Abstract
This meta-analysis aimed to evaluate the efficacy of remimazolam, a newly introduced anesthetic, in preventing postoperative nausea and vomiting (PONV) compared to other anesthetics. A systematic search of studies published up to January 2024 in PubMed, Cochrane Library, and Embase was conducted to identify randomized controlled trials (RCTs) comparing remimazolam with other anesthetics in terms of PONV. Following data synthesis, outcomes were reported as the relative risk (RR) with 95% confidence interval (95% CI). Subgroup analyses were performed according to the type of comparator anesthetic. A total of 50 RCTs involving 9,193 participants were included. The results showed no significant difference in PONV incidence between remimazolam and other anesthetic agents (RR: 0.96, 95% CI: 0.80–1.13, P = 0.607). However, subgroup analysis demonstrated that a lower incidence of overall PONV was associated with remimazolam compared with inhalation anesthetics (n = 363; RR: 0.50; 95% CI: 0.34–0.73; P < 0.001). Conversely, a higher incidence of postoperative vomiting was associated with remimazolam compared with propofol (n = 3,860; RR: 1.41; 95% CI: 1.05–1.90; P = 0.024). Overall, there was no significant difference between remimazolam and other anesthetic agents in preventing PONV. However, subgroup analysis revealed that remimazolam was more effective than inhalation anesthetics in mitigating PONV, and it was inferior to propofol in preventing postoperative vomiting.
Similar content being viewed by others
Introduction
Remimazolam is a recently introduced benzodiazepine anesthetic that targets γ-aminobutyric acid type A (GABA_A) inhibitory neurotransmitter receptors in the central nervous system (CNS)1. Structurally similar to midazolam, remimazolam differs by featuring a distinctive carboxylic ester linkage, which allows for dose-dependent hydrolysis by non-specific tissue esterases, bypassing hepatic metabolism as required by midazolam1,2. Additionally, compared with the metabolites of midazolam, those of remimazolam have a 400-fold lower affinity for GABA_A receptors, resulting in pharmacological inactivity1.
The organ-independent metabolism of remimazolam, along with the production of inactive metabolites and its reversibility, results in a favorable pharmacokinetic profile characterized by an ultra-short half-life and a reduced risk of prolonged sedation, facilitating enhanced recovery after surgery (ERAS)1. These properties, combined with an increasing preference for outpatient surgical procedures, have driven the rapid global adoption of remimazolam, a trend expected to continue1.
Postoperative nausea and vomiting (PONV) is a prevalent and distressing complication, affecting 10–30% of surgical patients, with incidence rates as high as 80% in high-risk populations3. PONV is associated with postoperative complications, increased healthcare costs, and extended hospital stays, thus underscoring the importance of PONV prevention, particularly within ERAS protocols in contemporary surgical care4. In this context, the selection of anesthetics that can contribute to PONV prophylaxis is critical in clinical settings5.
Previous studies have demonstrated that benzodiazepine administration before anesthesia, during induction, or at the end of surgery can significantly reduce PONV4,6,7. In particular, midazolam has been shown to be effective in managing acute refractory emesis during chemotherapy4,6,7. However, compared with other benzodiazepines, the effect of remimazolam on PONV remains unclear. Therefore, in this study, we performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of remimazolam compared with other anesthetic agents in preventing PONV.
Methods
Search strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered in the PROSPERO database (ID CRD42024511038). Two independent investigators (S.J. and H.J.) systematically searched PubMed, Cochrane Library, and Embase for relevant studies published up to January 8, 2024, using the search strategy outlined in Supplemental Table S1−3.
Study selection
After removing duplicates, two reviewers (S.J. and H.R.) independently screened the titles and abstracts of identified articles to assess their eligibility. The inclusion criteria were: (1) trials comparing “remimazolam (remimazolam group)” with “other anesthetic agents (comparator group)” in the context of general anesthesia or sedation, (2) trials involving adult patients (≥ 18 years), and (3) trials reporting the incidence of postoperative nausea, postoperative vomiting, or PONV as primary or secondary outcomes. The initial search included both RCTs and observational studies; however, only RCTs were included in the final analysis to ensure greater result clarity. The exclusion criteria were: (1) studies that used a placebo as the control group and (2) articles not published in English. Subsequently, the reviewers (S.J. and H.R.) independently conducted full-text reviews and data extraction. Disagreements regarding study inclusion or exclusion were resolved through consensus or by consultation with a third reviewer (C.K.).
Outcome and risk-of-bias assessment
Data were extracted directly from the original text, tables, or figures of the articles. The following variables were collected: author, publication year, journal, country, study design, patient demographics, surgical procedure, type of anesthesia (sedation or general anesthesia), type and dosage of anesthetics, sample size, incidence of outcomes (postoperative nausea, postoperative vomiting, or PONV), and timing of outcome measurement.
The primary outcome was PONV incidence in the remimazolam group compared with the comparator group. Given that few patients experience vomiting without nausea, the incidence rates of postoperative nausea and PONV are generally comparable8. Therefore, when only nausea was reported without data on PONV, the incidence of PONV was regarded as equivalent to that of postoperative nausea8. In cases where only postoperative vomiting was reported, this was interpreted as the incidence of PONV9. Most studies did not distinguish between nausea and vomiting, instead reporting them collectively as PONV. However, in trials that separately reported postoperative nausea or vomiting, these outcomes were analyzed independently in subgroup analyses.
