Abstract
Utilizing renewable resources to produce epoxides as an alternative to petroleum-based sources is a growing trend. In this regard, several vegetable oils can serve as suitable alternatives. This study investigated the epoxidation of corn oil using sulfuric acid as a catalyst. Hydrogen peroxide and formic acid were used as the oxygen donor and carrier to form performic acid, which contains the oxirane ring functional group. Kinetic modeling was employed in this experiment to investigate the parameters that influence reaction rates, and it was used in conjunction with the genetic algorithm. The highest conversion to oxirane, at 70%, was achieved with a ratio of 1.5 mol of hydrogen peroxide, a temperature of 55 °C, and a catalyst loading of 3 g. The kinetic data indicate that the epoxide ring-opening reaction (k12 = 12.53 mol L-1min-1) occurs much more rapidly than the initial epoxidation step (k11 = 0.043 mol L-1min-1), with the model demonstrating good accuracy (R² = 0.85) and minimal error (0.14). The outcomes showed a high level of concordance between the simulation and experimental data, confirming the model’s validity.
Similar content being viewed by others
Introduction
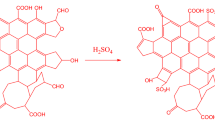
There is an increasing trend in the development of sustainable green materials due to growing environmental concerns, including the depletion of fossil fuels, as well as increased air pollution and the greenhouse effect1. Petrochemicals, such as resins, are widely used in engineering applications due to their valuable material properties, including high stiffness and strength2. However, these resins have significant limitations regarding biodegradability, processing cost, energy consumption, and health hazards3. Thus, it is vital to develop bio-based products from renewable and sustainable feedstocks4. Epoxidation is one of the most important reactions involving the addition of a double bond to a carbon-carbon bond. Unsaturated fatty acid esters are often performed in situ using the Prileschajew Reaction (performic acid method), which is already industrially performed on a large scale5. In this method, the unsaturated fatty acid reacts with performic acids generated in situ, for safety reasons, through the acid-catalyzed peroxidation of the respective organic acids with hydrogen peroxide6. The formation of performic acid occurs in the aqueous phase according to the following reaction, as shown in Fig. 1A.Formic acid reacts with hydrogen peroxide to produce performic acid and water in an equilibrium reaction. Performic acid is the active oxidizing agent for epoxidizing the double bonds in corn oil. The reaction conditions influence the amount of performic acid formed, directly affecting the efficiency of the epoxidation process.
Several studies have investigated the feasibility of utilising vegetable oils as feedstock instead of petroleum7,8. Corn oil was chosen for this study because it contains a high proportion of linoleic acid, offering more double bonds for epoxidation than vegetable oils richer in saturated fats, such as palm or coconut oil. Its high unsaturation level increases the likelihood of a greater oxirane yield, making it a suitable raw material for epoxide production. Moreover, corn oil is widely accessible, renewable, and generally affordable, supporting the aim of developing sustainable, bio-based chemical processes with the potential for practical scalability. These studies involve research on the epoxidation of vegetable oils and the variables that govern epoxidation. Homogeneous catalysts involve a reaction that forms a peracid, which is then used to react with fatty acid oil to produce its epoxide9. The unsaturated carbon double bond in fatty acid oil would be replaced by oxygen from the peracid form, thus producing an epoxide in the oil10. Hydrogen peroxide is the main oxygen donor to the fatty acid oil11. Unfortunately, since it is insoluble in organic matter, an oxygen carrier such as formic or acetic acid is needed12. Sulphuric acid plays an important role as a catalyst for the formation of peracid, as discussed by Vianello et al., who discovered that the difference in catalyst sulphuric acid loading has a significant effect on the rate of reaction and the thermal power generated13. Results show that both are increased proportionally with the catalyst weight loading, which suggests that sulphuric acid, with its strong catalytic activity, enhances the overall epoxidation process. Another study by Abolins et al.14 shows that the rate of the double bond increases gradually with the sulphuric acid catalyst loading, where 88.7% of the double bonds in tall oil fatty acid were oxidized and a 19.7% relative conversion to the oxirane ring was achieved. To date, corn oil has less potential for use as a raw material. However, an unsaturated fatty acid in corn oil is suitable for use as a raw material for epoxidation. Epoxidized corn oil is a potential alternative to conventional petroleum-based epoxy, serving as an intermediate for the synthesis of various chemical products15.
