Abstract
Non-small cell lung cancer (NSCLC) with EGFR mutations presents a unique challenge due to the development of oligometastasis during treatment with first-line tyrosine kinase inhibitors (TKIs). The optimal timing of radiotherapy in EGFR-mutant oligometastatic NSCLC remains debated. This study aims to investigate the timing of radiotherapy in relation to disease progression to optimize treatment outcomes. We conducted a retrospective analysis of stage IIIB-IV EGFR-mutant NSCLC patients who received first-line TKI therapy and developed oligoprogressive disease between January 2019 and October 2024. Two groups were compared: one receiving radiotherapy before disease progression (n = 41) and the other post-progression (n = 26). The primary outcomes were progression-free survival (PFS1), progression-free survival after radiotherapy (RT-PFS), and the duration of first-line TKI therapy. Statistical analyses were performed using Kaplan-Meier curves and Cox regression. A total of 67 patients were included. The median PFS1 was 13.8 months in the upfront consolidative radiotherapy group versus 9.8 months in the post-progression group (P = 0.391). The median duration of first-line TKI therapy was significantly longer in the post-progression radiotherapy group (20.3 months vs. 13.3 months, P < 0.001). Kaplan-Meier survival analysis showed a significant difference in TKI duration, suggesting delayed radiotherapy improved TKI duration. This retrospective study suggests that the timing of radiotherapy may influence the duration of first-line EGFR-TKI therapy in patients with oligometastatic NSCLC. Administering local therapy at the time of oligoprogression may help prolong TKI benefit without premature treatment escalation. However, given the study’s retrospective design and potential baseline imbalances, these findings should be interpreted with caution and require validation in future prospective trials.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related morbidity and mortality worldwide1. Oncogene-addicted NSCLC, particularly tumors harboring Epidermal growth factor receptor (EGFR) or (Anaplastic Lymphoma Kinase) ALK alterations, represents a distinct clinical and molecular subtype characterized by strong dependence on specific signaling pathways. Patients with these mutations often respond well to tyrosine kinase inhibitors (TKIs), which have become the standard first-line treatment2,3. However, despite initial treatment responses, disease progression remains a significant challenge, with many patients developing metastases. Oligometastasis, a condition where cancer has spread to a limited number of sites, has been recognized as an intermediate state between localized and widespread metastatic disease4.
Recent advances in understanding oligometastatic disease have opened new avenues for treatment, with local therapies, including radiotherapy, emerging as potential strategies to control metastatic sites while preserving the efficacy of systemic treatments5,6,7. Initially, local therapies in oligometastatic NSCLC were predominantly applied after disease progression, targeting oligoprogressive lesions6,8.And with the recognition of residual disease and the emergence of the concept of local consolidative therapy (LCT), there has been a paradigm shift towards earlier integration of local treatments9,10. However, the optimal timing of radiotherapy (RT)-whether before or after progression-remains unclear, especially in the context of EGFR-mutant NSCLC patients receiving first-line TKI therapy.
In this retrospective study, we aim to compare the outcomes of two different radiotherapy timing strategies in EGFR-mutant NSCLC patients with oligometastatic disease. By investigating the optimal timing of radiotherapy in the management of oligometastatic EGFR-mutant NSCLC, we hope to provide valuable insights that could inform treatment strategies and improve clinical outcomes in this specific cohort.
Results
Patient characteristics
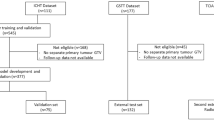
A total of 67 patients with or without oligometastatic (≤ 5 metastases and ≤ 3organs) NSCLC harboring the EGFR sensitizing mutation and treated with first line TKIs was enrolled between 2019 January to 2024 October (Fig. 1). Patients were divided into two groups based on the timing of radiotherapy intervention: upfront consolidative radiotherapy (n = 41) and post-progression radiotherapy (n = 26). Baseline characteristics for the entire cohort as well as for the two subgroups are summarized in Tables 1 and 2.