In one study, the total number of PONV events per patient was reported on a scale of 0 (none) to 310. An occurrence of 0 was regarded as the absence of PONV, whereas occurrences ranging from 1 to 3 were defined as the presence of PONV events for our analysis. For studies reporting outcomes at different time points, the earliest postoperative assessment was used9. Two researchers (S.J. and C.K.) independently assessed the risk of bias in the included studies using the Cochrane Risk of Bias 2 (RoB 2) tool for RCTs.
Data synthesis and subgroup analysis
All analyses were performed using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria), MedCalc version 18.11.6 (MedCalc Software bvba, Ostend, Belgium), and Comprehensive Meta-Analysis version 4.0 (Biostat Inc., Englewood, NJ, USA).
Data are expressed as the relative risk (RR) with 95% confidence interval (95% CI) and illustrated using forest plots. A random effects model was applied for overall group analysis to account for anticipated trial heterogeneity, including differences in the subjects, type of surgery, and use of anesthetics in the comparator group.
Subgroup analyses were conducted based on the following factors: (1) type of anesthetic used in the comparator group (propofol, inhalation agents, dexmedetomidine, midazolam, or etomidate), (2) type of anesthesia (sedation or general), (3) type of procedure (gastrointestinal [GI] endoscopy, bronchoscopy, or other surgical procedures), (4) participant age (adults [> 18 years] or elderly only), (5) participant sex (all or female only), (6) outcomes (postoperative nausea, postoperative vomiting, or PONV), and (7) remimazolam administration protocol (continuous infusion or intermittent bolus).
A meta-regression analysis was conducted to evaluate the effects of mean age, sex distribution, and coadministered opioids on the incidence of PONV. Coadministered opioids were classified according to the dominant agent; when both single-dose and continuous-infusion opioids were used, the infusion agent was used for classification. Sensitivity analysis was also performed on trials that designated PONV as the primary outcome, which was determined based on whether PONV was specified as the primary endpoint in the sample size calculation.
Heterogeneity was assessed using the I² statistic. For low heterogeneity (I² < 50%), a fixed effects model (Mantel-Haenszel method) was used. For moderate heterogeneity (50% ≤ I² < 75%), a random effects model was applied. Data were not pooled for high heterogeneity (I² > 75%). Publication bias was evaluated using funnel plots and Egger’s test. A P-value less than 0.05 was considered statistically significant.
Results
Literature search
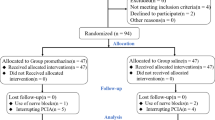
An initial search yielded 319 articles from the Medline, Embase, and Cochrane Library databases. After removing 68 duplicates, the titles and abstracts of the remaining 251 unique studies were reviewed. Of these studies, 63 studies were subjected to a full-text review, resulting in the identification of 50 eligible RCTs9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58. Excluded studies after the full-text review included 7 retrospective studies, 3 studies with inappropriate designs, and 3 studies with inadequate endpoints (Fig. 1). The PRISMA checklist is provided in Supplementary Table S4.
Study characteristics
The eligible studies, published between 2018 and 2023, involved a total of 9,193 participants9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58. Table 1 presents a summary of the key characteristics of the included RCTs. Among these trials, 10 RCTs focused on geriatric populations, with the elderly cohort defined by a varied age range from over 60 to over 75 years19,20,22,24,34,41,45,47,53,56. Nine RCTs specifically included female patients undergoing breast, thyroid, obstetric, or gynecologic surgery15,23,25,35,36,48,49,54,55. The comparators used for evaluating remimazolam in the studies were as follows: propofol in 37 RCTs9,11,13,14,15,16,17,18,19,20,21,22,23,24,26,28,29,30,31,33,35,36,37,39,40,41,43,48,49,50,51,52,53,55,56,57,58inhalation agents in 4 RCTs25,42,45,54dexmedetomidine in 5 RCTs10,27,32,34,47midazolam in 3 RCTs12,44,46and etomidate in 1 RCT38. A total of 24 trials enrolled patients receiving general anesthesia9,13,14,23,24,25,29,30,34,35,36,38,39,41,42,43,45,50,51,52,53,54,55,58and 26 trials included patients receiving procedural sedation10,11,12,15,16,17,18,19,20,21,22,26,27,28,31,32,33,37,40,44,46,47,48,49,56,57. In addition, 17 trials focused on patients undergoing GI endoscopy11,12,16,17,18,19,20,21,22,26,28,29,31,33,37,56,574 trials involved patients undergoing bronchoscopy9,10,39,44and 29 trials included patients undergoing other surgical procedures13,14,15,23,24,25,27,30,32,34,35,36,38,40,41,42,43,45,46,47,48,49,50,51,52,53,54,55,58. Notably, none of the trials targeted patients undergoing cardiac surgery. Among the reviewed RCTs, 33 trials collectively reported PONV as an outcome. Moreover, 18 trials specifically focused on postoperative nausea11,12,13,16,17,19,23,31,33,36,40,43,44,46,50,53,54,57and 17 trials examined postoperative vomiting9,11,12,13,16,19,23,31,33,36,40,43,44,46,50,54,57.
Risk of bias
The risk of bias for individual studies is presented in Fig. 2 and Supplemental Figure S5. In bias assessment, “some concerns” were identified in 3 RCTs for randomization, 2 RCTs for deviations from intended interventions, 3 RCTs for outcome measurement, and 19 RCTs for selective reporting. All included RCTs demonstrated a low risk of bias for missing outcome data. Overall, 25 trials were classified as having “some concerns” regarding bias, and the remaining 25 trials were categorized as having a “low risk”.
