Abstract
This study investigates the potential mechanisms by which Tris(2-butoxyethyl) phosphate (TBEP) may contribute to the development and progression of human malignancies. Fourteen representative cancer types were selected to explore the molecular pathways through which TBEP may exert its effects. By integrating network toxicology, molecular docking, and molecular dynamics (MD) simulations, we elucidated the underlying mechanisms of TBEP-related carcinogenicity. Potential targets associated with these malignancies were identified using multiple databases, including Public Chemical Database (PubChem), Search Tool for Interacting Chemicals (STITCH), SwissTargetPrediction, The Human Gene Database (GeneCards), the Online Mendelian Inheritance in Man (OMIM), and the Therapeutic Target Database (TTD). Core targets were further screened through STRING analysis and visualized using Cytoscape software. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were then performed to characterize the biological functions and pathways involved Three-dimensional structures of the core target proteins were retrieved from the Protein Data Bank (PDB), optimized using PyMOL (version 3.0.3), and subjected to molecular docking with AutoDock Vina to assess their binding affinities with TBEP. The stability of the resulting protein–ligand complexes was validated through MD simulations using GROMACS 2022. Finally, the relevance of the identified cancer types was confirmed using The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. Overall, our findings suggest that TBEP may promote cancer development by interacting with key targets such as SRC and CASP3 and modulating critical signaling pathways. This study provides new insights into the potential carcinogenic mechanisms of TBEP and offers a theoretical foundation for future prevention and therapeutic strategies.
Similar content being viewed by others
Introduction
Tris(2-butoxyethyl) phosphate (TBEP) is an organophosphate ester widely used as a flame retardant (FR) and plasticizer in consumer and industrial products such as electronics, textiles, and plastics. Due to its extensive application, TBEP has become a pervasive environmental contaminant, with measurable concentrations detected in dust, air, water, sediments, and living organisms1. Epidemiological studies have reported the presence of TBEP in household dust across multiple countries, indicating widespread global exposure2,3,4,5. In addition to dust, TBEP has also been identified in ambient air, soil, and drinking water6,7,8, allowing for human exposure through inhalation, ingestion, and dermal contact9,10. Increasing concern over its toxicological effects has prompted investigations into its potential carcinogenicity, neurotoxicity, reproductive toxicity, and endocrine-disrupting properties11.
Cancer is a multifactorial and complex disease characterized by uncontrolled cell proliferation and the ability to metastasize. According to the International Agency for Research on Cancer (IARC), approximately 20 million new cancer cases and 9.7 million cancer-related deaths, including non-melanoma skin cancers, were reported globally in 2022. Current estimates indicate that one in five individuals will be diagnosed with cancer during their lifetime, with cancer accounting for the deaths of about one in nine men and one in twelve women. Lung cancer was the most commonly diagnosed cancer worldwide in 2022, with approximately 2.5 million new cases (12.4%), followed by female breast cancer (11.6%), colorectal cancer (9.6%), prostate cancer (7.3%), and gastric cancer (4.9%). Lung cancer also had the highest mortality, causing 1.8 million deaths (18.7%), followed by colorectal (9.3%), liver (7.8%), breast (6.9%), and gastric cancers (6.8%). Gender-specific data further showed that breast cancer was the most prevalent malignancy among women, while lung cancer ranked highest among men in both incidence and mortality12. Environmental pollutants, including organophosphate flame retardants (OPFRs) such as TBEP, have emerged as potential contributors to cancer development, prompting concern regarding their carcinogenic properties13. In a study by Po-Ju Chen et al., TBEP was detected in 99.07% of lung cancer patients (n = 108), highlighting widespread exposure14. Given that dust and air are major exposure routes for OPFRs, their potential impact on respiratory health is of particular concern. Animal studies have shown that exposure to TBEP or triphenyl phosphate (TNBP) leads to pulmonary edema, thickened alveolar walls, oxidative stress, and immune-mediated lung damage15. Moreover, Shengwu Yuan et al. demonstrated that TBEP and tris(1,3-dichloro-2-propyl) phosphate (TDCPP) induced cytotoxicity, mitochondrial dysfunction, and DNA damage in human lung cancer cells via oxidative stress-related pathways16. These findings suggest that TBEP may contribute to respiratory system damage and play a role in lung cancer development. Beyond respiratory malignancies, TBEP exposure may also impact the digestive system. Previous studies have shown that per- and polyfluoroalkyl substances (PFAS) promote hepatocellular carcinoma via core oncogenes such as estrogen receptor 1 (ESR1)17. Similarly, Quaiser Saquib et al. reported the hepatotoxic and potentially carcinogenic effects of TBEP in human liver (HepG2) cells18. However, the influence of TBEP on other gastrointestinal malignancies, including esophageal, gastric, and colorectal cancers, remains largely unexplored. Given the likelihood of oral exposure to TBEP through contaminated water and bioaccumulation19, its role in digestive system cancers warrants further investigation. TBEP has also been implicated in endocrine disruption. Studies have shown that TBEP induces oxidative stress and alters steroidogenesis in Leydig cells (TM3)20, and increases 17β-estradiol and testosterone levels in human adrenal (H295R) cells21. In animal models, pubertal exposure to TBEP negatively affected male reproductive function in adulthood22. Yu-Che Ou et al. found significantly elevated urinary levels of TBEP and its metabolite di-(2-butoxyethyl) phosphate (DBEP) in patients with endometrial cancer compared to healthy controls, indicating active metabolism and possible involvement in hormone-related cancers23. Despite these associations, the link between TBEP exposure and hormone-dependent malignancies, including testicular, prostate, ovarian, endometrial, and breast cancers remains insufficiently characterized. In addition, OPFRs such as TBEP are frequently detected in human urine14,24,25, indicating substantial exposure to the urinary system. Yet, the relationship between TBEP and urological malignancies such as renal and bladder cancer is still unclear, representing another gap addressed in this study. Emerging animal data also suggest that early-life OPFR exposure may impair motor behavior and disrupt gene expression in the central nervous system. For instance, Yuanyuan Zheng et al. reported that TBEP inhibits motor neuron survival by interfering with extracellular matrix-receptor interactions26. While its neurotoxicity is increasingly evident, the effects of TBEP on malignancies of the nervous and musculoskeletal systems, such as gliomas and osteosarcomas (OSs), remain poorly understood. To date, studies investigating the role of TBEP in pan-cancer are scarce, and the underlying molecular mechanisms remain largely undefined.
Network toxicology is an interdisciplinary field that integrates bioinformatics, systems biology, and cheminformatics to provide a comprehensive framework for understanding how chemicals disrupt molecular networks and cellular functions, potentially leading to disease. A core component of this approach is the construction of molecular interaction networks that synthesize diverse data sources to elucidate the relationships between toxicants, their biological targets, and associated diseases27,28,29. For example, Yanggang Hong and colleagues systematically investigated the effects of bisphenol A on autoimmune diseases using a network toxicology-based strategy30. In contrast, molecular docking simulates the atomic-level binding interactions between small molecules and target proteins, offering mechanistic insights into how compounds such as TBEP may contribute to cancer initiation and progression31. In this study, we employed a network toxicology approach to predict key targets of TBEP toxicity, followed by molecular docking, molecular dynamics (MD) simulations, and bioinformatics validation. To the best of our knowledge, this is the first comprehensive investigation of TBEP-induced pan-cancer toxicity targets, providing a theoretical basis for assessing the health risks associated with OPFRs and plasticizers such as TBEP.
Materials and methods
Network toxicology analysis
Acquisition of TBEP structural information and toxicity prediction
The chemical structure and Simplified Molecular Input Line Entry System (SMILES) string of TBEP were retrieved from the Public Chemical (PubChem) database (https://pubchem.ncbi.nlm.nih.gov/). To predict its toxicological profile, the compound name “TBEP” was entered into the ProTox-IIdatabase (https://tox.charite.de/), and the SMILES string was submitted to Absorption, Distribution, Metabolism, Excretion and Toxicity Laboratory (ADMETlab) (https://admetmesh.scbdd.com/). The predicted toxicity results were then downloaded and archived for further analysis (Additional file 1).
Acquisition of the TBEP targets
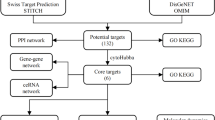
The SMILES string of TBEP was submitted to the Search Tool for Interacting Chemicals (STITCH) database (http://stitch.embl.de/cgi/input.p) and the SwissTargetPrediction database (http://www.swisstargetprediction.ch/), with the species limited to Homo sapiens. Predicted targets from the SwissTargetPrediction database were filtered to retain only those with a probability score greater than 0.1. Duplicate entries were removed, and the remaining targets were compiled to generate the final list of candidate TBEP-associated targets.
