Abstract
Urothelial carcinomas of the upper urinary tract (UTUC) are rare tumors with a high malignancy degree. Radical nephroureterectomy (RNU) with bladder cuff excision remains one of the standard treatments in clinically localized or locally advanced UTUCs. However, the role of cytoreductive RNU in treating clinically lymph node-positive (N+) UTUCs remains unclear. A nationwide retrospective study was conducted by the Taiwan UTUC Collaboration Group from July 1988 to June 2022. Patients with clinical N + UTUC before initiation of cancer therapy were included in this study. Initial clinically node-positive disease was noted in 288 (5.4%) of the 5,301 patients. Of the patients, 239 (83%) patients underwent RNU. UTUC-related mortality was markedly higher among patients not receiving RNU than among those who underwent surgery (69.4% vs. 36%). After adjusting for the effects of stepwise enrolled parameters, multivariate analysis showed that undergoing RNU (or not) and smoking (or not) were the only independent predictors of overall survival (OS). After adjusting for the effects of significant stepwise enrolled variables, multivariate analysis showed that RNU and smoking (or not) were the only independent predictors of cancer-specific survival (CSS). Our findings showed that RNU is associated with better OS and CSS in Taiwanese patients with N + UTUC. Common patient characteristics and most cancer characteristics were not related to the outcome. Our results provide new evidence on the efficacy of RNU for patients with N + UTUC, which could alter and guide the direction of future treatment guidelines.
Similar content being viewed by others
Introduction
Urothelial carcinoma of the upper urinary tract (UTUC) is a rare tumor with a high degree of malignancy that affects 1–4 patients in 100,000 people every year1,2. UTUC accounts for 5–10% of total reported urothelial carcinoma (UC)3,4. Similarly, the incidence of metastatic UTUC is relatively low (12–16%) at initial diagnosis1. UTUC has been reported as a multifactorial disease, similar to bladder cancer. Traditional risk factors include smoking, chronic exposure to aristolochic acid, and occupational exposure to carcinogenic chemicals in industries such as dye, rubber, and petrochemicals. As more evidence emerges, genomic factors have also been identified as playing a significant role in many urological cancers5. High-grade invasive UTUCs are commonly associated with regional lymph node metastasis in approximately one-third of cases6. Presence of cancer cells in lymph nodes indicates that the cancer has spread beyond the primary site and may metastasize to other parts of the body. Therefore, node-positive (N+) disease is generally considered as stage IV, an advanced condition requiring appropriate systemic treatment7. Lymph node metastasis is an important prognostic factor in patients with UTUC, resulting in a nearly threefold increase in the risk of cancer-specific mortality. Approximately two-thirds of patients with N + UTUC die of UTUC within five years8.
Radical nephroureterectomy (RNU) with bladder cuff excision is one of the gold standard treatments for clinically localized or locally advanced upper tract urothelial carcinoma (UTUC), whereas systemic chemotherapy or immunotherapy is the preferred treatment for patients with metastatic disease1. In muscle-invasive bladder cancer, radical cystectomy alone is considered an option for patients with cN1 disease who are ineligible for cisplatin-based chemotherapy, as well as for those with cN2–3 disease who achieve downscaling following systemic therapy7. Similarly, lymphadenectomy at the time of RNU is believed to provide therapeutic benefits, particularly in patients with muscle-invasive or locally advanced UTUC9,10. Notably, lymph node involvement is not an absolute contraindication for RNU, and aggressive surgical management has been associated with improved survival outcomes in selected patients11.
Although UTUC and bladder cancer share the same urothelial origin, their treatment paradigms differ, and the clinical evidence of cytoreductive RNU in clinical N + UTUC are limited. Given that systemic therapy is the recommended treatment for these patients, this study aimed to evaluate the potential survival benefit of cytoreductive RNU in a large cohort of clinical N + UTUC patients, utilizing data from a nationwide collaboration.
Methods
This nationwide study was conducted by the Taiwan UTUC Collaboration Group. The group comprises 19 Taiwanese hospitals. The study was approved by the Ethics Committee of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (approval number: 06-X36-105), and all methods were performed in accordance with the principles stated in the Declaration of Helsinki. The requirement for informed consent to participate was waived by the Institutional Review Board of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, due to the retrospective nature of the study. All personal identifiable information was removed before data analysis. We included patients with clinical N + UTUC before initiation of cancer therapy. Exclusion criteria included clinically negative lymph node metastasis; lack of a complete record of clinical staging, treatment, and oncological outcomes; concomitant distant metastases; and receiving palliative care instead of standard therapy. Finally, 288 patients were included in this study. Patients were categorized into two groups according to whether or not they had undergone cytoreductive RNU, namely RNU(+) and RNU(−), respectively.
