Mitochondrial respiration and fuel oxidation analysis
Oxygen consumption rates (OCRs) were measured in differentiated 3T3-L1 adipocytes seeded in XF Pro microplates per the manufacturer’s instructions (Seahorse Bioscience)36. After differentiation, cells were incubated in DMEM containing 25 mM glucose, 1 mM sodium pyruvate, and 4 mM glutamine for 1 h before analysis. OCRs were measured under different conditions using inhibitors such as oligomycin (2.5 μM), FCCP (2 μM), and antimycin A/rotenone (0.5 μM). Fuel oxidation was evaluated using the Seahorse XF Mito Fuel Flex Test Kit, which measures mitochondrial dependency and flexibility for substrates including glucose, glutamine, and fatty acids. Specific inhibitors (UK5099, BPTES, and Etomoxir) were applied to determine substrate dependency, flexibility, and overall metabolic capacity.
Statistical analysis
Data are expressed as mean ± SEM. Time-dependent differences were analyzed using two-way repeated-measures ANOVA followed by Bonferroni post hoc tests. Comparisons between groups were performed using the Mann–Whitney U test or Kruskal–Wallis test with Dunn’s multiple comparisons. Statistical significance was set at p < 0.05.
Additional information
To include, in this order: Accession codes (where applicable); Competing interests (mandatory statement). The corresponding author is responsible for submitting a competing interest statement on behalf of all authors. This statement must be included in the submitted article file.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sun, K., Kusminski, C. M. & Scherer, P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101. https://doi.org/10.1172/JCI45887 (2011).
Yang, A. & Mottillo, E. P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 477, 985–1008. https://doi.org/10.1042/BCJ20190468 (2020).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science https://doi.org/10.1126/science.aav2501 (2019).
Herker, E., Vieyres, G., Beller, M., Krahmer, N. & Bohnert, M. Lipid droplet contact sites in health and disease. Trends Cell Biol. 31, 345–358. https://doi.org/10.1016/j.tcb.2021.01.004 (2021).
Kimmel, A. R. & Sztalryd, C. The Perilipins: The perilipins: Major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu. Rev. Nutr. 36, 471–509. https://doi.org/10.1146/annurev-nutr-071813-105410 (2016).
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273. https://doi.org/10.1016/j.cmet.2006.07.001 (2006).
Rosen, E. D., Wu, C. J., Puigserver, P. & Spiegelman, B. M. Transcriptional regulation of adipogenesis. Genes Dev. 14, 1293 (2000).
Aon, M. A., Bhatt, N. & Cortassa, S. C. Mitochondrial and cellular mechanisms for managing lipid excess. Front. Physiol. 5, 282. https://doi.org/10.3389/fphys.2014.00282 (2014).
Kusminski, C. M. & Scherer, P. E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 23, 435–443. https://doi.org/10.1016/j.tem.2012.06.004 (2012).
Muoio, D. M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 159, 1253–1262. https://doi.org/10.1016/j.cell.2014.11.034 (2014).
Heinonen, S., Jokinen, R., Rissanen, A. & Pietilainen, K. H. White adipose tissue mitochondrial metabolism in health and in obesity. Obes. Rev. 21, e12958. https://doi.org/10.1111/obr.12958 (2020).
Green, C. R. et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 12, 15–21. https://doi.org/10.1038/nchembio.196192n (2016).
Jokinen, R., Pirnes-Karhu, S., Pietiläinen, K. H. & Pirinen, E. Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases–important players in mitochondrial metabolism and metabolic health. Redox Biol. 12, 246–263. https://doi.org/10.1016/j.redox.2017.02.011 (2017).
Chen, J. et al. Sirtuins: Key players in obesity-associated adipose tissue remodeling. Front. Immunol. 13, 1068986. https://doi.org/10.3389/fimmu.2022.1068986 (2022).
Ruderman, N. B. et al. AMPK and SIRT1: A long-standing partnership?. Am. J. Physiol. Endocrinol. Metab. 298, E751-760. https://doi.org/10.1152/ajpendo.00745.2009 (2010).
Corsetti, G. et al. Essential amino acids-rich diet decreased adipose tissue storage in adult mice: A preliminary histopathological study. Nutrients https://doi.org/10.3390/nu14142915 (2022).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465. https://doi.org/10.1038/s42255-021-00493-6 (2021).
Supruniuk, E., Żebrowska, E. & Chabowski, A. Branched chain amino acids—Friend or foe in the control of energy substrate turnover and insulin sensitivity?. Crit. Rev. Food Sci. Nutr. 63, 2559–2597. https://doi.org/10.1080/10408398.2021.1977910 (2021).
Sohn, B. H. et al. Isolation and identification of lipids from the silkworm (Bombyx mori) droppings. J. Korean Soc. Appl. Bi. 52, 336–341. https://doi.org/10.3839/jksabc.2009.060 (2009).