General patient characteristics
The median age was 59.1 years (range, 31–78), with 52.2% (n = 35) of patients aged < 60 years. All patients had adenocarcinoma histology, with EGFR mutations identified as follows: 38.8%(n = 26) exon 19 deletion, 47.8%(n = 32) exon 21 L858R, 7.5%(n = 5) exon 18, and 6%(n = 4) exon 20 mutations. The majority of patients had an ECOG performance status of 0–1 (94%, n = 63), with only 6% having a status of 2.
Regarding clinical features, most patients presented with T1-2 tumors (55.2%, n = 37) and N2-3 disease (88.1%, n = 59). A substantial proportion (68.7%, n = 46) had distant metastases (M1). Common metastatic sites included bone (31.3%, n = 21), brain (23.9%, n = 16), and pleura (11.9%, n = 8). Radiotherapy was primarily administered to the lung (74.2%, n = 49), followed by bone and brain.
In terms of treatment, 56.7% of patients received first-generation EGFR-TKIs, with the remainder receiving second- or third-generation TKIs. Half of the patients (47.8%, n = 35) underwent initial chemotherapy. Disease progression patterns were predominantly local (77.6%, n = 52), with fewer patients experiencing distant metastasis (17.9%, n = 12). The TRP rate is 7.5%. All characteristics are listed in Table 1.
Radiotherapy parameters of patients
A total of 67 patients received radiotherapy targeting lung, brain, or bone lesions, with detailed radiotherapy parameters summarized in Table S1.
Lung was the most commonly irradiated site, with 49 patients (73.1%) receiving thoracic radiotherapy. Among them, 45 patients (26.9%) received conventional radiotherapy (56–66 Gy in 28–33 fractions), and 4 (6%) patients received stereotactic body radiotherapy (SBRT) to lung lesions (50–60 Gy in 3–8 fractions).
Brain radiotherapy was administered to 9 patients (13.4%) using SBRT (45–50 Gy in 10 fractions), all with palliative intent.
Bone radiotherapy was performed in 9 patients (13.4%), including 5 patients treated with 30 Gy in 10 fractions and 4 patients receiving higher-dose regimens (40–50 Gy in 20–25 fractions), all with palliative intent.
Comparison between Pre- and Post-Progression radiotherapy groups
The baseline characteristics of patients in the upfront consolidative and post-progression radiotherapy groups were comparable, with no significant differences in key clinical variables, including age, gender, ECOG performance status, smoking history, EGFR mutation status, tumor staging (T, N, M stages), metastatic sites, radiotherapy sites, and prior chemotherapy use. Specifically, the distribution of EGFR mutations, including exon 19 deletions and exon 21 L858R mutations, was similar between the groups. Both groups also exhibited comparable tumor burden, with similar proportions of patients having T1-2 and N2-3 disease, as well as similar metastatic patterns. In particular, it should be noted that TRP were comparable between the two groups. Grade 1–2 TRP occurred in one patient (4.7%) in post-progression Group and three patients (6.5%) in upfront consolidative group. Grade ≥ 3 TRP occurred in no patients in post-progression Group and one patient (2.2%) in upfront consolidative group. These findings suggest that the baseline characteristics of the two groups were well-balanced, supporting the assumption that any observed differences in the duration of first-line TKI therapy can be attributed primarily to the timing of radiotherapy intervention, rather than other confounding factors. All characteristics are listed in Table 2.
Survival outcomes
Median follow-up time was 15.1 months. For the entire cohort, the median PFS1 was 11.7 months. It was 13.8 months (95% CI: 10.15–17.51) in the upfront consolidative radiotherapy group and 9.8 months (95% CI: 5.47–14.13) in the post-progression radiotherapy group (P = 0.391). And the median PFS2 in the post-progression radiotherapy group was 8.5months, we also analyzed the RT-PFS between the two groups and found no statistically significant difference (8.0 months, 95% CI: 6.37-9.63vs. 5.7 months, 95% CI:4.17–7.17, P = 0.122).
