Abstract
In a wide range of fields, including the pharmaceutical industry and forensic science, obtaining real-time results is paramount. Hence, new analytical techniques are being developed to be both simple and fast, providing results in a few minutes. Extractive-liquid Sampling Electron Ionization-Mass Spectrometry (E-LEI-MS) is proposed as a novel analytical approach that combines an ambient sampling with the high identification power of electron ionization, providing the results in less than five minutes. E-LEI-MS was employed to analyze 20 industrial drugs belonging to different therapeutic classes and pharmaceutical forms, to evaluate its ability to detect active pharmaceutical ingredients and excipients. All samples were analyzed without any pre-treatment. Additionally, the system was coupled to high-resolution MS and used to analyze 20 benzodiazepines. Among them, the six most commonly marketed benzodiazepines were used for cocktail fortification and analyzed as a residue on a glass surface to simulate the forensic scenario of benzodiazepines being used as rape drugs, where glass represents a crime scene sample. The accurate identification of these compounds demonstrates that E-LEI-MS can serve as a valuable screening technique in applications requiring the rapid acquisition of qualitative data.
Similar content being viewed by others
Introduction
Extractive-Liquid sampling Electron Ionization-Mass Spectrometry (E-LEI-MS) is a recently developed real-time analytical approach suitable for the direct analysis of a large number of compounds in different matrices1. E-LEI-MS capability to provide real-time data was tested in various applications, including the detection of pesticides in food, the determination of unknown compounds in artworks, and the detection of cocaine on banknotes1. Rapidity and accuracy are essential for developing novel analytical techniques in several fields, including pharmaceutical and forensic. A minimal environmental impact of the analysis is another crucial objective2. In this context, ambient ionization mass spectrometry stands out for its capacity to rapidly analyze samples in their native state under ambient conditions3, both in and out of the laboratory settings. Introduced for the first time in the mid-2000s4,5, ambient ionization techniques surpass traditional methods that involve laborious sample extraction procedures and lengthy analysis times when a fast response is on demand. Over the years, these techniques have been applied in various fields of research, such as forensic6,7,8, biomedical9, pharmaceutical10, and food sciences11,12. Real-time identification of Active Pharmaceutical Ingredients (APIs), excipients, and impurities in pharmaceutical products is an important screening tool for quality control testing and counterfeit detection. Desorption Electrospray Ionization mass spectrometry (DESI-MS), the first ambient ionization technique proposed in 20044, has been widely used for the analysis of pharmaceuticals in biological materials13, such as the distribution of drugs and their metabolites in tissues14. These results make DESI a useful tool for therapeutic drug monitoring. DESI-MS can also be used in drug discovery analysis15 and to identify active ingredients in pharmaceutical formulations16. Atmospheric Pressure Solid Probe (ASAP), an ambient ionization technique derived from DESI, was employed to identify sildenafil, a synthetic PDE5 inhibitor more commonly known as Viagra, in herbal medicines purchased online17. The ability to rapidly detect adulteration products is crucial in detecting counterfeit drugs and protecting human health.
Direct analysis in real-time mass spectrometry (DART-MS) was employed for the detection of illicit drugs, such as stimulants or hallucinogens, that are often used for adulterating other substances in criminal cases10. Benzodiazepines (BDZs), a well-known class of anxiolytic drugs, are the most frequently used substances in “drug-facilitated sexual assault” (DFSA) crimes18. Conventional forensic toxicology methods typically detect BZDs in biological matrices (blood, urine, hair) of DFSA victims19,20,21. However, BDZs have a short half-life, leading to rapid metabolism and excretion. Following drug administration, blood samples should ideally be collected within 24–48 hours18 and reliable results become challenging to obtain beyond 72 h22. The co-consumption of ethanol intensifies the psychomotor effects of these substances. Consequently, many cases of DFSAs occur in social venues where alcohol consumption is common, such as nightclubs, where cocktails can be easily adulterated with drugs. Given the challenges in detecting BDZs in biological matrices, it is valuable to evaluate alternative analytical approaches for detecting these drugs in drink residues directly on the crime scene22,23. The forensic science community shows increasing interest in ambient ionization MS due to its ability to rapidly analyze on-site samples with minimal or no pretreatment. However, ambient ionization MS techniques are coupled with an atmospheric pressure ionization source to perform the characteristic ionization/desorption process. E-LEI-MS combines ambient sampling, which characterizes Ambient Ionization Mass Spectrometry techniques, with Electron Ionization (EI)1. The use of EI enhances identification power by enabling direct comparison of experimental spectra with well-established EI spectral libraries. The E-LEI-MS principle is based on the direct extraction of analytes from the sample surface wetted by small drops of a suitable solvent. Once in the liquid phase, the analytes are immediately aspirated by the high vacuum of the EI source. The coupling of an EI source with the liquid phase was demonstrated through the studies on Direct Electron Ionization (DEI) interface24 and Liquid Electron Ionization (LEI) interface25. –26 The E-LEI-MS configuration has recently been modified, allowing E-LEI-MS to vaporize and analyze molecules with a medium-high boiling point, such as the active ingredients of certain pharmaceutical drugs. This paper presents two applications of E-LEI-MS: the detection of APIs and excipients in 20 real samples of different drug formulations and two tablets purchased online as supplements, and the identification of old and new BDZs by analyzing standard solutions and fortified cocktails. These analyses represent applications of the E-LEI-MS system in pharmaceutical and forensic areas. The ability to identify APIs and excipients supports its potential use as screening tool for pharmaceutical quality control and counterfeit drug detection. Furthermore, the detection of BDZs in fortified cocktail residues simulates their illicit use, demonstrating the role of E-LEI-MS in forensic investigations related to DFSA cases.