Identification of cancer-related targets
To identify targets associated with various cancer types, the following keywords were used to query multiple disease-related databases: “lung cancer, esophageal cancer, gastric cancer, colorectal cancer, skin cancer, testicular cancer, prostate cancer, ovarian cancer, endometrial cancer, breast cancer, kidney cancer, bladder cancer, glioma, and OS.” Searches were conducted in The Human Gene Database (GeneCards) (https://www.genecards.org/), the Online Mendelian Inheritance in Man (OMIM) database (https://omim.org/), and the Therapeutic Target Database (TTD) (http://db.idrblab.net/ttd/). From the GeneCards database, targets were selected based on a “Relevance score” greater than 10. For cancer types with fewer associated genes, a lower threshold of a relevance score greater than 1 was applied. All genes listed under the “Gene Map” section in the OMIM database were included, along with targets retrieved from the TTD database. After removing duplicates, all identified targets were combined to generate a final set of “cancer-related targets.”
Identification of intersection targets and construction of the regulatory network
Targets that overlapped between the previously identified “TBEP-associated targets” and “cancer-related targets” were defined as “intersection targets.” These intersection targets represent potential molecular mediators through which TBEP may contribute to the development of various cancers. To visualize the interactions, network relationship files and node attribute files were generated and imported into Cytoscape software for the construction of the TBEP-target regulatory network.
Construction of the protein-protein interaction (PPI) network and identification of core targets
The intersection targets were uploaded to the STRING database (https://string-db.org/) with the “Multiple Proteins” option selected and species restricted to Homo sapiens. The minimum required interaction score was initially set to medium confidence (> 0.4); for cancer types with simpler PPI networks, this threshold was lowered to medium confidence (> 0.15). Isolated nodes without interactions were removed. A PPI network was then generated to evaluate the interactions among intersection targets, and the resulting network data were downloaded for further analysis. The network file was imported into Cytoscape software, where the Network Analyzer tool was used to calculate topological parameters including degree, betweenness centrality, and closeness centrality for each node. Nodes were ranked according to their degree values, and the top five nodes with the highest degree were identified as core targets.
Gene function and pathway enrichment analysis
To elucidate the biological functions of the intersection targets, a comprehensive Gene Ontology (GO) enrichment analysis was conducted, encompassing biological processes (BP), cellular components (CC), and molecular functions (MF). A significance threshold was applied, with both p-values and adjusted p-values set at 0.05. Terms meeting these criteria were selected and visualized using bubble charts to provide detailed insights into the primary biological roles of the intersection targets. In parallel, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed to identify key signaling pathways potentially involved in TBEP-induced carcinogenesis. The top 30 significantly enriched pathways (p and adjusted p < 0.05) were similarly visualized via bubble charts. Both GO and KEGG analyses were performed using R Studio software (version 4.4.1).
Molecular docking of TBEP with core target proteins
To elucidate the molecular interactions between TBEP and key oncogenic proteins, molecular docking analyses were performed to characterize binding patterns and affinities. The molecular structure of TBEP was retrieved from the PubChem compound database, while three-dimensional crystallographic structures of the core target proteins were obtained from the RCSB Protein Data Bank (PDB, https://www.rcsb.org/). Protein structures were preprocessed using PyMOL (version 3.0.3) to remove water molecules and native ligands. The refined protein models were imported into AutoDock Tools 1.5.7 for preparation, including addition of hydrogen atoms, assignment of partial charges, and merging of non-polar hydrogens. Molecular docking simulations were conducted with AutoDock Vina, treating TBEP as a flexible ligand and the protein receptors as rigid. The conformation with the lowest binding free energy was considered the most favorable binding pose. Docking results were further analyzed and visualized using PyMOL (version 3.0.3), the Proteins Plus web platform (https://proteins.plus/), and the Protein-Ligand Interaction Profiler (PLIP, https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index).
MD simulation
Following molecular docking, selected core target–ligand complexes underwent MD simulations to evaluate the stability of their interactions under physiological conditions. Simulations accounted for temperature, pressure, and electrostatic effects using GROMACS 2022. The GAFF force field was applied to the small molecule ligands, while proteins were modeled using the AMBER14SB force field combined with the TIP3P water model. Protein and ligand structures were merged to construct the simulation system, which was subjected to isothermal–isobaric (NPT) ensemble conditions with periodic boundary settings. All hydrogen bonds were constrained via the LINCS algorithm with a 2 fs integration timestep. Electrostatic interactions were calculated using the Particle Mesh Ewald (PME) method with a 1.2 nm cutoff, and non-bonded interactions were truncated at 10 Å and updated every 10 steps. Temperature was maintained at 298 K using the velocity-rescaling thermostat, and pressure was controlled at 1 bar with the Berendsen barostat. The system was equilibrated through 100 ps each of isothermal-isochoric (NVT) and NPT simulations, followed by a 100 ns production run with conformations saved every 10 ps. Trajectory analyses, including root mean square deviation (RMSD), radius of gyration (Rg), buried solvent- accessible surface area (Buried SASA), and binding conformation superposition, were performed using Visual Molecular Dynamics (VMD) and PyMOL. Additionally, binding free energy calculations were conducted via the Molecular Mechanics Poisson–Boltzmann Surface Area (MM-PBSA) method using the gmmpbsa program.
Bioinformatics analysis and validation of key targets
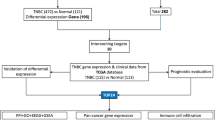
Gene expression differential analysis, prognostic assessment, and immune infiltration analysis were performed for the selected cancers using the SangerBox platform (version 3.0; http://vip.sangerbox.com/home.html)32. For gene expression analysis, all available samples from The Cancer Genome Atlas (TCGA) and the Genotype-Tissue Expression (GTEx) project were included, excluding samples with zero expression. A rank-sum test was used to evaluate differential expression between normal and tumor tissues. Prognostic analysis also included all TCGA and GTEx samples with non-zero expression, setting a minimum follow-up duration of 30 days. Overall survival differences between high and low gene expression groups were assessed.
Immune infiltration analysis was performed using the CIBERSORT algorithm on TCGA and GTEx samples, excluding zero-expression samples, to investigate the relationship between gene expression and immune cell populations.
Statistical analysis
All data analyses and visualizations were conducted using RStudio software (version 4.4.1). Statistical significance was defined as p < 0.05.
Results
Impact of TBEP on lung cancer
To investigate the potential molecular mechanisms by which TBEP influences lung cancer, we identified overlapping targets between TBEP-associated proteins and lung cancer-related genes, constructed protein interaction networks, and pinpointed key regulatory proteins.
Screening of intersection targets for TBEP-induced lung cancer
A total of 76 potential TBEP targets were retrieved from the STITCH and SwissTargetPrediction databases. Concurrently, 2,810 lung cancer-related targets were obtained from GeneCards, OMIM, and TTD. The intersection of these datasets yielded 45 common targets, representing potential molecular mediators of TBEP-induced lung cancer. These intersection targets are illustrated in a Venn diagram (Fig. 1A).
(A): The Venn diagram illustrating the common targets between TBEP and lung cancer. A total of 45 common targets were identified between TBEP and lung cancer. (B): PPI network diagram of core targets of lung cancer. (C): GO analysis of the intersection gene of TBEP and lung cancer. (D): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and lung cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
Using the STRING database with a medium confidence threshold (> 0.4), a PPI network was constructed comprising 44 nodes interconnected by 155 edges. The network was visualized in Cytoscape to analyze its topological properties, producing an intuitive interaction map (Fig. 1B). Node importance was reflected by color intensity, with redder nodes indicating higher degree values and greater potential significance. Based on degree centrality ranking, five core targets implicated in TBEP-related lung cancer were identified: SRC, CASP3, ERBB2, PTGS2, and MTOR. Among these, SRC showed the highest degree value and was prominently highlighted in bright red, emphasizing its central regulatory role within the network.
These findings highlight SRC and related key proteins as central mediators in the network of TBEP targets associated with lung cancer, providing focal points for further mechanistic exploration.
Impact of TBEP on digestive system malignant tumors
To explore TBEP’s potential involvement in digestive system cancers, we identified overlapping targets with esophageal, gastric, and colorectal cancers, constructed PPI networks, and highlighted key regulatory proteins.
Identification of intersection targets for TBEP-induced digestive system malignant tumors
A total of 4,891 esophageal cancer-related targets, 2,716 gastric cancer-related targets, and 2,739 colorectal cancer-related targets were obtained from the GeneCards, OMIM, and TTD. Intersection with TBEP-related targets revealed 56, 32, and 36 common targets for esophageal, gastric, and colorectal cancers, respectively. These overlaps are illustrated by Venn diagrams in Fig. 2A–C.
