Abstract
The tumor microenvironment (TME), particularly CD8+ T cell infiltration, critically influences high-grade serous ovarian cancer (HGSOC) progression and treatment response. The development and management of cancer depend heavily on CD8+ T cells. Identifying non-invasive predictors of TME immune status is crucial. We investigated whether clinicopathologic characteristics and peripheral blood parameters could predict CD8+ T infiltration in TME of HGSOC. Two independent cohort were analyzed: (1) A multicenter tissue microarray (TMA) cohort of 105 epithelial ovarian cancer cases revealed that high CD8+ T cell density in tumor parenchyma, stroma, or whole tissue was significantly associated with good prognosis. (2) A retrospective cohort of 95 HGSOC patients from West China Second University Hospital (2016–2020) demonstrated that peripheral blood lymphocytes, globulin (GLB), lactate dehydrogenase (LDH), and low-density lipoprotein (LDL) correlated with CD8+ T cell infiltration in TME. These findings support non-invasive blood markers as predictors of tumor immune status and highlight chemotherapy’s role in enhancing CD8+ T cell recruitment.
Similar content being viewed by others
Introduction
The most common and lethal gynecological cancer of ovarian is high-grade serous ovarian carcinoma (HGSOC)1,2. The typical approach for treating HGSOC involves using surgery to reduce the size of tumors along with chemotherapy based on platinum. Although initial remission is often achieved, the majority of patient relapse, with most eventually developing resistance to chemotherapy and succumbing to the disease. Despite extensive biological research and clinical trials spanning many years, as well as the integration of bevacizumab and poly (ADP-ribose) polymerase inhibitors (PARPi) into treatment regimens, there has been no significant improvement in the overall survival rates for HGSOC patients3,4.
It has been reported that tumor microenvironment can not only modulate the invasion, expansion of malignant cell, but also affect the chemotherapy, radiotherapy, and oncologic antibody targeting therapy efficiency of cancer5,6,7,8. CD8+ effector T cells is one of the major components in the tumor microenvironment9. HGSOC patients with abundant CD8+ tumor-infiltrating lymphocytes (TILs) are associated with favorable clinical outcomes. Three main spatial patterns of CD8+ tumor-infiltrating phenotypes have been described previously, infiltrated, excluded, and desert: (1) The infiltrated phenotype is characterized by CD8+ T cells infiltration into the tumor epithelium; (2) the excluded phenotype is defined by the accumulation of infiltrating CD8+ T cells in the stroma and (3) the desert phenotype refers to situations where CD8+ T cells are either absent or present in very low numbers10,11,12. A significant difference disparity in progression-free survival (PFS) between desert phenotype and the other two phenotypes had been observed12. Activated CD8+ T cells infiltration into the tumor epithelium can specifically recognize tumor cells and eliminate them through the release of perforin-granzyme as well as Fas–Fas ligand pathways to induce apoptosis13,14. Although tumor stroma CD8+ T cells may not directly engage in tumor cell killing activities but have the capacity to modulate stroma function and consequently regulate tumor progression and therapeutic response6. This suggests that understanding the spatial distribution of tumor-infiltrating CD8+ T cells, holds both biological and clinical significance.
Peripheral blood indicators, such as tumor, inflammatory, and lipid metabolism markers, are also linked to the prognosis of cancer15,16. Peripheral blood markers and tumor-infiltrating immune cells (TICs) have been demonstrated to be useful prognostic indicators for ovarian cancer17. Clinicopathological characteristics are linked with peripheral lymphocyte populations, such as T cells (CD3+, CD3+CD4+, CD3+CD8+, and CD8+CD28+), regulatory T cells (Tregs, CD4+CD25+CD127−), natural killer cells (NK cells, CD3−CD56+), and B cells (CD19+)18. Additionally, data from two large cohorts in the US and UK suggest that blood cholesterol and triglyceride levels may be inversely associated with ovarian cancer risk19.
To date, peripheral blood parameters and tumor immunological status in HGSOC have not been extensively examined. To comprehensively map CD8+ T cell spatial dynamics and their peripheral correlates, we combined a multicenter tissue microarray (TMA) cohort for phenotypic classification with a clinical cohort for blood parameter analysis. We conducted a retrospective study of 95 HGSOC patients to examine the connection among CD8+ T cells in the tumor microenvironment and peripheral blood parameters such as tumor markers, inflammatory markers, and lipid metabolism indicators. Our aim was to provide evidence for monitoring the immune status of the HGSOC microenvironment through fundamental indices.