Materials and methods
System configuration
The current E-LEI-MS configuration (Fig. 1) is a modified version of the setup described by A. Arigò1 adapted for a 7010 triple quadrupole (QqQ) mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) and a 7200 Accurate-Mass Quadrupole Time-of-Flight (Agilent Technologies, Santa Clara, CA, USA) both equipped with an EI source. The modifications made to the previous version improved the entrance of the liquid flow to the vacuum condition of the QqQ-MS and Q-ToF-MS. The vacuum guarantees the proper ionization efficiency but it also determines the aspiration capacity of the E-LEI-MS to suck the analyte solution from the sample surface to the EI source. The E-LEI-MS apparatus includes a solvent-release mechanism based on a syringe pump (KD Scientific syringe pump, KD Scientific Inc., Holliston, MA, USA; equipped with a 1-mL syringe, Hamilton, Bonaduz, Switzerland). The solvent is pumped through a Teflon tube into a tee connection intersected by the sampling tip. The sampling tip is the E-LEI-MS core component and consists of two coaxial tubes. The inner tubing (Fig. 1, inside capillary) differs slightly in the two system configurations:
-
E-LEI-QqQ-MS system: 20 cm length, 40 μm I.D., 375 μm O.D. (Polymicro Technologies, Phoenix, USA).
-
E-LEI-Q-ToF-MS system: 30 cm length, 50 μm I.D., 375 μm O.D. (Polymicro Technologies, Phoenix, USA).
The outer tubing (Fig. 1, outside capillary) is a peek tube measuring 8 cm in length with 450 μm I.D. and 660 μm O.D. (Postnova Analytics GmbH, Landsberg, Germany) leaving a gap between the tubes for solvent delivery onto the sample surface. The sample is positioned on a metal support, aligning the opening of the sampling tip, where the solvent flows, directly above it. The inside capillary reaches the on-off valve, a MV201 manual microfluid 3-port valve (LabSmith, Livermore, USA), where another silica capillary (Fig. 1, inlet capillary), connects the valve to the MS, acting as an extension of the inside capillary. The inlet capillary (Fig. 1) differs slightly in the two system configurations:
-
E-LEI-QqQ-MS system: 25 cm length, 40 μm I.D., 375 μm O.D. (Polymicro Technologies, Phoenix, USA).
-
E-LEI-Q-ToF-MS system: 30 cm length, 50 μm I.D., 375 μm O.D. (Polymicro Technologies, Phoenix, USA).
The necessity of employing different capillaries in the two configurations depends on the disparate suction force exerted by the vacuum pumps in the QqQ-MS and QTof-MS instruments. Therefore, the replacement of capillaries is necessary to adapt the E-LEI-MS system to different vacuum conditions. To further support vacuum strength, a new component inspired by the LEI interface25 was integrated into the system: the vaporization microchannel (VMC). The VMC, positioned before the high-vacuum ion source, facilitates the vaporization and transport of the liquid extract containing the analytes into the ion source24. In the E-LEI-MS system, the VCM consists of a tube (530 μm I.D.; 600 μm O.D.; 24 cm length; Alltech Associates Inc., Deerfield, USA) that passes through a heated region of the MS (transfer line) from the two-way junction, the component that allows for the connection between the inlet capillary and the VMC, to the EI source.
Chemicals
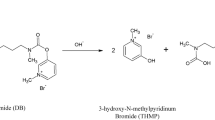
The E-LEI-MS system analyzed twenty commercially available pharmaceuticals containing 16 APIs (Supplementary Table S1 online). The standard solutions of twenty BDZs (Supplementary Table S2 online) in MeOH were provided by the Toxicology Laboratory A.S.T. AV1, located in Pesaro, Italy. The standard solutions were provided at different concentrations (20, 100 and 1000 mg/L). 20 µL of BDZ standard solutions were analyzed as dried spots on a watch glass surface. Six of them (clobazam, clonazepam, diazepam, flunitrazepam, lorazepam, and oxazepam) were used to fortify a gin tonic cocktail purchased in a local bar (at a concentration of 20 mg/L and 100 mg/L) to simulate a real adulteration of a cocktail in a DFSA. The six BDZs were selected for their common use as anxiolytic drugs. 20 µL of adulterated cocktails were spotted on a watch glass surface and analyzed as dried spots. Acetonitrile was used as E-LEI-MS solvent.
The analyzed pharma samples included both brand-name and generic drugs, covering different therapeutic classes and pharmaceutical formulations (no. 14 tablets; no. 1 lozenge; no. 1 gel; no. 1 soft gel; no. 1 granules; no. 1 injectable solution). For the extraction process acetonitrile (ACN; VWR BDH Chemicals® HiPerSolv Chromanorm; Milan, Italy), ethanol (EtOH; VWR BDH Chemicals® HiPerSolv Chromanorm; Milan, Italy), ultrapure water produced by a Millipore Direct-Q 3 UV system (Millipore Corp.; Milan, Italy), and a mixture of ultrapure water and EtOH 1:1 (v/v) were used as solvents (Supplementary Table S3 online). The solvent was chosen according to the chemical-physical characteristics of the APIs. Two pharmaceuticals in the form of tablets were purchased as dietary supplements from a website and analyzed using the E-LEI-Q-ToF-MS system for the potential detection of counterfeits.
Methods
Pharmaceutical formulations analysis
The analyses of twenty commercially available pharmaceutical formulations were performed using the E-LEI-QqQ-MS system in full scan acquisition mode within a mass range of m/z 83–550. The source and transfer line temperatures were adjusted according to the chemical-physical characteristics of the APIs, as shown in Supplementary Table S3. Setting the correct temperature is necessary for the efficient vaporization of the analytes. Different solvents (Supplementary Table S3 online) were used according to the solubility of the target compounds. During the dissolution step, the system was set in a no-aspiration status using the on-off valve, and the selected solvent was applied to the sample surface for two minutes at a flow rate of 5 mL/min. This procedure allowed the formation of a solvent droplet, facilitating the analyte’s transition into the liquid phase. The sampling started when the valve was opened allowing the aspiration of the solution from the sample surface to the MS. Sampling time was set to 30–60 s, depending on the instrument’s response and the pharmaceutical formulation composition. A reduced sampling time was employed to analyze samples with a powdery matrix or analytes with high boiling points. For the identification of APIs and excipients contained in the real samples, the National Institute of Standards and Technology (NIST) Mass Spectral Search Program (Version 2.3 prepared by NIST Mass Spectrometry Data Center) was used. The Human Metabolome Database (HMDB) website (Human Metabolome Database (hmdb.ca)) was also consulted for the identification of APIs spectra that were not available in the NIST library (Supplementary Table S3 online). The analyses of Diclofenac, Ibuprofen and Tadalafil formulation and the supplements tables were carried out at 260 °C as source temperature, covering a mass range of m/z 60–600, with a quench flow of 4 mL/min. The experimental spectra obtained by the E-LEI-Q-ToF-MS system were investigated using the Agilent MassHunter Unknows Analysis software.
