Abstract
The current research focused on the chronic effect of arsenic (As3+, As III) on the reproductive system in male rats. Fifty male rats (190 ± 10 g) were separated into five groups to assess Arsenic induced physiological, hormonal and histopathological impairments. Following the dissection, blood, vital organs, accessory reproductive, testicular and hypothalamic tissues were obtained. Blood plasma was isolated and preserved for determining oxidative stress and performing hormonal analysis, while epididymis was used for sperm parameters assessment, and testis were subjected to histological analysis. The results revealed a significant decrease in percent sperm motility, sperm viability, and daily sperm production rate in high-dose treatment groups (25 and 50 mg/L). The concentrations of catalase, superoxide dismutase, and peroxidase were found to be significantly reduced. At the same time, a remarkable increase in reactive oxygen species (ROS) and thiobarbituric acid reactive species (TBARs) and was evident in a dose-dependent manner. Similarly, significantly low amounts of plasma testosterone, follicle-stimulating hormones (FSH), and luteinizing hormone (LH) were noted among treatment groups after exposure to arsenic. Histopathological findings showed remarkable multiple degenerations in seminiferous tubules including tubular atrophy, Leydig cells hypoplasia, proliferative marginal neoplasia, severe necrosis and acute germ cell aplasia leading to Sertoli cell-only syndrome. Fluorescent immune-cyto-chemical data confirmed a dose-proportional lowered quantitative expression in the number of gonadotrophins releasing hormone (GnRH) like immunoreactive (IR) neurons. Analysis of the comet assay revealed intensified nuclear denaturation and sperm DNA fragmentation in As3+ treated groups vs. the control. “Arsenic exposure induces oxidative stress, disrupts hormonal balance, impairs spermatogenesis, and causes testicular damage and DNA fragmentation, ultimately leading to reduced male fertility.”
Similar content being viewed by others
Introduction
Arsenic contamination is a global environmental concern, because As3+ (As III) has a highly toxic and carcinogenic nature, leading to environmental and agriculture implications, water and food related serious health effects in man and various animals1,2. Human exposure occurs through two major sources, either geologically or via anthropogenic activities3,4. Atmospheric air, drinking water, and food sources are the main contributors to arsenic (As) contamination and toxicity5. Industrial use of As is associated with its presence in wood preservatives, insecticides, fungicides, paints, herbicides, and cotton desiccants. In the past, chemicals based on arsenic have been added to feed for pigs and poultry to improve colour, increase feed efficiency, and encourage growth. However, because of arsenic toxicity and possible health hazards, their use has been restricted6. Arsenic exists mostly in inorganic forms as arsenite (As III) and arsenate (As V), which are toxic to humans and terrestrial animals. Investigations reported that As in reduced (As III) and oxidized (As V) forms are readily absorbed and assembled in the body’s tissues and fluids7.
The toxic actions of arsenic in animal bodies are thought to be mediated by the deactivation of enzymes contributing to the regulation of cellular pathways, DNA synthesis, and repair, the number of which lies around 2008. Chronic as well as acute arsenic poisoning has been documented in the literature, being associated with vomiting, nausea, abdominal discomforts, diarrhea, encephalopathy, and peripheral neuritis9. Multisystem diseases are linked with chronic exposure to arsenic, which initiates successive pathological conditions in the body, inducing skin10, , bladder, kidney, and lung cancer, and non-tumorous disorders such as cardiovascular problems and clogging pulmonary disease6,11. In addition, arsenic has been linked to numerous issues in the body’s organ systems, including the reproductive, endocrine, and neurological systems12,13. One of the most frequent arsenic induced neurotoxicity is peripheral neuropathy, mimicking the Guillain-Barré syndrome14.
The endocrine system performs an important part in maintaining reproductive functions in animals via feedback mechanisms that control gonadotropin and gonadal secretions through the hypothalamic pituitary gonadal (HPG) axis15. Arsenic is known to accumulate in the gonads, where it interferes with gonadal development and disrupts normal functions16, involved in the inhibition of testicular steroidogenesis by altering the activities of spermatogenetic enzymes17, and was reported to induce testicular abnormalities in teddy goat bucks18. Previous research showed that As III interferes with estrogen production in the body by disrupting its normal signaling pathways19. In vitro studies by20 also demonstrated the provoked apoptotic (related to programmed cell death) and cytotoxicity activities in the Sertoli of mice. As established earlier, heavy metals intoxication including cadmium and arsenic interferes with the blood-testis barrier and impairs spermatogenesis via oxidative stress, apoptosis, inflammation, hypoandrogenism (impairment of the hypothalamus or pituitary gland), homeostatic imbalance and downregulation of genetic components, see Bhardwaj et al., 202421.
Cadmium and other heavy metals potentially elicit cytotoxic responses in testicular elements corresponding to elevated MDA and FRAP inadequacy22. Consolidated proteome and metabolic profiling affirmed the arsenic interplay via ERK/AKT/NF-kB dependent cascade divergence in key male fertility associated regulatory mechanisms. Biomarker analysis of arsenic disrupted androgenic male fertility linked mechanisms indicating that redundant reactive oxygen species (ROS) cause damage to certain biomolecules like proteins and DNA23. Excessive ROS are known to interrupt the neuronal communication among GnRH hormone expressing neurons and axonal extremities located in the arcuate and infundibular nucleus in the brain to block the excitatory impulses to the adeno-hypophysial gonadotropic cells24,25.
Considering previous research, this study aims to investigate the chronic effects of arsenic (As III) on male reproductive function in rats, focusing on the male reproductive system in rats, with a focus on understanding its physiological, hormonal, and histopathological impact. The research sought to evaluate how prolonged arsenic exposure affects oxidative stress levels, antioxidant enzyme activity, and key reproductive hormones such as testosterone, FSH, and LH. Additionally, the study aimed to assess changes in sperm parameters including motility, viability, and production rate, as well as to identify structural damage in testicular tissue through histological and immune-cytochemical analysis.
Materials and methods
Chemicals
Sodium arsenite (NaAsO2, CAS-7784-46-5) was bought from Sigma-Aldrich, Taufkirchen, Germany. Four preparations of dissolved sodium arsenite in double distilled water having concentrations of 1, 5, 25 and 50 mg/L were prepared.
Animals
Fifty male Sprague Dawley rats, PND (postnatal days) 60–90 having an average body weight of 190 ± 10 g, were utilized in the experiment. The rats were obtained from the Animal house, Department of Animal Sciences, Quaid-I-Azam University Islamabad. They were kept in stainless-steel cages within properly ventilated rooms, maintained at 20–26 °C, and placed in a 12-hour light/dark cycle during the whole experiment. They had ad-libitum access to water (tap) and standard laboratory made food. All experimental procedures with the animals were sanctioned by the Ethical Committee of the Zoology Department (Reference no. Zoo-2024/04) and were conducted following international standard regulations and guidelines. The authors confirm that the study was done and is reported in full accordance with ARRIVE guidelines (https://arriveguidelines.org).