The following patient characteristics were included in the database: sex, age, American Society of Anesthesiologists (ASA) physical status classification, other chronic illnesses, and smoking status. Tumor-specific parameters and treatments were recorded as follows: tumor size, number, location, grade, and stage; concurrent carcinoma in situ; lymphovascular invasion; histological variants; lymph node status; surgical margin; and adjuvant chemotherapy. Tumor staging was defined according to the 2010 American Joint Committee on Cancer TNM staging system, and tumor grade was determined based on the 2004 World Health Organization classification. RNU, chemotherapy, and other rescue therapy (if needed) were recorded in detail. Combined bladder cancer was defined as either metachronous or synchronous non–muscle-invasive bladder cancer diagnosed prior to RNU; therefore, radical cystectomy was not indicated.
After therapy, patients were regularly followed-up, including physical, laboratory, and radiological examinations, according to standard guidelines. In Taiwan, regular clinical follow-up of cancer patients usually lasts at least 5 years, mainly based on the payment regulations of our national health insurance. If it exceeds five years, we still encourage patients to continue to follow up in the outpatient clinic even if there is no special coverage from health insurance. During the follow-up period, if any patient fails to return for a follow-up visit as agreed, they will be contacted by phone for a follow-up visit. Follow-up treatment of cancer is an area that our country attaches great importance to and is also a necessary condition for the evaluation of medical centers. If the patient’s condition is stable, the follow-up interval will be gradually extended from weeks to months, and then to annual follow-up. If the condition changes during follow-up, it may vary depending on the specific circumstances of each case. In this study, the patients’ clinical visit records were our data source. If a patient was found to be missing regular clinical follow-ups, they were contacted by phone and asked to return to the hospital where the surgery was performed. The patient’s most recent clinic visit was used as the reference point to assess outcomes.
Survival status was confirmed by data stringing with the National Health Insurance Research Database. Cause of death was determined based on the death certificate and/or the last discharge note (if the patient died at the hospital).
Baseline characteristics between groups were compared using Pearson’s chi-square test for categorical variables and independent-samples t-tests for continuous variables. To address baseline imbalances and reduce confounding, we applied propensity score-based overlap weighting, which assigns greater weights to patients with the highest probability of receiving either treatment, thereby emphasizing the region of covariate overlap between groups. Propensity scores were estimated using a logistic regression model that included clinical T and N stages, tumor size, ECOG performance status, and other clinically relevant variables.
The overlap-weighted cohort was used for all adjusted outcome analyses. Overlap weighting was chosen over traditional matching because it retains the full sample, minimizes variance inflation, and better addresses covariate imbalance in observational studies with large baseline differences. Kaplan–Meier survival curves were generated to estimate overall survival (OS) and cancer-specific survival (CSS), and comparisons between groups were made using weighted stratified log-rank tests. Cox proportional hazards models were used to estimate hazard ratios (HRs) for prognostic outcomes, both in univariate and multivariate analyses. Variables with p < 0.1 in univariate analysis were included in multivariate models.
For exploratory endpoints, such as follow-up duration and adjuvant therapy usage, we used stepwise linear regression, with entry and stay criteria set at 0.05 and 0.1, respectively. All statistical tests were two-tailed, and significance was set at p < 0.05. Statistical analyses were performed using IBM SPSS version 26 (IBM Corp., Armonk, NY, USA).
Results
Between July 1988 and June 2022, 5,301 patients underwent RNU in the participating hospitals and were retrospectively analysed. Initial clinically positive lymph nodes were noted in 288 (5.4%) of the 5,301 patients. A total of 288 patients with clinically node-positive UTUC were included, with 239 (83%) undergoing RNU(+) and 49 (17%) receiving chemotherapy alone without surgery. Of those, 127 (44.1%) were males and 161 (55.9%) were females. Among those with N + UTUC and receiving RNU, 147 (61.5%) underwent lymph node dissection, with a postoperative pathological N + rate of 43%, and 139 (58.2%) received combined chemotherapy. The median age was 68 [interquartile range (IQR): 59.7–74.8] years, and the median follow-up period was 19.1 (IQR 8.1–48.6) months. The demographic, clinical, and pathological profiles of the 288 patients stratified by RNU are shown in Table 1.
Before overlap weighting, a total of 288 patients were analysed, including 239 who underwent radical nephroureterectomy [RNU(+)] and 49 who did not [RNU(−)]. The two groups showed several significant differences in baseline characteristics. Patients in the RNU(−) group were more likely to have smaller tumors (< 3 cm; 73.5% vs. 24.7%, p < 0.001), higher clinical T stage (cT3/cT4: 73.5% vs. 45.2%, p = 0.001), and more advanced clinical N stage (cN2/cN3: 65.3% vs. 43.9%, p = 0.01). Additionally, all patients in the RNU(−) group received chemotherapy, with 100% receiving palliative regimens, while the RNU(+) group had a broader distribution of chemotherapy types, including neoadjuvant, adjuvant, and salvage. The RNU(−) group also had a significantly higher UTUC-related mortality rate (69.4% vs. 36%, p < 0.001), while non-UTUC and unknown-cause mortality were similar between groups. These differences highlight a potential selection bias, with patients in the non-surgical group generally presenting with more aggressive disease and poorer prognostic features.