There was a statistically significant difference in the duration of first-line EGFR-TKI therapy between the two groups. Patients in the post-progression RT group had a longer TKI duration compared to the upfront consolidative RT group (median 20.3 vs. 13.3 months; p < 0.001, log-rank test), as shown by Kaplan–Meier analysis. These results suggest that the timing of RT intervention has a significant impact on the duration of first-line TKI therapy, delaying RT intervention associated with prolonged TKI duration (Fig. 2).
To further explore whether the effect of radiotherapy timing varied by treatment site, subgroup analyses were conducted according to irradiated sites, including lung, brain, and bone. As shown in Figure S1, TKI duration was significantly longer and statistically significant for lung radiotherapy in Figure S1(B), and a similar trend was observed in Figure S1(A), although not statistically significant.
Univariate and multivariate analyses of the duration of first-line TKI therapy
Univariable Cox regression analysis revealed that factors significantly associated with prolonged TKI duration included primary tumor (HR = 0.46, 95% CI: 0.26–0.81, P = 0.008), lower T stage (T1-2 vs. T3-4, HR = 2.09, 95% CI: 1.24–3.54, P = 0.006) and first generation TKI (1 vs. 2, HR = 2.16, CI: 1.09–4.26, P = 0.027). In multivariable Cox regression analysis, primary tumor (HR = 0.39, 95% CI: 0.21–0.71, P = 0.002) and lower T stage (HR = 2.53, 95% CI: 1.40–4.59, P = 0.002) were independently associated with prolonged TKI duration (Table 3). These findings indicate that primary tumor, lower T stage, and initial chemotherapy are critical predictors of longer TKI treatment duration.
Discussion
The management paradox in EGFR-mutant oligometastatic NSCLC lies in balancing the potential benefits of early local control against preserving systemic therapy efficacy. In this context, several studies have explored the progression patterns of oligometastatic disease in EGFR-mutant NSCLC. Al-Halabi et al.11 conducted a retrospective analysis of 49 EGFR-mutant metastatic NSCLC patients treated with TKI therapy, finding that lung was the most common site for both metastasis and disease progression, with 45% of patients experiencing progression at the primary site. Yoshida et al.12 similarly reported that isolated progression, most commonly in the lung, was associated with prolonged survival after TKI failure. Li et al.13 showed that oligometastatic patients often progress as oligoprogression (72.7%), with disease mainly confined to the residual metastatic sites, especially in the lung(60.6%).Another retrospective study of EGFR-mutant NSCLC patients demonstrated similar findings, among the 60 patients with oligoresidual disease, 44 (73%) experienced disease progression confined to the oligoresidual sites14.
These findings suggest that in EGFR-positive NSCLC, recurrence predominantly affects the primary tumor site, and oligometastatic EGFR-mutant NSCLC patients are more likely to develop oligoprogression, particularly at the lung primary site. This may indicate a biologically indolent behavior in this subset of patients, raising the question of whether early radiotherapy intervention could be beneficial for controlling progression and prolonging the effectiveness of systemic therapy in this group.
Based on this, current ASTRO/ESTRO guidelines recommend definitive local therapy for oligometastatic disease5, landmark trials support this recommendation15,16,17,18, but did not differentiate between oligo-progressors and extensively progressed patients. Our study specifically challenges the paradigm in this context of EGFR-driven biology, aiming to investigate the optimal timing of radiotherapy intervention in EGFR-mutant oligometastatic NSCLC patients. According to comparing outcomes between patients receiving radiotherapy during stable disease and after oligoprogression, our findings demonstrate that deferring radiotherapy until oligoprogression extends first-line TKI duration by 7 months (20.3 vs. 13.3 months, P < 0.001), providing a rational for addressing the clinical dilemma of"when to ablate locally” in first-line EGFR-TKI therapy populations.