Experiment setup
Rats were randomly assigned into five equal groups, having ten rats per group (n = 10/ group). Group I was considered as control group G-I (0 mg/L), and Groups II, III, IV, and V were supplied with 1 mg/L, 5 mg/L, 25 mg/L and 50 mg/L of dissolved sodium arsenite accordingly through drinking water ad libitum for 120 consecutive days. In this study an additional higher dose of 50 mg/L was added to a previous set of 1 mg, 5 mg and 25 mg/l Sodium arsenite of by Huang and co-workers26 in a detailed metabolomic and proteomic analysis of arsenic induced testicular toxicities.
Dissection and collection of blood and tissue
On the day of dissection, rats were weighed and sacrificed, followed by collection of blood in heparinized syringes. Animals were euthanized using an overdose of sodium pentobarbital administered intraperitoneally. Blood samples were centrifuged at 1000 g for 15 min, followed by plasma isolation and storage at − 20 °C for further biochemical analysis. Testes and epididymis were isolated and weighted after washing in normal saline. The left testis was rinsed with ice-cold saline and stocked in a freezer at − 20 °C for subsequent analysis of DSP (daily sperm production). The left side epididymis was washed by rinsing it with a sterile saline solution to remove debris, weighed, and minced for sperm analysis. The right testicular tissue was preserved in 10% formalin for histological analysis.
Body weight (BW) and organs weights
The body weight of each rat was recorded both earlier and later in the trial. The organs, including the testes, epididymides, seminal vesicles, and prostate glands, were excised, carefully clipped of excess fat, blotted dry with filter paper, and weighed individually using an electronic balance (Electronic Compact Scale SF-400 C, Germany).
Sperm motility and viability
The method previously used by David et al., (2019)27 was practiced with little modifications. Briefly, to assess viability and motility, a small section of the cauda epididymis was removed and ground in 2 ml of 40 °C saline solution. A 10-microliter sample from the prepared homogenate was then positioned on a Neubauer chamber, and at least 5 fields were examined using a high-power camera linked to the microscope at 40× magnification (Nikon Model YS 100, UK).
Daily sperm production (DSP)
The frozen testes were liquified and processed for DSP analysis using a method previously practiced by David et al., (2022)28. The observations for counting late spermatids were made using a microscope (40× magnification, Nikon Model YS 100, UK), and 3 readings were taken to obtain the average number of spermatids28.
Biochemical assessment
The blood plasma stored at − 20° was utilized for the measurement of antioxidant enzymes such as catalase (CAT) by a previously documented procedure by Din et al., (2024)29, peroxidase (POD) using a previous standard protocol of Kaker et al., (1985)30 and superoxide dismutase (SOD) following the standard protocol of Kaker et al., (1985)30. MDA levels (measured via Thiobarbituric acid reactive species, TBARS assay) and reactive oxygen species (ROS) both were assessed by using methods previously documented using Wright et al., (1981)31.
Hormone investigation
The blood plasma was used for the measurement of reproductive hormones, by performing an ELISA (enzyme-linked immunosorbent assay) through commercially accessible kits for Testosterone (Biocheck Inc, USA), Luteinizing hormone (LH) and follicle stimulating hormone (FSH) (Rat FSH Cat # E-EL-R0391 and Rat LH Cat # E-EL-R0026, ELISA Kits, Elabscience, USA) using a microplate reader (Platos R 496; AMP Diagnostic, USA). The entire samples were assessed in a single assay and findings were presented as ng/ml for testosterone and in international units (IU) for LH and FSH. All the hormones were analysed using the standard protocol by David et al., (2019)26.
Testis and epididymis histology
Testicular and epididymal tissues placed in formalin after animal dissection were dehydrated, followed by fixation, mounting on wooden blocks, and microtomy with the help of the microtome (Thermo Fisher Scientific, Shandon Finesse 325, UK) according to the procedure of Omirinde et al., (2021)32. Briefly, 1 μm thick sections were cut, stretched, and fixed onto a glass slide. 24 h later, slides of the testicular and epididymal tissues were stained with Eosin and mounted with DPX. Through a light microscope (LM) (Nikon, 187842, Japan), the prepared slides having testicular (tubular and lumen diameter, as well as epithelial height) and epididymal tissues were analyzed at 40X magnification. Microphotography was performed using Leica DM LB2 (Wetzlar, Germany) equipped with a digital camera. The software Next-generation Image Processing for Scientific Imaging simply called ImageJ2, version 2.9.0 (https://imagej.net/software/imagej2/). was utilized for the assessment of various morphology parameters and the interpretation of the results33.
Fluorescent immunohistochemistry
Expression of GnRH in rat hypothalamus
Rats were dissected after cervical dislocation. Their brains were immediately removed and hypothalamic were isolated and fixed by immersing in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 72 h. The tissue was placed in paraffin and sliced into 15-µm serial sections using a rotary microtome. Every 30th section (spaced 150 μm apart) was then adorned onto slides34. Fluorescence labelled immunohistochemistry was analysed following the procedure by David et al.., (2022)26 with slight modifications. Sections were washed in 0.02 M potassium phosphate-buffered saline (KPBS; pH 7.4) between steps. Shortly, after dewaxing the paraffin-embedded sections, heat-induced epitope retrieval was done for 20 min using 1× Tromethamine-ethylenediaminetetraacetic acid (Tris-EDTA) at 90 °C. Peroxidase function was stopped by incubating the slices in 2% H2O2 in methanol for 20 min. To minimize background staining from antibody-tissue interactions, the slices were incubated for 30 min in a blocking solution containing 0.02 M KPBS, 0.5% Triton, and 2% goat serum. The slices were then placed in an incubator overnight at 4 °C using blocking buffer, followed by incubation with primary rabbit polyclonal antibodies against GnRHR (ab202848; 1:250; Abcam, Cambridge, United Kingdom) for 1 h at 37 °C. Primary antibody treated sections were re-incubated with goat anti-mouse IgG (H + L) Alexa Fluor 488 containing 5mM sodium azide (AY1001: Invitrogen, Oregon, USA) diluted at 1:800 in Tris-buffered saline plus 5% bovine serum albumin (BSA). Gelvatol (polymer of ethanol) was prepared by dissolving 10.5 g of polyvinyl alcohol in 12 mL of glycerol, 26 mL of distilled water, and 53 mL of Tris (pH 8.5) under low heat for 6 h. The mixture was then stored overnight in the refrigerator and centrifuged for 15 min at 5000 rpm. The resulting aliquots were placed at 4 °C. The sections were mounted, and the slides were left to dry overnight in the dark at 4 °C before being examined under fluorescence microscopy.
Photography and data analysis
Images were taken through an Optikam (4083) B5 digital camera attached to an Optica B-383FL Epi-Fluorescence microscope (Ponteranica, Italy).
Comet assay
The comet assay is a highly sensitive procedure applied to assess DNA damage at the single-cell level. In this assay, sperm cells collected from the rat epididymis were subjected to metal ion stress and embedded in agarose gel (normal melting agarose 1%). A lysis buffer was prepared by adding 2.5 M NaCl, 100 mM EDTA, 10 mM Tris (pH 10), 1% Triton X-100, and 10 mM DTT. Sperms were lysed to remove membranes, and then subjected to electrophoresis for 20 min at 25 V. The DNA fragments were visualized under a microscope, where damaged DNA appears as a “comet” with a distinct tail, indicating the extent of DNA strand breaks or fragmentation induced by metal exposure. DNA damage in the sperms collected from cauda epididymis was measured in terms Comet Number (N), Tail moment (µm) and Tail DNA (%) by using the method described in35.