Kinetic studies on the epoxidation of various vegetable oils have been reported with major contributions made by Rangarajan etal16., Mungroo et al.17, Chou and Chang18, and Campanella et al.19. These studies provided insight into the reaction kinetics for homogenous systems with little consideration given to the aspect of ring opening of an epoxide. The increasing demand for environmentally sustainable processes in the chemical industry highlights the importance of developing greener methods for utilizing renewable resources, such as linoleic acid-rich corn oil. This abundant raw material has significant potential for conversion into epoxidized derivatives, which are used in various industrial applications, including polymers and coatings12. However, traditional epoxidation methods often rely on hazardous reagents, including concentrated acids, and generate unwanted byproducts, raising concerns about environmental and safety issues. Additionally, existing processes frequently struggle to achieve high oxirane content with minimal degradation, limiting their efficiency and scalability. Therefore, a more sustainable and efficient approach is needed to address these challenges.
This research presents a catalytic epoxidation process for linoleic acid-derived corn oil using an in situ performic acid mechanism, offering a novel and sustainable solution. The innovation lies in the in situ generation of performic acid directly within the reaction system, eliminating the need for pre-synthesized peracids and reducing environmental impact. Employing a specially designed catalyst enhances reaction selectivity, increases oxirane content, and minimizes side reactions. Additionally, optimizing reaction parameters ensures high efficiency and scalability, representing a significant advancement in green chemistry for producing epoxidized oils from renewable resources. This study aims to investigate both the kinetics of oleic acid epoxidation and the subsequent ring opening. Therefore, this study aims to (1) investigate the effect of temperature, catalyst loading, and hydrogen peroxide molar ratio on epoxidation reaction rate and (2) develop a kinetic model using a genetic algorithm to determine the rate constant of epoxidation of corn oil. This study fills the gap in understanding the catalytic epoxidation of linoleic acid-rich corn oil using in situ performic acid. Unlike previous studies on less unsaturated oils, this work focuses on optimizing reaction conditions for high linoleic content, with a novel approach to analyzing catalyst performance, oxirane yield, and reaction behavior to support sustainable epoxide production.
Materials and methods
Materials
Corn oil, formic acid, hydrogen peroxide, sulphuric acid, acetic acid, and crystal violet were purchased from Merck Sdn Bhd and QReC Sdn Bhd, as summarized in Table 1.
Experimental set-up
In the epoxidation process, a 1-liter beaker with a stirrer and thermometer was employed. A precisely controlled thermostatic water bath was utilized to maintain the required temperature. The mixture was then gradually heated to a prescribed temperature within the range of 35 to 95 °C while agitating at a defined speed between 50 and 300 rpm. Subsequently, a 50% hydrogen peroxide solution was added dropwise to the mixture. Process safety is a significant concern when working with peroxides and peracids due to the potential formation of detonable mixtures at high concentrations of active oxygen, which could lead to explosions upon heating. Continuous monitoring of the reaction temperature was implemented throughout the reaction period to prevent the vaporization of formic acid and to mitigate the risk of a runaway reaction.
Relative Conversion Oxirane (RCO)
Experiments were conducted to determine the relative conversion to oxirane (RCO) by calculating the oxirane oxygen content (OOC) theoretically and by using a direct titration of a hydrobromic acid solution to indicate the degree of conversion in the palm oil, thereby experimentally determining the OOC. The RCO, in Eq. 1, is determined based on the OOC value, which is determined theoretically in Eq. 2 and experimentally in Eq. 3.
Here, \(\:{X}_{0}\) is the initial iodine value, \(\:{\text{A}}_{\text{i}}\) is the molar mass of iodine, \(\:{A}_{o}\) is the molar mass of oxygen, \(\:N\:\)is the normality of HBr, \(\:V\) is the volume of the HBr solution used for the blank in milliliters (mL), \(\:V\) is the volume of HBr solution used for titration, and \(\:W\) is the weight of the sample.
Fourier Transform Infrared (FTIR) Spectroscopy
The analytical technique used for the epoxidized corn oil sample analysis was FTIR. FTIR is widely used in various fields because of its rapid analysis and versatility. The vibrational characteristics of amino acids and cofactors, which are vulnerable to microscopic structural modifications, are investigated through FTIR spectroscopy. The FTIR model used was a Bruker Vertex 70. The purpose of FTIR was to detect the strengths of these absorptions as well as the frequencies at which the sample absorbs. The strength and frequency of sample absorption are the axes in the two-dimensional graphic called the spectrum20.