While PFS1 and RT-PFS did not show statistically significant differences between the two groups, these findings provide complementary context to the observed difference in TKI duration. The absence of significant separation in PFS1 may be due to the fact that all patients initially responded to first-line EGFR-TKI, resulting in similar early disease control. In addition, variability in radiotherapy targets and timing may have attenuated differences in RT-PFS. Although PFS1 and RT-PFS are conventional survival metrics, TKI duration reflects real-world treatment continuity and decision-making. Therefore, we believe these endpoints should be interpreted together, with TKI duration offering additional practical insight into the benefit of delayed radiotherapy in this setting.
Our cohort analysis revealed three key findings. First, the prolonged TKI usage in the post-progression group (20.3 months, 95% CI: 15.10–25.43 vs. 13.3 months, 95% CI: 9.98–16.65, P < 0.001) suggests that the timing of radiotherapy may influence the evolutionary trajectory of resistant clones, with the potential to extend the duration of effective TKI therapy. This supports an evolutionary model where early ablation of TKI-sensitive clones accelerates selection of resistant populations. Second, several studies suggest that oligometastatic NSCLC often progresses at limited sites without a rapid increase in overall tumor burden19,20. In our study, the median 9.8-month interval (95% CI: 5.47–14.13) to oligoprogression implies that EGFR-mutant tumors may present a “golden window” for delayed intervention, offering a distinct opportunity for treatment strategies in patients with slower progression compared to rapid progressors. Finally, lung-targeted radiotherapy independently predicted longer TKI duration (HR = 0.46, 95% CI: 0.26–0.81, P = 0.008), potentially reflecting better control of primary resistant niches in the lung compared to metastases in other anatomical sites, such as bone or adrenal glands. This is consistent with Sun et al.‘s recent study21, which found that combining thoracic radiotherapy with TKI therapy prolonged PFS and OS (mPFS: 17.1 vs. 10.6 months, P = 0.004; mOS: 34.4 vs. 26.2 months, P = 0.029), with treatment-related adverse events (TRAEs) remaining manageable. These findings further support the potential of consolidative radiotherapy to enhance disease control and prolong the efficacy of targeted therapy. These findings highlight the significance of radiotherapy timing and anatomical specificity in optimizing the management of EGFR-mutant NSCLC.
To evaluate the statistical power of our findings, we conducted a post-hoc power analysis. Based on the observed TKI durations and standard deviations between the two groups, with a two-sided significance level of 0.05 and a sample size of 67, the statistical power was calculated to exceed 80%, indicating that our study had sufficient power to detect meaningful differences.
However, our study does have several limitations. First, due to the retrospective design and incomplete documentation, TRAEs, particularly radiation-related toxicities, we only counted the incidence of TRP, and did not consider other adverse effects systematically. Although prior meta-analyses have shown a manageable toxicity profile (e.g., 3.8% incidence of TRP)22,23, real-world safety data from our cohort remains lacking. Secondly, the sample size was relatively small, and the non-randomized design may introduce selection bias. Thirdly, the relatively short follow-up period limits our ability to assess long-term survival outcomes such as overall survival (OS).
Our results suggest that the timing of radiotherapy should be individualized according to disease dynamics. Initiating treatment with systemic EGFR-TKI therapy during the oligometastatic phase, followed by radiotherapy at the time of oligoprogression, may be an effective strategy to prolong TKI duration. Nevertheless, we acknowledge that the retrospective design, non-randomized group allocation, and baseline imbalances may introduce potential bias. Therefore, our conclusions should be interpreted with caution. Future multicenter, prospective trials with larger sample sizes and longer follow-up are essential to confirm the survival benefit associated with delayed radiotherapy. Furthermore, additional studies are warranted to refine radiotherapy protocols to further improve outcomes in patients with EGFR-mutant oligometastatic NSCLC, including treatment modality, dose, fractionation, and target site selection.
Conclusion
This study demonstrates that the timing of radiotherapy in patients with EGFR-mutant oligometastatic NSCLC has a significant impact on the duration of first-line EGFR-TKI therapy. Administering radiotherapy after the onset of oligoprogression was associated with a substantially longer duration of TKI treatment compared to early radiotherapy intervention. These findings highlight the potential clinical value of optimizing treatment sequencing to maximize the efficacy of targeted therapies and improve patient outcomes. However, given the retrospective design and limited sample size, these conclusions should be interpreted with caution.