Statistical analysis
Statistical analysis was conducted using IBM® SPSS® Statistics 23. The data was analyzed with one-way analysis of variance (ANOVA) followed by post hoc Tukey’s. The data satisfied the assumptions of normality and equal variances, we proceeded with parametric analysis using one-way ANOVA followed by Tukey’s post hoc test. All the results were expressed as mean ± SEM, and the significance level was set at p < 0.05.
Results
Effect of arsenic on body and organs weight
Although minor fluctuations in organ weights were observed across groups, these differences were not statistically significant (p > 0.05).” (Table 1). A decrease in testicular weight with increasing arsenic concentration vs. the control was seen, although the change was not significant (Table 1). A non-significant increase in epididymal weights was seen among arsenic treated groups. Non-significant differences were observed in the mean weight of the prostate gland and the seminal vesicle after treatment with arsenic. A decrease in the weights of the liver, kidney, and adrenal gland was noticed among various treatments while a non-significant increase in the heart weight of animals exposed to high doses of arsenic was observed.
Effect of arsenic on sperm mobility and viability
Arsenic exposure resulted in a significant reduction in both sperm motility (p < 0.05) and sperm viability (p < 0.01), with effects observed in a concentration-dependent manner (Table 2). These findings indicate a detrimental impact of arsenic on sperm function, potentially compromising reproductive health. Additionally, higher arsenic doses resulted in more pronounced decreases in both motility and viability, highlighting the toxic effects of the compound on male fertility.
Effect of arsenic on the daily sperm production (DSP) rate
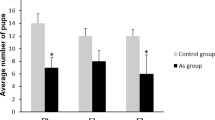
A notable reduction (p < 0.001) in the DSP rate was recorded in the arsenic-treated groups (G2 (p < 0.01), G3 (p < 0.001), G4 (p < 0.001), and G5 (p < 0.001)) vs. the control (Table 2). This suggests that arsenic exposure significantly impairs the dose-proportional DSP function. Furthermore, higher arsenic concentrations were associated with a greater reduction in DSP.
Effect of arsenic on biochemical parameters
All the results for the biochemical analysis have been summarized in Table 2. The exposure to arsenic led to a strong decline in SOD (p < 0.001) activity within the blood plasma of all the experimental groups vs. the control. However, a significant decrease in SOD levels was seen when G3 was compared with G2 (p < 0.01) and G4 (p < 0.05). A significantly lowered level of catalase was noticed among G2 (p < 0.05), and highly significantly lowered CAT levels (p < 0.001) were found in all other high arsenic dose-treated groups (G3, G4, and G5). Significant changes were also evident within groups. A notable decrease (p < 0.001) in POD was also seen in all arsenic treated groups. However, there was no significant change seen when a comparison was made between G2 with G3, and G4 with G5.
Rising levels of ROS production were evident in all the arsenic treated groups G2 (p < 0.05), G3 (p < 0.01), G4 (p < 0.001), and G5 (p < 0.001) vs. the control group. A significant change was also observed within all groups; however, a non-significant difference was seen between G2 and G3, G3 and G4, and G4 and G5. The MDA levels (measured via TBARS assay) were increased among all arsenic treated groups vs. the control (Table 2).
Effect of arsenic on hormone analysis
A non-significant variation in plasma testosterone levels was seen in the group G2 vs. the control, however, a significant reduction in testosterone levels was observed in G3–G5 (p < 0.05 to p < 0.001). Furthermore, non-significant changes were noted within groups. A non-significant decrease in LH and FSH concentrations was recorded among G2 and G3 groups as compared to the control. A significant decline in both the hormones was evident in G4 (p < 0.001) and G5 (p < 0.001) vs. the control group (Table 2). The declined values of gonadotropins strongly affirmed the immunoreactive outcomes in the same study where significant (p < 0.001 and 0.0001) decrease in GnRH like immunoreactivity in all treated groups specially group 3 to group 5 was also observed. These data revealed a close link between disruption of HPG axis via GnRH suppression, less produced gonadotropins and altered steroidogenesis.
Histomorphometry analysis
Effect of arsenic on testis morphology
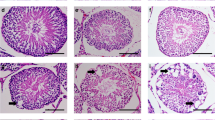
Testicular histology revealed that the control group (G1) had tightly organised seminiferous tubules (STs) with abundant morphologically normal mature spermatozoa in the lumen. As shown in Fig. 1, the seminiferous germinal epithelium, aligned with spermatogonia (Sg), primary and secondary spermatocytes, round spermatids (RS) and elongated spermatids (ES) in a characteristic spermatogenic process (Fig. 1A, B). Leydig cells of various shapes with large blood vessels were present in the interstitial spaces surrounding the seminiferous tubules. The caput and cauda epididymis flushing with plenty of mature spermatozoa (Fig. 1C, D, E). Group 2 (G2. 1 mg/l) seemed comparable to G1 with no visible deformations (Fig. 2A, B, C, D). Group 3 (G3. 5 mg/l) represented slightly disorganized seminiferous tubules with expanded diameter and increased luminal space, and less compact germinal epithelium, (Fig. 3A, B). Seminiferous tubules were less distant and highly constricted with an observable decrease in extra tubular spaces affecting the compact organization and functioning of Leydig cells. Caput and cauda epididymis were moderately supplied with spermatozoa (Fig. 3C, D).
Histological analysis of testicular and epididymal tissues in control group rats. (A) Testis showing compact seminiferous tubules with thick epithelium and elongated spermatids. (B) Higher magnification of seminiferous tubules with visible primary, secondary, round, and elongated spermatids. (C) Caput epididymis containing numerous healthy mature spermatozoa. (D) Higher magnification of caput epididymis. (E) Cauda epididymis filled with mature spermatozoa. Hematoxylin and eosin (H&E) staining; original magnifications: (A, C, E) ×20, (B, D) ×40. Sg spermatogonia, SC spermatocytes, St seminiferous tubule, LC Leydig cells.
(G2, sodium arsenite 1 mg/l); (A) No visible effect of 1 mg/l dose. Seminiferous tubules showing normal germinal epithelium (SG) spermatogonia, (SC) spermatocytes, (ES) elongated spermatids, (LC) Leydig cells; (B) Higher magnification of F; (C) Caput with numerous spermatozoa; (D) Cauda. H&E (×20) (×40).
Testicular sections of Group 4 (G4. 25 mg/l) had remarkable morphological alterations with unusual marginal regions molding the round to ellipsoidal form of seminiferous tubules. Highly proliferative areas were indicated with densely populated less specialized cells referring to germinal epithelial hyperplasia in oviform margins but less compaction in normal seminiferous epithelium (Fig. 4A, B). Degenerated Leydig cells and low number of elongated spermatids were evident in lumens. Similarly, Caput and Cauda epididymis appeared oligospermic with comparably less populated spermatozoa and thin epithelium (Fig. 4C, D).
