Abstract
The textbook outcome (TO) is a short-term outcome that integrates postoperative outcomes to evaluate the quality of surgery. In gastric cancer surgery, most TO data have been reported from Western countries. We aimed to analyze TO in Korea, where gastric cancer is prevalent and evaluate its effect on long-term survival. Based on the parameters defined by the Dutch Upper Gastrointestinal Cancer Audit (DUCA), we evaluated the TO, 5-year overall survival (OS), and independent prognostic factors stratified according to the TNM stage. The TO was achieved in 74.6% patients. Significant differences were observed in the 5-year OS rate between groups achieving TO and non-TO groups regardless of stage. The TO and ≥ 30 retrieved lymph nodes was an independent prognostic factor in patients with stage II/III tumors. The median number of retrieved lymph nodes required for D2 dissection increased depending on the tumor stage, from 38 in stage IA, whereas in distal and total gastrectomy D2 lymph node dissection was 44 and 55 in stages II and III, respectively. Therefore, evaluation of more than 30 lymph nodes should be considered to achieve optimal radical gastric cancer surgery.
Similar content being viewed by others
Introduction
Cancer is a leading cause of death worldwide and poses significant clinical, economic, and social problems1. Patients, society, healthcare providers, and hospitals are all interested in the assessment of surgical quality2,3. Traditionally, the quality of cancer surgery has been evaluated based on morbidity, mortality, and oncological outcomes4,5. However, the application of multiple rather than individual parameters for the assessment of oncological surgery quality has been proposed. The concept of a “textbook outcome” (TO), which integrates parameters reflecting the short-term outcome of surgery, was first proposed for colon cancer in the Netherlands6. This concept has gradually been applied to other surgical interventions, and the parameters have then been adjusted accordingly7,8,9,10.
The Dutch Upper Gastrointestinal Cancer Audit (DUCA) group initially suggested 10 parameters for gastric cancer surgery in 2017. They showed that the variation between hospitals and TO improved from 22.5% in 2011 to 40.0% in 201411. The Population Registry of Esophageal and Stomach Tumors of Ontario (PRESTO) also reported a close relationship between TO and survival12,13.
Several studies have evaluated the application of TO for gastric cancer in Western countries11,12,13,14,15,16. However, only a few reports derive from Eastern Asia, where gastric cancer is prevalent17,18. Thus, our aim was to evaluate the application of the TO following gastric surgery in a high-volume center in Korea using the original parameters suggested by the DUCA group and its association with long-term survival.
The DUCA group suggests the excision of a minimum of 15 lymph nodes in the resected specimen as a parameter of TO. According to the current Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) tumor–node–metastasis (TNM) classification (the 8th edition was used as a reference in this study), the evaluation of a minimum of 15 lymph nodes is recommended for N staging19,20. D2 lymph node dissection has been accepted as a standard procedure for locally advanced gastric cancer21,22,23. However, the minimal number of retrieved lymph nodes can influence prognosis and stage migration, and some studies have suggested that it would be desirable to excise more nodes24,25,26,27. In the present study, our aim was to investigate whether the evaluation of 15 excised lymph nodes may represent a suitable parameter for the assessment of surgical quality as a measure of successful TO.
Methods
Patients and study design
This study included all consecutive patients aged ≥ 18 years old who underwent gastrectomy for primary gastric adenocarcinoma at the Gastric Cancer Center at Seoul National University Hospital (SNUH) between January 2014 and December 2016, and included long-term survival data. Patients with metastatic or remnant gastric cancer were excluded. This was a retrospective cohort study; however, all complications were prospectively assessed and collected at weekly meetings at the SNUH28,29. Furthermore, we obtained long-term survival data from the national office of Statistics Korea.
The study was approved by the Institutional Review Board of Seoul National University Hospital, Korea (No. 2006-173-1135). The need for informed consent was waived by the Institutional Review Board of Seoul National University Hospital. No identifying information or images of participants are included in this manuscript. All methods were performed in accordance with relevant guidelines and regulations, and with the Declaration of Helsinki.
Original TO parameters
The original 10 parameters of the DUCA group were used: (a) curative resection: radical resection according to the surgeon after surgery; (b) absence of intraoperative complications: no transfusion, no organ injury or open conversion during operation; (c) tumor-negative resection margins confirmed by a pathologist; (d) at least 15 lymph nodes retrieved; (e) no severe postoperative complications according to the Clavien–Dindo classification; (f) no interventions or reoperation within 30 days after surgery; (g) no readmission to the ICU within 30 days after surgery; (h) no prolonged hospital stay 21 days or less; (i) no mortality within 30 days post-procedure; and (j) no readmission within 30 days after discharge.