Kinetic modelling of epoxidation of corn oil
Equations 4 and 5 describe the epoxidation process, while Eq. 6 shows the ring opening of epoxidized corn oil by in situ hydrolysis. FA, HP, PF, CO, and ECO refer to formic acid, hydrogen peroxide, performic acid, corn oil, and epoxidized corn oil, respectively. The rate constant is based on OOC, representing epoxidized oleic acid. Several assumptions underpin this model: (1) The epoxidation process occurs in a single phase, eliminating the need for distribution constants to characterize different species in aqueous and oil phases; (2) All involved reactions are homogeneous processes.
A genetic algorithm was formulated to solve ordinary differential equations (ODEs). This inherently parallel, self-learning system is adept at managing linear and nonlinear equations. Integrating with artificial intelligence and machine learning, genetic algorithms hold substantial importance in optimization and kinetic studies21. The kinetic model for epoxidation and epoxide ring degradation uses key rate constants k11, k12, k2, and k3. The corresponding rate equations have been formulated, resulting in a system of simultaneous differential equations as detailed in the following equation:
The validity of the parameters was confirmed by minimizing the error (e), which is quantified as the difference between the simulation and experimental outcomes, as defined in Eq. 14.
Where \(\:{\text{ELO}}_{\text{i}}^{\text{sim}}\) and\(\:{\text{}\text{ELO}}_{\text{i}}^{\text{exp}}\) denote the estimated and experimental epoxy concentrations, \(\:i\) is the ith data point, and \(\:n\) is the total number of data points in the simulations and experiments. The closer the value of \(\:e\) to zero, the better the agreement between the simulation and experimental values.
Results and discussion
Influence of temperature on the rate of epoxidation
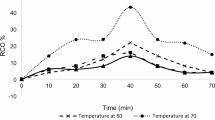
The temperatures needed to be adjusted started at 55 °C, 65 °C, and lastly, 75 °C. Data were collected during the experiment based on the observations made. RCO was calculated using the weight of the sample taken and the amount of hydrogen bromide used. As shown in Fig. 1B, the RCO changed over time. Specifically, at a temperature of 55 °C, the RCO reading increased significantly until the twentieth minute, after which it immediately decreased. Then, for a temperature of 65 °C, the optimum RCO had already been achieved at the tenth minute, and the RCO reading slowly decreased by two points below the optimum RCO achieved just then. Lastly, at a temperature of 75 °C, the same as the previous temperature, the optimum RCO was reached for the tenth time, and the temperature then moderately decreased.
Vegetable oil in situ epoxidation typically requires temperatures below 70 °C, as high temperatures during epoxidation lead to excessive epoxy ring-opening events. Furthermore, peroxy acids can explode at a temperature of 80–85 °C and easily break down when heated; however, as this epoxide mixture contained sulphuric acid as the catalyst, the ideal temperature needed to be slightly higher than the standard range of temperature for epoxidation, which was from 50 to 80 °C since the catalyst did react together with a peracid that would facilitate formation regarding an active epoxidizing species. The long-term stability of the produced epoxide was not extensively addressed in this study. Oxirane groups are sensitive to moisture, heat, and acidic conditions, which can promote spontaneous ring-opening and degradation over time22. To preserve epoxide stability, storage under low temperatures, in dry conditions, and away from light or acidic environments is recommended.
Influence of hydrogen peroxide on the corn oil molar ratio
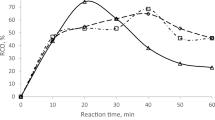
For the last parameter, the molar ratio of hydrogen peroxide was investigated. Hydrogen peroxide was used to react with formic acid during the epoxidation process to form a peracid, specifically peroxyformic acid. For the previous experiment, the ratio of hydrogen peroxide used was 1:1; however, for this parameter, the ratio would change to 0.5, 1.0, and 1.5 molar ratios. Based on Fig. 5, the highest yield of RCO was achieved at the twentieth minute, with a molar ratio of 1.5, and it subsequently decreased significantly thereafter. The highest RCO yield achieved in this parameter was approximately 43%; meanwhile, for the other molar ratios of 0.5 and 1.0, the RCO yields achieved were around 32% and 19%, respectively. At a molar ratio of 1.0, the graph fluctuated slightly.