(G4, sodium arsenite 25 mg/L); (A) High incidence of marginal germinal hyperplasia/neoplasia (arrow heads) in dilated tubules, degenerated germinal epithelium with lowered spermatogenesis, dispersed Leydig cells; (B) Higher magnification; circles indicate highly proliferative areas with giant cells; (C) Caput; (D) Cauda poorly filled with mature sperms, oligospermic. H&E (×20) (×40).
Group 5 (G5. 50 mg/l) showed profoundly inebriated and disorganized structural patterns in seminiferous tubules. A variety of deformed tubules were seen with degeneration, germinal epithelial atrophy, stasis, inflammation and necrosis. Disrupted Sertoli junctions with increased cellular spaces were observed (Fig. 5, A, B, C, D). Undifferentiated seminiferous tubules with absent lumen and atrophied germinal epithelium represented severe germ cell aplasia (Fig. 5E). Severe spermatozoa deficit (Fig. 5, F, G,H) was found in the caput and cauda.The morphometric records from all groups were represented in Table 3.
Testicular and epididymal histopathology in rats treated with sodium arsenite (50 mg/L). (A) Section showing multiple degenerated or atrophied seminiferous tubules () and germinal epithelial hypoplasia (arrowheads). (B–D) Severe atrophy, necrosis, and degeneration in seminiferous tubules. (E) Tubules with complete germ cell aplasia and Leydig cell hypoplasia (large arrows), with evident atrophy (). (F) Caput epididymis section showing marked reduction in sperm content. (G) High magnification of caput indicating severe acute oligospermia (circles). (H) Cauda epididymis with extremely low sperm presence. Hematoxylin and eosin (H&E) staining; original magnifications: (A–F, H) ×20; (G) ×40.Acronyms: St.d – degenerated seminiferous tubules.
Effect of arsenic on epidydimal histology
Arsenic exposure can lead to significant alterations in epididymal histology, causing damage to the epithelial cells. Histological changes may include degeneration of the epithelial lining. Prolonged exposure to arsenic has the potential to cause oxidative stress and inflammation, further compromising the structure and function of the epididymis. Figures 1, 2, 3 and 4, and 5 shows the dose dependent morphological changes induced by arsenic administration in adult rats for 120 days. A dose dependent disruption in tubular structure and increased lumen with reduced sperm concentration was evident among caput (Fig. 4) and cauda tissues (Fig. 5).
Effect of arsenic on fluorescent immunohistochemistry analysis
The effect of arsenic on central circuitry of HPG axis (Hypothalamic–Pituitary–Gonadal axis) was detected by using an antibody specific to the GnRH peptide. Quantitative expression of neurons immunoreactive to GnRH (GnRH-IR neurons) was observed in all animals of each group. Hypothalamic GnRH-IR expression in male rats across five groups was shown in representative photomicrographs (Fig. 6). A quantitative analysis of the individual and mean numbers of GnRH-IR neurons in each group was shown in Table 4. A decrease in the mean number of GnRH-IR expressions was observed in animals from group 1 to group 5 with an increasing concentration of administered arsenic. There was a significant (p < 0.001 and 0.0001) decrease in GnRH immunoreactivity in all groups. Similarly in group 3,4 and 5, a significant (p < 0.0001) decline in mean GnRH-IR neurons expressing immunoreactivity compared to group 2 was recorded. Likewise, a decreasing trend in mean GnRH-IR expression was also noticed between group 3 and group 5 (p < 0.0001) and between group 4 and group 5 (p < 0.01).
GnRH immunofluorescence in the hypothalamus of male rats exposed to sodium arsenite. Fluorescent micrographs showing gonadotropin-releasing hormone (GnRH) immunoreactivity in the hypothalamic region of rats treated with sodium arsenite at concentrations of 0 (a), 1 (b), 5 (c), 25 (d), and 50 mg/L (e) via drinking water. Alexa Fluor 488-conjugated mouse anti-GnRH secondary antibodies (Thermo Fisher Scientific, USA) were used for detection. Images were captured using an Optika fluorescence microscope at 20× magnification, with excitation at ~ 488 nm and emission at ~ 510–540 nm. Exposure time and gain were kept constant across all samples. Arrows indicate reduced GnRH expression with increasing arsenic concentration.
Effect of arsenic on comet assay
The analysis of the comet assay revealed a significant dose dependent rise in the number of comets in arsenic treatment groups (Table 4). In the comet assay, the metrics “% tail DNA” and “tail moment” are valid indicators of DNA fragmentation and genotoxic damage. These values measure the amount of DNA migration away from the cell nucleus during electrophoresis, which is a sign of DNA damage. A marked increase (p < 0.01) in the comet number was observed in the G3 (5 mg/L) group in comparison to the control. A highly significant rise (p < 0.001) in comet formation was evident in greater dosage treatment groups (25 and 50 mg/L) vs. the control. No significant changes were recorded in tail moment and percent tail DNA among the control and G1 and G2, however high dose treatment groups (25 mg/L and 50 mg/L) showed a noticeable rise in the movement of the tail and percent tail DNA in comparison to the control group as depicted in Table 4.
Discussion
Arsenic is harmful to animals and humans. It is considered to induce reproductive toxic effects due to its deposition in reproductive tissues, comparable to other heavy metals36. In line with the literature, the current work proved As toxicity for reproduction in male rats exposed to four different doses orally administered through drinking water.
In the existing study we analysed a decrease in testes and accessory organ weights including epididymis and prostate gland. Our studies were in line with previous research where testicular weight gradually decreased among the treated groups in comparison to the control group37. One of the possible reasons for this reduction might be the breakdown of necessary proteins disrupted by reactive oxygen species that are known to be associated with arsenic38. Studies have reported that arsenic induces hepatotoxicity and nephrotoxicity, which might be responsible for reducing the body weight39,40. In this study we found a decrease in sperm viability and motility in all treated groups, especially in the highest doses of 25 mg/l and 50 mg/l. The findings of the current research revealed that treatment of male rats with different concentrations of arsenic preparation induced lowered daily sperm count. The reduction in DSP reflects the overall compromised spermatogenic process in which arsenic and other heavy metals produce ROS in sperm. These ROS damage the DNA and cause many irreversible nuclear insults like karyorrhexis and karyolysis which are associated with apoptotic and necrotic cell death41. The spermatogenic product carries severe nuclear and structural impairments which result in low viability and motility. These findings are in line with the previous work of Souza et al. (2019)42, where adult rats experienced a reduction in daily sperm production rate when treated with arsenic43,44.