Methods
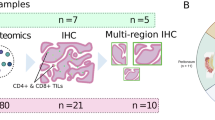
Study cohort of TMA
The ovarian cancer tissue microarray (TMA) (HOvaC151Su01) and the clinicopathological information including age, TNM stage, the International Federation of Gynecology and Obsterics (FIGO) stage, and survival state were obtained from Shanghai Outdo Biological Technology Co., Ltd. The TMA including 151 malignant human ovarian tissues in total. Excluding the exfoliated samples, a total of 105 epithelial ovarian cancer cases were eventually incorporated into our study. The study was approved by the Ethics Committee of Shanghai Outdo Biological Technology Co., Ltd. This TMA cohort focused on prognostic analysis, while the subsequent single-center cohort (Section "Patients and samples of HGSOC") investigated blood-based predictors of CD8+ infiltration.
Multiplex IHC staining
The mIHC staining employed the Opal kit (PerkinElmer, NEL811001KT). The TMA was placed in a 63℃ electric oven for an hour. Subsequently, steps such as dewaxing, antigen retrieval, elimination of endogenous peroxidase, and blocking were completed. Primary antibody was incubated at room temperature for 1 h. TBST was used to wash the slide, and the secondary antibody was incubated at room temperature for 10 min. Opal dye (1:100) was applied for 10 min after TBST washes. Microwave heating was employed to remove the complexes of primary and secondary antibodies bound to the antigen, leaving only the fluorescent dye. The step from primary incubate to microwave heating were repeated until all the indicators are marked completely. The primary antibodies used were CD8 (PA577, Abcarta), PanCK (PA125, Abcarta). Finally, DAPI staining was carried out and a fluorescence anti-quenching mounting medium was dropped to mount the slide.
Multiplex immunohistochemistry (mIHC) imaging and inform analysis
Slides were scanned using AKOYA PhenoImager HT microscope. Acquired images were analyzed with inForm software. Tissue segmentation was divided into tumor and stroma depend on the expression of CK. Cell segmentation was based on the distinct expression patterns of markers. Calculate the infiltration density of CD8+ cells based on the number of CD8+ cells infiltrated in the tissue and the tissue area.
Patients and samples of HGSOC
Patients with HGSOC at West China Second University Hospital were the subjects of a retrospective study that ran from January 1, 2016, to July 1, 2020. Histological evidence of HGSOC at the original tumor location was observed in all patients. Exclusion criteria were: (1) concurrent primary malignancies; (2) immunomodulatory therapy within 3 months prior to surgery; (3) autoimmune diseases or active infections; (4) incomplete clinical data. A total of 95 eligible patients were finally enrolled. Basic data about each patient’s characteristics were gathered. A summary of the clinical characteristics of individuals with HGSOC is provided in Table 1.
This study received ethical approval from the Regional Ethics Committee of West China Second University Hospital, Sichuan University (Approval No. 2021(Shen 069)). All experimental procedures were conducted in strict compliance with both the institutional ethical guidelines and international standards for human subject research as outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants prior to their enrollment in the study.
Laboratory measurements of peripheral blood parameters
Our research focused on a wide range of biomarkers, such as those related to lipid metabolism, inflammation, and tumors. The inflammatory indicators included neutrophils, monocytes, lymphocytes, platelets, the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), the monocyte-to-lymphocyte ratio (MLR), albumin (ALB), globulin (GLB), and lactate dehydrogenase (LDH). The levels of NLR, PLR, LMR and GLB as specific values, did not have a standard normal range. The tumor markers CA19-9 and CA125 were added. High-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A (ApoA), apolipoprotein B (ApoB), triglyceride (TG), and cholesterol (CHO) are lipid metabolism indices. The biomarkers in our study were measured in each patient before surgery or biopsy via hospital laboratory equipment for two weeks. In a biochemical test, LDH, GLB, ALB, HDL, LDL, ApoA, ApoB, TG, and CHO were assessed with a Siemens ADVIA Chemistry XPT instrument, whereas CA-125 and CA19-9 were measured with Siemens ADVIA Centaur XPT instrument. Platelets, lymphocytes, monocytes, and neutrophils were measured with a Sysmex XN9000 instrument. All the normal range were show in Table 2.