After applying overlap weighting, baseline characteristics between the RNU(+) and RNU(−) groups became well-balanced across most clinical variables, minimizing potential confounding. No statistically significant differences were observed in age (mean age: 66.8 vs. 65.7 years, p = 0.525), sex distribution (male: 42.5% vs. 57.1%, p = 0.111), smoking history (21.2% vs. 31.1%, p = 0.215), end-stage renal disease, history of other malignancies, or tumor location. The distributions of tumor size, clinical T and N stages, and multifocality were also comparable, indicating effective balancing of tumor burden and disease severity between the groups. However, differences in chemotherapy patterns remained due to treatment assignment. All patients in the RNU(−) group received palliative chemotherapy, whereas the RNU(+) group included patients treated with neoadjuvant, adjuvant, and salvage chemotherapy. Despite weighting, mortality outcomes remained significantly different: the RNU(−) group had a substantially higher UTUC-related mortality rate (64.6% vs. 32.3%, p = 0.001), while non-UTUC and unknown-cause mortality were similar between groups.
Univariate Cox regression analysis identified RNU(−), male sex, older age (≥ 70 years), smoking, advanced T and N stages, multifocal disease, and palliative chemotherapy as significant risk factors for worse OS. For CSS, risk factors included RNU(−), male sex, advanced N stage, and chemotherapy, particularly palliative chemotherapy (Table 2).
Multivariate analysis adjusting for stepwise-enrolled covariates revealed that RNU status and smoking were the only independent predictors of both OS and CSS. Specifically, patients who did not undergo RNU had significantly worse OS (HR 2.19, 95% CI 1.44–3.31, p < 0.001) and CSS (HR 1.90, 95% CI 1.05–3.46, p = 0.034), while smoking was also independently associated with poorer OS (HR 1.51, 95% CI 1.01–2.26, p = 0.044) and CSS (HR 1.48, 95% CI 1.01–2.17, p = 0.043). Additionally, chemotherapy with gemcitabine and cisplatin was significantly associated with poorer OS and CSS compared to no chemotherapy (Table 2).
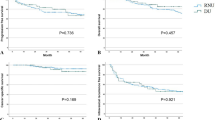
Before applying overlap weighting, the 1-, 3-, and 5-year overall survival (OS) rates in the RNU(+) group were 68.8%, 45.2%, and 38.5%, respectively, whereas the 1-, 2-, and 3-year OS rates in the RNU(−) group were 42.8%, 10.7%, and 0%, respectively. The cancer-specific survival (CSS) rates at 1-, 3-, and 5-year intervals in the RNU(+) group were 88.2%, 61.9%, and 41.5%, while the 1-, 2-, and 3-year CSS rates in the RNU(−) group were 71.7%, 52.4%, and 0%, respectively. After applying overlap weighting, the 1-, 3-, and 5-year OS rates in the RNU(+) group were 69.5%, 46.8%, and 37.9%, compared to 43.5%, 13.9%, and 0% at 1-, 2-, and 3-year intervals in the RNU(−) group. Similarly, the 1-, 3-, and 5-year CSS rates in the RNU(+) group were 84.6%, 58.6%, and 39.3%, while the 1-, 2-, and 3-year CSS rates in the RNU(−) group were 67.5%, 49.3%, and 0%, respectively. Figure 1 summarizes the association between RNU and cancer survival outcomes after overlap weighting.
Kaplan–Meier survival curves comparing overall and cancer-specific survival in clinical N + UTUC patients who received cytoreductive radical nephroureterectomy (RNU[+]) versus those who did not (RNU[–]). RNU Radical nephroureterectomy, UTUC Urothelial carcinomas of the upper urinary tract. Supplement Fig. 1. Kaplan–Meier survival curves comparing patients who received cytoreductive RNU [RNU(+)] vs. those who did not [RNU(–)] among clinical N + UTUC patients, stratified by chemotherapy status.
Discussion
In this propensity score-matched study, RNU for patients with clinical N + UTUC was associated with better oncological outcomes than systemic therapy alone. Moreover, the results provide new evidence for the efficacy of cytoreductive RNU in patients with clinical N + UTUC, suggesting its inclusion in the standard treatment for selected cases (with favorable factors, such as nonmultifocal disease and low ASA status). In addition, other factors, including the location and extent of the cancer and presence of comorbidities, need to be considered before performing RNU. In selected cases, chemotherapy may be recommended before or after surgery to improve outcomes.