Overall analysis
A pooled analysis of 9,193 participants indicated that the effect of remimazolam on PONV was comparable to that of other anesthetics (random effects model; RR: 0.96, 95% CI: 0.80–1.13, P = 0.607, I2: 21%; Fig. 3). The analysis revealed no significant publication bias, as evidenced by the funnel plot (Fig. 4A) and Egger’s test (P = 0.323).
Funnel plot. A: Overall analysis, B-E: Subgroup analysis—variations according to anesthetic comparators, F-G: Subgroup analysis—general anesthesia vs. procedural sedation, H-J: Subgroup analysis—variations according to surgical procedure types, K: Subgroup analysis—elderly patients only, L: Subgroup analysis—female patients only, M: Subgroup analysis—nausea only outcome, N: Subgroup analysis—vomiting only outcome, O: Subgroup analysis—continuous infusion, P: Subgroup analysis—intermittent bolus, Q: Sensitivity analysis—PONV as primary outcomes.
Subgroup analysis 1: variations according to anesthetic comparators
In comparison with propofol, remimazolam demonstrated a similar incidence of PONV (fixed effects model; n = 7,340; RR: 1.03, 95% CI: 0.89–1.20, P = 0.676, I2: 14%; Fig. 5). However, compared with inhalation anesthetics, remimazolam was associated with a lower incidence of PONV (fixed effects model; n = 363; RR: 0.50, 95% CI: 0.34–0.73, P < 0.001, I2: 33%). No significant difference in PONV incidence was observed between remimazolam and dexmedetomidine (fixed effects model; n = 504; RR: 0.94, 95% CI: 0.75–1.19, P = 0.621, I2: 9%) or between remimazolam and midazolam (fixed effects model; n = 853; RR: 0.86, 95% CI: 0.32–2.28, P = 0.757, I2: 0%). Analysis of etomidate was not feasible due to the presence of only a single RCT, which reported no PONV cases. Subgroup analyses for the propofol, inhalation, dexmedetomidine, and midazolam comparator groups showed no significant publication bias, as determined by Egger’s test (P = 0.113, 0.309, 0.864, and 0.141, respectively). The corresponding funnel plots are presented in Fig. 4B-E.
Subgroup analysis 2: general anesthesia vs. procedural sedation
Subgroup analysis stratified by anesthesia type (general anesthesia vs. procedural sedation) revealed that the effect of remimazolam on PONV did not differ significantly from that of other anesthetics in patients receiving either general anesthesia (fixed effects model; n = 3,137; RR: 0.91, 95% CI: 0.74–1.12, P = 0.368, I2: 30%; Supplemental Fig. S6) or procedural sedation (fixed effects model; n = 6,056; RR: 0.96, 95% CI: 0.83–1.12, P = 0.591, I2: 15%). No significant publication bias was detected, as demonstrated by funnel plots (Fig. 4F and G) and Egger’s test results (general anesthesia: P = 0.986, procedural sedation: P = 0.157).
Subgroup analysis 3: variations according to surgical procedure types
Subgroup analysis stratified by procedure type showed that the effect of remimazolam on PONV was comparable to that of other anesthetics among patients undergoing GI endoscopy (fixed effects model; n = 4,724; RR: 0.91, 95% CI: 0.76–1.11, P = 0.358, I2: 15%; Supplemental Fig. S7), bronchoscopy (fixed effects model; n = 858; RR: 1.02, 95% CI: 0.80–1.30, P = 0.875, I2: 44%), and other surgical procedures (fixed effects model; n = 3,611; RR: 0.94, 95% CI: 0.77–1.14, P = 0.542, I2: 28%). No significant publication bias was identified in subgroup analyses focusing on GI endoscopy, bronchoscopy, and other surgical procedures, as indicated by both funnel plots (Fig. 4H-J) and Egger’s test results (P = 0.342, 0.334, and 0.914, respectively).
Subgroup analysis 4: elderly patients
A review of 10 RCTs involving 1,692 geriatric patients excluded a study from the analysis due to the absence of PONV. Among these geriatric patients, no significant difference in PONV incidence was observed between those treated with remimazolam and those treated with other anesthetics (fixed effects model; n = 1,692; RR: 1.14, 95% CI: 0.88–1.49, P = 0.322, I² = 0%; Supplemental Fig. S8). The funnel plot (Fig. 4K) and Egger’s test (P = 0.638) indicated no significant publication bias.
Subgroup analysis 5: female patients
An analysis of 9 RCTs involving 1,061 female patients found no significant difference in the risk of PONV between remimazolam and other anesthetics (fixed effects model; n = 1,061; RR: 0.89, 95% CI: 0.61–1.30, P = 0.541, I² = 37%; Supplemental Fig. S9). Egger’s test revealed no evidence of publication bias (P = 0.092), with the corresponding funnel plot shown in Fig. 4L.
Subgroup analysis 6: nausea-only outcome
Postoperative nausea was specifically reported by 18 RCTs (Fig. 6). When compared with propofol, remimazolam exhibited no significant difference in postoperative nausea incidence (fixed effects model; 14 RCTs, n = 3,750; RR: 0.92, 95% CI: 0.76–1.11, P = 0.363, I² = 18%). One RCT comparing remimazolam with inhalation anesthetics showed a lower risk of postoperative nausea for remimazolam (n = 60; RR: 0.44, 95% CI: 0.23–0.86). Three RCTs comparing remimazolam to midazolam reported no significant difference in postoperative nausea incidence (fixed effects model; n = 853; RR: 0.86, 95% CI: 0.32–2.28, P = 0.757, I² = 0%). Due to a lack of RCTs reporting postoperative nausea as an outcome, analysis of dexmedetomidine and etomidate was not feasible. No publication bias was detected in subgroup analyses for propofol (Egger’s test: P = 0.208) and inhalation agents (Egger’s test: P = 0.141; funnel plot: Fig. 4M).