Materials and methods
Definition of oligometastatic and oligoprogressive disease
In this study, the classification of oligometastatic and oligoprogressive disease was based on the international consensus statement on synchronous NSCLC published in 201924. Oligometastatic disease was defined as the presence of five or fewer metastatic lesions in up to three organs, all of which were technically amenable to local radical treatment. The definition included intrathoracic metastases, such as contralateral lung nodules, pleural lesions, and chest wall involvement, as long as they were isolated, limited in number, and suitable for local therapy.
Oligoprogression was defined as limited progression (≤ 5 new or enlarging lesions) occurring during ongoing EGFR-TKI therapy, with the remainder of the disease remaining controlled. Patients were eligible if the progressive lesions were technically suitable for local treatment, such as radiotherapy or surgery.
We explicitly included patients with isolated thoracic progression (e.g., lung, mediastinum, chest wall) under the oligoprogressive category, in accordance with the consensus recommendation that anatomic location alone should not exclude patients from the oligometastatic spectrum if local treatment is feasible.
Acquisition of clinical data
We conducted a retrospective study on stage IIIB-IV EGFR-mutated NSCLC patients who received first-line TKI therapy and developed oligoprogression between January 2019 and October 2024 at the Affiliated Hospital of Qingdao university. This study was approved by the ethics committees of the Affiliated Hospital of Qingdao university, China (Reference No. QYFYWZLL29747). All experiments involving human participants were conducted in accordance with the Declaration of Helsinki and relevant institutional guidelines and regulations. Due to the retrospective nature of this study, the requirement for informed consent was waived by the Ethics Committee of the Affiliated Hospital of Qingdao University. The inclusion criteria were as follows: 1) Pathologically confirmed diagnosis of oligometastatic NSCLC with an activating EGFR mutation (including Exon19 deletion, Exon 21 L858R, Exon 18 and Exon 20 mutation);2) Initial response to first-line TKI therapy, followed by the development of oligoprogression or oligometastatic disease during ongoing TKI treatment. Of note, patients who received chemotherapy were only included if the chemotherapy was administered concurrently with EGFR-TKI as part of the initial treatment regimen. This approach was not considered a prior line of therapy; 3) Patients classified into two groups based on the timing of radiotherapy in relation to disease progression: (a) The post-progression group included patients who developed oligoprogression or oligometastasis during ongoing TKI therapy. Then they received radiotherapy, continuing the same TKI until further progression. (b) The upfront consolidative RT group comprised patients who had received at least 6–8 weeks (deemed effective) of continuous and effective TKI therapy and underwent consolidative radiotherapy before any evidence of disease progression. The same TKI therapy was continued after RT until oligometastases. Disease progression was rigorously defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by institutional investigators. All imaging was reviewed by board-certified radiologists, and ambiguous cases were discussed and confirmed in multidisciplinary tumor board (MDT) meetings.4) Age ≥ 18 years; 5) Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2. The exclusion criteria were as follows: (1) Incomplete Imaging Data or Irregular Follow-up; (2) Irregular Medication Use or Arbitrary Change of TKI; (3) With other malignancies or severe comorbidities. Oligoprogression was defined as the presence of ≤ 5 metastatic lesions in ≤ 3 organs. It’s important to note that patient enrollment criteria were determined by the course of the disease, and these patients eventually developed oligometastasis or oligoprogression, regardless of initial TNM staging. Some patients were initially diagnosed with stage IIIB or IIIC (M0), but were included in the study after progression to oligometastasis during EGFR-TKI treatment, at the time of radiotherapy, some patients remained in the locally advanced stage, while others had progressed to oligometastasis. A flowchart summarizing the patient grouping and treatment sequence is presented in Figure S2.