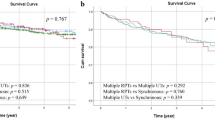
A previous report showed poor survival postoperatively for patients with nodal metastasis12. Patients with clinical N + UTUC should be offered neo-adjuvant chemotherapy as standard therapy; surgery must only be offered after a good response under the current guidelines13. In this study, although 58.2% of patients received chemotherapy, the rate of neoadjuvant chemotherapy was relatively low (13.8%). Among those who received chemotherapy, 29.7% underwent adjuvant chemotherapy, 3.3% as salvage therapy, while 11.3% received it as palliative treatment. While we cannot completely exclude the potential impact of adjuvant or palliative chemotherapy on OS and CSS, its effect should be considered comparable to that of patients who received chemotherapy alone without surgery. Therefore, for patients with N + UTUC, systemic chemotherapy should not be regarded as the sole standard treatment but rather as an adjunct to surgical intervention. In cases without contraindications, radical nephroureterectomy (RNU) should remain a viable alternative to systemic therapy. To further clarify the prognostic impact of RNU, we conducted a subgroup analysis excluding patients who received either neoadjuvant or adjuvant chemotherapy. The survival advantage of RNU persisted in this subset. Patients in the RNU(+) group demonstrated significantly higher OS and CSS compared to those managed without RNU, both in the neoadjuvant-free and adjuvant-free cohorts (p < 0.0001 for OS and p = 0.007 for CSS without neoadjuvant; p < 0.0001 for OS and p = 0.017 for CSS without adjuvant chemotherapy). These results confirm the robustness of our findings and are presented in Supplementary Fig. 1 due to space limitations in the main text. Therefore, chemotherapy does not appear to have had a major influence on the survival differences observed between groups.
In our cohort, 61.5% of patients who underwent RNU also received LND, with a postoperative pathological node-positive (pN+) rate of 43%. This relatively low rate of LND in clinically node-positive (cN+) patients raises concerns about variability in surgical practices, which may influence both staging accuracy and oncologic outcomes. Several factors may explain the discrepancy between clinical and pathological nodal status. First, preoperative imaging may overestimate lymph node involvement due to reactive or inflammatory adenopathy, leading to potential overstaging. Second, the extent of LND may be limited by intraoperative assessments or surgeon discretion, especially in patients with high operative risk or minimal radiologic suspicion. Third, the use of neoadjuvant chemotherapy, although limited, may have contributed to nodal downstaging. Finally, variability in pathological examination protocols and the presence of micrometastases not detected by routine methods may also account for underdetection.
Lymph node density and extranodal extension are powerful predictors of survival outcomes of patients with N + UTUC14,15. Therefore, lymph node dissection has been recommended as part of standard care, even in the absence of clinical evidence of nodal metastases16,17,18. It is vital to simultaneously perform RNU and lymphadenectomy, particularly in patients with nonmetastatic UTUC14,15,16,17,18. Cytoreductive RNU differs from standard RNU in its primary goal and patient selection. While standard RNU is performed as a curative treatment for localized UTUC, cytoreductive RNU is conducted in patients with advanced or metastatic UTUC to reduce tumor burden and potentially improve survival when combined with systemic therapy. Unlike standard RNU, which aims for complete tumor removal, cytoreductive RNU is a palliative or adjunctive strategy intended to enhance the effectiveness of systemic treatments and alleviate symptoms in selected patients19,20.
Importantly, while some cases were managed with palliative RNU to alleviate refractory symptoms such as hematuria, flank pain, or recurrent urinary tract infections, the majority of patients with nodal disease were treated with curative intent. Although the benefit of LND in locally advanced UTUC remains debated, the rate of LND in our cohort (61.5%) is notably higher than in many historical series, which reported rates of only 15–30%21. This finding emphasizes a relatively aggressive surgical approach in our multicenter real-world setting.
The incidence of lymph node metastasis increases with T stage and has been reported to be approximately 60% in locally advanced UTUC22. The observed pN + rate of only 43% in this cN + cohort highlights the limitations of conventional imaging modalities (CT or MRI) in accurately detecting nodal disease. These findings underscore the dual role of RNU in patients with cN + UTUC: as a potential definitive treatment for true node-positive disease and as a diagnostic intervention that enables accurate pathological staging, which is essential for guiding systemic therapy.
Image studies are very important in cancer diagnosis. Over the past decades, advancements in imaging techniques have significantly improved the diagnosis and risk stratification of UTUC23. CT urography remains the standard modality, offering high-resolution imaging with protocol optimization for urothelial evaluation. MR urography serves as a suitable alternative for patients with contraindications to iodinated contrast media. Additionally, 18 F-fluorodeoxyglucose PET/CT may improve detection of metastatic disease and nodal involvement. However, despite these advancements, limitations remain in the detection of micrometastases or distinguishing between malignant and reactive nodes. Standardized imaging protocols and enhanced radiologic interpretation are therefore critical to improving diagnostic accuracy and informing surgical planning in UTUC.