Subgroup analysis 7: vomiting-only outcome
Postoperative vomiting was specifically reported by 17 RCTs (Fig. 7). When compared with propofol in 13 RCTs involving 3,860 patients, remimazolam was associated with a higher incidence of postoperative vomiting (fixed effects model; n = 3,860; RR: 1.41, 95% CI: 1.05–1.90, P = 0.024, I² = 0%). However, a single RCT comparing remimazolam with inhalation anesthetics showed no difference in postoperative vomiting risk (n = 60; RR: 1.00, 95% CI: 0.28–3.63). Similarly, 3 RCTs involving 853 patients showed no significant difference in postoperative vomiting incidence between remimazolam and midazolam (fixed effects model; n = 853; RR: 0.82, 95% CI: 0.24–2.78, P = 0.750, I² = 0%). Egger’s test indicated no significant publication bias in subgroup analyses for propofol (P = 0.197) and inhalation agents (P = 0.890; funnel plot: Fig. 4N).
Subgroup analysis 8: continuous infusion vs. intermittent bolus of remimazolam
In subgroup analysis stratified by remimazolam administration protocol, the incidence of PONV did not differ significantly between remimazolam and other anesthetics in studies using either continuous infusion (fixed effects model; n = 4,795; RR: 0.94, 95% CI: 0.82–1.07, P = 0.478, I² = 21%) or intermittent bolus (fixed effects model; n = 4,398; RR: 0.87, 95% CI: 0.66–1.14, P = 0.483, I² = 24%; Supplemental Fig. S10). No evidence of publication bias was observed in subgroup analyses for continuous infusion and intermittent bolus, as demonstrated by funnel plots (Fig. 4O–P) and Egger’s test (P = 0.984 and 0.068, respectively).
Meta-regression analysis: effects of mean age, sex distribution, and coadministered opioids
In a random-effects meta-regression model using mean age as the moderator, the age coefficient was 0.01 (95% CI: − 0.01–0.03, P = 0.412), indicating no significant effect of age; the age-specific R² analog (proportion of between-study variance explained) was 0.03 (Supplemental Fig. S11).
When the proportion of male participants served as moderator, the coefficient was 0.01 (95% CI: 0.00–0.02, P = 0.236), demonstrating that sex distribution did not have a significant effect; the R² analog was less than 0.01 (Supplemental Fig. S11).
In a random-effects meta‐regression model with the type of coadministered opioids as a categorical moderator (reference: no opioid coadministration, 9 RCTs), the coefficients for remifentanil, alfentanil, fentanyl, and sufentanil were − 0.64 (95% CI: −1.40–0.12, P = 0.098, 22 RCTs), − 0.39 (95% CI: −1.15–0.37, P = 0.311, 8 RCTs), 0.33 (95% CI: −0.67–1.34, P = 0.516, 6 RCTs), and 0.03 (95% CI: −1.07–1.13, P = 0.959, 5 RCTs), respectively. None of them differed significantly from the reference, and the R² analog was 0.33.
Sensitivity analysis: PONV as the primary outcome
Three RCTs involving 309 patients specifically assessed PONV as the primary outcome. Sensitivity analysis demonstrated no significant difference in PONV risk between remimazolam and comparator anesthetics (random effects model; RR: 0.78, 95% CI: 0.39–1.59, P = 0.499, I² = 52%; Supplemental Fig. S12). Egger’s test revealed no evidence of publication bias (P = 0.385), with the corresponding funnel plot shown in Fig. 4Q.
Discussion
This meta-analysis of 50 RCTs involving 9,193 patients evaluated the efficacy of remimazolam compared with other anesthetic agents in preventing PONV. The overall analysis showed no significant difference in PONV prevention between remimazolam and other agents. However, substantial heterogeneity was observed across studies with respect to remimazolam dosage and administration protocol, coadministered medications, and patient populations. To address heterogeneity, subgroup analyses were performed. The results of the analyses revealed that remimazolam was more effective than inhalation anesthetics in mitigating PONV. Patients who received remimazolam instead of inhalation agents experienced reduced postoperative nausea; however, compared with propofol, remimazolam was associated with a high incidence of postoperative vomiting. The efficacy of remimazolam in preventing PONV did not differ according to patient age, sex, type of anesthesia, type of surgical procedure, or remimazolam administration protocol.
As ERAS protocols gain traction, there is a growing emphasis on the management of PONV. Previously regarded as a normal postoperative event, PONV is now recognized as a complication requiring proactive and strategic intervention4. This shift aligns with the overarching goal of enhancing perioperative care to facilitate more efficient recovery and improved patient outcomes. Established risk factors for PONV include female gender, non-smoking status, younger age, type and duration of surgery, history of PONV or motion sickness, and postoperative opioid use3,4,5. In our study, the statistically significant difference in PONV incidence observed between anesthetic agents suggests that anesthetic selection may warrant greater attention, particularly for patients with a high risk of PONV. Moreover, beyond statistical significance, this finding carries clinical relevance. Given that most established risk factors for PONV are non-modifiable, the choice of the anesthetic agent as a modifiable factor should be strategically optimized to support preventive efforts.