Arsenic is known to cross the blood testes barrier (BTB) and may induce apoptosis in maturing sperms through its cytotoxic effects45. Our findings also showed a disturbance in daily sperm production which is a possible consequence of the endocrine disruptive potential of arsenic and the interference with the hypothalamic-pituitary gonadal axis, declined testicular steroidogenesis, Leydig cells degeneration and insensitivity to hypophysial gonadotropins and lowered blood supply as demonstrated by previous research46,47. Arsenic may cross the BTB, where it induces oxidative stress in testes. Oxidative stress is characterized by the large-scale production of free radicals such as ROS and TBARs. The present study demonstrated a dose-dependent rise in the ROS level and acidic species, leading to spermatogenic arrest due to arsenic evoked cytotoxicity and accumulation in testes. Redox imbalance and altered antioxidant levels trigger lipid peroxidation (LPO), which damages lipid membranes, resulting in a decline of sperm motility and impaired spermatogenesis48. Regarding antioxidants, catalase (CAT) and superoxide dismutase (SOD) are key enzymes with free radical scavenging properties in testes49. Our study found an arsenic concentration related decrease in their levels across all arsenic treatments, although, by contrast, some studies have reported an increase in SOD levels50. In many tissues, exposure to arsenic can raise Superoxide Dismutase (SOD) activity as a compensatory mechanism for oxidative stress. However, in testicular tissue, it can lower SOD activity, which may contribute to reproductive harm. The distinct cellular and molecular milieu in the testes, which are especially susceptible to the harmful effects of arsenic, may be the cause of this variation in SOD response. In addition, elevated testicular temperature is linked to decreased SOD and catalase activity42. A declination in the peroxidase (POD) concentration inhibits the conversion of H2O2 into H2O via oxidation-reduction reactions, leading to cellular damage. Our findings suggest that arsenic accumulation in testicular tissues interferes with the activities of antioxidant enzymes51. Arsenic is known to interfere with the Nrf signaling pathway that is a crucial defense system against oxidative and electrophilic stress. The Nrf system keeps key regulatory factors such as Keap1 and Nrf2 transcription factors involved in detoxification and antioxidant defense gene expression. Arsenic intoxication blocks the normal degradation of Nrf by KEEP1-CLU3 and promotes its accumulation and translocation in the nucleus52.
Histological analysis of testicular tissues found a notable alteration in testicular organization among control and arsenic treated rats. The luminal and tubular diameter of STs were decreased with increasing doses of arsenic, which might be due to lowered production of testosterone as reported by Ge et al.. (2021)53. The arsenic-induced alterations in testes include reduction in interstitial space and increased tubular density rendered the Leydig cells irresponsive to pituitary gonadotropins. We also found contractions and degenerative changes within STs, and basement membrane impairments. These changes may be due to arsenic elicited oxidative stress in testicular tissues as previously predicted54. Animals administered high concentrations of arsenic (25-50 mg/L) showed a marked degenerative pattern in testes like Leydig cell hypoplasia, seminiferous epithelial atrophy, marginal hyperplasia and germ cell aplasia55. The effects that came after the disruption of the HPG axis were low steroidogenesis, oxidative stress, apoptosis and cell death. Sertoli cells play a major role in spermatogenic events. Our study is concomitant with previous work where Sertoli cells were found to undergo apoptosis when exposed in vitro to arsenic trioxide as described by Kim et al.56. Functional disruption of Sertoli cells disorganizes the germinal epithelium and causes spermatogenic arrest. Studies showed that As3+ exhibits direct binding ability to androgen receptor –NH2 or –COOH group termini to alter the normal N–C interaction. This binding downregulates the androgen receptor transcription and modulates RNA based silencing of androgen in mouse Sertoli cells57,58. Like arsenic, other heavy metals show implications not only in testicular maturation but also through ovarian inhibition in females. Heavy metals like cadmium induce apoptosis in follicular cells, inhibit ovarian steroidogenesis and evoke teratogenicity59.
A significant decrease in FSH and LH concentrations was seen among the entire As-exposed group in comparison to the control. A strong drop in the plasma testosterone level was witnessed; It was found to be associated with a decrease in the number of Leydig cells, ultimately causing spermatogenic arrest60. These results are concordant with earlier findings where arsenic exposure was shown to inhibit steroidogenesis in male and female rodents61. Findings of the current study corroborated the previous observations that arsenic may have a direct inhibitory effect on testes both at endocrinological and histological levels in mammals. We also observed lowered concentrations of testosterone (T) in arsenic treated groups. One of the possible causes of lowered T levels is that arsenic treatment changed the process of sperm formation by gene expression of those enzymes which are contributing to testosterone production62. The results of lowered testosterone concentrations in all the arsenic treated groups additionally suggested that the degeneration of Leydig cells might have occurred with a significant decrease in the Leydig cell population, providing a low level of substrate for gonadotropins and disturbing the process of spermatogenesis63.
To delineate the plausible cause of the observed inhibition of testicular activity, we examined whether arsenic exposure affects hypothalamic GnRH expression as part of the central regulatory circuitry of the HPG axis. It was fascinating to note that with increasing dosage of arsenic, the GnRH gradually decreased, which inhibited testicular testosterone due to a lowered GnRH-IR expression. Besides the GnRH inhibition, arsenic-induced testicular dysfunction might include numerous pathways like blocking of steroidogenic enzyme and androgen receptor transcriptional activity. Arsenic potentially targeted the important regulatory genes controlling the process of steroidogenesis like steroidogenic acute regulatory protein, StAR, 3β-HSD, 17β-HSD, LHR, and ABP64. Furthermore, inadequate antioxidant activity, as well as elevated ROS and MDA levels, contributed to the disruption of the HPG axis.
It has been demonstrated that arsenic can cross both the placenta and blood brain barriers65. In contrast to the current findings, Li et al. (2018)66 reported enhanced mRNA and protein expression of GnRH in the hypothalamus during puberty in female offsprings whose mother was exposed to arsenic during gestation42. The discrepancy in the GnRH expression between the two studies may pertain to the method of estimation, where the former study observed GnRH protein expression by enzymes linked immunosorbent assay (ELISA) and western blot, but the current study utilized the fluorescent immunocytochemical detection method. Moreover, the difference between these two observations may lie in gender difference that needs to be resolved. Lastly, a possible explanation for this disparity may also be the time, dose, and route of exposure between the two studies.
Arsenic induces genotoxic effects through breakage of DNA strands2. Arsenic exposure can cause sperm DNA damage via a variety of mechanisms, including oxidative stress, interference with DNA repair pathways, and disruption of epigenetic control. In the present study, we found that with an increase in dose in adult rats, an increase in the number of comets with a rise in % tail DNA. Similar results were reported previously where arsenic linked DNA damage was seen in leukocytes in the blood of mice67. Sperm counts, motility, and viability findings in our study substantially confirmed prior work by Irvine and colleagues67, who found that sperm DNA damage usually corresponded with poor seminal characteristics such as lower count and motility or aberrant morphology. One of the most significant impacts of arsenic genotoxicity is the inhibition of DNA repair. Arsenic’s reactive oxygen species impede two DNA repair processes: nucleotide excision repair and base excision repair. Mutagenic targets of arsenic involve specific base adjustment in 8-oxoguanine to produce modified nucleases68.