Immunohistochemical staining
Prior to immunohistochemistry (IHC) staining, the HGSOC tissue samples were embedded in paraffin and fixed in 4% paraformaldehyde (PFA). Two separate pathologists examined an H&E-stained tissue section to distinguish between tumor and stroma, which are referred to as regions of interest (ROI).
The tissue that was sectioned serially underwent staining using the CD8 antibody. Following the manufacturer’s instructions, CD8 (ZSGB-BIO, Catalog No. ZA-0508) primary antibodies and an ABC elite immunoperoxidase kit were used for immunohistochemical staining. The interface of tumor and normal tissue was identified by two different pathologists who were not aware of any of the clinical information pertaining to the patients. Computer-assisted calculations of the density of CD8+ T cells in both the tumor and stroma were carried out as described by Guifang Guo et al.20. These calculations were carried out with the assistance of 3DHISTECH CaseViewer and ImageJ v1.48, which is a Java-based image processing program that is in the public domain and was developed at the National Institutes of Health in Bethesda, Maryland, United States of America. Using the 3DHISTECH CaseViewer high throughput at 20 × , the median densities of CD8+ T cells in the tumor and stroma were 26.5/mm2 and 44.5/mm2, respectively. A number below the median was considered low expression, whereas a value above the median was considered high expression.
Statistical analysis
R 4.3.3 was used to conduct the statistical analysis. Using a chi-square test, differences in clinical factors based on CD8+ T cell expression levels in the tumor and stroma were evaluated. Tables 4, 5, and 6 provide all peripheral blood parameter values, which are given as the median (minimum‒maximum). P values less than 0.05 were considered significant.
Results
CD8 infiltration density tightly associated with patient prognosis
To investigate the role of CD8+ T cell infiltration density in epithelial ovarian cancer, we employed mIHC to detect the expression of cytokeratin (CK) and CD8. In this study, the CK-positive area was defined as the tumor parenchyma, and the CK-negative area was defined as the tumor stroma (Fig. 1A). Based on the localization of CD8+ T cells, they were classified into infiltration within the tumor parenchyma or infiltration within the stroma. The analysis revealed significant heterogeneity in the infiltration patterns of CD8+ T cells among different tissue samples, which were specifically manifested in various situations such as: complete absence of infiltration, low-grade infiltration, high infiltration within the tumor parenchyma, high infiltration within the stroma, and high infiltration in both the tumor parenchyma and stroma (Fig. 1B).
Localization, infiltration patterns of CD8+ T cells in ovarian cancer tissues and their prognostic significance. (A) The tumor area was defined by detecting CK expression using mIHC. The CK-positive area is the tumor parenchyma, and the CK-negative area is the tumor stroma. (B) Significant heterogeneity in infiltration patterns was observed based on the localization of CD8+ T cells in the tumor parenchyma and stroma. (C) Kaplan–Meier survival curve analysis of the infiltration density of CD8+ T cells in different regions with patients’ overall survival. (Left) Infiltration density in the tumor stroma; (Middle) Infiltration density in the tumor parenchyma; (Right) Infiltration density in the entire tissue area (total).
Subsequently, we conducted survival analyses on the infiltration density of CD8+ T cells in the tumor parenchyma, stroma, and the entire tissue area, respectively. The results showed that in all three analyses, a high infiltration density of CD8+ T cells was significantly associated with good prognosis of patients (Fig. 1C). In conclusion, this study suggests that the infiltration density of CD8+ T cells is closely related to patient prognosis, and the evaluation of their infiltration level has important clinical significance in predicting patients’ treatment response and prognosis.
Prognostic significance of CD8+ T cells and serum factors in HGSOC
To establish the clinical relevance of CD8+ T cell infiltration, we further explored predictors of CD8+ infiltration in our hospital-based cohort. In this study, 95 formalin-fixed paraffin-embedded (FFPE) HGSOC patient samples were selected. Histological sections were observed using HE staining to preliminarily distinguish between tumor parenchymal and stromal regions (Fig. 2A). Subsequently, serial sections were subjected to CD8 immunohistochemical staining. By comparing the HE-stained sections with the CD8-stained sections, the infiltration sites of CD8+ T cells (either within the tumor parenchyma or stroma) were accurately determined (Fig. 2B). Finally, based on clarifying the infiltration sites, CD8+ T cells were counted to evaluate their infiltration status in the corresponding regions (Fig. 2C).