(A): The Venn diagram illustrating the common targets of TBEP and esophageal carcinoma. A total of 56 common targets were identified between TBEP and esophageal carcinoma. (B): The Venn diagram illustrating the common targets of TBEP and gastric cancer. A total of 32 common targets were identified between TBEP and gastric cancer. (C): The Venn diagram illustrating the common targets between TBEP and colorectal cancer. A total of 36 common targets were identified between TBEP and colorectal cancer. (D): PPI network diagram of core targets of Esophageal cancer. (E): PPI network diagram of core targets of Gastric cancer. (F): PPI network diagram of core targets of Colorectal cancer. (G): GO analysis of the intersection gene of TBEP and Esophageal cancer. (H): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Esophageal cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (I): GO analysis of the intersection gene of TBEP and Gastric cancer. (J): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Gastric cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (K): GO analysis of the intersection gene of TBEP and Colorectal cancer. (L): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Colorectal cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
PPI networks were constructed using the STRING database, with the confidence threshold set to medium confidence (> 0.15) for gastric and colorectal cancers. The esophageal cancer network comprised 65 nodes and 420 edges; the gastric cancer network contained 32 nodes and 294 edges; and the colorectal cancer network included 36 nodes connected by 357 edges. These networks were visualized using Cytoscape, generating detailed PPI maps (Fig. 2D–F). Core TBEP targets implicated in digestive system malignancies were identified based on degree centrality. For esophageal cancer, the core targets were ESR1, BCL2, CASP3, SRC, and FOS; for gastric cancer, CASP3, SRC, MTOR, ERBB2, and MAPK14; and for colorectal cancer, SRC, CASP3, ERBB2, MTOR, and PTGS2. Among these, ESR1, CASP3, and SRC exhibited the highest degree values in esophageal, gastric, and colorectal cancers, respectively.
These results suggest that TBEP may influence digestive system cancers through key regulatory proteins such as ESR1, CASP3, and SRC, underscoring their potential as targets for further study.
Impact of TBEP on skin cancer
To investigate the molecular mechanisms by which TBEP may influence skin cancer, we identified overlapping targets, constructed PPI networks, and pinpointed key regulatory proteins.
Identification of intersection targets for TBEP-induced skin cancer
A total of 3,720 skin cancer-related targets were retrieved from GeneCards, OMIM, and TTD. Intersection with TBEP-associated targets yielded 40 common genes. These overlapping targets are depicted in a Venn diagram (Fig. 3A).
(A): The Venn diagram illustrating the common targets between TBEP and Skin Cancer. A total of 40 common targets were identified between TBEP and Skin Cancer. (B): PPI network diagram of core targets of Skin cancer. (C): GO analysis of the intersection gene of TBEP and Skin cancer. (D): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Skin cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
A PPI network was constructed using the STRING database with a medium confidence threshold (> 0.15), comprising 40 nodes connected by 412 edges. The network was visualized in Cytoscape, generating an interpretable interaction map (Fig. 3B). Degree centrality analysis identified five core TBEP targets associated with skin cancer: SRC, CASP3, ERBB2, MTOR, and PTGS2. SRC exhibited the highest degree value, highlighting its central role in the network.
These findings emphasize SRC and several other key proteins as central mediators in TBEP-related skin cancer, providing potential targets for further functional studies.
Impact of TBEP on male reproductive malignancies
To elucidate TBEP’s potential involvement in male reproductive cancers, we identified overlapping targets for testicular and prostate cancers, constructed PPI networks, and highlighted key hub genes.
Identification of intersection targets for TBEP-induced male reproductive malignancies
Using the GeneCards, OMIM, and TTD, 2,841 testicular cancer-related targets and 3,205 prostate cancer-related targets were retrieved. Intersection with TBEP-associated targets yielded 37 and 38 overlapping genes for testicular and prostate cancers, respectively. These intersections are depicted in Venn diagrams (Fig. 4A–B).
(A): The Venn diagram illustrating the common targets between TBEP and Testicular cancer. A total of 37 common targets were identified between TBEP and Testicular cancer. (B): The Venn diagram illustrating the common targets of TBEP and Prostate cancer. A total of 38 common targets were identified between TBEP and Prostate cancer. (C): PPI network diagram of core targets of Testicular cancer. (D): PPI network diagram of core targets of Prostate cancer. (E): GO analysis of the intersection gene of TBEP and Testicular cancer. (F): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Testicular cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (G): GO analysis of the intersection gene of TBEP and Prostate cancer. (H): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Prostate cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
PPI networks were generated using the STRING database. The testicular cancer network consisted of 36 nodes connected by 129 edges, while the prostate cancer network included 38 nodes linked by 138 edges. Both networks were visualized in Cytoscape, producing detailed PPI maps (Fig. 4C–D). Based on degree centrality, five core TBEP-related hub genes were identified for each cancer type. For testicular cancer, these were CASP3, ERBB2, SRC, ABL1, and PTGS2; for prostate cancer, CASP3, SRC, ERBB2, PTGS2, and MAPK8. CASP3 showed the highest degree centrality in both networks, indicating its prominent role.
These results suggest that CASP3 and related hub genes may be key mediators of TBEP’s effects in male reproductive system malignancies, warranting further investigation.
Impact of TBEP on common female malignancies
To assess TBEP’s potential role in common female malignancies, we identified overlapping targets for ovarian, endometrial, and breast cancers, constructed PPI networks, and determined key regulatory proteins.
Identification of intersection targets for TBEP-induced female malignancies
Using the GeneCards, OMIM, and TTD, 2,856 ovarian cancer-related targets, 1,936 endometrial cancer-related targets, and 3,421 breast cancer-related targets were retrieved. Intersection with TBEP-associated targets yielded 28, 29, and 38 overlapping genes for ovarian, endometrial, and breast cancers, respectively. These intersections are visualized in Venn diagrams (Figs. 5A–C).
(A): The Venn diagram illustrating the common targets between TBEP and Ovarian cancer. A total of 28 common targets were identified between TBEP and Ovarian cancer. (B): The Venn diagram illustrating the common targets between TBEP and Endometrial cancer. A total of 29 common targets were identified between TBEP and Endometrial cancer. (C): The Venn diagram illustrating the common targets between TBEP and Breast cancer. A total of 38 common targets were identified between TBEP and Breast cancer. (D): PPI network diagram of core targets of Ovarian cancer. (E): PPI network diagram of core targets of Endometrial cancer. (F): PPI network diagram of core targets of Breast cancer. (G): GO analysis of the intersection gene of TBEP and Ovarian cancer. (H): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Ovarian cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (I): GO analysis of the intersection gene of TBEP and Endometrial cancer. (J): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Endometrial cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (K): GO analysis of the intersection gene of TBEP and Breast cancer. (L): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Breast cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
PPI networks were constructed via the STRING database with a medium confidence threshold (> 0.15). The ovarian cancer network comprised 28 nodes connected by 254 edges; the endometrial cancer network included 29 nodes and 265 edges; and the breast cancer network contained 38 nodes linked by 353 edges. These networks were visualized in Cytoscape, generating detailed PPI diagrams (Fig. 5D–F). Degree centrality analysis identified five core TBEP targets in each cancer type. For ovarian cancer, these were CASP3, SRC, ERBB2, CDK1, and MTOR; for endometrial cancer, SRC, CASP3, ERBB2, MTOR, and CDK1; and for breast cancer, CASP3, SRC, ERBB2, MTOR, and PTGS2. CASP3, SRC, and CASP3 showed the highest degree centrality in ovarian, endometrial, and breast cancers, respectively.
These findings suggest that CASP3, SRC, and related hub proteins may serve as critical mediators in TBEP-associated female malignancies, providing potential targets for further investigation.
Impact of TBEP on urological malignancies
To explore TBEP’s potential involvement in urological cancers, we identified overlapping targets for kidney and bladder cancers, constructed PPI networks, and highlighted key hub proteins.
Identification of intersection targets for TBEP-induced urological malignancies
Using GeneCards, OMIM, and TTD, 3,743 kidney cancer-related targets and 2,897 bladder cancer-related targets were obtained. Intersection with TBEP-associated targets yielded 37 and 35 overlapping genes for kidney and bladder cancers, respectively. These overlaps are illustrated in Venn diagrams (Fig. 6A–B).
(A): The Venn diagram illustrating the common targets between TBEP and Kidney cancer. A total of 37 common targets were identified between TBEP and Kidney cancer. (B): The Venn diagram illustrating the common targets between TBEP and Bladder cancer. A total of 35 common targets were identified between TBEP and Bladder cancer. (C): PPI network diagram of core targets of Kidney cancer. (D): PPI network diagram of core targets of Bladder cancer. (E): GO analysis of the intersection gene of TBEP and Kidney cancer. (F): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Kidney cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (G): GO analysis of the intersection gene of TBEP and Bladder cancer. (H): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Bladder cancer. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
PPI networks were constructed using the STRING database with a medium confidence threshold (> 0.15). The kidney cancer network comprised 37 nodes connected by 354 edges, and the bladder cancer network included 35 nodes linked by 354 edges. These networks were visualized in Cytoscape, producing detailed PPI maps (Fig. 6C–D). Degree centrality analysis identified five core TBEP targets for each cancer type. For kidney cancer, these were CASP3, SRC, ERBB2, MTOR, and MAPK14; for bladder cancer, SRC, CASP3, ERBB2, MTOR, and MAPK14. CASP3 and SRC were the most central targets in both malignancies, highlighting their potential regulatory significance.
These findings indicate that CASP3, SRC, and related proteins may play crucial roles in TBEP-associated urological cancers, warranting further functional studies.
Impact of TBEP on neuromuscular malignancies
To assess the potential role of TBEP in neuromuscular cancers, we identified overlapping targets for glioma and OS, constructed PPI networks, and pinpointed key hub proteins.