We strongly advocate for RNU combined with lymph node dissection (LND) in patients exhibiting suspicious nodal involvement in upper tract urothelial carcinoma (UTUC) for several compelling reasons. Firstly, RNU with LND provides critical pathological information, facilitating accurate staging and informing subsequent systemic therapy decisions. Secondly, conventional imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) have limited sensitivity in detecting lymph node metastases, particularly in cases involving small, inflammatory, or micrometastatic nodes. While positron emission tomography/computed tomography (PET/CT) offers improved detection capabilities, it is not without limitations, including false positives and negatives due to factors like inflammatory uptake and physiological urinary excretion24. Therefore, histopathological evaluation through LND remains the most reliable method for assessing nodal status and guiding treatment planning. Our findings suggest that cytoreductive RNU offers significant benefits in this patient population. Achieving negative nodal status post-RNU may indicate potential for cure and improved survival, especially when followed by appropriate adjuvant systemic therapy tailored to the definitive pathological T stage. Furthermore, complete surgical resection of metastatic nodes during RNU can lead to curative outcomes in select N + UTUC cases. Even in scenarios where complete nodal resection is unfeasible, cytoreductive RNU provides accurate pathological staging, enabling targeted adjuvant systemic or radiation therapy to address residual micrometastatic disease. Thus, cytoreductive RNU serves as a pivotal component of a multimodal treatment strategy for nodal metastatic UTUC. While a direct comparison between RNU alone and systemic therapy alone could theoretically determine the superior approach, such an intention-to-treat analysis may yield inferior oncological outcomes, rendering it clinically uninformative for this specific patient population.
Overall, based on the preoperative diagnosis, the prognosis of patients with clinical N + UTUC who underwent surgery was significantly better than that of patients who did not. Ouzzane et al.25 reported that 20% of UTUC patients with preoperative clinically negative lymph nodes (cN0) had postoperative N + lymph nodes. The five-year CSS of patients with pathologically negative lymph nodes (pN0) was 81%, whereas that of patients with positive lymph nodes (pN1/2) was 47% (p < 0.001)25. These results indicate the impact of lymph node status and oncological outcomes from RNU treatment on UTUC. Nonetheless, the impact of RNU on the prognosis of patients with N + UTUC remains unclear. Pelcovits et al.12 reported 1-, 5-, and 8-year OS rates of 63.7%, 24.2%, and 18.7%, respectively, after RNU with lymph node dissection for isolated lymph node involvement (pN + M0). Use of neoadjuvant chemotherapy increased during the study period (from 6.7 to 14.2%), whereas that of adjuvant chemotherapy remained stable (42.7–44.3%)12. Compared to Pelcovits et al.12, there was a higher rate of neoadjuvant chemotherapy and lower rate of adjuvant chemotherapy use in our study, but these differences were not significant. OS rates reported in our study were better than those in previous reports, suggesting that for patients with N + UTUC receiving RNU, either preoperative neoadjuvant chemotherapy or postoperative chemotherapy are potentially helpful. Nevertheless, evidence for isolated lymph node metastasis is still lacking. Morton et al.26 reported the outcomes of isolated lymph node metastasis in bladder cancer after surgical resection. The 5- and 7-year OS of the entire cohort were 20% and 17%, respectively. Multifocality was the only tumor characteristic related to the outcomes.
According to the limited evidence, cytoreductive surgery may provide a survival benefit in select patients with metastatic UTUC when combined with systemic therapy in previous reports. A contemporary analysis of the National Cancer Database found that CRS after multiagent chemotherapy significantly improved median OS compared to chemotherapy alone (13.7 vs. 10.8 months), with cytoreductive surgery being an independent predictor of better outcomes27. Similarly, data from the SEER database showed that nephroureterectomy combined with ST significantly prolonged OS in patients with localized primary tumors (T1-T2, median OS: 20 vs. 10 months), but this benefit was not observed in those with more advanced primary tumors (T3-T4, median OS: 12 vs. 10 months)28. These findings suggest that cytoreductive nephroureterectomy may be particularly beneficial in patients with lower-stage primary tumors but may have limited impact in those with more advanced disease. In addition to surgery and chemotherapy, emerging evidence suggests that systemic inflammatory markers, such as the systemic inflammatory response index SIRI and pan-immune inflammatory value PIV, may serve as potential prognostic indicators for bladder cancer. Their potential association with UTUC also warrants further investigation29,30.
In our cohort, cytoreductive RNU was associated with improved OS and CSS in patients with clinically N + UTUC. Given the generally poor prognosis associated with N + UTUC, these findings underscore the importance of a multimodal treatment approach to improve survival outcomes. Our results suggest that RNU should be considered a pivotal diagnostic and therapeutic component in the management of Taiwanese patients with N + UTUC. Incorporating surgical intervention into the treatment algorithm for this high-risk population may confer a meaningful survival benefit and should be taken into account in clinical decision-making.