The TO was achieved when all indicators were met. At our institution, surgery was performed by four surgeons during the study period. All types of surgical interventions, such as open, laparoscopic, and robotic surgery, were included. Examination of flash-frozen specimens of the proximal and distal resection margins was performed routinely following stomach resection of all regular surgeries. Tumor-negative resection margins were defined when the final pathology report reported the absence of tumor cells at the resection margin. Postoperative complications of grade II or higher were considered severe.
The TO and non-TO was compared for all patients and for patients of stages II/III (considering that most gastric cancer in Korea is diagnosed at the early stage. The baseline characteristics of the TO and non-TO groups were evaluated to determine differences in clinical characteristics. We compared the long-term survival between the TO and non-TO groups and investigated whether TO and clinicopathological variables were independent factors.
Determination of the required number of lymph nodes resected per patient to satisfy D2 dissection outcomes.
To obtain the required number of lymph nodes resected per patient to satisfy D2 dissection, we evaluated the number of lymph nodes retrieved in each stage. For patients with stage II/III gastric cancer, in addition to the total number of lymph nodes retrieved from D2 dissection, we estimated the number of lymph nodes retrieved from D1 dissection by totaling the number of lymph nodes presumably resected from all N1 level stations22. This calculation was possible because the surgical specimen handling in the operating room included dividing lymph nodes according to the topographic classification of lymph node stations and the pathology report also includes data on topographic lymph node metastasis.
Statistical analysis
Statistical analyses were performed using R software (v.4.2.2, Vienna, Austria). The TO and non-TO were compared using the Student’s t-test for continuous variables and the chi-square or Fisher’s exact test for categorical variables, as appropriate. The adjusted Kaplan–Meier curves and log-rank tests were analyzed to compare the 5-year OS between the TO and non-TO groups for all patients, for stage I patients, and for stage II/III patients. All variables with significant p-values from the univariate analysis were included in a multivariate model to determine the association between 5-year OS and the clinical variables. Statistical significance was set at P < 0.05.
Results
TO by original parameters
In total, 2,379 patients underwent gastrectomy for primary gastric cancer at the SNUH between January 2014 and December 2016. After excluding 125 patients with stage IV cancer and 59 patients with remnant gastric cancer, 2,195 patients were included in the analysis.
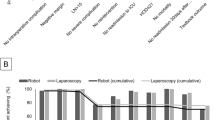
A TO was achieved in 1,637 (74.6%) patients: 1,204 (77.6%) in stage I and 433 (67.2%) in stages II/III. The results of all parameters included in the TO group are shown in Fig. 1. At all stages, the most significant single violating parameter in achieving TO was the occurrence of severe postoperative complications, which occurred in 22.6% of all patients, in 19.5% of stage I patients, and in 30.1% of patients at stages II/III.
Baseline characteristics of patients
Characteristics of the TO and non-TO groups are shown in Table 1. Patients achieving TO and non-TO were stratified into stages I and II/III for comparison. Regardless of the stage, significant differences in sex and operation type between both groups was observed. Furthermore, combined resection, tumor location, and size were significantly different between the TO and non-TO groups, except for tumor stage I.
Five-year OS between TO and non-TO
For all patients, satisfactory 5-year postoperative survival data was available. The survival analysis of all patients, and for patients with stage I and stage II/III tumors, is shown in Fig. 2. The 5-year OS rates in TO and non-TO patients were as follows: 89.6% vs. 79.6% (p < 0.001) for all patients, 96.3% vs. 91.6% for patients with stage I tumor (p < 0.001), and 73.2% vs. 62.1% (p = 0.002) for those with stage II/III tumor.
Multivariate analysis of clinicopathologic factors and 5-year OS
Regardless of the TNM stage, TO and age were independent variables for OS (Table 2). Sex was an additional independent factor only for patients at stage I. In patients with stage II/III tumors, the lymph node dissection level, pathological T and N stages, number of retrieved lymph nodes, and size of tumor were associated with OS.