EGFR mutation and radiotherapy
Genomic DNA was extracted from paraffin-embedded specimens obtained by percutaneous needle biopsy or bronchoscopy in patients with accessible lesions. For patients with only cytological samples available, methanol-fixed cytologic specimens from pleural effusions or bronchial lavage fluid were used for DNA extraction. EGFR mutation analysis was performed using the ARMS method or direct sequencing, and the specific mutations, including EGFR exon 19 deletions (19del) and EGFR exon 21 L858R point mutations, were identified.
The decision to administer local radiation therapy was made by a multidisciplinary team (including oncologists, radiation oncologists, radiologists, pulmonologists, and other relevant specialists). The radiotherapy plan was developed by taking into account the patient’s overall health, tumor size and location, and other clinical parameters, including organ function.
Patients in the upfront consolidative RT group received radiotherapy during ongoing first-line EGFR-TKI therapy before any radiographic evidence of progression. In these cases, oligometastatic lesions are usually asymptomatic. The MDT team will consider radiotherapy intervention for patients with the following conditions: 1) Stabilized disease following EGFR-TKI treatment: Patients had achieved disease control (either stable disease or partial response on TKIs for at least 6–8 weeks. 2) Residual lesions identifiable on imaging: Despite systemic disease control, imaging revealed residual oligometastatic lesions that were potentially aggressive, persistent, or located at clinically relevant sites. Radiotherapy was added in accordance with current ASTRO/ESTRO guidelines, which recommend the addition of local consolidative therapy (LCT) after initial systemic control in synchronous oligometastatic NSCLC. LCT at this stage aims to reduce tumor burden, eliminate residual foci, and potentially improve PFS by delaying systemic failure5. In this group, radiotherapy was applied to selected oligometastatic lesions rather than all visible lesions.
Patients in the post-progression group received radiotherapy after developing oligoprogressive disease while on EGFR-TKI therapy. The decision to proceed with local treatment in this setting was based on the following MDT-assessed criteria: (1) Lesion accessibility and technical feasibility: Only lesions amenable to safe and effective delivery of radiation, based on anatomical location and dosimetric constraints, were considered for treatment. (2) Symptom control: Radiotherapy was prioritized in patients with symptomatic lesions, including those causing pain, cough, hemoptysis, or neurological symptoms, even in the absence of radiographic progression. (3) Oligometastatic disease burden: Patients with limited number (≤ 5) of metastatic lesions and controlled primary tumor were considered eligible for local therapy, in accordance with the definition of oligometastatic disease. All RT decisions were finalized only after MDT consensus, balancing the potential clinical benefit against risks and patient-specific factors.
Primary tumors were treated with either hypofractionated radiotherapy (50–60 Gy in 3–8 fractions) or conventionally fractionated radiotherapy (56–66 Gy in 28–33 fractions). Bone metastases were treated with conventional fractionated radiation therapy (30 Gy/10 F or 40–50 Gy/20–25 F depends on radiotherapy site), and brain metastases were treated with conventional fractionated radiation therapy (45–50 Gy/10F).
Follow-up and treatment response assessment
Follow-up time was calculated as the interval from the initiation of first-line EGFR-TKI therapy to the date of radiologically confirmed disease progression, as defined by RECIST version 1.1. All patients in the study experienced progression, and no censoring was applied. Patients were evaluated every 3 months until disease progression. Routine surveillance imaging included chest and abdominal CT scans, as well as brain MRI. Bone scans were performed when bone metastasis was suspected, and PET scans were conducted when systemic progression was suspected. Treatment response, local relapse, and distant metastasis were recorded.