Identification of intersection targets for TBEP-induced neuromuscular malignancies
A total of 3,855 glioma-related and 2,732 OS-related targets were retrieved from the GeneCards, OMIM, and TTD. Intersection with TBEP-associated targets identified 39 common genes for glioma and 31 for OS. These intersections are visualized in Venn diagrams (Fig. 7A–B).
(A): The Venn diagram illustrating the common targets between TBEP and Glioma. A total of 39 common targets were identified between TBEP and Glioma. (B): The Venn diagram illustrating the common targets between TBEP and Osteosarcoma. A total of 31 common targets were identified between TBEP and Osteosarcoma. (C): PPI network diagram of core targets of Glioma. (D): PPI network diagram of core targets of Osteosarcoma. (E): GO analysis of the intersection gene of TBEP and Glioma. (F): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Glioma. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission. (G): GO analysis of the intersection gene of TBEP and Osteosarcoma. (H): Enrichment analysis of the top 30 KEGG of the intersection genes of TBEP and Osteosarcoma. Data source: KEGG database (Kanehisa et al., 2024). ©2024 Kanehisa Laboratories. Used with permission.
PPI network and identification of core targets
PPI networks were constructed using the STRING database with a medium confidence threshold (> 0.15). The glioma network comprised 39 nodes linked by 401 edges, while the OS network included 31 nodes connected by 304 edges. These networks were visualized using Cytoscape, producing detailed interaction maps (Fig. 7C–D). Degree centrality analysis identified five core TBEP targets for each cancer type. For glioma, these were SRC, CASP3, PTGS2, MAPK14, and ERBB2; for OS, CASP3, ERBB2, SRC, MTOR, and MAPK14. SRC and CASP3 exhibited the highest degree values in the glioma and OS networks, respectively.
These findings highlight SRC, CASP3, and other key proteins as central regulators in TBEP-associated neuromuscular malignancies, offering promising targets for further investigation.
GO and KEGG pathway enrichment analysis
To elucidate the potential molecular mechanisms by which TBEP may contribute to the development of various malignant tumors, we conducted GO and KEGG pathway enrichment analyses on the overlapping target genes between TBEP and each cancer type33,34,35. In lung cancer, 888 GO terms were significantly enriched (P < 0.05), including 767 BPs, 37 CCs, and 84 MFs, alongside 81 significantly enriched KEGG pathways. Detailed enrichment results for other malignancies are provided in Supplementary Table S1. For each cancer, the top 10 significantly enriched GO terms in BP, CC, and MF categories were selected and visualized as bubble plots (Lung cancer—Figure 1C; Esophageal cancer—Figure 2G; Gastric cancer—Figure 2I; Colorectal cancer—Figure 2K; Skin cancer—Figure 3C; Testicular cancer—Figure 4E; Prostate cancer—Figure 4G; Ovarian cancer—Figure 5G; Endometrial cancer—Figure 5I; Breast cancer—Figure 5K; Kidney cancer—Figure 6E; Bladder cancer—Figure 6G; Glioma—Figure 7E; OS—Figure 7G). In these plots, bubble size and bar length correspond to the number of enriched genes per GO term, while color intensity indicates statistical significance, with darker red representing greater enrichment. Similarly, the top 30 significantly enriched KEGG pathways were visualized in bubble plots for each cancer type (Lung cancer—Figure 1D; Esophageal cancer—Figure 2H; Gastric cancer—Figure 2J; Colorectal cancer—Figure 2L; Skin cancer—Figure 3D; Testicular cancer—Figure 4F; Prostate cancer—Figure 4H; Ovarian cancer—Figure 5H; Endometrial cancer—Figure 5J; Breast cancer—Figure 5L; Kidney cancer—Figure 6F; Bladder cancer—Figure 6H; Glioma—Figure 7F; OS—Figure 7H).
In the GO BP analysis, lung and esophageal cancers were predominantly enriched in the regulation of membrane potential. Gastric, prostate, ovarian, and endometrial cancers showed enrichment in responses to xenobiotic stimuli, while colorectal, testicular, and kidney cancers were mainly associated with responses to lipopolysaccharide. Skin and bladder cancers were enriched in cellular responses to chemical stress, and breast cancer, glioma, and OS were linked to rhythmic processes.
For CC terms, lung and esophageal cancers were primarily associated with the synaptic membrane; gastric, testicular, prostate cancers, and glioma with the neuronal cell body; colorectal cancer and OS with the glutamatergic synapse; skin cancer with endocytic vesicles; ovarian, endometrial, breast, and kidney cancers with the basal plasma membrane; and bladder cancer with the organelle outer membrane. Regarding MF terms, lung cancer targets were chiefly enriched in neurotransmitter receptor activity, whereas targets in other malignancies were mainly involved in protein serine/threonine kinase activity. KEGG pathway analysis indicated significant enrichment in multiple signaling pathways across the cancers. Lung and esophageal cancers were notably enriched in the neuroactive ligand–receptor interaction pathway; gastric, skin, prostate, ovarian, endometrial, and kidney cancers showed enrichment in Kaposi sarcoma-associated herpesvirus infection; and colorectal, testicular, breast, bladder cancers, glioma, and OS were predominantly enriched in pathways related to proteoglycans in cancer. Comprehensive details of the GO and KEGG enrichment analyses are provided in Supplementary Table S2.
Molecular Docking of TBEP with core target proteins
Our analysis revealed a notable overlap among core targets implicated across the various cancers examined, suggesting that TBEP may promote tumorigenesis and progression by modulating shared critical genes and signaling pathways. Notably, SRC and CASP3 consistently emerged as central hub proteins across all cancer types. Degree value ranking further highlighted SRC and CASP3 as the top one or two targets in most of the malignancies analyzed. Based on these results, we prioritized molecular docking studies on SRC and CASP3, while also performing complementary docking analyses on other key targets to provide a comprehensive understanding of TBEP’s molecular interactions.
To elucidate the interactions between TBEP and the identified core targets, molecular docking analyses were performed. All docking simulations yielded negative Vina scores, indicating favorable binding free energies and suggesting spontaneous, stable interactions between TBEP and these proteins. The docking poses with the lowest free energy, representing the most stable conformations, were selected for visualization (Fig. 8). A summary of the docking results is provided in Table 1. TBEP established multiple stable molecular interactions with core targets, including hydrogen bonds, hydrophobic contacts, and, in some cases, salt bridges, underscoring its strong binding potential. For example, TBEP formed five hydrogen bonds with three amino acid residues (SER94, ASP99, SER101) of SRC, with bond lengths ranging from 2.7 to 3.4 Å (Fig. 8A), alongside hydrophobic interactions with THR103 and GLU128. The binding free energy of the TBEP-SRC complex was calculated as − 3.5 kcal/mol. Similarly, TBEP interacted with CASP3 via four hydrogen bonds involving residues GLN203, HIS207, TYR385, and TRF387, with bond distances between 2.28 and 3.5 Å (Fig. 8B), hydrophobic contacts with HIS237 and TRP340, and a salt bridge with ARG341. The TBEP–CASP3 complex exhibited a binding free energy of − 4.6 kcal/mol. Docking analyses for TBEP with other core targets—including ERBB2 (Fig. 8C), PTGS2 (Fig. 8D), MTOR (Fig. 8E), ESR1 (Fig. 8F), BCL2 (Fig. 8G), FOS (Fig. 8H), MAPK14 (Fig. 8I), ABL1 (Fig. 8J), MAPK8 (Fig. 8K), and CDK1 (Fig. 8L)—are detailed in Table 1. These docking models highlight the intimate molecular interactions between TBEP and its core targets, providing valuable structural insights and emphasizing the potential role of TBEP in modulating molecular pathways involved in diverse cancers.
Molecular docking interactions of TBEP with core cancer-related proteins. (A):TBEP binds SRC via five hydrogen bonds (SER94, ASP99, SER101; 2.7–3.4 Å) and hydrophobic interactions with THR103 and GLU128. (B): TBEP forms four hydrogen bonds (GLN203, HIS207, TYR385, TRP387; 2.28–3.5 Å) and hydrophobic contacts with HIS237, TRP340, plus a salt bridge with ARG341 in CASP3. (C): TBEP interacts hydrophobically with seven residues (VAL64, LEU67, PHE71, VAL164, LEU167, PHE171, ILE175) in ERBB2, without hydrogen bonds or salt bridges. (D): In PTGS2, TBEP establishes five hydrogen bonds (GLN203, HIS388, HIS207, TYR385, TRP387; 3.1–3.2 Å), hydrophobic interactions with four residues, and two salt bridges (HIS207, HIS388). (E): TBEP forms three hydrogen bonds (ARG116, GLU104; 3.2–3.4 Å), hydrophobic contacts with three residues, and a salt bridge with HIS99 in MTOR. (F): TBEP binds ESR1 through one hydrogen bond (GLY390; 2.9 Å) and hydrophobic interactions with seven residues. (G): In BCL2, TBEP establishes four hydrogen bonds (LYS53, LYS101, GLU49; 2.2–3.6 Å), hydrophobic contacts with four residues, and a salt bridge with ARG362. (H): TBEP engages FOS via five hydrogen bonds (LYS69, LEU7; 2.1–3.0 Å), a hydrophobic contact with TYR65, and a salt bridge with LYS69. (I): TBEP interacts with MAPK14 through two hydrogen bonds (ASP88, ARG23; 3.1–3.3 Å), hydrophobic interactions with three residues, and a salt bridge with LYS69. (J): TBEP forms six hydrogen bonds (ARG362, SER385, ASN336; 3.0–3.3 Å), hydrophobic contacts with seven residues, and a salt bridge with ARG362 in ABL1. K: In MAPK8, TBEP forms five hydrogen bonds (THR21, GLY35, ALA36, ASN156, SER155, LYS153; 3.2–6.3 Å) and hydrophobic interactions with three residues. L: TBEP binds CDK1 via three hydrogen bonds (GLN132, TYR15; 3.2–3.4 Å) and hydrophobic interactions with seven residues.