Benzodiazepine analysis
The optimal conditions for the E-LEI-MS analysis of BDZs were investigated by analyzing the standard solutions of bromazepam, clobazam, clonazepam, chlordiazepoxide, diazepam, flunitrazepam, lorazepam, nitrazepam and oxazepam in MeOH at a concentration of 100 mg/L. These analyses were conducted in flow injection and full scan acquisition modes. The parameters investigated were the source temperatures (280 °C and 350 °C), the signal-to-noise ratios at different mass ranges (m/z 60–400; m/z 80–400; m/z 90–400), and the solvent to use as the liquid phase (ACN and MeOH).
Clobazam, clonazepam, diazepam, flunitrazepam, lorazepam, and oxazepam were used to fortify gin tonic cocktails at concentrations of 100 mg/L and 20 mg/L.22
Standard solutions and fortified cocktails were spiked on a watch glass surface and dried for the E-LEI-MS analysis. The spot of 20 µL of the standard solution covered an area of 10 cm2 on the watch glass surface, due to the volume expansion of the solvent (MeOH). The spot of 20 µL of the fortified gin tonic has been observed to cover 1 cm² of the watch glass surface. In this case, the matrix spread across a minor surface because of its complex composition based on ethanol, sparkly water, sugar, and flavours. The analyses of the spots were carried out with VMC and source temperatures of 400 °C and 350 °C, respectively. Extraction and aspiration processes were the same as for pharmaceutical formulation analyses. ACN was selected as the proper solvent. The BDZs were identified by comparing experimental spectra with the NIST library. The identification of bentazepam and cinazepam was obtained by comparing the experimental spectra with an online database and using Molecular Structure Correlator software. The detection of the drugs in the gin tonic fortified at 20 mg/L was enabled by a lab-made library obtained collecting HR experimental spectra of the BDZs using the E-LEI-QToF-MS system.
Results and discussion
Pharmaceutical application
The E-LEI-MS system analyzed pharmaceuticals dissolving APIs and excipients from the formulation surface. Eight APIs out of sixteen were correctly identified by comparing the experimental spectra with those in the NIST library. Three parameters can be considered to establish the correctness of the identification using the NIST library: match factor, reverse match (r. match) factor and probability. The match factor value is a measure of the similarity between the experimental spectrum and the library spectrum on a scale of 0 to 1000. The identifications of six APIs recorded a spectral match equal to or greater than 680, with only two instances where the match value was < 550. A value equal to or greater than 680 can be considered a fair match. However, a spectrum with many peaks may yield lower match values in the hit list compared to a spectrum with fewer peaks. This has been observed in a spectrum of coeluted compounds or a spectrum with noisy baselines, as could be in the case of ambient sampling. In these conditions, it may be more useful to consider the value of the r. match.
The r. match factor values are used to express the similarity of two spectra, ignoring the differences between them and focusing on the similarity, excluding the peaks of the experimental spectrum that are not in the reference spectrum of the library. As the match factor, the r. match value was calculated on a scale of 0 to 1000. During API identification, a r. match value equal to or greater than 773 was recorded, with two cases where the match value was < 590.
Finally, the probability value expresses the correctness of any result in the list obtained by comparing the experimental spectrum with the library, it was calculated based on the differences between the hits. The identification of APIs does not necessarily take this parameter into account.
The APIs identified in the corresponding pharmaceutical formulation (Supplementary Table S1 online) using the NIST library were: tadalafil, ibuprofen, diclofenac, paracetamol, betamethasone, metformin, and acetylsalicylic acid. The comparison of the experimental spectrum and HMDB spectrum allowed the identification of levocetirizine. However, not all pharmaceutical formulations were suitable for the analysis of the E-LEI-MS system and other APIs were not recognized. Indeed, tablets of scopolamine and pantoprazole had a gastro-resistant structure. This pharmaceutical formulation was designed for slow release and the APIs were not extracted from the sample surface because ACN was unsuitable to dissolve the tablet coating for its specific and resistant composition.
Ultrapure water was also employed as an E-LEI-MS solvent, facilitating the dissolution of the sugar coating of the scopolamine tablet. However, the thickness of the coating prevented the extraction of the scopolamine, and no signal was recorded. The direct extraction of the API from the core of the tablet was not a viable option in this context, as the inner part was composed of a powdery substance that was incompatible with the E-LEI-MS system. The E-LEI-MS system is generally compatible with powder analysis; however, in this case, the solvent droplet for analyte extraction was not formed on the inner part of the tablet because the matrix was too dry. Consequently, the absence of this microdroplet compromised the suitability of the analysis with the E-LEI-MS system. The analysis of the pantoprazole tablet presented comparable difficulties to the analysis of scopolamine. ACN was the only solvent used for this application as it was able to form a droplet on the sample surface for the E-LEI-MS extraction process. However, the pantoprazole was not extracted from the coating due to the complexity of the matrix. In support of this finding, the experimental spectrum did not match with the predicted pantoprazole spectrum, which is not presented in the NIST library, but it did with the triethyl acetate spectrum. The triethyl acetate is an excipient of the tablet of pantoprazole. The core of the tablet of pantoprazole was unsuitable for the E-LEI-MS analysis process, as the tablet of scopolamine. The tablet of bilastine was also unsuitable for the analysis with the E-LEI-MS system because the sample, formed by thickeners, emulsifiers, and anticaking agents, completely absorbed the ACN from the syringe pump. Additionally, the ACN was unsuitable for dissolving the butamirate lozenge formed by sugar, artificial sweeteners, and thickeners. The analysis of telmisartan was performed under an EI source temperature of 350 °C because of the high boiling point of the API (771.9 ± 70 °C). The ACN was selected as the E-LEI-MS solvent because the telmisartan molecule was insoluble in water. The experimental spectrum of the telmisartan tablet showed some differences from the HMDB-predicted spectrum. Although the experimental spectrum corresponded to those in the library spectrum, the relative ion abundances differed. The tablet of glimepiride was investigated using EtOH as solvent and at an EI source temperature of 350 °C to support the vaporization of the API. No signal was recorded using ACN. The experimental spectrum did not match with the HMDB-predicted spectrum and the fragments recorded were not attributed to any other excipient of these tablets. The identification of levocetrizine was also performed using the HMDB-predicted spectrum. A comparison of the experimental and predicted spectra reveals the presence of common peaks: m/z 201, 89, 203, 187, 271, 285. The observed discrepancy in the intensity of 285 ions does not raise concern, as it is a predicted spectrum, thereby affirming the validity of the identification of levocetirizine.