Required number of lymph nodes to be resected per patient to satisfy successful D2 dissection
The median number of retrieved lymph nodes (interquartile range) for each patient in our study was 38.0 (30.0–48.0) for stage IA, 41.0 (33.0–51.0) for stage IB, 42.0 (31.0–55.0) for stage IIA, 44.0 (33.0–56.0) for stage IIB, 45.0 (37.0–57.0) for stage IIIA, 51.0 (40.0–63.0) for stage IIIB, and 53.5 (43.0–61.0) for patients with stage IIIC tumors (Fig. 3). No patients with stage II/III tumors had < 15 retrieved lymph nodes.
The median number of lymph nodes retrieved from D2 dissection in patients with stage II/III tumors was 44.0 (35.0–55.0) for distal gastrectomy and 55.0 (45.0–68.0) for total gastrectomy. (Table 3) The median number of lymph nodes retrieved from presumed D1 dissection in patients with stage II/III tumors was 31.0 (24.0–40.0) for distal gastrectomy and 38.0 (30.0–48.0) for total gastrectomy.
Discussion
Several studies evaluating TO have been conducted, but different parameters have been used12,13,14,15,16. The PRESTO group did not include any parameters of curative resection or intra-operative events compared with the DUCA group12,13. In addition, studies from East Asia excluded other parameters; for instance, the definition of severe complications was Clavien–Dindo grade III or higher rather than grade II or higher30,31. Since the purpose of the TO is that of quality assessment, a unified, standardized parameter should be analyzed. For a fair comparison with studies using Western data, we applied the definition of DUCA first proposed for gastric cancer surgery.
The percentage of TO ranged from 22 to 41.1% in studies from Western countries. In our study, a TO of 74.6% was observed for all patients, and 67.2% was achieved for patients with stage II/III tumors. These results may have been influenced by the many differences between western and eastern countries. In Korea, because there are relatively more early-stage gastric cancer patients, it is easier to achieve oncologic parameters such as curative resection and tumor-negative resection margins. Compared with the Western countries, body weight is relatively lower, making it easier to achieve parameters related to complications. Lastly, similar to the previous results showing that high volume centers have higher TO, our hospital, with an extensive number of patients who undergo gastric cancer surgery, seemed to have achieved a higher rate of TO. Considering the various factors that can affect TO, it is not appropriate to simply compare our results with those of Western countries; however, analyzing the TO of a high volume center in area where gastric cancer is prevalent may be meaningful for comparisons in future studies.
In our study, the parameter “no severe complications” was not significantly different from that reported in studies from Western countries32. This parameter was the most significant single violating parameter to achieve TO in our patients. In our patients, the parameters “no intervention,” “no readmission to ICU,” “no prolonged hospital stay,” “no postoperative mortality,” and “no readmission after discharge” were associate with more severe complications. This, reducing the incidence of severe complications is necessary to achieve TO.
Other parameters regarding oncological results, including curative resection and tumor-negative resection margins, were achieved in most stage II/III patients (98.91% for “curative resection” and 97.20% for “tumor-negative resection margins”). Although this may be attributed to the higher number of cases of early stages tumors in which curative resection is possible. For curative resection, we performed a careful preoperative evaluation of the resectability (sometimes involving a consultation with multi-disciplinary team, if necessary). Furthermore, we routinely verified the frozen resection margins after stomach transection, which most likely improved the oncological outcome parameters of TO33,34.
Similar to previous studies, our results showed significant differences in 5-year OS between the TO and non-TO groups, regardless of tumor stage. (12–16) In the multivariate analysis of clinicopathological factors, TO was an independent factor that affected the 5-year OS in all patients (stages I to III).
According to the DUCA group, retrieving ≥ 15 lymph nodes was the most significant parameter violated in failure to achieve TO. This result (57.1%) was clearly different from ours in which 100% of patients had stage II/III tumors. It is well known that the pathologist’s efforts to identify lymph nodes in the specimen can result variability in the number of lymph nodes identified35. According to well-designed randomized control trials for advanced gastric cancer, KLASS 02 from Korea and CLASS 01 from China that compared the results of laparoscopic versus open distal gastrectomy with D2 dissection for advanced gastric cancer, the mean number of retrieved lymph nodes was 46.6–47.4 in KLASS-02 compared with 36.1–36.9 in CLASS 0136,37. The differences in the results in these studies may be due to the variable pathological evaluation protocols of lymph nodes in these two countries. However, the number of retrieved lymph nodes in KLASS-02 was not much different from our data.