The antiemetic properties of benzodiazepines have been more extensively studied in chemotherapy populations than in surgical populations59. The most recent guidelines from the American Society of Clinical Oncology include a moderate-level recommendation for the use of lorazepam or alprazolam as an adjunct in managing chemotherapy-induced nausea and vomiting refractory to standard prophylaxis60,61.
Although the precise antiemetic mechanism remains uncertain, a prevailing hypothesis posits that benzodiazepines may inhibit dopaminergic signaling in the chemoreceptor trigger zone, thereby impeding the emetic reflex59,62,63. This inhibition may occur through the suppression of dopamine synthesis, release, and postsynaptic activity59,62,63. However, it remains unclear whether this dopamine reduction results directly from the effect of benzodiazepines or indirectly via inhibition of adenosine reuptake59,62,63. Additionally, benzodiazepines may attenuate serotonin release, and their anxiolytic properties are thought to contribute to antiemetic effects59,62,63.
Remimazolam, a novel benzodiazepine, is expected to have similar antiemetic effects1. Existing studies on the effect of benzodiazepines on PONV in surgical patients have been limited, often exploring their use as an adjunct or premedication59. Therefore, the use of remimazolam as a primary anesthetic agent may offer more comprehensive insights into the role of benzodiazepines in PONV management for these patients.
In the present study, the overall pooled analysis revealed no significant difference in the incidence of PONV between remimazolam and other anesthetics. However, a more detailed subgroup analysis of 4 RCTs involving 363 subjects demonstrated a significantly lower incidence of PONV with remimazolam compared with inhalation agents. Inhalation agents are well known as potent triggers of emetogenesis64,65. Regardless of the specific volatile anesthetic, such as isoflurane, sevoflurane, or desflurane, approximately 1 in 3 or 4 patients is estimated to experience PONV following general anesthesia with these agents64,65. In this study, 30.8% of patients who received inhalation agents experienced PONV, consistent with the findings of prior research. In contrast, only 15.5% of those treated with remimazolam reported PONV, showing a significantly lower incidence.
A proposed mechanism for inhalation agent-induced emesis involves the enhancement of serotonin type 3 (5-HT3) signaling in both peripheral and central regions66. Specifically, the area postrema/nucleus of the solitary tract (AP/NTS) in the brainstem, along with vagal afferent fibers from the gastrointestinal tract, are key players in this process66. Activation of these pathways by inhalation agents triggers the emetic reflex, resulting in nausea and vomiting. Additionally, inhalation anesthetics may impair gastrointestinal function, which could further contribute to emesis66. A subgroup analysis focusing solely on postoperative nausea identified inhalation agents as inferior to remimazolam. However, when assessing postoperative vomiting alone, no significant difference was observed between remimazolam and inhalation agents. This discrepancy may be attributed to the inclusion of only a single RCT with a small sample size of 60 subjects in this subgroup analysis. Additional large-scale studies are necessary to accurately delineate the effects of remimazolam and inhalation agents on nausea and vomiting separately.
In this study, although no significant difference was found in overall PONV or postoperative nausea only between propofol and remimazolam, subgroup analysis of postoperative vomiting only demonstrated that it was reduced with propofol compared with remimazolam. Propofol is considered an effective antiemetic even though its precise mechanism of action remains uncertain67. One hypothesis suggests that propofol may exert antiemetic effects by antagonizing serotonin activity, thereby inhibiting pathways responsible for nausea and vomiting67,68. Another proposed mechanism indicates that propofol reduces synaptic transmission in the olfactory cortex, leading to a decrease in the release of excitatory amino acids such as glutamate and aspartate, which may be associated with the emetic response67,68. Currently, large-scale prospective studies comparing the incidence of PONV as a primary outcome between remimazolam and propofol are limited. A recent meta-analysis, which included 11 studies published up to July 2023 and focused on patients receiving general anesthesia, found no significant difference in the incidence of PONV between remimazolam and propofol (odds ratio: 1.04, 95% CI: 0.70–1.56); however, compared with inhalation agents, remimazolam demonstrated a lower PONV rate (odds ratio: 0.25, 95% CI: 0.13–0.47)69. In a retrospective propensity-matched study involving 666 patients with a primary focus on PONV, Suzuki et al. found that PONV was significantly reduced with propofol compared with remimazolam70. Specifically, propofol was associated with a lower incidence of overall PONV (21% vs. 35%, P < 0.001), postoperative nausea (21% vs. 35%, P < 0.001), and postoperative vomiting (9% vs. 16%, P = 0.009). Our findings also suggest that the profile of propofol may be more favorable than that of remimazolam in the prevention of postoperative vomiting, indicating its potential superiority as an antiemetic. However, further research is needed to draw definitive conclusions regarding the relative effects of propofol and remimazolam on PONV.