MD simulation of SRC-TBEP and CASP3-TBEP complexes
To further assess the stability of the SRC–TBEP and CASP3–TBEP complexes, MD simulations were performed. The root mean square deviation (RMSD) profiles for both complexes (SRC–TBEP: Fig. 9A; CASP3–TBEP: Fig. 9D) gradually stabilized over time, indicating stable binding interactions between the proteins and TBEP. Similarly, the radius of gyration (Rg) values (SRC–TBEP: Fig. 9B; CASP3–TBEP: Fig. 9E) stabilized throughout the simulation, reflecting the maintenance of compact molecular conformations. The buried solvent-accessible surface area (Buried SASA), which indicates the extent of intermolecular contact, also plateaued during the simulations (SRC–TBEP: Fig. 9C; CASP3–TBEP: Fig. 9F), suggesting a consistent contact interface between TBEP and the proteins. Superimposition of representative conformations (SRC–TBEP: Fig. 9G; CASP3–TBEP: Fig. 9H) revealed a high degree of overlap for TBEP within the binding sites, demonstrating stable ligand positioning. Finally, binding free energy calculations using the MM-PBSA method were conducted on equilibrated trajectories. The calculated ΔE_MM-PBSA values were − 56.04 ± 4.06 kJ/mol for SRC–TBEP and − 82.24 ± 2.51 kJ/mol for CASP3–TBEP, indicating strong and favorable binding affinities for both complexes.
(A): Root Mean Square Deviation (RMSD) of the SRC-TBEP Complex, SRC Core Target Protein, and TBEP Ligand; (B): Radius of Gyration (Rg) of the SRC-TBEP Complex; (C): The buried solvent accessible surface area(Buried SASA) between TBEP and the core target protein SRC; (D): Root Mean Square Deviation (RMSD) of the CASP3-TBEP Complex, CASP3 Core Target Protein, and TBEP Ligand; (E): Radius of Gyration (Rg) of the CASP3-TBEP Complex; (F): The buried solvent accessible surface area(Buried SASA) between TBEP and the core target protein CASP3; (G): Superimposition of SRC-TBEP Simulated Conformations; (H): Superimposition of CASP3-TBEP Simulated Conformations.
Verification of key targets through network bioinformatics
Expression of core targets in cancer tissues
To investigate differential expression of key targets between cancerous and normal tissues, we analyzed the expression profiles of SRC and CASP3 across various cancers using data from TCGA. Additionally, ESR1 and BCL2 expression levels were assessed specifically in esophageal cancer. As illustrated in Fig. 10A, SRC expression was significantly elevated in lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), combined gastric and esophageal cancer (STES), gastric cancer (STAD), colon adenocarcinoma (COAD), colorectal adenocarcinoma (COADREAD), rectal adenocarcinoma (READ), skin cutaneous melanoma (SKCM), testicular germ cell tumors (TGCT), prostate adenocarcinoma (PRAD), ovarian serous cystadenocarcinoma (OV), invasive breast carcinoma (BRCA), clear cell renal cell carcinoma (KIRC), chromophobe renal cell carcinoma (KICH), glioma (GBMLGG), and low-grade glioma (LGG), compared to corresponding normal tissues. Similarly, CASP3 expression (Fig. 10B) was significantly higher in LUAD, LUSC, esophageal carcinoma (ESCA), STES, STAD, COADREAD, COAD, READ, SKCM, TGCT, PRAD, OV, uterine corpus endometrial carcinoma (UCEC), BRCA, KIRC, kidney pan-cancer cohort (KIPAN), bladder urothelial carcinoma (BLCA), GBMLGG, and LGG than in normal tissues. Furthermore, ESR1 and BCL2 expression levels were notably elevated in ESCA compared to normal esophageal tissue (Fig. 10C–D).
(A): Analysis of the expression differences of SRC core targets in tumor tissues and normal tissues. (B): Analysis of the expression differences of CASP3 core targets in tumor tissues and normal tissues, (C): Analysis of the expression differences of ESR1 core targets in Esophageal cancer and Normal tissues. (D): Analysis of the expression differences of BCL2 core targets in Esophageal cancer and Normal tissues.
Association between core target expression and disease prognosis
To evaluate the relationship between core target expression levels and patient survival across various cancer types, we conducted survival analyses using data from TCGA. As shown in the forest plots (Fig. 11A), SRC expression was significantly associated with prognosis in PRAD, KIRC, GBMLGG, and BLCA (P < 0.05). Specifically, elevated SRC expression predicted poorer clinical outcomes in PRAD and KIRC (hazard ratio [HR] > 1), whereas higher expression correlated with improved survival in GBMLGG and BLCA (HR < 1). Similarly, increased CASP3 expression (Fig. 11B) was linked to reduced survival in GBMLGG, LGG, and KIPAN, but predicted better prognosis in SKCM. For ERBB2 (Fig. 11C), high expression was negatively associated with survival in GBMLGG, LGG, and OV (HR > 1), while exhibiting a protective effect in KIPAN, KIRC, and KICH (HR < 1). Further prognostic analyses were performed for additional core targets, including PTGS2 (Fig. 11D), MTOR (Fig. 11E), MAPK14 (Fig. 11F), CDK1 (Fig. 11G), ABL1 (Fig. 11H), MAPK8 (Fig. 11I), ESR1 (Fig. 11J), BCL2 (Fig. 11K), and FOS (Fig. 11L). The detailed survival associations for these targets are depicted in Figs. 11D–L.
(A): Forest plot of the impact of SRC core targets on the prognosis of malignant tumor diseases. (B): Forest plot of the impact of CASP3 core targets on the prognosis of malignant tumor diseases. (C): Forest plot of the impact of ERBB2 core targets on the prognosis of malignant tumor diseases. (D): Forest plot of the impact of PTGS2 core targets on the prognosis of malignant tumor diseases. (E): Forest plot of the impact of MTOR core targets on the prognosis of malignant tumor diseases. (F): Forest plot of the impact of MAPK14 core targets on the prognosis of malignant tumor diseases. (G): Forest plot of the impact of CDK1 core targets on the prognosis of malignant tumor diseases. (H): Forest plot of the impact of ABL1 core target on the prognosis of testicular cancer disease. (I): Forest map of the impact of MAPK8 core targets on the prognosis of prostate cancer diseases. (J-L): Forest map of the effects of ESR1, BCL2 and FOS core targets on the prognosis of esophageal cancer.
Impact of core targets on disease immune cell infiltration
The association between core target gene expression and immune cell infiltration was examined across multiple cancer types using immune profiling data. As shown in Fig. 12A, SRC expression was significantly correlated with infiltration of specific immune cell subsets, particularly M0 macrophages, resting CD4 + memory T cells, and naïve B cells. Notably, SRC expression positively correlated with M0 macrophage infiltration in LUAD, LUSC, STAD, TGCT, and BRCA. Similarly, CASP3 expression exhibited strong associations with M1 macrophages and resting memory CD4 + T cells across various tumors (Fig. 12B). In particular, 12 cancer types, including LUAD, LUSC, and STAD, showed a robust positive association between CASP3 levels and M1 macrophage infiltration. Focusing on ESCA, ESR1 expression positively correlated with infiltration by M1 macrophages and resting dendritic cells (Fig. 12C). Similarly, BCL2 expression was associated with increased levels of resting mast cells and monocytes (Fig. 12D). Collectively, these results suggest that these core targets may contribute to TBEP-mediated modulation of the tumor immune microenvironment across different cancers.
(A): Analysis of immune infiltration of SRC core targets in malignant tumors. (B): Analysis of immune infiltration of CASP3 core targets in malignant tumors. (C): Analysis of immune infiltration of ESR1 core targets in esophageal cancer. (D): Analysis of immune infiltration of BCL2 core targets in esophageal cancer.
Discussion
FRs are widely used in diverse products worldwide, including construction materials, textiles, and electronic devices. However, growing evidence of the toxicity associated with traditional brominated FRs—especially polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs)—has prompted their ban in many countries. In response, OPFRs have been increasingly adopted as alternative compounds36. TBEP is one such OPFR that is pervasive in the environment, detected in air, water, and soil samples. Studies have demonstrated that TBEP possesses carcinogenic, neurotoxic, reproductive toxic, and endocrine-disrupting properties37. Additionally, recent research highlights the bioaccumulative potential of OPFRs, including TBEP38,39. Without adequate regulatory intervention, TBEP is poised to pose a significant threat to human health, representing not only an environmental concern but also a critical public health issue. In this study, we applied a combination of network toxicology and molecular docking approaches to elucidate the potential molecular mechanisms by which TBEP may contribute to the initiation and progression of human malignancies.