As shown in Fig. 3, number of retrieved lymph node increased as the tumor stage increased. The median number of dissected lymph node for stage IA expected to be dissected was a minimum of 38 and the 25% interquartile value of lymph nodes retrieved in stage IA was 30. Additionally, as shown in Table 3, the number of lymph nodes located in the level 1 nodes (N1 level stations) was 31.0 for distal gastrectomy and 38.0 for total gastrectomy, respectively. Therefore, 15 retrieved lymph nodes would be insufficient to support the radicality of gastrectomy. Additionally, our multivariate analysis revealed significantly improved survival outcomes after retrieval of ≥ 30 lymph nodes. This finding is consistent with several previous studies recommending the retrieval of at least 25 lymph nodes to avoid stage migration and improve oncological outcomes.
We believe that the number of retrieved lymph nodes is affected by the dissection of operator and the evaluation of the pathologist. Furthermore, differences between our results and other studies from Western countries and China may have originated from the pathology evaluation. In our study, the TO and the number of retrieved lymph nodes affected the OS. An accurate evaluation of lymph nodes not only by surgeon but also by the pathologist is expected to increase the TO, which in turn, increases survival, thereby improving the quality of gastric cancer surgery.
A limitation of this study was that this was a single-center retrospective cohort study. The TO is an indicator used to evaluate the quality of surgery, and it would be necessary to compare the TO across hospitals in Korea. We used single-center data for accurate analysis of TO, parameters and OS, therefore, we expect to be able to collect and collect data from multiple centers for a future study. In particular, the high number of retrieved lymph nodes observed in our cohort may reflect the characteristics of a high-volume center and may not be generalized to institutions with different surgical volumes or practices. In addition, we acknowledge that direct comparison of the TO with Western countries is difficult due to the differences in patient populations. Nonetheless, this study provides data on the TO results in area where gastric cancer is highly prevalent and the TO was stratified according to tumor stage for comparison of advanced gastric cancer. To overcome differences in patient distribution, we relied on a benchmark study involving multiple centers from all over the world. Although our study population did not receive immunotherapy, we acknowledge that the increasing use of immune checkpoint inhibitors may influence the role of lymph node dissection in the future. Further research is needed to evaluate this potential interaction.
Conclusion
We achieved a TO of 74.6% for all patients with gastric cancer, and a TO of 77.6% for patients with stage I tumors and 67.2% for those with stage II/III using the DUCA criteria. The TO was an independent prognostic factor for 5-year OS. Evaluation of more lymph nodes should be considered to achieve optimal radical gastric cancer surgery.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to patient privacy concerns, but are available from the corresponding author on reasonable request.
References
Mattiuzzi, C. & Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob Health. 9, 217–222 (2019).
Dijs-Elsinga, J. et al. Choosing a hospital for surgery: the importance of information on quality of care. Med. Decis. Mak. 30, 544–555 (2010).
Lohr, K. N. (ed) Medicare: A Strategy for Quality Assurance: Volume II Sources and Methods (National Academies, 1990).
Baiocchi, G. L. et al. International consensus on a complications list after gastrectomy for cancer. Gastric Cancer. 22, 172–189 (2019).
Learn, P. A. & Bach, P. B. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med. Care. 48, 1041–1049 (2010).
Kolfschoten, N. E. et al. Focusing on desired outcomes of care after colon cancer resections: hospital variations in ‘textbook outcome’. Eur. J. Surg. Oncol. 39, 156–163 (2013).
van Roessel, S. et al. Textbook outcome: nationwide analysis of a novel quality measure in pancreatic surgery. Ann. Surg. 271, 155–162 (2020).
Merath, K. et al. A multi-institutional international analysis of textbook outcomes among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma. JAMA Surg. 154, e190571 (2019).
Gorgec, B. et al. Assessment of textbook outcome in laparoscopic and open liver surgery. JAMA Surg. 156, e212064 (2021).
Ten Berge, M. G. et al. Textbook outcome as a composite outcome measure in non-small-cell lung cancer surgery. Eur. J. Cardiothorac. Surg. 59, 92–99 (2021).
Busweiler, L. A. et al. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br. J. Surg. 104, 742–750 (2017).
Levy, J. et al. Gastrectomy case volume and textbook outcome: an analysis of the population registry of esophageal and stomach tumours of Ontario (PRESTO). Gastric Cancer. 23, 391–402 (2020).
Levy, J. et al. Textbook outcome and survival in patients with gastric cancer: an analysis of the population registry of esophageal and stomach tumours in Ontario (PRESTO). Ann. Surg. 275, 140–148 (2022).