Identification and characterization of CD8+ T cells populations. (A) Regions of interest (ROIs): tumor (T) and stroma (S). (B) Representative CD8-stained IHC image. Scale bar: 100 μm. (C) Representative images of different density of CD8+ T cells in the tumor (T) and stroma (S). Scale bar:50μm. (D) The density of CD8+ T cells in the tumor (T) showed a tendency that correlated with the level of TG in the blood, with a coefficient of 0.28 (P = 0.010). (E) The density of CD8+ T cells in the stroma (S) showed a tendency that correlated with the level of TG in the blood, with a coefficient of 0.13 (P = 0.23).
We sought to explore the prognostic role of the basic characteristic, and the prognostic factors included in this study in HGSOC. Through univariate Cox analysis, chemosensitivity, lymphocytes and ApoA, were found to be significantly associated with the prognosis of HGSOC. Forest plots provide a straightforward and intuitive way to present the statistical outcomes of various factors, as depicted in Fig. 3A: chemosensitivity (HR = 2.03, P < 0.05), lymphocytes (HR = 0.56, P < 0.05), ApoA (HR = 4.07, P < 0.05). following adjustment for other confounding variables, lymphocytes and ApoA remained independent predictors of OS in the multivatiate Cox regression analysis (Fig. 3B lymphocytes (HR = 0.52, P < 0.05), ApoA (HR = 3.87, P < 0.05)).
The prognostic role of the basic characteristic and the whole index included in this study in HGSOC. (A) Univariate cox analysis of the basic characteristic and the whole index included in the cohort. (B) Multivariate cox analysis of the basic characteristic and the whole index included in the cohort.
Correlations of basic characteristics with CD8+ T cells within the tumor microenvironment
The expression of CD8+ T cells in the tumor revealed that individuals who underwent neoadjuvant chemotherapy had a greater degree of CD8+ T cell expression (35.1% vs. 64.9%, P < 0.05). Age and CD8+ T cell frequency in the tumor or stroma did not correlate, according to our findings. Furthermore, we were unable to discover any connection between FIGO stage and the number of CD8+ T cells in the stroma or tumor. There was no difference in the degree of chemosensitivity of CD8+ T cells in the tumor or stroma. Table 3 displays the outcomes.
Correlations of tumor markers with CD8+ T cells within the tumor microenvironment
No significant associations were observed between CD8+ T cell infiltration and tumor markers (CA125, CA19-9) in either tumor or stroma compartments (Table 4). The infiltration of CD8+ T lymphocytes in the stroma (P = 0.615) and tumor (P = 0.427) did not correlate with CA125 levels. Moreover, we discovered no connection between CA19-9 and CD8+ T cell infiltration in the stroma (P = 0.363) or tumor (P = 0.147). Table 4 displays the outcomes.
Correlations of inflammatory markers with CD8+ T cells within the tumor microenvironment
Lymphocyte density in the tumor correlated with CD8+ T cell density (P = 0.049), but not in the stroma (P = 0.248). The number of CD8+ T lymphocytes in the stroma (P < 0.05) and tumor (P < 0.05) increased with increasing GLB. There was a correlation (P < 0.05) between the density of CD8+ T cells in the stroma and the blood LDH level. We discovered that neither in the tumor nor in the stroma did the infiltration of CD8+ T cells exhibit any correlation with neutrophils, monocytes, platelets, the NLR, the PLR, the MLR, or ALB (Table 5).
Correlations of lipid metabolism markers with CD8+ T cells within the tumor microenvironment
Among all the lipid metabolism indicators tested, LDL was the uniquely associated with CD8+ T cells. LDL and CD8+ T cell expression in the stroma were related (P < 0.05) (Table 6). Furthermore, the concentration of CD8+ T cells in the tumor showed a positive correlation with TG levels (coefficient = 0.28, P < 0.05) (Fig. 2D). Stromal CD8+ T cell density also tended toward a positive association with TG, although this was not statistically significant (coefficient = 0.13, P = 0.23) (Fig. 2E).
Discussion
Our TMA data confirmed that CD8+ infiltration is a prognostic biomarker in epithelial ovarian cancer. Crucially, we identified peripheral lymphocytes, GLB, LDH, and LDL as accessible predictors of this infiltration. This synergy between tissue-based prognosis and blood-based monitoring offers clinical utility. However, understanding the underlying biological mechanisms linking these peripheral markers to TME immune status is crucial for maximizing the impact of these findings.