In the case of cefixime, a cephalosporin antibiotic, the total absence of characteristic fragments in the experimental spectrum could be attributable to degradation, poor vaporization or ionization. A molecule of cefixime has both amino and carboxylic groups in its structure, which give it a good mass spectrometric response either in the positive ionization or in the negative ionization, using electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI)27. HPLC methods have been developed for detecting antibiotics, including cefixime. It can be deduced that the EI process is most likely unsuitable for these compounds.
Some excipients were identified by investigating the experimental spectra with the NIST library or using the Agilent MassHunter Unknows Analysis software. These include triacetin in a tadalafil tablet, macrogol 600 in a diclofenac soft gel, benzyl alcohol in a diclofenac injectable solution, and triethyl citrate in both pantoprazole and acetylsalicylic tablets.
Tadalafil and supplements analysis
Tadalafil belongs to the phosphodiesterase type 5 (PDE5) inhibitor group. This class of drugs is used to treat erectile dysfunction. Sildenafil, better known as Viagra, is the most famous API in this category. The successful identification of tadalafil extracted from the surface of a pharmaceutical tablet performed using the E-LEI-QqQ MS configuration demonstrates the ability of this system to efficiently vaporize molecules with high boiling points following the introduction of VMC into the configuration. An extensive examination of the tadalafil tablet was conducted using the E-LEI-QToF-MS system. Delay, meaning the time between the valve opening and the signal detection, and signal duration were one minute and a half and two minutes, respectively (Fig. 2A). This outcome corresponds with the capacity of the E-LEI-MS system to obtain real-time results. The tadalafil tablets had a film formed by triacetin, and its fragments were visible in the experimental spectrum (Fig. 2B). A separation process is not included in the E-LEI-MS analysis; however, the Agilent MassHunter Unknowns Analysis software is enabled to distinguish the signals of coeluted compounds through ion deconvolution (Fig. 2C). Using this software, both excipient and API were identified with a match of 54 and 66, respectively. These match values could be attributed mainly to the exclusion of some m/z from the acquisition mass range (e.g. m/z 43 and 44, for triacetin and tadalafil, respectively) and to threshold and deconvolution parameters. Moreover, the identification of tadalafil and triacetin was investigated with the E-LEI-Q-ToF-MS system to distinguish the spectra of the two compounds enhanced by the specificity of the accurate mass (Fig. 2C). The interest in tadalafil and related drugs results from the high prevalence of counterfeits. In Italy, the PDE5 inhibitor drugs can only be bought with a doctor’s prescription. Two tablet products (indicated as tablet 1 and tablet 2) were purchased as vitaminic supplements for erectile dysfunction from legitimate websites. The selected tablets were chosen because their description referred somehow to the pharmaceutical action of the drug class to which tadalafil belongs; then, it was hypothesized a different composition from what was declared on the selling website. Analysis of tablet 1 did not reveal any trace of tadalafil or similar API. Tablet 2, contrary to what was declared on the website, arrived in a blister, that listed the ingredients, including sildenafil (Supplementary Fig. S1 online). The presence of this API was confirmed by analysis using the E-LEI-MS system. Therefore, tablet 2 was officially a drug, and it should not be on sale as a supplement. This result highlights the significant potential of E-LEI-MS as a screening technique for detecting counterfeit products. The capacity to identify sildenafil in less than two minutes from the beginning of the sampling process could support forensic laboratories, which are often subjected with substantial workloads required by time-consuming analysis procedures.
Extracted ions chromatogram of m/z 103.0394, accurate mass of a triacetin fragment (excipient of tadalafil tablet), and m/z 389.1377, accurate mass of a tadalafil fragment (API) (A). Comparison between the experimental spectrum of the tadalafil tablet (red) and the tadalafil spectrum of the NIST library (blue). The fragments of triacetin can be noted in the red spectrum (B). Deconvolution of triacetin and tadalafil signals performed by Agilent MassHunter Unknowns Analysis software (C).
Diclofenac and impurity A analysis
Diclofenac belongs to the therapeutic class of non-steroidal anti-inflammatory drugs (NSAIDs). This API was analyzed in four different pharmaceutical formulations: tablets, softgel capsules, gel, and injectable solutions. Diclofenac was present in three different salified forms, according to the shelf life of the pharmaceutical formulations (Supplementary Table S1 online). The tablets and the injectable solution contained diclofenac sodium, while the soft capsule and the gel contained diclofenac epolamine and diclofenac diethylamine, respectively. The spectrum of non-salified diclofenac was the only one present in the NIST library. The analysis of the softgel capsule was conducted releasing the solvent on its inner surface because the E-LEI-MS analysis of the intact pharmaceutical formulation didn’t allow the direct extraction of the active ingredient. The outer coating of a softgel capsule is composed primarily of gelatin, which is designed to encapsulate the active ingredient in liquid form. Consequently, the E-LEI-MS analysis was performed on the broken pharmaceutical formulation and the extraction process took place on the inner surface of the capsule, as it is in direct contact with the API. After examining the experimental spectrum, it is possible to discern the presence of diclofenac and the fragments attributed to an excipient (Supplementary Fig. S2 online). The identification of both compounds was confirmed by comparing the experimental spectrum with the NIST library. Diclofenac was the spectrum with the best match values: match factor 809, r. match factor 845, and probability 95.9%. Some fragments (m/z 89, 103, 117, 133 and 177) were present in the experimental spectrum but were not assigned to the API. The NIST library identified them as the spectrum of macrogol 600, an excipient of the softgel capsule of diclofenac, and reported in NIST as 2-[2-[2-[2-[2-(2-ethoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethanol. The identification of this excipient gave a match value and a r. match value of 526 and 710, respectively (Supplementary Fig. S2 online). A small amount of gel containing 1% diclofenac was distributed on the surface of the metal sample holder. The sampling process was completed in 30 s, and the signal was recorded in less than one minute. The identification of diclofenac was achieved by comparing the spectrum with the NIST library, resulting in a match value of 926, a r. match value of 942, and a probability of 96.8% (Supplementary Fig. S3 online).