Sperm DNA integrity has a substantial impact on fertility and early embryo development. Sperm DNA fragmentation (SDF) is linked to lower fertilization rates, developmental arrest, preimplantation embryonic lethality and miscarriage. According to studies, SDF can impact embryo development following the maternal zygotic transition, perhaps causing apoptosis and making it harder to reach the blastocyst stage. Given the facts shown above, lifestyle adjustments, antioxidant therapy, and treatment of underlying medical conditions are required to lower the risk of sperm DNA damage and boost fertility. Future research should standardize DNA fragmentation testing, study novel antioxidant therapy, and look into the function of sperm DNA damage in recurrent pregnancy loss69.
In the current study we focused solely on male rats, which may not fully represent the results of arsenic treatments on the reproductive system of males in other species, including humans. Neither did the study include long-term follow-up to assess potential recovery or permanent damage following arsenic exposure. Additionally, the mechanism by which arsenic induces DNA damage in sperm and disrupts hormonal regulation was not further explored. Lastly, the study’s dose-dependent effects were observed, but a broader range of doses or more detailed exposure timelines might provide a more comprehensive understanding of arsenic’s impact.
Conclusion
Chronic arsenic exposure significantly disrupts the reproductive system in male rats, causing declines in sperm motility, viability, and production, especially at higher doses (25 and 50 mg/L). Increased oxidative stress, marked by elevated levels of ROS and lipid peroxidation, alongside reduced antioxidant enzyme levels, suggests that oxidative damage is involved in reproductive toxicity. As3+ exposure also lowered the levels of reproductive hormones, including testosterone, FSH, and LH, further impairing reproductive functions. Histological analysis revealed damage to the testicular structure, with disorganized seminiferous tubules and decreased spermatocyte concentration, indicating disrupted spermatogenesis. Additionally, reduced gonadotropin-releasing hormone GnRH immunoreactive neurons and DNA damage in sperm cells highlight arsenic’s detrimental effects on reproductive health. These findings suggested that arsenic exposure leads to spermatogenic arrest and impaired testicular function, ultimately affecting male fertility. Future studies should explore potential protective agents or therapeutic interventions to mitigate arsenic-induced reproductive toxicity. Additionally, long-term and low-dose exposure models should be investigated to better mimic real-world environmental conditions.
Data availability
The data will be available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
One-way analysis of variance
- As:
-
Arsenic
- BSA:
-
Bovine serum albumin
- BTB:
-
Blood testes barrier
- CAT:
-
Catalase
- °C:
-
Centigrade
- DNA:
-
Desoxyribonucleic acid
- DSP:
-
Daily sperm production
- ELISA:
-
Enzymes linked immunosorbent assay
- FSH:
-
Follicle stimulating hormones
- GnRH:
-
Gonadotrophin releasing hormone
- H2O:
-
Water
- H2O2 :
-
Hydrogen peroxide
- HPG:
-
Hypothalamic pituitary gonadal
- HPBS:
-
Potassium phosphate-buffered saline
- IR:
-
Immunoreactive
- IU:
-
International unit
- KPBS:
-
Potassium phosphate-buffered saline
- LH:
-
Luteinizing hormone
- LPO:
-
Lipid peroxidation
- mg/mL:
-
Microgram per litter
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Sodium dismutase
- TBARs:
-
Thiobarbituric acid reactive species
- Tris–EDTA:
-
Tromethamine-ethylenediaminetetraacetic acid
References
Abbas, G. et al. Arsenic (As) uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public. Health. 15, 59. https://doi.org/10.3390/ijerph15010059 (2018).
Bhardwaj, J. K. & Panchal, H. Quercetin mediated Attenuation of cadmium induced oxidative toxicity and apoptosis of spermatogenetic cells in caprine testes in vitro. Environ. Mol. Mutagen. 62 (6), 374–384 (2021).
Cardoso, A. P. F., AlEryani, L. & States, J. C. Arsenicinduced carcinogenesis: the impact of MiRNA dysregulation. Toxicol. Sci. 165, 284–290. https://doi.org/10.1093/toxsci/kfy128 (2018).
Panchal, H. & Bhardwaj, J. K. Quercetin supplementation alleviates cadmium induced genotoxicity-mediated apoptosis in caprine testicular cells. Biol. Trace Elem. Res. 202, 1–14. https://doi.org/10.1007/s12011-023-04038-8 (2024).
Bundschuh, J. et al. Seven potential sources of arsenic pollution in Latin America and their environmental and health impacts. Sci. Total Environ. 780, 1–17. https://doi.org/10.1016/j.scitotenv.( (2021).
Ratnaike, R. N. Acute and chronic arsenic (As) toxicity. Postgrad. Med. J. 79, 391–396. https://doi.org/10.1136/pmj.79.933.391 (2003).
Hong, Y. S., Song, K. H. & Chung, J. Y. Health effects of chronic arsenic (As) exposure. J. Prev. Med. Public. Health. 47, 245–252. https://doi.org/10.3961/jpmph.14.035 (2014).
Jomova, K. et al. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 31, 95–107. https://doi.org/10.1002/jat.1649 (2011).
Barton, A. L. & McLean, B. An unusual case of peripheral neuropathy possibly due to arsenic toxicity secondary to excessive intake of dietary supplements. Ann. Clin. Biochem. 50, 496–500. https://doi.org/10.1177/0004563212473276 (2013).
Flora, S. J. S. Preventive and therapeutic strategies for acute and chronic human arsenic (As) exposure. Arsenic Drinking Water Food. 341–370. https://doi.org/10.1007/978-981-13-8587-2_13 (2020).
Ahmed, S. et al. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicol. Sci. 141, 166–175. https://doi.org/10.1093/toxsci/kfu113 (2014).
Abdul, K. S. M., Jayasinghe, S. S., Chandana, E. P. S., Jayasumana, C. & De Silva, P. M. C. S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 40, 828–846 (2015). https://doi.org/10.1016/j.etap.2015.09.016 (2015).
Flora, S. J. & Agrawal, S. Arsenic, cadmium, and lead. In: Reproductive and Developmental Toxicology, 537–566Academic Press, (2017). https://doi.org/10.1016/B978-0-12-804239-7.00031-7
Goddard, M. J., Tanhehco, J. L. & Dau, P. C. Chronic arsenic poisoning masquerading as LandryGuillainBarré syndrome. Electromyogr. Clin. Neurophysiol. 32, 419–423 (1992). https://europepmc.org/article/med/1396293
Bikal, P. & Bhardwaj, J. K. N-acetyl-l-cysteine mediated Attenuation of cadmium induced oxidative stress and apoptosis in ovarian antral follicles in vitro. Environ. Toxicol. 1–11. https://doi.org/10.1002/tox.24505 (2025).
Adeogun, A. E. et al. Impact of arsenic on male and female reproductive function: A review of the pathophysiology and potential therapeutic strategies. NaunynSchmiedebergs Arch. Pharmacol. 12-, 1–22. https://doi.org/10.1007/s00210-024-03452-6 (2024).
Sarkar, M., Chaudhuri, G. R., Chattopadhyay, A. & Biswas, N. M. Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J. Androl. 5, 27–31. https://doi.org/10.1097/00049258-200301000-00005 (2003).