Our study provides compelling evidence linking TBEP to the initiation and progression of several common human malignancies. This research innovatively explores the potential molecular regulatory mechanisms by which TBEP influences cancer development. Specifically, we identified key molecular pathways and five core targets involved in TBEP’s effects across various malignancies. Notably, SRC and CASP3 emerged consistently as core targets in all cancers examined, frequently ranking as the top two in terms of importance. This suggests that TBEP may promote tumorigenesis through shared targets, pathways, and mechanisms across diverse cancer types. Consequently, SRC and CASP3 could serve as broadly responsive molecular biomarkers for environmental stress, with potential applications in cross-tumor predictive models of exposure-related cancer risk. These biomarkers could be valuable tools in environmental health surveillance and translational toxicology. SRC, a non-receptor tyrosine kinase and established oncogene, regulates cell proliferation, differentiation, migration, invasion, angiogenesis, and drug resistance40. Its oncogenic effects are mediated through signaling pathways including PI3K/AKT, STAT3, FAK, and MAPK. Aberrant SRC activation is common in cancer cells, promoting tumor growth and metastasis38,41,42. For instance, SRC promotes the proliferation and metastasis of lung cancer cells by activating EGFR, STAT3, and FAK signaling43,44. Moreover, SRC inhibition has been shown to reverse chemotherapy resistance and improve chemosensitivity45,46. Elevated SRC expression correlates with increased invasiveness in esophageal, gastric, and colorectal cancers, where it drives cell migration via STAT3, ERK, and FAK pathways47,48,49. In BLCA, SRC is significantly upregulated and closely related to cisplatin resistance; it supports tumor survival by reprogramming energy metabolism, particularly glycolysis and the pentose phosphate pathway50. Additionally, SRC family kinases contribute to glioma development, therapeutic resistance, and recurrence by enhancing the migratory capacity of glioma stem cells51. In OS, SRC activation is associated with angiogenesis, especially in anoikis-resistant cells. Specifically, SRC-driven MAPK signaling is critical for angiogenesis and represents a promising therapeutic target for OS metastasis52. These findings align with our identification of SRC, MAPK14, and CASP3 as core targets in OS, underscoring their central roles in TBEP-associated tumorigenesis.
CASP3 is a key protease responsible for executing apoptosis, and its dysregulation, such as reduced expression or functional inhibition, can enable cancer cells to evade programmed cell death, thereby facilitating tumorigenesis. Suppression or disruption of the apoptotic pathway is a hallmark of various malignancies53. In particular, inhibition of CASP3 activity has been linked to increased resistance to chemotherapy in lung adenocarcinoma and gastric cancer, thereby reducing treatment efficacy54,55. Although CASP3 is primarily known for its apoptotic role, it can paradoxically promote tumor cell migration and invasion through non-apoptotic mechanisms under certain conditions56. Moreover, CASP3 expression levels correlate significantly with patient prognosis in some cancers; decreased CASP3 expression often associates with advanced tumor stages, poor clinical outcomes, and reduced survival rates57. Emerging evidence suggests that CASP3 not only mediates apoptosis but also contributes to tumorigenesis and malignant transformation. For example, activation of CASP3 can induce the transformation of human fibroblasts into malignant cells, a process involving the translocation of the downstream effector endonuclease G (EndoG). EndoG then activates SRC and STAT3 signaling pathways, thereby promoting tumor cell proliferation and survival58. In conclusion, CASP3 has a dual role in cancer biology: while it facilitates apoptosis, it can also drive tumorigenesis through non-apoptotic pathways, particularly via activation of the SRC-STAT3 axis58. This dual functionality supports our findings that CASP3, alongside SRC, serves as a central target implicated in the pathogenesis and progression of multiple cancer types.
It is important to recognize that SRC and CASP3 perform distinct roles depending on the cancer type. In hormone-sensitive cancers, Elsberger et al. reported that all eight members of the SRC kinase family (SFK) are expressed in breast tissues, with SRC kinase showing the highest expression in breast cancer and correlating with poorer patient survival59. Moreover, SRC is implicated in resistance to endocrine therapy in breast cancer60. Previous research has demonstrated that SRC-3, in complex with TRAF4, promotes ovarian cancer cell proliferation, migration, invasion, and differentiation through activation of the PI3K/AKT pathway; notably, elevated SRC expression is associated with increased chemotherapy resistance61,62. Additionally, studies have identified the CXCL1-LCN2 axis as an activator of SRC signaling, inducing epithelial-mesenchymal transition (EMT) and thereby enhancing prostate cancer cell migration and tumor invasiveness63. SRC, particularly SRC-3, also potentiates the transcriptional activity of estrogen receptors (ER) and androgen receptors (AR), driving the progression of breast and prostate cancers64,64. CASP3 supports cancer cell survival under stress by promoting cytoprotective autophagy and facilitating the DNA damage response66. In immune-driven cancers, SRC kinase plays a direct role in modulating anti-infective and anti-tumor immune responses by regulating immune cell phagocytosis, migration, inflammatory signaling, and survival. SRC-mediated reactive oxygen species (ROS) production, cytokine release, and intercellular communication can contribute to the formation of either pro-inflammatory or immunosuppressive tumor microenvironments67. CASP3 may exert a dual role in immune-related cancers by inducing tumor cell apoptosis while also modulating immune signaling pathways to influence anti-tumor immunity65,66,67,68. Consequently, TBEP may further contribute to the development and progression of various cancers through its regulatory effects on SRC and CASP3 activities69.
Therefore, it is reasonable to hypothesize that the expression levels of these core genes differ between cancer patients and healthy individuals. Validation using data from TCGA confirmed that the expression of these key targets in tumor tissues from the studied cancer types was significantly altered compared to normal tissues, underscoring their critical roles in tumorigenesis. These differential expression patterns highlight the potential utility of these genes as diagnostic biomarkers and reinforce their value as therapeutic targets70. Additionally, we investigated the relationship between core target expression and patient prognosis, as well as their impact on immune cell infiltration. The consistency between network toxicology predictions and bioinformatics validation provides compelling evidence supporting the pathophysiological involvement of TBEP in the development and progression of these malignancies.
Following the molecular docking analysis, MD simulations were performed to assess the stability of the docked complexes. The relatively low binding free energies (ΔE_MM-PBSA) for the SRC–TBEP and CASP3–TBEP complexes indicate strong binding affinities, supporting the potential of these interactions. While these computational docking results suggest promising binding between TBEP and the target proteins, it is important to recognize that binding affinity predictions alone do not confirm functional bioactivity. Experimental validation is required to corroborate these findings. Notably, to our knowledge, this study is the first systematic integration of network toxicology and molecular docking approaches to explore pan-cancer mechanisms associated with TBEP exposure. From a translational medicine perspective, the identification of core regulatory targets offers promising sites for novel drug development and opens avenues for precision medical interventions. From a public health standpoint, our findings provide crucial guidance for mitigating FR exposure through three key strategies: (1) establishing exposure limit standards for FRs, (2) promoting the development of environmentally friendly alternative materials, and (3) strengthening regulatory oversight across the entire lifecycle of FRs. The implementation of these measures is essential to reducing environmental toxicant risks and advancing public health goals.
Despite the robust theoretical foundation of this study, several limitations must be acknowledged. The conclusions are currently based solely on in silico analyses, necessitating further validation through in vitro and in vivo experiments to confirm the predicted molecular interactions and biological effects. The broad, multi-cancer scope and the physicochemical properties of TBEP—such as low water solubility, high lipophilicity, and bioaccumulative potential—pose challenges for experimental modeling, potentially limiting its behavior in conventional biological systems. Moreover, TBEP exposure typically occurs at chronic, low doses in the environment, meaning standard acute toxicity assays may not accurately reflect its long-term biological impact. These factors highlight the need for physiologically relevant exposure models incorporating long-term dosing and toxicokinetic profiling to better understand TBEP’s effects.
Another limitation of our current computational approach is the lack of dose–response modeling and threshold estimation. The observed binding affinities do not necessarily translate into functional bioactivity, particularly in the absence of defined exposure concentrations required to trigger biological effects. Incorporating dose-dependent experimental validation and quantitative modeling would substantially enhance the predictive accuracy and practical relevance of this framework.
Despite these challenges, the integration of network toxicology with empirical data represents a promising direction for future research. Prioritizing the synergy between computational predictions and experimental validation will strengthen the biological and translational significance of the findings. Furthermore, while core targets such as SRC and CASP3 are implicated across multiple cancers due to their roles in immune regulation, apoptosis, and endocrine signaling, the heterogeneity of their upstream regulators and downstream effectors across distinct tumor microenvironments warrants further mechanistic studies, so as to elucidate specific regulatory axes and improve targeted intervention strategies.