The diclofenac injection solution was analyzed as a 20 µL dry spot on the sample holder. The experimental spectrum reported the fragments of both API and an excipient (Supplementary Fig. S4 online). The NIST identified diclofenac with a match value of 589, r. match value of 598. Benzyl alcohol, an excipient of the injectable solution, was identified with a match value of 560, and a r. match value of 812. However, the NIST assigned the best match to 2 H-Indol-2-one, 1-(2,6-dichlorophenyl)-1,3-dihydro (match value 742, r. match valued 791, Supplementary Fig. S4 online) which was also found and investigated in the analysis of diclofenac tablets.
Tablets of diclofenac lacked a film coating, and they were composed of multiple layers that facilitated the gradual release of the active ingredient over time. The original and generic tablets are similar in composition, differing in some excipients. Consequently, the results obtained are comparable. The delay time of the signal is always between 30 s and 1 min. The experimental spectra had similar match values for diclofenac and impurity A (also called diclofenac amine), present in the NIST library as 2 H-Indol-2-one, 1-(2,6-dichlorophenyl)-1,3-dihydro, 645 and 616, respectively (Supplementary Fig. S5 online). The diclofenac amide (impurity A) is a degradation product of diclofenac, which results from photo-transformation or spontaneous cyclization in strongly acid pH conditions. It can be considered a potential prodrug or an impurity formed during the synthesis of diclofenac. The two compounds share the same fragments, except m/z 295, which was absent in the impurity A spectrum and corresponds to the molecular ion of diclofenac. In addition, fragment m/z 277 displayed a lower relative intensity in the diclofenac spectrum than in the impurity A spectrum, consisting of its molecular ion. An examination of the experimental spectrum suggested the existence of both compounds in the sample, as evidenced by the presence of m/z 295 ion and the high relative intensity of m/z 277 ion (Supplementary Fig. S5 online). Diclofenac was present in the tablets as diclofenac sodium, which spectrum was absent in the NIST library. The GC-MS spectrum of diclofenac sodium recorded by Yilmaz and Ciltas was identical to that of impurity A28. Consequently, analyses were conducted on these tablets using the E-LEI-Q-ToF-MS system, which enables the identification of compounds based on the accurate masses of their molecular ions. The extracted ions signals of the accurate masses m/z 295.0160, m/z 277.0054, and m/z 318.0078, which are the molecular ions of diclofenac, impurity A, and diclofenac sodium, respectively, demonstrated the simultaneous presence of all compounds, hence the experimental spectrum was the addition of the intensity of their fragments (Fig. 3). The difference in the relative abundance of each signal may depend on the sampling point on the tablet surface.
Forensic application
The BDZs are a group of drugs having a common molecular structure composed of a benzene ring fused to a seven-membered diazepine ring and a phenyl ring attached to the 5-position of the diazepine ring19. They are very renowned and consumed for their use as anxiolytic drugs. The twenty BDZs selected for this study had comparable molecular weights and high boiling points (Supplementary Table S2 online). Because of their similar chemical structure, only ten of them were employed to evaluate the optimal parameters for the analyses. The analysis of the standard solutions at a concentration of 100 mg/L, performed in flow injection mode, suggested that source and transfer line temperatures of 350 °C and 400 °C, respectively, would facilitate the vaporization of these compounds. The mass range of m/z 90–400 was selected to provide the optimal signal-to-noise ratio, in accordance with the BZD molecular weights, except for cinazepam, which has a molecular weight higher than 400 g/mol. The BZD analyses allow for the exploitation of accurate mass in the identification process. Nineteen out of twenty BDZs were properly identified as spots, after spiking 20 µL of standard solutions on a watch glass surface and drain, covering an area of 12 cm2. After extracting for two minutes, sampling occurred for 30 s. In 16 cases, the recognition occurred by comparison with spectra contained in the NIST library. The failure to recognize clonazolam was probably a consequence of the low concentration of the standard solution (20 mg/L) distributed on a surface of approximately 12 cm2. It is important to consider that the sample may not be distributed uniformly on the watch glass surface. The fragments in the experimental spectrum did not match the NIST spectrum because they differed in relative intensity and were low in absolute intensity. For diclazepam, the spectrum was not present in the NIST library. When it was not possible to make a direct comparison, due to the absence of the NIST spectrum, online databases were consulted. A comparison of the experimental spectrum with the EI spectrum of diclazepam of the MoNA - MassBank of North America website showed a match of three fragments out of four. The spectra of bentazepam and cinazepam were also absent from the NIST library. Bentazepam was identified using mass calculator software, which processed the accurate mass of the molecular ion to facilitate a correlation with the molecular formula of the compound of interest. The spectral information on the PubChem website allowed the identification of cinazepam, and four fragments of the experimental spectrum could be attributed to this compound. Two noteworthy cases are those of clonazepam and nitrazepam. In the clonazepam experimental spectrum, some fragments did not match the NIST spectrum of this compound. These fragments were either absent or differently intense compared to the clonazepam spectrum. However, their presence can be explained as these ions belong to 7-aminoclonazepam, a derivative of clonazepam, which was probably present in the standard solution (Supplementary Fig. S6 online). A comparable case was observed investigating the spectrum of nitrazepam, where the intensity of the m/z 222 ion also indicated the presence of aminonitrazepam (Supplementary Fig. S7 online). Fragments not belonging to the compound were also observed in the experimental spectrum of lormetazepam. No compound could be attributed to these ions. Nevertheless, the identification of lormetazepam through comparison with the NIST library was successfully achieved with a match factor of 646, an r. match of 569, and a probability of 49.6%.