In Fig. 2, the graph of the 1.5 molar ratio of hydrogen peroxide immediately decreased after it passed the highest epoxidation yield. This likely occurred due to the instability of the oxirane ring and may also be attributed to the side reaction that resulted from the excess hydrogen peroxide. This result was also quite contradictory to the finding from23, which, according to the data, obtained a larger proportion of RCO when the hydrogen peroxide concentration was raised. The oxirane ring showed poor stability at the lowest mole ratio of 1:1 between hydrogen peroxide and oleic acid.
Influence of catalyst loading on the rate of epoxidation
In this experiment, sulphuric acid was used as the catalyst, and the weight of sulphuric acid was measured to determine the ideal amount of catalyst needed to reach the highest epoxidation yield. Based on Fig. 3, the graph for the catalyst loading of 3 g of sulphuric acid shows that the RCO yield reached its highest point, exceeding 70%. As the graph decreased, the RCO values remained the same at the twentieth and thirtieth minutes, indicating that the duration required to reach a lower point was longer than for other amounts of catalyst loading. For the 6 g and 9 g of sulphuric acid, the RCO yield didn’t achieve its high value, and both graphs just moderately went down after barely reaching their optimum point. Too many catalysts wouldn’t help the experiment reach its ideal RCO value; yet, the best amount proven in this experiment was only 3 g of sulfuric acid.
However, this finding contradicts the statement that the reaction time required to obtain the maximal conversion of oxirane value decreased when the acid concentration was raised from 1 to 2 g. Additionally, it was noted that glycol production increased as the acid concentration rose. Higher oxirane cleavage and a proportionally lower oxirane value were seen when the catalyst loading was raised to 3%. Therefore, a 2 g loading of sulphuric acid produced the best conversion to oxirane. As for the corn oil used in the experiment, the amount of sulphuric acid needed was the lowest among the others, which was only 3 g of catalyst. Too much sulphuric acid could lead to an oxirane ring opening and produce an unintended epoxide24. In this study, corn oil, a renewable and biodegradable resource, was used as the raw material, promoting bio-based feedstocks and supporting waste reduction initiatives. However, a comprehensive life cycle assessment (LCA) and waste management evaluation would be necessary for future work to thoroughly assess the environmental benefits of using corn oil for epoxide production.
FTIR characterization
Figure 4 shows the FTIR spectra show clear differences between the corn oil before and after epoxidation. A strong peak near 1650 cm¹ is observed in the original corn oil, which corresponds to the C = C stretching vibration from unsaturated fatty acids. After epoxidation, the intensity of this peak decreases, indicating that the double bonds have reacted. A new absorption band appears around 820–850 cm-1 in the epoxidized sample, assigned to the C–O–C stretching of the oxirane group, confirming the formation of epoxide structures. Small changes are also seen in the C–H stretching region between 2850 and 2950 cm¹, suggesting slight modifications in the fatty acid chains. Overall, the FTIR results provide supporting evidence for successful epoxidation, complementing the wet chemical analysis for RCO determination25.
Kinetic modelling of epoxidation of corn oil
The ideal reaction conditions for the epoxidation process were determined using kinetic modeling with MATLAB software; the reaction rate values, k, are listed in Table 2. For every chemical, the experimental data’s reaction rates, k, match the initial concentration. For the reaction rate \(\:{k}_{11}\) The rate was a second slower than \(\:{k}_{12}\). This was because the reaction only formed performic acid and its byproduct, water. The rate constant k11 (0.043 mol L-1 min-1) represents the epoxide formation, while k12 (12.53 mol L-1 min-1) reflects the consumption rate of intermediates in a secondary reaction pathway. The significantly higher value of k12 suggests that this step is much faster, which may impact the overall epoxide yield if not controlled.
The constants k2 (0.110 mol L-1min-1) and k3 (0.066 mol L-1min-1) correspond to the rates of epoxide formation and degradation through ring-opening, respectively. The lower value of k3 indicates a slower degradation rate, which is beneficial for preserving the oxirane content. The R2 value of 0.85 indicates a reasonable agreement between the experimental and simulated data, demonstrating the model’s ability to capture the reaction kinetics, though some minor discrepancies remain. The low sum of error (0.14) also supports the model’s reliability in describing the process. These results highlight the efficiency of the epoxidation process while identifying areas where further model refinement could enhance predictive accuracy.