Mei, H. et al. Possible mechanisms by which silkworm faeces extract ameliorates adenine-induced renal anaemia in rats. J. Ethnopharmacol. https://doi.org/10.1016/j.jep.2020.113448 (2021).
Kim, M. W. et al. Anti-obesity effects of the larval powder of steamed and lyophilized mature silkworms in a newly designed adult mouse model. Foods https://doi.org/10.3390/foods12193613 (2023).
Song, J. et al. Phytol from Faeces Bombycis alleviated migraine pain by inhibiting Nav1.7 sodium channels. J. Ethnopharmacol. 306, 116161. https://doi.org/10.1016/j.jep.2023.116161 (2023).
Matsuda, H. et al. Suppressive effects of Bombycis Feces (Bombyx feces) and Bombyx batryticatus (stiff silkworm) extracts on blood glucose level elevation in disaccharides-loaded rats. Tradit. Kampo Med. https://doi.org/10.1002/tkm2.1368 (2023).
Lackey, D. E. et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 304, E1175–E1187. https://doi.org/10.1152/ajpendo.00630.2012 (2013).
Shao, J. et al. BCAA catabolism drives adipogenesis via an intermediate metabolite and promotes subcutaneous adipose tissue expansion during obesity. bioRxiv. https://doi.org/10.1101/2022.08.18.504380 (2022).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619. https://doi.org/10.1038/s41586-019-1503-x (2019).
Ma, Q.-X. et al. BCAA–BCKA axis regulates WAT browning through acetylation of PRDM16. Nat. Metab. 4, 106–122. https://doi.org/10.1038/s42255-021-00520-6 (2022).
Yoneshiro, T. et al. Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. Elife https://doi.org/10.7554/eLife.66865 (2021).
Nogueiras, R. et al. Sirtuin 1 and Sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 92, 1479–1514. https://doi.org/10.1152/physrev.00022.2011 (2012).
Kang, Y. M. et al. Cheong-sang-gyeon-tong-tang improves hepatic steatosis by regulating cholesterol metabolism. Mol. Cell. Toxicol. 20, 1001–1010. https://doi.org/10.1007/s13273-024-00426-w (2024).
Kim, K. Y., Kang, Y. M., Kim, T. I., Kim, Y. J. & Kim, K. Oncheong-eum alleviated hepatic lipid accumulation and intestinal barrier disruption in nonalcoholic steatohepatitis model. Mol. Cell. Toxicol. 21, 239–250. https://doi.org/10.1007/s13273-024-00444-8 (2025).
Lee, J. et al. Adipogenic effects of Ostreae Testa water extract on white adipocytes. Mol. Cell. Toxicol. 20, 159–165. https://doi.org/10.1007/s13273-023-00335-4 (2024).
Lee, H. G., Hur, J., Won, J. P. & Seo, H. G. Heme oxygenase-1 mediates the inhibitory effect of ginseng (Panax ginseng) leaf extract on differentiation in 3T3-L1 adipocytes. Mol. Cell. Toxicol. 20, 699–708. https://doi.org/10.1007/s13273-023-00408-4 (2024).
Lee, Y. K. et al. Perilipin 3 deficiency stimulates thermogenic beige adipocytes through PPARalpha activation. Diabetes 67, 791–804. https://doi.org/10.2337/db17-0983 (2018).
Park, J. H. et al. Inactivation of EWS reduces PGC-1alpha protein stability and mitochondrial homeostasis. Proc. Natl. Acad. Sci. USA 112, 6074–6079. https://doi.org/10.1073/pnas.1504391112 (2015).
Lee, Y. K. et al. Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model. Eur. J. Pharmacol. 957, 175946. https://doi.org/10.1016/j.ejphar.2023.175946 (2023).
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF 2019R1F1A1050505, NRF 2022R1F1A1064221 and RS-2025-00513591), the National Research Council of Science & Technology (NST) grant from the Korean government (MIST) (CAP21024-000), and a grant from the Korea Institute of Oriental Medicine (KSN2511030).
Funding
Basic Science Research Program through the National Research Foundation of Korea (NRF), NRF 2019R1F1A1050505 and NRF 2022R1F1A1064221, National Research Council of Science & Technology (NST) grant from the Korean government (MIST), CAP21024-000, Korea Institute of Oriental Medicine grant, KSN2511030, National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT),RS-2025-00513591
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.K. Lee and J.H. Park; Funding acquisition, Y.K. Lee and J.H. Park; Investigation, Y.K. Lee., U.C. Shin., S. Muthamil., C-H. Bae., S-W. Kim., J.M. Oh., Ji-Hyo. Lyu., and H-J. Jang.; Writing—original draft, Y.K. Lee and J.H. Park; Writing—review & editing, Y.K. Lee and J.H. Park. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, Y.K., Lyu, J., Shin, U.C. et al. Extract of Bombycis Feces suppressed 3T3-L1 adipogenesis resulting in the regulation of fatty acid-dependent energy consumption. Sci Rep 15, 30419 (2025). https://doi.org/10.1038/s41598-025-14700-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14700-x