Following the notable identification power of the E-LEI-QToF-MS system in the analysis of BDZs, this capability was also investigated to detect these compounds in more complex matrices. BDZs are the drugs most frequently used in the DFSA as stunners18. MS analytical methods commonly used in forensic chemistry involve searching for these substances in blood, urine and hair samples from potential victims of violence19. However, detecting these drugs in biological matrices is complicated because they are rapidly metabolized and excreted from the body18,23. Another aspect to consider is that the complexity of the biological matrices requires laborious preparation steps and time-consuming analysis procedures. Due to their popularity, the BDZs are frequently compounds of interest for developing adulteration substance detection methods in different matrices29. The detection of BDZ in beverages can be considered as a complementary analytical approach to those already used for biological samples. Indeed, in drinks used as a means of drug administration, BDZs are not metabolized. Therefore, rapid detection of these substances in beverages22,23 may prove to be a rapid screening tool for drug identification in DFSA cases. In this sense, E-LEI-MS could be used as a screening technique, acting directly on the residue found at the crime scene, without any extraction process or sample preparation. To prove the capacity of the E-LEI-MS for the detection of drugs in beverage residues, six BDZs were used to fortify cocktails and simulate their illicit use in DFSA. Clobazam, clonazepam, diazepam, flunitrazepam, lorazepam, and oxazepam were chosen because of their popularity and being often, associated with cases of DFSA18,30. To simulate a sample found on a crime scene, gin tonic aliquots were fortified with BZDs at two levels of concentrations, 20 and 100 mg/L according to the amount of drug needed to obtain the effects on the victims22. These values should not be considered as limits of quantification or detection. Quantitative parameters were not evaluated during analysis because of the qualitative nature of the technique. Consequently, comparison with other techniques for rapid detection and quantification of this type of substance is challenging29,31. 20 µL of the adulterated gin tonic solutions were placed on a watch glass surface dried for simulating a glass residue and analyzed. The spot of adulterated gin tonic covered a surface of ≈ 1 cm2. Signals were recorded with an average delay of 30 s, confirming the real-time aspect of this technique. As an example, Fig. 4A shows the results coming from gin tonic adulterated with diazepam. The comparison with the NIST library allowed the correct identification of the BDZs contained in cocktails at a concentration of 100 mg/L (Fig. 4B). However, the use of the NIST library was not successful in identifying all the BDZs in gin tonic adulterated at 20 mg/L. Consequently, a lab-made library of BDZs HR-spectra, recorded during the optimization parameter analyses, has been realized using Library Editator software. In a E-LEI-MS analysis, the analytes are aspirated depending on their distribution in the spot, the position of the sampling tip, and the quantity extracted in the solvent droplet. These factors may be the cause of sensitivity problems which explains why some BDZs were not recognized by the NIST library. The lab-made library allowed a better investigation of results from analyses of cocktails fortified at a concentration of 20 mg/L because this approach involved a comparison based on the accurate mass of HR spectra. Instead, the NIST library relies on the nominal mass for determining the match value between two spectra. All BDZs of gin tonic spots fortified with BDZs at a concentration of 20 mg/L were identified through a comparison of the experimental spectra with the lab-made HR library which was performed by the Agilent MassHunter Unknowns Analysis software. As an example, the diazepam detection is reported in Fig. 4C.
Greenness evaluation
Compliance with green chemistry parameters is becoming increasingly crucial for developing new analytical techniques. At the same time, several tools have been designed to evaluate the environmental impact of the analytical process32. The greenness evaluation of the previous E-LEI-MS configuration1 was calculated using the AGREE green assessment tool33obtaining a good result. A new assessment was made considering the application proposed with the E-LEI-Q-ToF-MS system, which should be regarded as the less green of the two setups used. The score obtained (Fig. 5) is comparable to that of the previous configuration, suggesting that the E-LEI-MS system is highly green despite the instrumentation utilised. A notable limitation is the absence of automation in the analysis process, which remains entirely manual. The development of the E-LEI-MS as a portable system may result in the enhancement of this aspect.
Another metric widely employed is the Complex Modified GAPI (ComplexMoGAPI)34. This tool adds a numeric value to the coloured pentagrams of previous tools, such as the green analytical procedure index (GAPI) and the Complex GAPI. The E-LEI-Q-TOF-MS score obtained using the ComplexMoGAPI open source sets the system to an acceptable greenness range (Fig. S8A). However, this score does not highlight some features of the E-LEI-MS system, such as the capability to provide real-time results, the rapidity of the analysis, and the use of solvent in a microliter range. This evaluation tool is probably unsuitable for a prototypical system because it currently contains many not-applicable fields, supporting more conventional techniques.
The Click Analytical Chemistry Index (CACI) is a novel approach that promotes simplicity and practicality of the analytical process35. The CACI score of the E-LEI-Q-ToF-MS evaluation labels this system as highly practical (Fig. S8B). As in the AGREE evaluation, the lack of an automated process negatively impacts the final assessment.
Overall, the potential of the E-LEI-MS as an environmentally friendly technique is confirmed by all tools.