In the HGSOC cohort, patients receiving neoadjuvant chemotherapy exhibited a significantly greater number of CD8+ T cells in the tumor. This aligns with growing evidence that certain chemotherapeutics, particularly platinum-based agents, can induce immunogenic cell death (ICD) in tumor cells. ICD releases damage-associated molecular patterns (DAMPs) like HMGB1 and ATP, which activate dendritic cells and promote the priming and recruitment of effector T cells, including CD8+ T cells, into the tumor bed20,21,22. Chemotherapy has been shown in the past to cause effector immune cells to invade tumors21. When paired samples from 14 HGSOC patients were immunohistochemically stained before and after neoadjuvant chemotherapy, the patients’ postchemotherapy CD8+ T cell infiltration was significantly greater than that of the control group22. Moreover, tumors with chemosensitive HGSOC demonstrate significantly greater infiltration of immune effector cells than those with chemoresistant HGSOC23. These results indicate that chemotherapy is essential for influencing the immunological milieu and encouraging immune effector cell penetration into the tumor, which increases the therapeutic effectiveness of HGSOC. A potential therapeutic strategy is the combination of immunotherapy and chemotherapy as a neoadjuvant treatment.
We found that higher peripheral blood lymphocyte counts were associated with higher tumor CD8+ T cell counts. This association may reflect a more robust systemic immune capacity. Higher circulating lymphocyte counts, particularly CD8+ T cells and their precursors, potentially provide a larger pool of effector cells capable of being recruited to the TME. Effective trafficking requires chemokine gradients (e.g., CXCL9, CXCL10, CCL5) produced within the tumor and intact endothelial adhesion molecules (e.g., ICAM-1, VCAM-1)24,25. A larger peripheral pool, coupled with favorable tumor-derived signals, could facilitate greater intratumorally infiltration. The main elements of antitumor immunity in the tumor microenvironment include ex vivo migrating antigen-presenting cells, CD8+ T cells, and CD4 + T cells. The trafficking of immune cells to the tumor is a tightly regulated process that involves adhesion to endothelial cells, trans endothelial migration, infiltration through the extracellular matrix, and ultimately homing. T cell recruitment to the tumor location is largely controlled by the vascular network within the tumor. Circulating peripheral blood T cells undergo functional reprogramming due to immunosuppressive cues within the microenvironment. Effector or helper T cell populations are driven toward continuous differentiation and exhaustion, leading to the activation of dormant Treg cells that dampen immune responses26. The peripheral blood lymphocyte subset composition has been shown to be a predictive marker of immune checkpoint inhibitor efficacy in the treatment of non-small cell lung cancer27. Moreover, recent prospective clinical studies have demonstrated that the analysis of peripheral blood lymphocyte subsets can reliably predict tumor prognosis, with results typically preceding the use of tumor markers by approximately three months28.
Moreover, our findings revealed a strong association between better CD8+ T cell accumulation in both the tumor and stroma and increased GLB levels. GLB encompasses immunoglobulins produced by plasma cells. Elevated GLB levels might indicate heightened systemic humoral immune activity or inflammation29. While the precise role of immunoglobulins in T cell infiltration is complex, potential mechanisms include: (1) Antibody-dependent cellular cytotoxicity (ADCC) mediated by IgG antibodies coating tumor cells, which could facilitate NK cell and macrophage recruitment/activation, indirectly shaping the TME and potentially enhancing T cell function; (2) Formation of immune complexes that activate complement or Fc-receptor bearing cells, contributing to inflammation and immune cell recruitment25. GLB is typically categorized as α1−, α2−, β−, or γ-GLB, which are synthesized primarily by the liver. γ-GLB is produced and released by plasma cells. In certain instances, they may be elevated in tumors. ScRNA-seq and spatial transcriptomics studies have been conducted to elucidate the role of tumor cell subpopulations that secrete immunoglobulins in the mechanism of tumor invasion in small cell lung cancer. According to the results, individuals in the small cell lung cancer cohort who had prolonged immunoglobulin expression had worse overall survival (OS) and progression-free survival (PFS)30.
Interestingly, we observed a significant correlation between higher LDH levels and greater stromal CD8+ T cell infiltration. LDH is a marker of cellular turnover and glycolysis. Elevated serum LDH is often associated with high tumor burden, tissue damage, and hypoxic, glycolytic TME. While high lactate levels (a consequence of glycolysis) are generally immunosuppressive and can inhibit CD8+ T cell function31,32, the association with stromal infiltration here might reflect a different aspect. Increased LDH could signify greater tumor cell death or stromal cell metabolic activity within the TME, potentially releasing factors that attract immune cells to the stroma. Alternatively, infiltrating immune cells themselves contribute to LDH release upon activation or death33. The specific link between circulating LDH and stromal T cell density warrants further mechanistic investigation to dissect cause and effect.