In our reanalysis using overlap weighting to balance baseline differences, RNU remained a robust and independent predictor of both OS and CSS in patients with node-positive UTUC, reinforcing our central conclusion that cytoreductive RNU provides meaningful survival benefits even in the setting of advanced nodal disease. Notably, smoking status also emerged as an independent prognostic factor associated with worse outcomes in the adjusted Cox models. This observation aligns with prior studies indicating that smoking not only promotes urothelial carcinogenesis but may also impair immune surveillance, increase mutational burden, and reduce responsiveness to systemic therapies31. Rink et al.31 demonstrated that current smokers had significantly worse CSS after RNU, independent of tumor stage or grade, while Chen et al. reported higher risks of recurrence and progression among smokers. While our primary focus remains the survival benefit conferred by RNU, the observed impact of smoking underscores the importance of incorporating smoking cessation strategies into the holistic management of UTUC. These findings support enhanced patient counselling and highlight the potential role of modifiable risk factors in improving long-term outcomes.
In our subsequent analysis, we applied the overlap weighting technique to mitigate the inherent limitations of our retrospective study design. Overlap weighting offers several key advantages. It retains the entire cohort without reducing sample size, ensuring maximal statistical power. Unlike traditional propensity score matching, it avoids arbitrary exclusion of cases and eliminates the risk of poor matches or extreme weights. By emphasizing patients with the greatest clinical equipoise, overlap weighting achieves superior covariate balance between groups and approximates the conditions of a randomized controlled trial (RCT). Furthermore, it minimizes model dependence and reduces variance in effect estimation. Together, these features enhance the internal validity of our findings and provide a more rigorous and interpretable comparison of treatment outcomes.
The present study has several limitations. First, the retrospective nature of this study and its reliance on a database limited the availability of individual patient details. Consequently, we cannot fully exclude the possibility of selection bias. Moreover, the lack of prospective validation further underscores the need for cautious interpretation of our findings. Second, an extensive centralized pathological review could not be conducted due to the multiple institutions involved. However, our preliminary multicenter pathology review indicated moderate agreement between cancers with regard to most cancer-related factors, including UTUC grading and staging32. Third, some therapeutic records were unavailable for patients in the study population. Fourth, there were differences in patients’ underlying health status between groups, which may have affected their survival. Furthermore, routine patient care and surgical practice may have varied between hospitals and/or may have changed over time. Further prospective studies with larger numbers of cases and detailed patient records are required to confirm these results.
Conclusion
This multicenter, retrospective cohort study demonstrated that cytoreductive RNU is independently associated with improved OS and CSS in Taiwanese patients with clinically N + UTUC, even after rigorous adjustment using overlap weighting. These findings highlight the potential survival benefit of incorporating surgical resection into the treatment algorithm for select patients with nodal disease, particularly when combined with systemic therapy.
Importantly, tumor multifocality and smoking history emerged as key prognostic factors, emphasizing the need for individualized treatment planning. While the presence of nodal metastasis has traditionally signaled the need for systemic therapy alone, our results support a multimodal approach, where RNU serves not only as a therapeutic modality but also as a critical staging tool that guides subsequent management.
Given the limitations of preoperative imaging in accurately assessing nodal status, RNU with lymph node dissection may play an essential role in both therapeutic and diagnostic contexts. Prospective studies are warranted to validate these findings and clarify optimal patient selection criteria for cytoreductive surgery in N + UTUC.
Data availability
Data available on request to the corresponding author (Dr. Yao-Chou Tsai, email: tsai1970523@yahoo.com.tw) due to restrictions.
References
Rouprêt, M. et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 79, 62–79. https://doi.org/10.1016/j.eururo.2020.05.042 (2021).
Killock, D. New standard for localized UTUC. Nat. Rev. Clin. Oncol. 17, 275. https://doi.org/10.1038/s41571-020-0354-6 (2020).
van Doeveren, T., van der Mark, M., van Leeuwen, P. J., Boormans, J. L. & Aben, K. K. H. Rising incidence rates and unaltered survival rates for primary upper urinary tract urothelial carcinoma: A Dutch population-based study from 1993 to 2017. BJU Int. 128, 343–351. https://doi.org/10.1111/bju.15389 (2021).
Zeng, S. et al. Impact of previous, simultaneous or intravesical recurrence bladder cancer on prognosis of upper tract urothelial carcinoma after nephroureterectomy: A large population-based study. Transl Androl. Urol. 10, 4365–4375. https://doi.org/10.21037/tau-21-758 (2021).
Russo, P. et al. Relationship between loss of Y chromosome and urologic cancers: new future perspectives. Cancers (Basel). 16, 3766. https://doi.org/10.3390/cancers16223766 (2024).