Conclusion
The E-LEI-MS system stands out from traditional ambient ionization MS techniques due to its unique combination of ambient sampling and electron ionization (EI). This novel analytical approach offers a high identification power, satisfactory reproducibility, and total absence of matrix effects, as the EI process ensures that ionization occurs without the influence of the surrounding molecules. Although the E-LEI-MS is not comparable to other more sensitive targeted approaches for identifying substances in various matrices29,30,31this technique is promising because of its capacity for real-time analysis. A key advantage of E-LEI-MS is its rapid analysis time, less than five minutes from extraction to signal recording, eliminating the need for sample pretreatment. Additionally, the recent introduction of the VMC, inspired by the LC-LEI-MS interface, allows the analysis of compounds with higher boiling points. The E-LEI-MS produces qualitative results, but not quantitative, because the aspiration force during the sampling step is determined by the high vacuum in the ionization chamber, and the absolute amount of analyte that reaches the EI source is not calculable. The ability to analyze many compounds on different surfaces enables the application of the E-LEI-MS in different fields, including the pharmaceutical industry, and forensic science. The E-LEI-MS system was successfully tested for detecting and identifying APIs and excipients in real pharmaceutical formulations. The inability to detect certain APIs was attributed to drug formulation design and, in some cases, molecular structure constraints. However, these findings confirm the broad applicability of E-LEI-MS for identifying diverse compounds across multiple matrices. The E-LEI-MS is best positioned as a complementary screening tool, to traditional laboratory procedures which, while more sensitive, are also time-consuming and labor-intensive. The case report of the sildenafil tablet purchased on a legitimate website as a dietary supplement highlights the system’s efficacy in detecting counterfeit drugs. These findings indicate the promising potential of E-LEI-MS in quality control tests and fraud detection within the pharmaceutical industry. Additionally, E-LEI-MS has shown promise in forensic investigations, particularly in detecting illicit drugs. The detection of BDZs in fortified gin tonic demonstrates its potential for crime-scene analysis, where real-time results are critical for rapid decision-making solutions. These findings highlight E-LEI-MS as a powerful, fast, and reliable screening technique, with promising applications in pharmaceutical, forensic, and regulatory settings.
Data availability
Raw data used in the generation of Figs. 2, 3 and 4, S1-S7 were submitted as separate files but are not publicly available due to the prototypal nature of the system herein presented and the specificity of software needed for data processing (Mass Hunter Workstation Qualitative Analysis Version B.07.00 Build 7.0.7024.0 Copyright 2014 Agilent Technologies, Inc; Mass Hunter Workstation Qualitative Analysis Version 10.0 Build 10.0.10305.0 Copyright 2006-2018 Agilent Technologies, Inc; Mass Hunter Workstation Qualitative Analysis Unknowns Analysis Version 10.2 Build 10.2.733.8 Copyright 2006-2019 Agilent Technologies, Inc) but are available from the corresponding author upon reasonable request.
References
Arigò, A. et al. Extractive–liquid sampling electron ionization–mass spectrometry (E–LEI–MS): A new powerful combination for direct analysis. Sci. Rep. 13, 6429. https://doi.org/10.1038/s41598-023-33647-5 (2013).
Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013 (2018).
Murray, K. K. et al. Definition of terms relating to mass spectrometry (IUPAC Recommendations). Pure Appl. Chem. 85, 1515–1609. https://doi.org/10.1351/PAC-REC-06-04-06 (2013).
Takáts, Z., Wiseman, J. M., Gologan, B. & Cooks, R. G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 30, 6471–6473. https://doi.org/10.1126/science.1104404 (2004).
Cody, R. B., Laraméè, J. A. & Durst, H. D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77, 2297–2302. https://doi.org/10.1021/ac050162j (2005).
Forbes, T. P., Sisco, E., Staymates, M. & Gillen, G. DART-MS analysis of inorganic explosives using high temperature thermal desorption. Anal. Method. 34, 49988–44996. https://doi.org/10.1039/C7AY00867H (2017).
Wojtowicz, A. & Wietecha-Poslunszny, R. DESI-MS analysis of human fluid and tissue for forensic applications. Appl. Phys. A. 125, 312. https://doi.org/10.1007/s00339-019-2564-2 (2019).
Sisco, E. & Forbes, T. P. Forensic applications of DART-MS: A review of recent literature. Forensic Chem. 22, 100294. https://doi.org/10.1016/j.forc.2020.100294 (2021).
Harkin, C. et al. On-tissue chemical derivatization in mass spectrometry imaging. Mass. Spec. Rev. 41, 645–898. https://doi.org/10.1002/mas.21680 (2021).
Cardoso-Palacios, C. & Lanekoff, I. Direct Analysis of Pharmaceutical Drugs Using Nano-DESI MS. J. Anal. Methods Chem., 3591908, (2016). https://doi.org/10.1155/2016/3591908 (2016).
Beneito-Cambra, M. et al. Ambient (desorption/ionization) mass spectrometry methods for pesticide testing in food: A review. Anal. Methods. 12, 4831. https://doi.org/10.1039/D0AY01474E (2020).
Lv, Y., Zhao, J., Xue, H. & Ma, Q. Ambient ionization mass spectrometry for food analysis: recent progress and applications. TrAC 178, 117814. https://doi.org/10.1016/j.trac.2024.117814 (2024).
Mainero Rocca, L., L’Episcopo, N., Giordiani, A. & Staderini, A. Direct multiclass desorption electrospray ionization-tandem mass spectrometry method for the analysis of sleep inducers and ototoxic drug in dried blood spots. Rapid Commun. Mass. Spectrom. 36, 9265. https://doi.org/10.1002/rcm.9265 (2022).
Mesa Sanchez, D. et al. Mass spectrometry imaging of diclofenac and its metabolites in tissues using nanospray desorption electrospray ionization. Anal. Chim. Acta. 1233, 340490. https://doi.org/10.1016/j.aca.2022.340490 (2022).
Soudah, T., Zoabi, A. & Margulis, K. Desorption electrospray ionization mass spectrometry imaging in discovery and development of novel therapies. Mass. Spectrom. Rev. 42, 751–778. https://doi.org/10.1002/mas.21736 (2021).
Taylor, A. J. et al. Classification of tablet formulation by desorption electrospray ionization mass spectrometry and transmission Raman spectroscopy. J. Chemometrics. 36, 3412. https://doi.org/10.1002/cem.3412 (2022).
Monser, D. et al. Fast and semiquantitative screening for sildenafil in herbal over-the-counter formulations with atmospheric pressure solid analysis probe (ASAP) to prevent medicinal adulteration. J. Pharm. Biomed. Anal. 214, 114720. https://doi.org/10.1016/j.jpba.2022.114720 (2022).
Skov, K., Johansen, S. S., Linnet, K. & Nielsen, M. K. K. A review on the forensic toxicology of global drug-facilitated sexual assaults. Eur. Rev. Med. Pharmacol. Sci. 26, 183–197. https://doi.org/10.26355/eurrev_202201_27767 (2022).