Figure 5 illustrates a notable discrepancy between the simulation and the OOC experiment. The simulation graph was quite low, with its highest point only reaching below 0.6 OOC. Meanwhile, the experiment graph showed that the OOC could reach a higher value, almost 0.8, yet after the twentieth minute, it significantly decreased. This could be happening because of the purity of the solutions used or the efficiency of the epoxidation reaction. The deviations between the simulation and experimental results, especially near the peak oxirane content, are mainly due to simplified modeling assumptions that do not fully capture side reactions and oxirane degradation in linoleic acid-rich corn oil. Secondary ring-opening reactions, oxirane instability, and minor experimental variations may also contribute to the discrepancies. While the model predicts the overall trend well, the observed gaps highlight the need for more detailed kinetic mechanisms in future work.
Conclusion
In conclusion, with the aid of a catalyst, the epoxidation of corn oil was accomplished effectively by utilizing hydrogen peroxide and formic acid as oxygen donors and carriers. This experiment aimed to determine whether it would be feasible to convert the unsaturated fatty acids in corn oil into epoxides, thereby enabling its use in various industrial applications. Those two objectives were successfully achieved by completing all the parameters to observe their effect on the epoxidation process. As for the simulation and experiment graphs, it can be seen that the experiment reached a higher OOC than the simulation, likely due to the high efficiency of the epoxidation process. The FTIR results showed the presence of distinctive absorption bands for the epoxide and the stretching vibration band of the oxirane group (C-O-C). Based on the study, the highest RCO (70%) was achieved at a ratio of 1.5 mol of hydrogen peroxide, a temperature of 55 °C, and a catalyst loading of 3 g. In this study, kinetic modeling was performed using the ODE45 solver, based on the Runge-Kutta method. It was found that the function fits with the simulation and experimental results.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Azmi, I. S. et al. Synthesis of bio-polyol from epoxidized palm oleic acid by homogeneous catalyst. J. Elastomers Plast. 0, 1–13. https://doi.org/10.1177/00952443221147029 (2022).
Sardon, H., Mecerreyes, D., Basterretxea, A., Avérous, L. & Jehanno, C. From lab to market: current strategies for the production of biobased polyols. ACS Sustain. Chem. Eng. https://doi.org/10.1021/acssuschemeng.1c02361 (2021).
Biermann, U., Bornscheuer, U. T., Feussner, I., Meier, M. A. R. & Metzger, J. O. Fatty acids and their derivatives as renewable platform molecules for the chemical industry, Angew. Chemie - Int. Ed. 60, 20144–20165. https://doi.org/10.1002/anie.202100778 (2021).
Varma, N., Wadatkar, H., Salve, R., Kumar, T. V. & Impact Advancing Sustainable Agriculture: A Comprehensive Review of Organic Farming Practices and Environmental J. Exp. Agric. Int. 46 695–703. https://doi.org/10.9734/jeai/2024/v46i72623. (2024).
Jalil, M. J. et al. Selective epoxidation of crude oleic acid-Palm oil with in situ generated performic acid 7 152–155. (2018).
de Haro, J. C., Rodríguez, J. F., Pérez, Á. & Carmona, M. Incorporation of Azide groups into bio-polyols. J. Clean. Prod. 138, 77–82. https://doi.org/10.1016/j.jclepro.2016.05.012 (2016).
Alzahrani, H. A. & Bravo-Suárez, J. J. In situ Raman spectroscopy study of silver particle size effects on unpromoted Ag/α-Al2O3 during ethylene epoxidation with molecular oxygen. J. Catal. 418, 225–236. https://doi.org/10.1016/j.jcat.2023.01.016 (2023).
Marriam, F., Irshad, A., Umer, I. & Arslan, M. Vegetable oils as bio-based precursors for epoxies. Sustain. Chem. Pharm. 31, 100935. https://doi.org/10.1016/j.scp.2022.100935 (2023).
Sinadinović-Fišer, S., Janković, M. & Borota, O. Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chem. Eng. Process. Process. Intensif. 62, 106–113. https://doi.org/10.1016/j.cep.2012.08.005 (2012).
Kurańska, M. & Malewska, E. Waste cooking oil as starting resource to produce bio-polyol - analysis of transesteryfication process using gel permeation chromatography. Ind. Crops Prod. 162, 1–8. https://doi.org/10.1016/j.indcrop.2021.113294 (2021).
Gunawan, E. R., Suhendra, D., Arimanda, P. & Asnawati, D. Murniati, epoxidation of terminalia Catappa L. Seed oil: optimization reaction. South. Afr. J. Chem. Eng. 43, 128–134. https://doi.org/10.1016/j.sajce.2022.10.011 (2023).