Our study has several limitations. First, there was considerable heterogeneity in the design of the RCTs included in this meta-analysis, such as variations in PONV evaluation (timing and outcome, including nausea only, vomiting only, or overall PONV), PONV prophylaxis protocol, anesthetic administration method, flumazenil use, and patient population. Second, heterogeneity was observed in the administration protocol of remimazolam across the included trials. In particular, variations in the allowed rescue dosing and the duration of sedation made it difficult to accurately quantify and stratify the total amount of remimazolam administered. To approximate the impact of cumulative exposure, subgroup analyses were conducted according to the type of procedure (general anesthesia vs. procedural sedation) and the administration method (continuous infusion vs. single bolus with intermittent rescue dosing). However, this approach may not have fully accounted for differences in total remimazolam exposure. Further well-designed studies are warranted to clarify the dose-dependent effects of remimazolam on PONV. Third, various perioperative drugs coadministered during surgery could influence the incidence of PONV. In particular, opioid use is a well-established risk factor3,4,5. To account for these drugs, a meta-regression analysis was performed incorporating opioid use and the type of opioid administered. However, this analysis could not fully capture the effects of all coadministered perioperative drugs. Table 1 presents a summary of the coadministered medications reported in each trial. Fourth, only a limited number of studies reported PONV as the primary outcome, with the majority addressing it as a secondary outcome. This underscores the need for rigorously designed, large-scale RCTs that specifically focus on PONV as the primary outcome to establish more definitive conclusions. Lastly, most RCTs were conducted in East Asia, resulting in a predominance of Asian participants, which may restrict the generalizability of the findings to other ethnic groups. Future RCTs in more diverse regions are essential to provide more comprehensive insights applicable to various populations.
In conclusion, remimazolam was not significantly different from other anesthetic agents in overall PONV prevention. However, subgroup analysis revealed differing effects: remimazolam was associated with a lower incidence of PONV compared with inhalation anesthetics, and was associated with a higher incidence of postoperative vomiting compared with propofol.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.
References
Colao, J. & Correa, D. R. Rapidly metabolized anesthetics: novel alternative agents for procedural sedation. J Anesth. Clin. Res 7, 690 (2016).
Kim, S. H. & Fechner, J. Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 75, 307–315 (2022).
Canakci, E. et al. Prevalence study for postoperative nausea vomiting: A training hospital example. Niger J. Clin. Pract. 24, 1633–1640 (2021).
Song, J. W. Efficacy of remimazolam in reducing postoperative nausea and vomiting: a superior alternative anesthetic for total intravenous anesthesia? Korean J. Anesthesiol 77, 409–410 (2024).
Gupta, R. & Soto, R. Prophylaxis and management of postoperative nausea and vomiting in enhanced recovery protocols: expert opinion statement from the American society for enhanced recovery (ASER). Perioper Med 5, 4 (2016).
Mandalà, M. et al. Midazolam for acute emesis refractory to dexamethasone and granisetron after highly emetogenic chemotherapy: a phase II study. Support Care Cancer. 13, 375–380 (2005).
Lee, Y., Wang, J. J., Yang, Y. L., Chen, A. & Lai, H. Y. Midazolam vs Ondansetron for preventing postoperative nausea and vomiting: a randomised controlled trial. Anaesthesia 62, 18–22 (2007).
Zhang, Y. et al. Effect of opioid-free anesthesia on the incidence of postoperative nausea and vomiting: A meta-analysis of randomized controlled studies. Medicine 102, E35126 (2023).
Zhou, Y. Y. et al. Efficacy and safety of remimazolam besylate in bronchoscopy for adults: A multicenter, randomized, double-blind, positive-controlled clinical study. Front Pharmacol 13, 1005367 (2022).
Chen, X., Xin, D., Xu, G., Zhao, J. & Lv, Q. The efficacy and safety of remimazolam tosilate versus Dexmedetomidine in outpatients undergoing flexible bronchoscopy: A prospective, randomized, blind, Non-Inferiority trial. Front Pharmacol 13, 902065 (2022).
Wang, C. et al. Safety and effectiveness of the combination of remimazolam tosilate and Propofol in gastroscopy: a multicenter, randomized controlled, single-blind clinical trial. Front Pharmacol 14, 1124667 (2023).
Rex, D. K. et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and Midazolam in patients undergoing colonoscopy. Gastrointest. Endosc. 88, 427–437e6 (2018).
Doi, M. et al. Efficacy and safety of remimazolam versus Propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase iib/iii trial. J. Anesth. 34, 543–553 (2020).
Mao, Y., Guo, J., Yuan, J., Zhao, E. & Yang, J. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: A randomized controlled trial comparing remimazolam with Propofol. Drug Des. Devel Ther. 16, 1199–1209 (2022).
Zhang, S., Wang, J., Ran, R., Peng, Y. & Xiao, Y. Efficacy and safety of remimazolam Tosylate in hysteroscopy: A randomized, single-blind, parallel controlled trial. J. Clin. Pharm. Ther. 47, 55–60 (2022).
Chen, S. et al. The efficacy and safety of remimazolam Tosylate versus Propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am. J. Transl Res. 12, 4594–4603 (2020).
Shi, W. et al. Efficacy and safety of the Remimazolam-Alfentanil combination for sedation during gastroscopy: A randomized, Double-blind, Single-center controlled trial. Clin. Ther. 44, 1506–1518 (2022).
Yao, Y. et al. Discharge readiness after remimazolam versus Propofol for colonoscopy: A randomised, double-blind trial. Eur. J. Anaesthesiol. 39, 911–917 (2022).
Lu, K. et al. Remimazolam versus Propofol for deep sedation/anaesthesia in upper Gastrointestinal endoscopy in elderly patients: A multicenter, randomized controlled trial. J. Clin. Pharm. Ther. 47, 2230–2236 (2022).
Hu, B. et al. Effect of remimazolam tosilate on respiratory depression in elderly patients undergoing gastroscopy: A multicentered, prospective, and randomized study. Drug Des. Devel Ther. 16, 4151–4159 (2022).