Dal Cero, M. et al. Textbook outcome and survival after gastric cancer resection with curative intent: a population-based analysis. Eur. J. Surg. Oncol. 48, 768–775 (2022).
Sedlak, K. et al. Union is strength: textbook outcome with perioperative chemotherapy compliance decreases the risk of death in advanced gastric cancer patients. Eur. J. Surg. Oncol. 48, 356–361 (2022).
Cibulas, M. A. et al. Impact of textbook oncologic outcome attainment on survival after gastrectomy: a review of the National cancer database. Ann. Surg. Oncol. 29, 8239–8248 (2022).
Park, S. H. et al. Epidemiology of gastric cancer in korea: trends in incidence and survival based on Korea central cancer registry data (1999–2019). J. Gastric Cancer. 22, 160–168 (2022).
Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075 (2018).
Amin, M. B. et al. AJCC Cancer Staging Manual (Springer International Publishing, 2018).
Waddell, T. et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 40, 584–591 (2014).
Kim, T. H. et al. Korean practice guidelines for gastric cancer 2022: an evidence-based, multidisciplinary approach. J. Gastric Cancer. 23, 3–106 (2023).
Japanese Gastric Cancer A. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 26, 1–25. (2023).
NCC Network. Gastric Cancer. (2023). Version 1. (2023). Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
Okajima, W. et al. Prognostic impact of the number of retrieved lymph nodes in patients with gastric cancer. J. Gastroenterol. Hepatol. 31, 1566–1571 (2016).
Liu, Y. Y. et al. Does a higher cutoff value of lymph node retrieval substantially improve survival in patients with advanced gastric cancer?—Time to embrace a new digit. Oncologist 22, 97–106 (2017).
Biondi, A. et al. Does a minimum number of 16 retrieved nodes affect survival in curatively resected gastric cancer? Eur. J. Surg. Oncol. 41, 779–786 (2015).
Kong, S. H. et al. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann. Surg. 255, 50–58 (2012).
Lee, K. G. et al. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien-Dindo system. J. Gastrointest. Surg. 18, 1269–1277 (2014).
Kim, T. H. et al. The comprehensive complication index (CCI) is a more sensitive complication index than the conventional Clavien-Dindo classification in radical gastric cancer surgery. Gastric Cancer. 21, 171–181 (2018).
Chen, J. Y. et al. Textbook outcome, chemotherapy compliance, and prognosis after radical gastrectomy for gastric cancer: a large sample analysis. Eur. J. Surg. Oncol. 48, 2141–2148 (2022).
Roh, C. K. et al. Textbook outcome and survival of robotic versus laparoscopic total gastrectomy for gastric cancer: a propensity score matched cohort study. Sci. Rep. 11, 15394 (2021).
Baiocchi, G. L. et al. Incidence and grading of complications after gastrectomy for cancer using the GASTRODATA registry: a European retrospective observational study. Ann. Surg. 272, 807–813 (2020).
Watanabe, A. et al. Intraoperative frozen section analysis of margin status as a quality indicator in gastric cancer surgery. J. Surg. Oncol. 127, 66–72 (2023).
Berlth, F. et al. Prognostic impact of frozen section investigation and extent of proximal safety margin in gastric cancer resection. Ann. Surg. 272, 871–878 (2020).
Lemmens, V. E. et al. Lymph node examination among patients with gastric cancer: variation between departments of pathology and prognostic impact of lymph node ratio. Eur. J. Surg. Oncol. 37, 488–496 (2011).
Lee, H. J. et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann. Surg. 270, 983–991 (2019).
Yu, J. et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA 321, 1983–1992 (2019).
Acknowledgements
This study was supported by a grant from a research fund from Mario & Chunim Lim and Bokson Yang at Seoul National University Hospital (grant number 3020200160) and the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (grant No. HA21C0036).
Author information
Authors and Affiliations
Contributions
YSC, SRK, HKY: study design, performance of the study, data collection, statistical anaylsis, interpretation and manuscript writing, JSK: data collection, manuscript writing; YJK, YSS, HSL, WHK: performance of the study; SHK, DJP, HJL: performance of the study and interpretation, All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cho, YS., Kim, S., Kim, J. et al. Textbook outcome of gastric cancer surgery and lymph node evaluation as its parameter to improve long-term survival. Sci Rep 15, 34159 (2025). https://doi.org/10.1038/s41598-025-14971-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14971-4