Conclusion
Our findings indicate that TBEP may contribute to the occurrence and development of multiple malignancies by affecting core targets such as SRC and CASP3, along with shared signaling pathways. This study explores the possible mechanistic associations between TBEP exposure and the development of diverse cancer types, providing valuable insights to advance environmental health research and inform public health policy. Employing an interdisciplinary approach that integrates network toxicology, molecular docking, MD simulations, and bioinformatics analyses, we offer a novel perspective on the role of TBEP in cancer progression. We anticipate that these findings will lay the groundwork for future investigations into the influence of environmental factors on cancer onset and progression. Additionally, by deepening our understanding of these associations, we aim to facilitate the development of more effective prevention and therapeutic strategies, thereby contributing to significant advancements in public health.
Data availability
All data are publicly available. The database used in this article, as well as the tools for biological information verification and their links, are presented as follows. PubChem: https://pubchem.ncbi.nlm.nih.gov/ProTox: https://tox.charite.de/ADMETlab: https://admetmesh.scbdd.com/STITCH: http://stitch.embl.de/SwissTargetPrediction: http://www.swisstargetprediction.ch/GeneCards: https://www.genecards.org/OMIM: https://omim.org/Therapeutic Target Database (TTD): http://db.idrblab.net/ttd/SangerBox (Version 3.0) : http://vip.sangerbox.com/home.html.
Abbreviations
- TBEP:
-
Tris(2-butoxyethyl) phosphate
- MD:
-
molecular dynamics
- PubChem:
-
Public Chemical Database
- STITCH:
-
Search Tool for Interacting Chemicals
- GeneCards:
-
The Human Gene Database
- OMIM:
-
Online Mendelian Inheritance in Man
- TTD:
-
Target Database
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- PDB:
-
Protein Data Bank
- TCGA:
-
The Cancer Genome Atlas
- GTEx:
-
Genotype-Tissue Expression
- FR:
-
flame retardant
- IARC:
-
International Agency for Research on Cancer
- OPFRs:
-
organophosphate flame retardants
- TNBP:
-
triphenyl phosphate
- TDCPP:
-
tris(1,3-dichloro-2-propyl) phosphate
- PFAS:
-
per- and polyfluoroalkyl substances
- ESR1:
-
estrogen receptor 1
- HepG2:
-
human liver cells
- TM3:
-
Leydig cells
- H295R:
-
human adrenal cells
- DBEP:
-
di-(2-butoxyethyl) phosphate
- Oss:
-
osteosarcomas
- SMILES:
-
Simplified Molecular Input Line Entry System
- ADMETlab:
-
Absorption, Distribution, Metabolism, Excretion and Toxicity Laboratory
- PPI:
-
Protein-Protein Interaction
- BP:
-
biological processes
- CC:
-
cellular components
- MF:
-
molecular functions
- PLIP:
-
Protein-Ligand Interaction Profiler
- NPT:
-
isothermal–isobaric
- PME:
-
Particle Mesh Ewald
- NVT:
-
isothermal-isochoric
- RMSD:
-
root mean square deviation
- Rg:
-
radius of gyration
- Buried SASA:
-
buried solvent-accessible surface area
- VMD:
-
Visual Molecular Dynamics
- MM-PBSA:
-
Molecular Mechanics Poisson–Boltzmann Surface Area
- LUAD:
-
lung adenocarcinoma
- LUSC:
-
lung squamous cell carcinoma
- STES:
-
combined gastric and esophageal cancer
- STAD:
-
gastric cancer
- COAD:
-
colon adenocarcinoma
- COADREAD:
-
colorectal adenocarcinoma
- READ:
-
rectal adenocarcinoma
- SKCM:
-
skin cutaneous melanoma
- TGCT:
-
testicular germ cell tumors
- PRAD:
-
prostate adenocarcinoma
- OV:
-
ovarian serous cystadenocarcinoma
- BRCA:
-
invasive breast carcinoma
- KIRC:
-
clear cell renal cell carcinoma
- KICH:
-
chromophobe renal cell carcinoma
- GBMLGG:
-
glioma
- LGG:
-
low-grade glioma
- ESCA:
-
esophageal carcinoma
- UCEC:
-
uterine corpus endometrial carcinoma
- KIPAN:
-
kidney pan-cancer cohort
- BLCA:
-
bladder urothelial carcinoma
- HR:
-
hazard ratio
- PBDEs:
-
polybrominated diphenyl ethers
- PCBs:
-
polychlorinated biphenyls
- EndoG:
-
endonuclease G
- SFK:
-
SRC kinase family
- EMT:
-
epithelial-mesenchymal transition
- ER:
-
estrogen receptors
- AR:
-
androgen receptors
- ROS:
-
reactive oxygen species
References
Chupeau, Z. et al. Organophosphorus flame retardants: A global review of indoor contamination and human exposure in Europe and epidemiological evidence. Int. J. Environ. Res. Public. Health. 17, 6713 (2020).
Tan, H. et al. Organophosphate flame retardants in house dust from South China and related human exposure risks. Bull. Environ. Contam. Toxicol. 99, 344–349 (2017).
Chen, Y. et al. A review on organophosphate flame retardants in indoor dust from china: implications for human exposure. Chemosphere 260, 127633 (2020).
He, C. et al. Organophosphate and brominated flame retardants in Australian indoor environments: levels, sources, and preliminary assessment of human exposure. Environ. Pollut. 235, 670–679 (2018).
Van Den Eede, N., Dirtu, A. C., Neels, H. & Covaci, A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 37, 454–461 (2011).
Kim, U. J., Wang, Y., Li, W. & Kannan, K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the united States. Environ. Int. 125, 342–349 (2019).
Li, J. et al. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 54, 53–61 (2014).
Lee, S., Jeong, W., Kannan, K. & Moon, H. B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 103, 182–188 (2016).
Hou, R., Xu, Y. & Wang, Z. Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 153, 78–90 (2016).
Wei, G. L. et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ. Pollut. 196, 29–46 (2015).
Guo, Y. et al. Organophosphorus flame retardants and their metabolites in paired human blood and urine. Ecotoxicol. Environ. Saf. 268, 115696 (2023).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Hong, Y. et al. Integrative in Silico analysis of per-and polyfluorinated alkyl substances (PFAS)-associated molecular alterations in human cancers: A multi-cancer framework for predicting toxicogenomic disruption. Int. J. Surg. 10, 1097 (2025).
Chen, P. J. et al. Association between exposure to organophosphate flame retardants and epidermal growth factor receptor expression in lung cancer patients. Thorac. Cancer. 15, 1805–1814 (2024).
Meng, Y. et al. Alkyl organophosphate flame retardants (OPFRs) induce lung inflammation and aggravate OVA-simulated asthmatic response via the NF-кB signaling pathway. Environ. Int. 163, 107209 (2022).
Yuan, S. et al. Cytotoxicity, mitochondrial impairment, DNA damage and associated mechanisms induced by Tris (1, 3-dichloro-2-propyl) phosphate and Tris (2-butoxyethyl) phosphate in A549 cells. Sci. Total Environ. 787, 147668 (2021).
Hong, Y. et al. Decoding per-and polyfluoroalkyl substances (PFAS) in hepatocellular carcinoma: A multi-omics and computational toxicology approach. J. Transl Med. 23, 504 (2025).
Saquib, Q. et al. Tris (2-butoxyethyl) phosphate (TBEP): A flame retardant in solid waste display hepatotoxic and carcinogenic risks for humans. Chemosphere 296, 133977 (2022).
Yao, C., Yang, H. & Li, Y. A review on organophosphate flame retardants in the environment: occurrence, accumulation, metabolism and toxicity. Sci. Total Environ. 795, 148837 (2021).
Jin, Y., Chen, G. & Fu, Z. Effects of TBEP on the induction of oxidative stress and endocrine disruption in Tm3 Leydig cells. Environ. Toxicol. 31, 1276–1286 (2016).
Liu, X., Ji, K. & Choi, K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 114–115, 173–181 (2012).
Pan, H. Y. et al. Exposure to Tris (2-butoxyethyl) phosphate induces abnormal sperm morphology and testicularáhistopathology in male rats. Ecotoxicol. Environ. Saf. 241, 113718 (2022).
Ou, Y. C. et al. Lifestyle factors and urine levels of organophosphorus flame retardants in endometrial cancer: insights from a case-control study. Environ. Health Preventative Med. 29, 63 (2024).
Wang, L. J. et al. Effects of urinary organophosphate flame retardants in susceptibility to attention-deficit/hyperactivity disorder in school-age children. Ecotoxicol. Environ. Saf. 287, 117281 (2024).
Cheng, F. J. et al. Levels of organophosphate flame retardants and their metabolites among 391 volunteers in taiwan: difference between adults and children. Front. Public. Health. 11, 1186561 (2023).
Zheng, Y. et al. Organophosphate flame retardants Tris (2-butoxyethyl) phosphate (TBEP) and Tris (2-chloroethyl) phosphate (TCEP) disrupt human motor neuron development by differentially affecting their survival and differentiation. Sci. Total Environ. 948, 174772 (2024).