Zigeuner, R. & Pummer, K. Urothelial carcinoma of the upper urinary tract: surgical approach and prognostic factors. Eur. Urol. 53, 720–731. https://doi.org/10.1016/j.eururo.2008.01.006 (2008).
National Comprehensive Cancer Network. Bladder Cancer. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (2023).
Roscigno, M. et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur. Urol. 53, 794–802. https://doi.org/10.1016/j.eururo.2008.01.008 (2008).
Winer, A. G. et al. Prognostic value of lymph node yield during nephroureterectomy for upper tract urothelial carcinoma. Urol. Oncol. 35, e9. https://doi.org/10.1016/j.urolonc.2016.11.002 (2017).
Seisen, T. et al. Contemporary role of lymph node dissection at the time of radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 35, 535–548. https://doi.org/10.1007/s00345-016-1764-z (2017).
Margulis, V. et al. Outcomes of radical nephroureterectomy: A series from the upper tract urothelial carcinoma collaboration. Cancer 115, 1224–1233. https://doi.org/10.1002/cncr.24135 (2009).
Pelcovits, A. et al. Outcomes of upper tract urothelial carcinoma with isolated lymph node involvement following surgical resection: implications for multi-modal management. World J. Urol. 38, 1243–1252. https://doi.org/10.1007/s00345-019-02897-2 (2020).
EAU-ASCO Penile Cancer Guidelines. Presented at the EAU Annual Congress Milan (2023).
Fajkovic, H. et al. Prognostic value of extranodal extension and other lymph node parameters in patients with upper tract urothelial carcinoma. J. Urol. 187, 845–851. https://doi.org/10.1016/j.juro.2011.10.158 (2012).
Raza, S. J. et al. Lymph node density for stratification of survival outcomes with node positive upper tract urothelial carcinoma. Can. J. Urol. 26, 9852–9858 (2019).
Roscigno, M. et al. Lymphadenectomy at the time of nephroureterectomy for upper tract urothelial cancer. Eur. Urol. 60, 776–783. https://doi.org/10.1016/j.eururo.2011.07.009 (2011).
Lughezzani, G. et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology 75, 118–124. https://doi.org/10.1016/j.urology.2009.07.1296 (2010).
Nazzani, S. et al. Rates of lymph node invasion and their impact on cancer specific mortality in upper urinary tract urothelial carcinoma. Eur. J. Surg. Oncol. 45, 1238–1245. https://doi.org/10.1016/j.ejso.2018.12.004 (2019).
Seisen, T. et al. Efficacy of systemic chemotherapy plus radical nephroureterectomy for metastatic upper tract urothelial carcinoma. Eur. Urol. 71, 714–718. https://doi.org/10.1016/j.eururo.2016.11.012 (2017).
Baard, J. et al. Contemporary patterns of presentation, diagnostics and management of upper tract urothelial cancer in 101 centres: the clinical research office of the endourological society global upper tract urothelial carcinoma registry. Curr. Opin. Urol. 31, 354–362. https://doi.org/10.1097/MOU.0000000000000899 (2021).
Lee, H. Y. et al. Is lymph node dissection necessary during radical nephroureterectomy for clinically node-Negative upper tract urothelial carcinoma?? A Multi-Institutional study. Front. Oncol. 12, 791620. https://doi.org/10.3389/fonc.2022.791620 (2022).
Kikuchi, E. et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J. Clin. Oncol. 27, 612–618. https://doi.org/10.1200/JCO.2008.17.2361 (2009).
Nakai, H. et al. Imaging of upper tract urothelial carcinoma. Radiographics 44, e240056. https://doi.org/10.1148/rg.240056 (2024).
Anjos, D. A. et al. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J. Nucl. Med. 48, 764–770. https://doi.org/10.2967/jnumed.106.036350 (2007).
Ouzzane, A. et al. The impact of lymph node status and features on oncological outcomes in urothelial carcinoma of the upper urinary tract (UTUC) treated by nephroureterectomy. World J. Urol. 31, 189–197. https://doi.org/10.1007/s00345-012-0983-1 (2013).
Morton, E. et al. Urothelial carcinoma of the bladder with isolated lymph node metastasis: natural history and outcomes following surgical resection. Urol. Oncol. 41, e7 https://doi.org/10.1016/j.urolonc.2022.11.003 (2023).
Pollock, G. et al. Postoperative and survival outcomes after cytoreductive surgery in the treatment of metastatic upper tract urothelial carcinoma. Urology 153, 244–249. https://doi.org/10.1016/j.urology.2021.01.017 (2021).
Morra, S. et al. Survival benefit of nephroureterectomy in systemic therapy exposed metastatic upper tract urinary urothelial carcinoma patients. World J. Urol. 42, 343. https://doi.org/10.1007/s00345-024-05057-3 (2024).
Russo, P. et al. SIRI as a biomarker for bladder neoplasm: utilizing decision curve analysis to evaluate clinical net benefit. Urol. Oncol. https://doi.org/10.1016/j.urolonc.2025.01.007 (2025).