Zhang, Y. et al. Benzodiazepines in complex biological matrices: Recent updates on pretreatment and detection methods. J. Pharm. Anal. 13, 442–462. https://doi.org/10.1016/j.jpha.2023.03.007 (2023).
Gupta, S. & Samal, N. Application of direct analysis in real-time mass spectrometry (DART-MS) in forensic science: A comprehensive review. Egypt. J. Forensic Sci. 12, 17. https://doi.org/10.1186/s41935-022-00276-4 (2022).
Pérez Orts, M., van Asten, A. & Kohler, I. The evolution toward designer benzodiazepines in Drug-Facilitated sexual assault cases. J. Anal. Toxicol. 47, 1–25. https://doi.org/10.1093/jat/bkac017 (2023).
Famiglini, G., Termopoli, V., Palma, P. & Cappiello, A. Liquid chromatography-electron ionization tandem mass spectrometry with the Direct-EI interface in the fast determination of diazepam and flunitrazepam in alcoholic beverages. Electrophoresis 37, 1048–1054. https://doi.org/10.1002/elps.201500517 (2016).
D’Aloise, P. & Chen, H. Rapid determination of flunitrazepam in alcoholic beverages by desorption electrospray ionization-mass spectrometry. Sci. Justice. 52, 2–8. https://doi.org/10.1016/j.scijus.2011.08.007 (2012).
Cappiello, A., Famiglini, G., Palma, P. & Siviero, A. Liquid Chromatography-Electron ionization mass spectrometry: Fields of application and evaluation of the performance of a Direct-EI interface. Mass. Spectrom. Rev. 24, 978–989. https://doi.org/10.1002/mas.20054 (2005).
Termpoli, V., Famiglini, G., Palma, P., Piergiovanni, M. & Cappiello, A. Atmospheric pressure vaporization mechanism for coupling a liquid phase with electron ionization mass spectrometry. Anal. Chem. 89, 2049–2056. https://doi.org/10.1021/acs.analchem.6b04646 (2017).
Grasselli, G., Arigò, A., Palma, P., Famiglini, G. & Cappiello, A. Latest developments in direct and Non-Direct LC-MS methods based on liquid electron ionization (LEI). Crit. Rev. Anal. Chem. 24, 1–18. https://doi.org/10.1080/10408347.2024.2381543 (2024).
Meng, F., Chen, X., Zeng, Y. & Zhong, D. Sensitive liquid chromatography-tandem mass spectrometry method for the determination of cefixime in human plasma: Application to a Pharmacokinetic study. J. Chromatogr. B. 819, 277–282. https://doi.org/10.1016/j.jchromb.2005.02.015 (2005).
Yilmaz, B. & Ciltas, U. Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods. J. Pharm. Anal. 5, 153–160. https://doi.org/10.1016/j.jpha.2014.10.005 (2015).
Merone, G. M. et al. Fast Quantitative LC-MS/MS Determination of Illicit Substances in Solid and Liquid Unknown Seized Sample. Anal. Chem., 93, 16308–16313, (2021). https://doi.org/10.1021/acs.analchem.1c03310
Grela, A., Gautam, L. & Cole, M. D. A multifactorial critical appraisal of substances found in drug facilitated sexual assault cases. Forensic Sci. Int. 292, 50–60. https://doi.org/10.1016/j.forsciint.2018.08.034 (2018).
Locatelli, M. et al. Fabric-Phase sorptive membrane array as a noninvasive in vivo sampling device for human exposure to different compounds. Anal. Chem. 93, 1957–1961. https://doi.org/10.1021/acs.analchem.0c04663 (2021).
Locatelli, M. et al. Green profile tools: Current status and future perspectives. Adv. Sample Prep. 6, 100068. https://doi.org/10.1016/j.sampre.2023.100068 (2023).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE-Analytical greenness metric approach and software. Anal. Chem. 92, 14, 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887 (2020).
Monsour, F. R., Plotka-Wasylka, J., Locatelli, M. & Modified, G. A. P. I. (eds) (MoGAPI) Tool and Software for the Assessment of Method Greenness: Case Studies and Applications. Analytica 5, 451–457, (2024). https://doi.org/10.3390/analytica5030030.
Monsour, F. R., Bedair, A. & Locatelli, M. Click analytical chemistry index as a novel concept and framework, supported with open source software to assess analytical methods. Adv. Sample Prep. 14 https://doi.org/10.1016/j.sampre.2025.100164 (2025).
Acknowledgements
This work has been funded by the European Union - NextGenerationEU within the framework of PNRR 4 Mission - Component 2 - Investment 1.1 under the Italian Ministry of University and Research (MUR) program “PRIN 2022” – grant number 202224R9NL – Reliable and rapid profiling of pesticides in Cannabis sativa L. by real-time in electron ionization detection (REI) and gas chromatography-mass spectrometry technique, with particular emphasis on the miniaturization and automation of sample preparation procedures - CUP: H53D23003760001. The authors are grateful to Agilent Technologies for providing the MS instrumentation and Laboratorio di Tossicologia, A.S.T. AV1, 61122 Pesaro, Italy for providing and preparing the BDZ standards and samples.
Funding
This work has been funded by the European Union - NextGenerationEU within the framework of PNRR 4 Mission - Component 2 - Investment 1.1 under the Italian Ministry of University and Research (MUR) program “PRIN 2022” – grant number 202224R9NL – Reliable and rapid profiling of pesticides in Cannabis sativa L. by real-time in electron ionization detection (REI) and gas chromatography-mass spectrometry technique, with particular emphasis on the miniaturization and automation of sample preparation procedures - CUP: H53D23003760001.
Author information
Authors and Affiliations
Contributions
A. A.: Funding, conceptualization, Methodology, Data curation, Writing−review and editing, Supervision. G. N.: Formal analysis, Data acquisition, Validation, Writing−review and editing. G. F.: Data curation, Supervision, Review. C. R. and M. A.: Resources. A. C.: Supervision, Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nevola, G., Arigò, A., Famiglini, G. et al. Drug screening in pharmaceutical and forensic applications using extractive-liquid sampling electron ionization-mass spectrometry (E-LEI-MS). Sci Rep 15, 30578 (2025). https://doi.org/10.1038/s41598-025-14772-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14772-9