Xin, Y., Chu, T., Wang, J. & Xu, A. Sedative effect of remimazolam combined with alfentanil in colonoscopic polypectomy: a prospective, randomized, controlled clinical trial. BMC Anesthesiol. 22, 262 (2022).
Liu, F. et al. Effect of remimazolam tosilate on the incidence of hypoxemia in elderly patients undergoing Gastrointestinal endoscopy: A bi-center, prospective, randomized controlled study. Front. Pharmacol. 14, 1131391 (2023).
Choi, J. Y. et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J. Clin. Anesth. 82, 110955 (2022).
Zhang, J., Wang, X., Zhang, Q., Wang, Z. & Zhu, S. Application effects of remimazolam and Propofol on elderly patients undergoing hip replacement. BMC Anesthesiol. 22, 118 (2022).
Park, C. G. et al. Impact of remimazolam versus Sevoflurane anesthesia on cerebral oxygenation and intracranial pressure during gynecological laparoscopy with mild hypercapnia. Med. Sci. Monit. 29, e941315 (2023).
Wei, A. et al. The safety and efficacy of remimazolam Tosylate combined with Propofol in upper Gastrointestinal endoscopy: A multicenter, randomized clinical trial. PLoS One. 18, e0282930 (2023).
Lee, S. et al. Comparison of oxygen reserve index according to the remimazolam or Dexmedetomidine for intraoperative sedation under regional anesthesia-A single-blind randomized controlled trial. Front. Med. 10, 1288243 (2023).
Cao, Y. H. et al. Remimazolam tosilate sedation with adjuvant sufentanil in Chinese patients with liver cirrhosis undergoing gastroscopy: A randomized controlled study. Med. Sci. Monit. 28, e936580 (2022).
Shi, F., Chen, Y., Li, H., Zhang, Y. & Zhao, T. Efficacy and safety of remimazolam tosilate versus Propofol for general anesthesia in cirrhotic patients undergoing endoscopic variceal ligation. Int. J. Gen. Med. 15, 583–591 (2022).
Luo, L. et al. Comparative study about different doses of remimazolam in short laparoscopic surgery: A randomized controlled Double-Blind trial. Ther. Clin. Risk Manag. 19, 829–837 (2023).
Cui, X. et al. Efficacy and safety of different doses of remimazolam tosilate applied in upper Gastrointestinal endoscopy: A prospective randomized controlled Double-Blind trial. Drug Des. Devel Ther. 17, 2889–2896 (2023).
Kim, H. et al. Comparison of remimazolam and Dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: a randomized clinical trial. Reg. Anesth. Pain Med. 49, 110–116 (2024).
Dong, S. A. et al. A randomized, controlled clinical trial comparing remimazolam to Propofol when combined with alfentanil for sedation during ERCP procedures. J. Clin. Anesth. 86, 111077 (2023).
Liao, Y. Q., Min, J., Wu, Z. X. & Hu, Z. Comparison of the effects of remimazolam and Dexmedetomidine on early postoperative cognitive function in elderly patients with gastric cancer. Front. Aging Neurosci. 15, 1123089 (2023).
Wang, L. et al. Cardiopulmonary adverse events of remimazolam versus Propofol during cervical conization: A randomized controlled trial. Drug Des. Devel Ther. 17, 1233–1243 (2023).
Matsumoto, A. et al. Remimazolam’s effects on postoperative nausea and vomiting are similar to those of Propofol after laparoscopic gynecological surgery: A randomized controlled trial. J. Clin. Med. 12, 5402 (2023).
Zhang, K. et al. Effects of opioid-free Propofol or remimazolam balanced anesthesia on hypoxemia incidence in patients with obesity during Gastrointestinal endoscopy: A prospective, randomized clinical trial. Front. Med. 10, 1124743 (2023).
Huang, X. et al. The difference in mean arterial pressure induced by remimazolam compared to etomidate in the presence of Fentanyl at tracheal intubation: A randomized controlled trial. Front. Pharmacol. 14, 1143784 (2023).
Pan, Y. et al. Comparison of Remimazolam-Flumazenil versus Propofol for rigid bronchoscopy: A prospective randomized controlled trial. J. Clin. Med. 12, 257 (2022).
Zhao, N. et al. Moderate sedation by total intravenous remimazolam-alfentanil vs. propofol-alfentanil for third molar extraction: A prospective randomized controlled trial. Front. Med. 9, 950564 (2022).
Yang, J. J. et al. Effect of remimazolam on postoperative delirium in older adult patients undergoing orthopedic surgery: A prospective randomized controlled clinical trial. Drug Des. Devel Ther. 17, 143–153 (2023).
Song, S. W., Jang, Y. N., Yoon, M. W. & Jeon, Y. G. Quality of recovery in patients administered remimazolam versus those administered an inhalant agent for the maintenance of general anesthesia: a randomized control trial. BMC Anesthesiol. 22, 226 (2022).
Oh, E. J. et al. Comparison of Propofol vs. remimazolam on emergence profiles after general anesthesia: A randomized clinical trial. J. Clin. Anesth. 90, 111223 (2023).
Pastis, N. J. et al. Safety and efficacy of remimazolam compared with placebo and Midazolam for moderate sedation during bronchoscopy. Chest 155, 137–146 (2019).
Lee, S., Kang, H. Y., Ahn, Y. N. & You, A. H. Comparison of the incidence of postoperative acute kidney injury following the administration of remimazolam or Sevoflurane in elderly patients undergoing total knee arthroplasty: A randomized controlled trial. J. Pers. Med. 13, 789 (2023).