Among lipid markers, higher LDL levels were uniquely associated with increased stromal CD8+ T cell density. Cholesterol metabolism is intricately linked with immune cell function. Tumor-infiltrating lymphocytes require cholesterol for membrane synthesis and signaling during activation and proliferation. LDL serves as a major cholesterol carrier. It is plausible that increased LDL reflects a systemic lipid profile that supports the metabolic demands of activated lymphocytes trafficking to or proliferating within the tumor stroma34. Furthermore, cholesterol derivatives (oxysterols) can modulate immune responses through receptors like LXR, although their specific impact on T cell infiltration needs further study35,36.
This study demonstrates that neoadjuvant chemotherapy enhances CD8+ T cell infiltration in HGSOC tumors, potentially via immunogenic cell death. Peripheral blood lymphocytes (reflecting a larger effector cell pool and systemic capacity), GLB (indicating humoral immune activity/inflammation), LDH (signifying tumor burden/stress/metabolic activity), and LDL (potentially supporting lymphocyte metabolic demands) correlate with stromal/tumor CD8+ T cell densities, suggesting their utility as minimally invasive predictors of tumor immune status. The proposed biological mechanisms underlying these associations provide a framework for interpreting these findings and guide future research. These findings support further exploration of chemotherapy-immunotherapy synergy and blood-based immune monitoring in HGSOC, and motivate deeper investigations into the specific molecular pathways linking these peripheral markers to TME immune architecture.
Conclusion
This study demonstrates that neoadjuvant chemotherapy enhances CD8+ T cell infiltration in HGSOC tumors. Peripheral blood lymphocytes, GLB, LDH and LDL correlate with stromal/tumor CD8+ T cell densities, suggesting their utility as minimally invasive predictors of tumor immune status. These findings support further exploration of chemotherapy-immunotherapy synergy and blood-based immune monitoring in HGSOC.
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
References
Xu, J. et al. Single-cell RNA sequencing reveals the tissue architecture in human high-grade serous ovarian cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 28, 3590–3602 (2022).
Chowdhury, S. et al. Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer. Cell 186, 3476-3498.e3435 (2023).
Yang, L. et al. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 47, 1–11 (2022).
Kandalaft, L. E., Dangaj Laniti, D. & Coukos, G. Immunobiology of high-grade serous ovarian cancer: Lessons for clinical translation. Nat Rev Cancer. 22, 640–656 (2022).
Zhang, A. W. et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 173(1755–1769), e1722 (2018).
Wang, W. et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165, 1092–1105 (2016).
Binder, D. C., Fu, Y. X. & Weichselbaum, R. R. Radiotherapy and immune checkpoint blockade: potential interactions and future directions. Trends Mol. Med. 21, 463–465 (2015).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Hwang, W. T., Adams, S. F., Tahirovic, E., Hagemann, I. S. & Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol. 124, 192–198 (2012).
Xu, A. M. et al. Spatiotemporal architecture of immune cells and cancer-associated fibroblasts in high-grade serous ovarian carcinoma. Sci. Adv. 10, eadk8805 (2024).
Hornburg, M. et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell 39, 928-944.e926 (2021).
Desbois, M. et al. Integrated digital pathology and transcriptome analysis identifies molecular mediators of T-cell exclusion in ovarian cancer. Nat. Commun. 11, 5583 (2020).
Barry, M. & Bleackley, R. C. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2, 401–409 (2002).
Golstein, P. & Griffiths, G. M. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol. 18, 527–535 (2018).
Chen, J. Q. et al. Matched analysis of detailed peripheral blood and tumor immune microenvironment profiles in bladder cancer. Epigenomics 16, 41–56 (2024).
Jørgensen, N., Lænkholm, A. V., Sækmose, S. G., Hansen, L. B. & Hviid, T. V. F. Peripheral blood immune markers in breast cancer: Differences in regulatory T cell abundance are related to clinical parameters. Clin. Immunol. (Orlando, Fla). 232, 108847 (2021).
Zhang, W., Ling, Y., Li, Z., Peng, X. & Ren, Y. Peripheral and tumor-infiltrating immune cells are correlated with patient outcomes in ovarian cancer. Cancer Med. 12, 10045–10061 (2023).