The primary endpoint of this study was the duration of first-line EGFR-TKI therapy, defined as the time from the initiation of TKI therapy until the need for a change in treatment due to disease progression; The secondary endpoints were progression-free survival (PFS1), defined as the time from the initiation of TKI therapy to the first disease progression; progression-free survival (PFS2), defined as the time from the first disease progression to subsequent progression; PFS2 was only calculated for patients in the post-progression RT group, as it was defined as the time from first disease progression to second progression or death. Patients in the upfront consolidative RT group had not experienced their first progression at the time of radiotherapy and were therefore not eligible for PFS2 analysis; and radiotherapy progression-free survival (RT-PFS), defined as the time from the initiation of radiotherapy to disease progression; severe treatment-related pneumonitis (TRP), defined as an inflammatory condition of the lungs resulting from cancer therapies such as thoracic radiation therapy, targeted therapies (e.g., EGFR tyrosine kinase inhibitors), or immune checkpoint inhibitors. It is characterized by inflammation of lung tissue, which can lead to symptoms like cough, shortness of breath, and fever.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM). Descriptive statistics were used to summarize the baseline characteristics of patients. Categorical variables were compared using the Chi-square test, and continuous variables were expressed as mean ± standard deviation or median with interquartile range, depending on the data distribution.
Survival analysis was conducted using Kaplan-Meier curves and compared using the log-rank test. For univariate analysis, Cox proportional hazards models were applied to evaluate the association between baseline characteristics and survival outcomes. Variables with a P value < 0.10 (two-sided) in univariate analysis were included in the multivariate Cox proportional hazards model, using a forward selection procedure. In multivariate analysis, P values < 0.05(two-sided) were considered statistically significant.
A post hoc power analysis was performed to evaluate whether the study had adequate power to detect the observed difference in TKI duration between the two groups, using a two-sided significance level of 0.05.
Data availability
The datasets generated and/or analyzed during the current study contain patient-sensitive information and are not publicly available due to confidentiality and ethical restrictions. However, de-identified data may be available from the corresponding author upon reasonable request and with approval from the institutional ethics committee.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- EGFR:
-
Epidermal growth factor receptor
- TKI:
-
Tyrosine kinase inhibitor
- PFS:
-
Progression-free survival
- RT-PFS:
-
Radiotherapy progression-free survival
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- SBRT:
-
Stereotactic body radiotherapy
- TRAEs:
-
Treatment-related adverse events
- ASTRO:
-
American Society for Radiation Oncology
- ESTRO:
-
European Society for Radiotherapy and Oncology
- HR:
-
Hazard ratio
References
Meza, R. et al. Lung cancer incidence trends by gender, race and histology in the united states, 1973–2010. PLoS One. 10 (3), e0121323 (2015).
Wu, Y. L. et al. Alectinib in resected ALK-Positive Non-Small-Cell lung cancer. N Engl. J. Med. 390 (14), 1265–1276 (2024).
Rotow, J. & Bivona, T. G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer. 17 (11), 637–658 (2017).
Weichselbaum, R. R. & Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 8 (6), 378–382 (2011).
Iyengar, P. et al. Treatment of oligometastatic Non-Small cell lung cancer: an ASTRO/ESTRO clinical practice guideline. Pract. Radiat. Oncol. 13 (5), 393–412 (2023).
Gan, G. N. et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving Crizotinib. Int. J. Radiat. Oncol. Biol. Phys. 88 (4), 892–898 (2014).
Wang, X. S. et al. Randomized trial of First-Line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-Mutated Non-Small cell lung cancer. J. Natl. Cancer Inst. 115 (6), 742–748 (2023).
Weickhardt, A. J. et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J. Thorac. Oncol. 7 (12), 1807–1814 (2012).
Rossi, S. et al. Survival outcome of tyrosine kinase inhibitors beyond progression in association to radiotherapy in oligoprogressive EGFR-mutant non-small-cell lung cancer. Future Oncol. 15 (33), 3775–3782 (2019).
Xu, Q. et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with First-Line EGFR-TKIs. J. Thorac. Oncol. 13 (9), 1383–1392 (2018).
Al-Halabi, H. et al. Pattern of failure analysis in metastatic EGFR-Mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J. Thorac. Oncol. 10 (11), 1601–1607 (2015).
Yoshida, T. et al. RECIST progression patterns during EGFR tyrosine kinase inhibitor treatment of advanced non-small cell lung cancer patients harboring an EGFR mutation. Lung Cancer. 90 (3), 477–483 (2015).