Alvear, M. et al. Epoxidation of light olefin mixtures with hydrogen peroxide on TS-1 in a laboratory-scale trickle bed reactor: transient experimental study and. Chem. Eng. Sci. 118467. https://doi.org/10.1016/j.ces.2023.118467 (2023).
Vianello, C., Piccolo, D., Salzano, E. & Maschio, G. Preliminary study of epoxidation of soybean oil in stirred tank reactor: the effect of the mixing program, 57 (2017).
Fridrihsone, A., Abolins, A. & Kirpluks, M. Screening life cycle assessment of tall oil-based polyols suitable for rigid polyurethane foams. Energies 13, https://doi.org/10.3390/en13205249 (2020).
Adnan, N. N., Hafidzal, M., Hanafi, M., Razak, N. H. & Yusof, F. A. Study on mechanical and thermal properties of plasticizer with epoxidation waste cooking oil, 1 34–41. (2025).
Rangarajan, B., Havey, A., Grulke, E. A. & Culnan, P. D. Kinetic parameters of a Two-Phase model for in situ epoxidation of soybean oil, 72 1161–1169. (1995).
Mungroo, R., Pradhan, N. C., Goud, V. V. & Dalai, A. K. Epoxidation of Canola oil with hydrogen peroxide catalyzed by acidic ion exchange resin, JAOCS. J. Am. Oil Chem. Soc. 85, 887–896. https://doi.org/10.1007/s11746-008-1277-z (2008).
Chou, T. & Chang, J. Acetic acid as an oxygen carrier between two, 37–41. (2007).
Campanella, A., Baltanás, M. A., Capel-Sánchez, M. C., Campos-Martín, J. M. & Fierro, J. L. G. Soybean oil epoxidation with hydrogen peroxide using an amorphous ti/sio 2 catalyst. Green. Chem. 6, 330–334. https://doi.org/10.1039/B404975F (2004).
Neswati, N. & Nazir Combination of temperature and time in epoxidation for producing epoxidized palm oil as source of bio polyol, IOP conf. Ser. Earth Environ. Sci. 757, 012069. https://doi.org/10.1088/1755-1315/757/1/012069 (2021).
Jumain Jalil, M., Azfar Izzat Aziz, M., Nuruddin Azlan Raofuddin, D., Suhada Azmi, I. & Heiry Mohd Azmi, M. Saufi md. Zaini, I. Mariah ibrahim, Ring-Opening of epoxidized waste cooking oil by hydroxylation process: optimization and kinetic modelling. ChemistrySelect 7, 1–9. https://doi.org/10.1002/slct.202202977 (2022).
Rahim, N. H., Jalil, M. J., Mubarak, N. M., Azmi, I. S. & Anbuchezhiyan, G. Catalytic epoxidation of unsaturated fattyacids in palm stearin via in situ peracetic acids mechanism. Sci. Rep. 15(1), 4789 (2025).
Jalil, M. J. et al. Optimization of epoxidation Palm-Based oleic acid to produce polyols. Chem. Chem. Technol. 16, 66–73. https://doi.org/10.23939/chcht16.01.066 (2022).
Yan, W. et al. Opportunities and emerging challenges of the heterogeneous Metal-Based catalysts for vegetable oil epoxidation. ACS Sustain. Chem. Eng. 10, 7426–7446. https://doi.org/10.1021/acssuschemeng.2c00617 (2022).
Dominguez-Candela, I., Lerma-Canto, A., Cardona, S. C., Lora, J. & Fombuena, V. Physicochemical characterization of novel epoxidized vegetable oil from Chia seed oil. Mater. (Basel). 15, 1–19. https://doi.org/10.3390/ma15093250 (2022).
Author information
Authors and Affiliations
Contributions
Intan Suhada Azmi, Siti Nadzirah Abd Manaf, Amnani Shamjuddin, and Mohd Azril Riduan: Data curation. Mohd Jumain Jalil: conceptualization and methodology. Amine Aymen Assadi, Nabisab Mujawar Mubarak, and Nadeem A Khan reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Azmi, I.S., Jalil, M.J., Manaf, S.N.A. et al. Catalytic epoxidation of linoleic acid derived corn oil via in situ performic acid mechanism. Sci Rep 15, 32041 (2025). https://doi.org/10.1038/s41598-025-15010-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15010-y