Li, X., Tian, M., Deng, Y., She, T. & Li, K. Advantages of sedation with remimazolam compared to Midazolam for the removal of impacted tooth in patients with dental anxiety. J. Oral Maxillofac. Surg. 81, 536–545 (2023).
Deng, C. M. et al. Effect of intraoperative remimazolam on postoperative sleep quality in elderly patients after total joint arthroplasty: a randomized control trial. J. Anesth. 37, 511–521 (2023).
Zhang, F. et al. Remimazolam Tosylate combined with Low-Dose Propofol improves sedation and safety in hysteroscopy. Drug Des. Devel Ther. 16, 4101–4108 (2022).
Yue, L. et al. Remimazolam versus Propofol in combination with Esketamine for surgical abortion: A double-blind randomized controlled trial. Clin. Transl Sci. 16, 1606–1616 (2023).
Kim, E. J. et al. Comparison of postoperative nausea and vomiting between remimazolam and Propofol in patients undergoing oral and maxillofacial surgery: a prospective randomized controlled trial. BMC Anesthesiol. 23, 132 (2023).
Lee, H. J. et al. Comparison of the recovery profile of remimazolam with Flumazenil and Propofol anesthesia for open thyroidectomy. BMC Anesthesiol. 23, 147 (2023).
Yang, L. et al. Clinical trial comparing remimazolam with Propofol during intravenous anesthesia: A prospective randomised clinical trial. Comb. Chem. High. Throughput Screen. 27, 1544–1550 (2024).
Toyota, Y. et al. Remimazolam-based anesthesia with Flumazenil allows faster emergence than propofol-based anesthesia in older patients undergoing spinal surgery: A randomized controlled trial. Medicine 102, E36081 (2023).
Hari, Y. et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J. Anesth. 36, 265–269 (2022).
Huang, Y. et al. Efficacy and safety of remimazolam compared with Propofol in hypertensive patients undergoing breast cancer surgery: a single-center, randomized, controlled study. BMC Anesthesiol. 23, 409 (2023).
Guo, J. et al. Remimazolam tosilate compared with Propofol for Gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 22, 180 (2022).
Xu, C. et al. Efficacy and Safety of Remimazolam Besylate Combined with Alfentanil in Painless Gastroscopy: A Randomized, Single-Blind, Parallel Controlled Study. Contrast Media Mol Imaging 7102293 (2022). (2022).
Luo, W. et al. Efficacy and safety of remimazolam tosilate versus Propofol in patients undergoing day surgery: a prospective randomized controlled trial. BMC Anesthesiol. 23, 182 (2023).
Javaherforoosh Zadeh, F. Is lorazepam effective at preventing nausea and vomiting after laparoscopic cholecystectomy? A randomized controlled trial. Acta Anaesth. Belg. 68, 131–135 (2017).
Hesketh, P. J. et al. Antiemetics: ASCO guideline update. J. Clin. Oncol. 38, 2782–2797 (2020).
Au, E. et al. The effect of perioperative benzodiazepine administration on postoperative nausea and vomiting: a systematic review and meta-analysis of randomised controlled trials. Br. J. Anaesth. 132, 469–482 (2024).
Majumdar, J. R. et al. Midazolam’s effect on post operative nausea and vomiting and discharge times in outpatients undergoing cancer related surgery. AANA J. 87, 179 (2019).
Habib, A. S. Midazolam–an anti-emetic? Anaesthesia 57, 725 (2002).
Apfel, C. C., Stoecklein, K. & Lipfert, P. PONV: a problem of inhalational anaesthesia? Best Pract. Res. Clin. Anaesthesiol. 19, 485–500 (2005).
Zhong, W. et al. Mechanisms of nausea and vomiting: current knowledge and recent advances in intracellular emetic signaling systems. Int. J. Mol. Sci. 22, 5797 (2021).
Horn, C. C., Wallisch, W. J., Homanics, G. E. & Williams, J. P. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur. J. Pharmacol. 722, 55–66 (2014).
Kim, E. G., Park, H. J., Kang, H., Choi, J. & Lee, H. J. Antiemetic effect of Propofol administered at the end of surgery in laparoscopic assisted vaginal hysterectomy. Korean J. Anesthesiol. 66, 210–215 (2014).
Collins, G. G. S. Effects of the anaesthetic 2,6-diisopropylphenol on synaptic transmission in the rat olfactory cortex slice. Br. J. Pharmacol. 95, 939–949 (1988).
Kim, S. Y., Sim, K. M., Na, H. S., Koo, B. W. & Shin, H. J. Effect of remimazolam for general anesthesia on postoperative nausea and vomiting: A systematic review and meta-analysis. Anaesthesiologie 73, 685–693 (2024).
Suzuki, Y., Kawashima, S., Makino, H., Doi, M. & Nakajima, Y. Comparison of postoperative nausea and vomiting between remimazolam and propofol: a propensity score-matched, retrospective, observational, single-center cohort study. Korean J. Anesthesiol. 76, 143–151 (2023).
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.J., J.Y., H.C., H.R.Database Searching: S.J., H.J.Study Selection and Review: S.J., H.R., C.K.Risk-of-Bias Assessment: S.J., C.K.Data Synthesis and Statistical Analysis: S.J., H.R.Original Draft Writing: S.J., H.C., H.R.Review and Editing: J.Y., H.J., S.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ri, HS., Jeon, S., Yeo, J. et al. Efficacy of remimazolam in preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Sci Rep 15, 29236 (2025). https://doi.org/10.1038/s41598-025-14976-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14976-z