Tao, W. et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal radix curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 145, 1–10 (2013).
Jiashuo, W., Zhang, F., Zhuangzhuang, L., Weiyi, J. & Yue, S. Integration strategy of network Pharmacology in traditional Chinese medicine: A narrative review. J. Tradit Chin. Med. 42, 479–486 (2022).
Hong, Y. Advancing network toxicology in environmental pollutants analysis: key considerations and future perspectives from. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2025.179698 (2025).
Hong, Y. et al. Environmental triggers and future risk of developing autoimmune diseases: molecular mechanism and network toxicology analysis of bisphenol A. Ecotoxicol. Environ. Saf. 288, 117352 (2024).
Chandran, K., Zochedh, A., Sukumaran, S., Sultan, A. B. & Kathiresan, T. Quantum chemical calculation, topological analysis, biological evaluation and molecular Docking of allo-ocimenol against breast cancer. Int. J. Quantum Chem. 124, e27299 (2024).
Shen, W. et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. Imeta 1, e36 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Wang, X. & Song, F. The neurotoxicity of organophosphorus flame retardant Tris (1, 3-dichloro-2-propyl) phosphate (TDCPP): main effects and its underlying mechanisms. Environ. Pollut. 346, 123569 (2024).
Che, L. et al. Integrated network toxicology and population-based analysis uncovers organophosphate flame retardant exposure as a risk factor for hepatic steatosis and fibrosis: mechanistic and clinical insights. Int. J. Surg. https://doi.org/10.1097/js9.0000000000002680 (2025).
Zhang, Q. et al. Plant accumulation and transformation of brominated and organophosphate flame retardants: A review. Environ. Pollut. 288, 117742 (2021).
Huang, Y., Liu, J., Yu, L., Liu, C. & Wang, J. Gonadal impairment and parental transfer of Tris (2-butoxyethyl) phosphate in zebrafish after long-term exposure to environmentally relevant concentrations. Chemosphere 218, 449–457 (2019).
Roskoski, R. Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 94, 9–25 (2015).
Wang, Y., Cao, H., Chen, J. & McNiven, M. A. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol. Biol. Cell. 22, 1529–1538 (2011).
Irby, R. B. & Yeatman, T. J. Role of Src expression and activation in human cancer. Oncogene 19, 5636–5642 (2000).
Chung, B. M. et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene 28, 1821–1832 (2009).
Liu, W., Liang, Y., Chan, Q., Jiang, L. & Dong, J. CX3CL1 promotes lung cancer cell migration and invasion via the src/focal adhesion kinase signaling pathway. Oncol. Rep. 41, 1911–1917 (2019).
Li, A. & Wang, R. Inhibition of Src attenuates stemness and reverses cisplatin resistance of non-small-cell lung cancer cells. Bull. Exp. Biol. Med. 178, 460–466 (2025).
Ochi, N. et al. Src mediates ERK reactivation in gefitinib resistance in non-small cell lung cancer. Exp. Cell. Res. 322, 168–177 (2014).
Jiang, Y. et al. TOPK promotes metastasis of esophageal squamous cell carcinoma by activating the Src/GSK3β/STAT3 signaling pathway via γ-catenin. BMC Cancer. 19, 1264 (2019).
Peng, L. et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the src/fak pathway. Carcinogenesis 30, 1660–1669 (2009).
Huang, Y. H. et al. Src-FAK signaling mediates Interleukin 6-Induced HCT116 colorectal cancer epithelial-mesenchymal transition. Int. J. Mol. Sci. 24, 6650 (2023).
Gong, Y. et al. SRC enhanced cisplatin resistance in bladder cancer by reprogramming Glycolysis and Pentose phosphate pathway. Commun. Biol. 8, 36 (2025).
Han, X. et al. The role of Src family kinases in growth and migration of glioma stem cells. Int. J. Oncol. 45, 302–310 (2014).
Gao, Z. et al. Anoikis-resistant human osteosarcoma cells display significant angiogenesis by activating the Src kinase-mediated MAPK pathway. Oncol. Rep. 41, 235–245 (2019).
Li, B. X. et al. Novel pathways of HIV latency reactivation revealed by integrated analysis of transcriptome and target profile of Bryostatin. Sci. Rep. 10, 3511 (2020).
Su, T. J. et al. Oncogenic miR-137 contributes to cisplatin resistance via repressing CASP3 in lung adenocarcinoma. Am. J. Cancer Res. 6, 1317–1330 (2016).
Lin, Y. F. et al. Targeting the XIAP/caspase-7 complex selectively kills caspase-3-deficient malignancies. J. Clin. Invest. 123, 3861–3875 (2013).
Bhatia, V., Mula, R. V. & Falzon, M. Parathyroid hormone-related protein regulates integrin α6 and β4 levels via transcriptional and post-translational pathways. Exp. Cell. Res. 319, 1419–1430 (2013).
Wang, Z. et al. Prognostic significance of mRNA expression of casps in gastric cancer. Oncol. Lett. 18, 4535–4554 (2019).
Zhu, C., Fan, F., Li, C. Y., Xiong, Y. & Liu, X. Caspase-3 promotes oncogene-induced malignant transformation via EndoG-dependent Src-STAT3 phosphorylation. Cell. Death Dis. 15, 486 (2024).
Elsberger, B. et al. Breast cancer patients’ clinical outcome measures are associated with Src kinase family member expression. Br. J. Cancer. 103, 899–909 (2010).
Hiscox, S. & Nicholson, R. I. Src kinase: A therapeutic opportunity in endocrine-responsive and resistant breast cancer. Expert Rev. Endocrinol. Metab. 6, 423–435 (2011).
Wang, Y., Luo, X., Wu, N., Liao, Q. & Wang, J. SRC-3/TRAF4 facilitates ovarian cancer development by activating the PI3K/AKT signaling pathway. Med. Oncol. 40, 76 (2023).
Pengetnze, Y., Steed, M., Roby, K. F., Terranova, P. F. & Taylor, C. C. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem. Biophys. Res. Commun. 309, 377–383 (2003).
Lu, Y. et al. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell. Commun. Signal. 17, 118 (2019).
Gelman, I. H. Androgen receptor activation in castration-recurrent prostate cancer: the role of Src-family and Ack1 tyrosine kinases. Int. J. Biol. Sci. 10, 620–626 (2014).
Li, L., Deng, C. X. & Chen, Q. SRC-3, a steroid receptor coactivator: implication in cancer. Int. J. Mol. Sci. 22, 4760 (2021).
Samarasekera, G. et al. Caspase 3 and caspase 7 promote cytoprotective autophagy and the DNA damage response during non-lethal stress conditions in human breast cancer cells. PLoS Biol. 23, e3003034 (2025).
Byeon, S. E. et al. The role of Src kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 512926 (2012). (2012).
Zhou, Z., Xu, S., Jiang, L., Tan, Z. & Wang, J. A systematic pan-cancer analysis of CASP3 as a potential target for immunotherapy. Front. Mol. Biosci. 9, 776808 (2022).
Hong, Y. et al. Genomic evidence based on EQTL data implicates endocrine disruptors as environmental risk factors for Estrogen receptor-positive breast cancer. Int. J. Surg. https://doi.org/10.1097/JS9.0000000000002642 (2025).
Hong, Y. et al. Integrative genomic and single-cell framework identifies druggable targets for colorectal cancer precision therapy. Front. Immunol. 16, 1604154 (2025).
Acknowledgements
We are grateful to PubChem, ADEMTlab, STITCH, SwissTargetPrediction, GeneCards, OMIM, TTD, PDB and TCGA and other databases for providing all the public data. We are also grateful to every researcher and participant who has contributed to these studies.
Funding
The National Natural Science Foundation of China (62461006), the Natural Science Foundation of Guangxi province(2022GXNSFAA035513), the Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation(2024GXNSFBA010032), the Youth Science Foundation of Guangxi Medical University (GXMUYSF202420), the National Key Clinical Specialty Construction Project and the Guangxi Key Clinical Specialty Construction Project funded this research.
Author information
Authors and Affiliations
Contributions
H.T.L and P.Y.H conceived and designed the study and reviewed the final manuscript. B.Q.H and D.M.H prepared and reviewed drafts of the publication. X.H.O and H.T.L obtained and evaluated the data and produced the images and tables. Z.Y.X and S.K.L designed and authorized approval of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study exclusively utilized publicly accessible databases, including PubChem, ADMETlab, STITCH, SwissTargetPrediction, GeneCards, OMIM, and the Therapeutic Target Database (TTD). As no human participants, identifiable personal data, or animal subjects were involved, and all data analyzed were obtained from open-access sources, this research did not require approval from an institutional ethics committee or Institutional Review Board (IRB). This aligns with prevailing guidelines for studies employing de-identified, publicly available datasets.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, H., Huang, P., Huang, B. et al. Mechanistic insights into the effects of Tris-2-butoxyethyl phosphate on multiple cancers using network toxicology and molecular docking. Sci Rep 15, 28781 (2025). https://doi.org/10.1038/s41598-025-14733-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14733-2