Zubair, M., Ahmad, M., Jamil, H. & Deeba, F. Toxic effects of arsenic on semen and hormonal profile and their amelioration with vitamin E in teddy goat bucks. Andrologia 48, 1220–1228. https://doi.org/10.1111/and.12564 (2016).
Chatterjee, A. & Chatterji, U. Arsenic abrogates the estrogensignaling pathway in the rat uterus. Reprod. Biol. Endocrinol. 8, 80. https://doi.org/10.1186/1477-7827-8-80 (2010).
Kim, K. W., Chanpiwat, P., Hanh, H. T., Phan, K. & Sthiannopkao, S. Arsenic geochemistry of groundwater in Southeast Asia. Front. Med. China. 5 https://doi.org/10.1007/s11684-011-0158-2 (2011).
Bhardwaj, J. K., Siwach, A., Sachdeva, D. & Sachdeva, S. N. Revisiting cadmiuminduced toxicity in the male reproductive system: an update. Arch. Toxicol. 98, 3619–3639. https://doi.org/10.1007/s00204-024-03871-7 (2024).
Li, T. et al. Allcause mortality risk associated with longterm exposure to ambient PM2·5 in china: A cohort study. Lancet Public. Health. 3, 470–477. https://doi.org/10.1016/S2468-2667 (2018).
Bhardwaj, J. K., Palliwal, A. & Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 35, 22823. https://doi.org/10.1002/jbt.22823 (2021).
Agarwal, A., Saleh, R. A. & Bedaiwy, M. A. Role of reactive oxygen species in the pathophysiology of human reproduction. Int. J. Fertil. Steril. 79, 829–843. https://doi.org/10.1016/S0015-0282(02)04948-8 (2003).
Spaziani, M. et al. Hypothalamopituitary axis and puberty. Mol. Cell. Endocrinol. 520 https://doi.org/10.1016/j.mce.2020.111094 (2021).
Huang, Q. et al. Integrated proteomics and metabolomics analysis of rat testis: mechanism of arsenicinduced male reproductive toxicity. Sci. Rep. 6, 9–12. https://doi.org/10.1038/srep32518 (2016).
David, M., Ain, Q., ul, Ahmad, M., Zaman, W. & Jahan, S. A biochemical and histological approach to study antifertility effects of methanol leaf extract of asplenium dalhousiae hook. In adult male rats. Andrologia 51, 1–9. https://doi.org/10.1111/and.13262 (2019).
David, M. et al. Determination of possible contraceptive potential of methanolic leaf extract of mentha longifolia L. in adult male rats: A biochemical and histological study. Toxicol. Res. 11, 951–961. https://doi.org/10.1093/toxres/tfac075( (2022).
Din Muhammad, H. M., Anjum, M. A. & Naz, S. Siliconmediated alleviation of salinity stress in Petunia (Petunia hybrida) by modulation of morphological, physiological and biochemical indices. J. Soil. Sci. Plant. Nutr. 12, 215–229. https://doi.org/10.1007/s42729-024-01681-5 (2024).
Kakkar, P., Das, B. & N Viswanathan, P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 130 (2). https://doi.org/10.1097/YCO.0b013e3280117733 (1984).
Wright, J. R., Colby, H. D. & Miles, P. R. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch. Biochem. Biophys206.296-304. (1981). https://doi.org/10.1016/0003-9861(81)90095-3
Omirinde, J. O., Olukole, S. G. & Oke, B. O. Nerve and glial cell expressions in the testes and epididymides of different age groups of cane rat (Thryonomys swinderianus). J. Microsc Ultrastruct. 9, 67–75. https://doi.org/10.4103/jmau.jmau_6_20 (2021).
Louiza, D. et al. A comparative histological study of testicles and epididymis in pets (dog and cat) and wild animals (genet and mongoose). Egypt. Acad. J. Biol. Sci. Histol. Histochem. 15, 193–207. https://doi.org/10.21608/eajbsd.2023.302658 (2023).
Huan, M. A. O. & Yan, L. I. Research progress of manganese exposure on abnormal GnRH release in hypothalamus affecting reproductive function. J. Environ. Occup. Med. 40, 107–110. https://doi.org/10.11836/JEOM22296 (2023).
Ain, Q. U., David, M., Ijaz, M. U. & Jahan, S. Assessment of antiandrogenic and antispermatogenic activity of Hedera Nepalensis in adult male rats. Andrologia 54, 213–223. https://doi.org/10.1111/and.14353 (2022).
Sun, H. J. et al. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 69, 148–158. https://doi.org/10.1016/j.envint.2014.04.019 (2014).
Zubair, M., Ahmad, M. & Qureshi, Z. I. Review on arsenicinduced toxicity in male reproductive system and its amelioration. Andrologia 49, 1–8. https://doi.org/10.1111/and.12791 (2017).
Majhi, C. R. et al. Effects of acetaminophen on reactive oxygen species and nitric oxide redox signaling in kidney of arsenicexposed rats. Food Chem. Toxicol. 49, 974–982. https://doi.org/10.1016/j.fct.2011.01.003 (2011).
Dopp, E., von Recklinghausen, U., DiazBone, R., Hirner, A. V. & Rettenmeier, A. W. Cellular uptake, subcellular distribution and toxicity of arsenic compounds in methylating and nonmethylating cells. Environ Res 110, 435–442 https://doi.org/10.1016/j.envres.2009.08.012(2010).
Kumar, R. et al. Design, synthesis and screening of triazolopyrimidine-pyrazole hybrids as potent apoptotic inducers. Arch. Pharm. Chem. Life Sci. 350, e1700137. https://doi.org/10.1002/ardp.201700137 (2017).
Pant, N., Murthy, R. C. & Srivastava, S. P. Male reproductive toxicity of sodium arsenite in mice. Reprod. Toxicol. 23, 399–403. https://doi.org/10.1191/0960327104ht467oa (2004).
Souza, A. C. F. et al. Combined effects of arsenic exposure and diabetes on male reproductive functions. Andrology 7, 730–740. https://doi.org/10.1111/andr.12613 (2019).
Jana, K., Jana, S. & Samanta, P. K. Effects of chronic exposure to sodium arsenite on hypothalamopituitarytesticular activities in adult rats: possible an estrogenic mode of action. Reprod. Biol. Endocrinol. 4, 9. https://doi.org/10.1186/1477-7827-4-9 (2006).
Tewari, K. Fluoride and/or arsenic toxicity in mice testis with formation of giant cells and subsequent recovery by some antidotes. (2005). 19.167-180 doi:200-530-11189.
Tariba Lovaković, B. Cadmium, arsenic, and lead: elements affecting male reproductive health. Curr. Opin. Toxicol. 19, 7–14. https://doi.org/10.1016/j.cotox.2019.09.005 (2020).
Tanaka, A. et al. Comparative study of the toxic effects of gallium arsenide, indium arsenide and arsenic trioxide following intratracheal instillations to the lung of Syrian golden hamsters. Fukuoka Acta Med. 91, 21–33 (2000). https://europepmc.org/article/med/10714013
Khaki, A. Antioxidative effects of Citro flavonoids on spermatogenesis in rat. Afr. J. Pharm. Pharmacol. 5, 721–725. https://doi.org/10.5897/AJPP11.277 (2011).