Ye, S. et al. Peripheral lymphocyte populations in ovarian cancer patients and correlations with clinicopathological features. J. Ovarian Res. 15, 43 (2022).
Trabert, B. et al. Ovarian cancer risk in relation to blood cholesterol and triglycerides. Cancer Epidemiol., Biomark. Prev.: Publ. Am. Assoc. Cancer Res., Cosponsored Am. Soc. Prev. Oncol. 30, 2044–2051 (2021).
Lisandrelli, R., Winkler, M., Trajanoski, Z. & Lamberti, G. Studying immunogenic cell death in human colorectal cancer organoids. Onco. Targets. Ther. 18, 705–715 (2025).
Liu, M. et al. Improved T-cell immunity following neoadjuvant chemotherapy in ovarian cancer. Clin. Cancer Res. 28, 3356–3366 (2022).
Sun, J. et al. Immuno-genomic characterisation of high-grade serous ovarian cancer reveals immune evasion mechanisms and identifies an immunological subtype with a favourable prognosis and improved therapeutic efficacy. Br. J. Cancer 126, 1570–1580 (2022).
Walther, F. et al. High ratio of pCXCR4/CXCR4 tumor infiltrating immune cells in primary high grade ovarian cancer is indicative for response to chemotherapy. BMC Cancer 22, 376 (2022).
He, Z. et al. The ability of clinically relevant chemotherapeutics to induce immunogenic cell death in squamous cell carcinoma. Front. Biosci. (Landmark edition). 29, 158 (2024).
Abbott, D. H. et al. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Human reproduction (Oxford, England). 32, 923–936 (2017).
Dangaj, D. et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell 35(885–900), e810 (2019).
Miao, K. et al. peripheral blood lymphocyte subsets predict the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Front. Immunol. 13, 912180 (2022).
Song, L. et al. Changes in peripheral blood regulatory T Cells and IL-6 and IL-10 levels predict response of pediatric medulloblastoma and germ cell tumors with residual or disseminated disease to craniospinal irradiation. Int. J. Radiat. Oncol. Biol. Phys. 111, 479–490 (2021).
Aghara, H. et al. Unraveling the gut-liver-brain axis: Microbiome, Inflammation, and emerging therapeutic approaches. Mediators Inflamm. 2025, 6733477 (2025).
Wu, F. et al. Deciphering the role of immunoglobulin secreting malignant lineages in the invasive frontiers of small cell lung cancer by scRNA-seq and spatial transcriptomics analysis. Cell Discov. 9, 123 (2023).
Ding, J., Karp, J. E. & Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 19, 353–363 (2017).
Seth, P. et al. Deletion of lactate dehydrogenase-a in myeloid cells triggers antitumor immunity. Can. Res. 77, 3632–3643 (2017).
“Significant Activity” for ADC in Ovarian Cancer. Cancer discovery. 2021; 11: Of3.
Feingold, K. R. Lipid and lipoprotein metabolism. Endocrinol. Metab. Clin. North Am. 51, 437–458 (2022).
Borràs, C. et al. Restoring cholesterol efflux in vascular smooth muscle cells transitioning into foam cells through Liver X receptor activation. Biomed. Pharmacother. = Biomed. Pharmacother. 188, 118178 (2025).
Sun, L. et al. LRP11 promotes stem-like T cells via MAPK13-mediated TCF1 phosphorylation, enhancing anti-PD1 immunotherapy. J. Immunother. Cancer 12, e008367 (2024).
Acknowledgements
Not applicable.
Funding
We thank the support from the National Natural Science Foundation of China (82301923), the Sichuan Provincial Key Research and Development Projects (2024YFFK0268), the Technology Innovation R&D Project of Chengdu Science and Technology Bureau (2024-YF05-00191-SN) and the Horizontal Science and Technology Project of Sichuan University (23H0221, 23H0222).
Author information
Authors and Affiliations
Contributions
J.M. wrote the manuscript text, H.X. and X.L. designed and supervised the implementation of the entire study; L.Y. and J.M. analyzed the data. Y.C. and X.Z. collected the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mai, J., Yang, L., Chen, Y. et al. Prediction of CD8+ T cell infiltration in the tumor microenvironment of HGSOC patients. Sci Rep 15, 30518 (2025). https://doi.org/10.1038/s41598-025-14720-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14720-7