Li, X. Y. et al. Analysis of progression patterns and failure sites of patients with metastatic lung adenocarcinoma with EGFR mutations receiving First-line treatment of tyrosine kinase inhibitors. Clin. Lung Cancer. 21 (6), 534–544 (2020).
Miyawaki, T. et al. Association between oligo-residual disease and patterns of failure during EGFR-TKI treatment in EGFR-mutated non-small cell lung cancer: a retrospective study. BMC Cancer. 21 (1), 1247 (2021).
Conibear, J. et al. Study protocol for the SARON trial: a multicentre, randomised controlled phase III trial comparing the addition of stereotactic ablative radiotherapy and radical radiotherapy with standard chemotherapy alone for oligometastatic non-small cell lung cancer. BMJ Open. 8 (4), e020690 (2018).
Peng, P. et al. EGFR-TKIs plus stereotactic body radiation therapy (SBRT) for stage IV Non-small cell lung cancer (NSCLC): A prospective, multicenter, randomized, controlled phase II study. Radiother Oncol. 184, 109681 (2023).
Illini, O. et al. Multidisciplinary treatment of advanced or metastatic ALK-positive non-small cell lung cancer: Real-world data on brigatinib combined with local therapy. Med. (Baltim). 104 (17), e42297 (2025).
Elamin, Y. et al. OA22.04 BRIGHTSTAR local consolidative therapy with brigatinib in tyrosine kinase Inhibitor-Naïve ALK-Rearranged metastatic NSCLC. J. Thorac. Oncol. 18(11), S96 (2023).
Lang, P., Gomez, D. R. & Palma, D. A. Local ablative therapies in oligometastatic NSCLC: new data and new directions. Semin Respir Crit. Care Med. 41 (3), 369–376 (2020).
Chen, Y. H., Ho, U. C. & Kuo, L. T. Oligometastatic disease in Non-Small-Cell lung cancer: an update. Cancers (Basel) 14(5), 1350 (2022).
Sun, H. et al. Thoracic radiotherapy improves the survival in patients with EGFR-Mutated Oligo-Organ metastatic Non-Small cell lung cancer treated with epidermal growth factor Receptor-Tyrosine kinase inhibitors: A multicenter, randomized, controlled, phase III trial. J. Clin. Oncol. 43 (4), 412–421 (2025).
Li, X. et al. Efficacy and safety of EGFR inhibitors and radiotherapy in locally advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 18 (27), 3055–3065 (2022).
Meng, Y. et al. Treatment-Related pneumonitis of EGFR tyrosine kinase inhibitors plus thoracic radiation therapy in patients with Non-Small cell lung cancer: A systematic review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 118 (2), 415–426 (2024).
Dingemans, A. C. et al. Definition of synchronous oligometastatic Non-Small cell lung Cancer-A consensus report. J. Thorac. Oncol. 14 (12), 2109–2119 (2019).
Acknowledgements
Not applicable.
Funding
This work was supported by the HUI LAN Public Welfare.
Author information
Authors and Affiliations
Contributions
H-JW and MZ established the study design. B-YZ and H-JW provided study materials and patients. MZ was responsible for the collection and assembly of data. MZ and QW performed data analysis and interpretation. All authors contributed to the manuscript writing, with final approval of the manuscript provided by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics declarations
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University, China (Reference No. QYFYWZLL29747). All experiments involving human participants were conducted in accordance with the Declaration of Helsinki and institutional guidelines and regulations. Given the retrospective nature of this study, the requirement for informed consent was waived by the Ethics Committee of the Affiliated Hospital of Qingdao University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, M., Wang, L., Wang, Q. et al. Evaluation of the optimal timing of radiotherapy in EGFR-Mutant oligometastatic NSCLC through a retrospective analysis of treatment sequences and survival outcomes. Sci Rep 15, 30650 (2025). https://doi.org/10.1038/s41598-025-15056-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15056-y