Russo, P. et al. Comparison of PIV and other immune inflammation markers of oncological and survival outcomes in patients undergoing radical cystectomy. Cancers (Basel). 16, 651. https://doi.org/10.3390/cancers16030651 (2024).
Rink, M. et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur. Urol. 63, 1082–1090. https://doi.org/10.1016/j.eururo.2013.01.042 (2013).
Chang, C. H. et al. Impact of pathology review in adverse histological characteristics and pT stages of upper tract urothelial cancer in a multicenter study. Front. Oncol. 11, 757359. https://doi.org/10.3389/fonc.2021.757359 (2021).
Acknowledgements
The authors would like to thank all members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration group: Allen W. Chiu, Bing-Juin Chiang, Chao-Hsiang Chang, Chao-Yuan Huang, Cheng-Huang Shen, Cheng-Kuang Yang, Cheng-Ling Lee, Chen-Hsun Ho, Che-Wei Chang, Chia-Chang Wu, Chieh-Chun Liao, Chien-Hui Ou, Chih-Chen Hsu, Chih-Chin Yu, Chih-Hung Lin, Chih-Ming Lu, Chih-Yin Yeh, Ching-Chia Li, Chi-Ping Huang, Chi-Rei Yang, Chi-Wen Lo, Chuan-Shu Chen, Chung-Hsin Chen, Chung-You Tsai, Chung-Yu Lin, Chun-Hou Liao, Chun-Kai Hsu, Fang-Yu Ku, Hann-Chorng Kuo, Han-Yu Weng, Hao-Han Chang, Hong-Chiang Chang, Hsiao-Jen Chung, Hsin-Chih Yeh, Hsu-Che Huang, Ian-Seng Cheong, I-Hsuan Alan Chen, Jen-Kai Fang, Jen-Shu Tseng, Jen-Tai Lin, Jian-Hua Hong, Jih-Sheng Chen, Jungle Chi-Hsiang Wu, Kai-Jie Yu, Keng-Kok Tan, Kuan-Hsun Huang, Kun-Lin Hsieh, Lian-Ching Yu, Lun-Hsiang Yuan, Hao-Lun Luo, Marcelo Chen, Min-Hsin Yang, Pai-Yu Cheng, Po-Hung Lin, Richard Chen-Yu Wu, See-Tong Pang, Shin-Hong Chen, Shin-Mei Wong, Shiu-Dong Chung, Shi-Wei Huang, Shuo-Meng Wang, Shu-Yu Wu, Steven Kuan-Hua Huang, Ta-Yao Tai, Thomas Y. Hsueh, Ting-En Tai, Victor Chia-Hsiang Lin, Wei-Chieh Chen, Wei-Ming Li, Wei-Yu Lin, Wen-Hsin Tseng, Wen-Jeng Wu, Wun-Rong Lin, Yao-Chou Tsai, Yen-Chuan Ou, Yeong-Chin Jou, Yeong-Shiau Pu, Yi-Chia Lin, Yi-Hsuan Wu, Yi-Huei Chang , Yi-sheng Lin, Yi-Sheng Tai, Yu-Khun Lee, Yuan-Hong Jiang, Yu-Che Hsieh, Yu-Chi Chen, Yu-Ching Wen, Yung-Tai Chen, Zhe-Rui Yang.
Author information
Authors and Affiliations
Contributions
Conceptualization, Tsai YC; methodology, Tsai YC; formal analysis and data curation, Wu SY, Huang CP, Chang CH, Huang SK, Tsing WH, Li WM, Ke HL, Chen IH, Lin JT, Tseng JS, Lin WR, Jiang YH, Lee YK, Wang SS, Li JR, Chen WC, Tai TE, Lin PH, Hsueh TY, Li HK, Chen PC, Huang CY, Chen YT, Wu CC, Huang HC, Lin WY, Weng HY, Lo CW and Tsai YC; investigation, Wu SY, Huang CP, Chang CH, Huang SK, Tsing WH, Li WM, Ke HL, Chen IH, Lin JT, Tseng JS, Lin WR, Jiang YH, Lee YK, Wang SS, Li JR, Chen WC, Tai TE, Lin PH, Hsueh TY, Li HK, Chen PC, Huang CY, Chen YT, Wu CC, Huang HC, Lin WY, Weng HY, Lo CW and Tsai YC; writing—original draft, Wu SY; writing—review and editing, Tsai YC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Shu-Yu Wu is a member of the editorial team, but he was not involved in the review process of this article.All other authors have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, SY., Huang, CP., Chang, CH. et al. Cytoreductive nephroureterectomy for treatment of upper urinary tract urothelial carcinoma initially diagnosed as node-positive. Sci Rep 15, 29481 (2025). https://doi.org/10.1038/s41598-025-14947-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14947-4