Kumar, S., Murarka, S., Mishra, V. V. & Gautam, A. K. Environmental & lifestyle factors in deterioration of male reproductive health. Indian J. Med. Res. 1.29-35 PMID 25673539 (2014).
Guvvala, P. R., Sellappan, S. & Parameswaraiah, R. J. Impact of arsenic(V) on testicular oxidative stress and sperm functional attributes in Swiss albino mice. Environ. Sci. Pollut Res. 23, 18200–18210. https://doi.org/10.1007/s11356-016-6870-3 (2016).
Kumar, P. et al. Multicomponent synthesis of some molecular hybrid containing thiazole pyrazole as apoptosis inducer. Drug Res. 68, 72–79. https://doi.org/10.1055/s-0043-116947 (2018).
Saleha Banu, B. et al. Vivo genotoxic effect of arsenic trioxide in mice using comet assay. Toxicol 162, 171–177. https://doi.org/10.1016/S0300-483X(01)00359-6 (2001).
Zeng, Q., Yi, H., Huang, L., An, Q. & Wang, H. Reduced testosterone and Ddx3y expression caused by longterm exposure to arsenic and its effect on spermatogenesis in mice. Environ. Toxicol. Pharmacol. 63, 84–91. https://doi.org/10.1016/j.etap.2018.08.012 (2018).
Ge, R. S., Li, X. & Wang, Y. Leydig cell and spermatogenesis. Adv. Exp. Med. Biol. 1288, 111–129. https://doi.org/10.1007/978-3-030-77779-1_6 (2021).
Chiou, T. J., Chu, S. T., Tzeng, W. F., Huang, Y. C. & Liao, C. Arsenic trioxide impairs spermatogenesis via reducing gene expression levels in testosterone synthesis pathway. Chem. Res. Toxicol. 21, 1562–1569. https://doi.org/10.1021/tx700366x (2008).
Mahavir, M. A., Sansthan, C., Kumar, R., Kumar, A. & Sansthan, M. C. Impact of arsenic on testosterone synthesis pathway and sperm production in mice. Innov. J. Med. Health Sci. 3, 185–189 (2013). http://www.innovativejournal.in/index.php/ijmhs
Kim, Y. J. et al. Arsenic trioxideinduced apoptosis in TM4 Sertoli cells: the potential involvement of p21 expression and p53 phosphorylation. Toxicol 285, 167–185. https://doi.org/10.12717/DR.2015.19.4.167 (2011).
Hirner, A. V. & Rettenmeier, A. W. Methylated meta l(loid) species in humans. In: Organometallics in Environment and Toxicology, Vol. 7 465–521 (2015). https://doi.org/10.1515/9783110436600-019
Luo, J. et al. Nasal delivery of nerve growth factor rescues hypogonadism by upregulating GnRH and testosterone in aging male mice. EBioMedicine 35, 295–306. https://doi.org/10.1016/j.ebiom.2018.08.021 (2018).
Smith, E., Smith, J., Smith, L., Biswas, T. & Correll, R. Arsenic in Australian environment: an overview. J. Environ. Sci. Health Tox Hazard. Subst. Environ. Eng. 38, 223–239. https://doi.org/10.1081/ese-120016891 (2003).
Wang, A., Holladay, S. D., Wolf, D. C., Ahmed, S. A. & Robertson, J. L. Reproductive and developmental toxicity of arsenic in rodents: a review. Int. J. Toxicol. 25, 319–331. https://doi10.1080/10915810600840776 (2006).
Rosenblatt, A. E. & Burnstein, K. L. Use of microbial consortia in bioremediation of metalloid polluted environments. Mol. EndocrinoL. 11, 891. https://doi.org/10.3390/microorganisms11040891 (2009).
Bhardwaj, J. K., Bikal, P. & Sachdeva, S. N. Cadmium as an ovarian toxicant: A review. J. Appl. Toxicol. 44, 129–147. https://doi.org/10.1002/jat.4526 (2024).
Ahotupa, M. & Huhtaniemi, I. Impaired detoxification of reactive oxygen and consequentoxidative stress in experimentally Cryptorchid rat testis. Biol. Reprod. 46, 1114–1118. https://doi.org/10.1095/biolreprod46.6.1114 (1992).
Jana, K., Jana, S. & Samanta, P. K. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod. Biol. Endocrinol. 4, 9. https://doi.org/10.1186/1477-7827-4-9 (2006).
Li, S. et al. Hum. Medicinal plants and natural products in amelioration of arsenic toxicity: a short review. Exp. Toxicol. 55, 349–354. https://doi.org/10.1080/13880209.2016.1235207 (2015).
Ma, W., Song, H., Das, S. K., Paria, B. C. & Dey, S. K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proceedings of the National Academy of Sciences. 100(5):2963-8. (2003). https://doi.org/10.1073/pnas.0530162100.
Irvine, D. S. et al. DNA integrity in human spermatozoa: relationships with semen quality. J. Androl. 21, 33–44. https://doi.org/10.1002/j.1939-4640.2000.tb03273.x (2000).
Esteves, S. C., Zini, A., Coward, R. M., Evenson, D. P., Gosálvez, J., Lewis, S. E.,… Humaidan, P. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia, 53(2), e13874. https://doi.org/10.1111/and.13874. (2021).
Agarwal, A., Majzoub, A., Baskaran, S., Selvam, M. K. P., Cho, C. L., Henkel, R.,… Shah, R. Sperm DNA fragmentation: a new guideline for clinicians. The world journal of men’s health, 38(4), 412. doi: 10.5534/wjmh.200128. (2020).
Acknowledgements
We are thankful to the Reproductive Physiology Laboratory, Department of Zoology, Quaid-i-Azam University, Islamabad, for supporting in this research.
Author information
Authors and Affiliations
Contributions
Sarwat Jahan, Syed Zahid Mahboob led the design the research work and approved study. Jawaher Alzahrani, Muhammad Ayub, Asif Kamal, Razia Virk, Syed Zahid Mahboob planned the study, executed the experimental work, analyzed the results and did the paper writeup. Kamran Ullah, Jawaher Alzahrani, Asif Kamal, Aziz Ullah, Muhammad Maaz Qureshi, Mehwish David, Hussain Badshah, Moona Nazish, Muhammad Tahir Naseem, Razia Virk helped in compiling the results, data interpretation and aided in writing the article. Kamran Ullah, Riffat Bano, Asif Kamal, Moona Nazish, Hussain Badshah, Aziz Ullah helped in compilation of results and reviewed the initial draft. Sarwat Jahan, Hussain Badshah and Muhammad Tahir Naseem reviewed and rephrased the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declaration
All rats handling and procedures were approved upfront by the Ethical Committee of the Zoology Department (Reference no. Zoo-2024/04) and were conducted following international standard regulations and guidelines. We confirming that the study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahboob, S.Z., Jahan, S., Badshah, H. et al. Reproductive toxicity in male rats induced by chronic arsenic exposure involves hormonal and structural changes. Sci Rep 15, 29037 (2025). https://doi.org/10.1038/s41598-025-14929-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14929-6