Abstract
Sensorineural hearing loss (HL) is becoming more prevalent among people of all ages. Several studies have suggested that insulin resistance (IR) could impact hearing. The aim of this work was to demonstrate the relationship between IR and HL based on the surrogate marker of metabolic score for IR (METS-IR). This cross-sectional study eventually enrolled 3,921 adults (weighted population: 43,438,988) based on National Health and Nutrition Examination Survey 1999–2018 (NHANES). Sensorineural HL was divided into low-frequency HL (LFHL), speech-frequency HL (SFHL), and high-frequency HL (HFHL) based on the better ear’s pure-tone average (PTA). We used multivariate logistic regression analysis to explore the association between METS-IR and risk of HL. 798 (weighted 16.3%), 1,124 (weighted 24.6%), and 2,043 (weighted 49.4%) participants with LFHL, SFHL, and HFHL were eventually included in this research, respectively. Individuals with METS-IR in the highest tertile had higher risks of SFHL with OR of 1.814 (95% CI: 1.180–2.790) than those in the lowest tertile. Linear relationships were found between METS-IR and sensorineural HL. In conclusions, our results suggested a positive association between METS-IR and the risk of sensorineural SFHL. Early management of IR might be beneficial for HL. Considering the limitations of cross-sectional studies, the evidence from longitudinal and follow-up studies are still needed.
Similar content being viewed by others
Introduction
Hearing is one of the most important human senses and is also essential for effective communication, emotional health, and quality of life1. Hearing loss (HL) is becoming more prevalent not only among older people but across people of all ages. Around 1.5 billion people in the world face HL currently, and this number is estimated to reach 2.5 billion by 20502. Although part of patients will benefit from hearing rehabilitation services, there is no doubt that HL has had physical and mental impacts on them2. Thus, identifying risk factors for HL is necessary to develop prevention and intervention strategies.
The association between metabolic diseases and HL has gradually received extensive attention in recent years3. Insulin resistance (IR), as the core pathological state of metabolic disorder, is characterized by the decreased response of cells to insulin4. IR is involved in the development of several metabolic diseases, such as non-alcoholic fatty liver disease, cardiovascular diseases, type 2 diabetes mellitus (T2DM), polycystic ovary syndrome, metabolic syndrome, and obesity5,6. IR has been reported to influence the auditory system via microvascular dysfunction and peripheral neuropathy7. The mechanisms mentioned above jointly constitute the ‘metabolism-vascular-nerve’ theoretical framework for exploring the relationship between IR and HL.
IR is not an isolated disease but a complex state closely intertwined with disorders of glycolipid metabolism, accumulation of visceral fat and chronic inflammation4. Some insulin sensitivity indicators that rely on insulin level testing are not suitable for promotion in primary medical institutions due to the standardization difficulties and high cost of insulin testing8. Triglyceride-glucose index (TyG), a non-insulin dependent indicator of IR, was also reported to have an L-shaped association with the risk of sensorineural HL9. Nevertheless, the definition of HL in their research was inconsistent with the World Report on Hearing and may have overestimated the sample size of patients with HL. Moreover, the calculation of TyG is so simple that IR could not be assessed accurately. Metabolic score for IR (METS-IR) is a new non-insulin dependent indicator, which is calculated based on fasting plasma glucose (FPG), triglyceride (TG), body mass index (BMI), and high-density lipoprotein cholesterol (HDL-C)8. Thus, METS-IR can simultaneously reflect the following pathological processes, including abnormal glucose metabolism, lipid metabolism imbalance and central obesity. This multi-index synergy enables METS-IR to quantify the overall burden of metabolic disorders more comprehensively. METS-IR, which relies on conventional metabolic indicators, avoids the shortcomings of insulin detection, making it more suitable for primary medical care or epidemiological research10. Therefore, the present research was designed to evaluate the association between IR and sensorineural HL based on METS-IR.
Results
Baseline characteristics
The sample in this research consisted of 3,921 participants (weighted population: 43,438,988) containing 1,984 (weighted 49.7%) males and 1,937 (weighted 50.3%) females. There were 798 (weighted 16.3%), 1,124 (weighted 24.6%), and 2,043 (weighted 49.4%) participants with LFHL, SFHL, and HFHL, respectively. Table 1 shows the baseline characteristics of the enrolled participants. Compared to those without HL, affected individuals were older, had higher rates of diabetes, hypertension, ototoxic medication use, and occupational noise exposure, and more likely to consume cigarette and alcohol. FBG and TG in the participants with HL were much higher than those in normal participants. Median METS-IR in the participants with LFHL, SFHL, and HFHL were 42.6, 43.4, and 42.4, which were higher than those in the individuals without HL (Table S1-3).
Relationships between METS-IR and HL
Table 2 presented the logistic regression analysis results. As a continuous variable, METS-IR was statistically significantly positively associated with the odds of LFHL, SFHL, and HFHL with or without adjusting for covariates. Then, the participants were divided into three groups based on METS-IR tertiles. The ranges of METS-IR tertiles 1–3 were < 36.3, 36.3≤ &<46.6, and ≥ 46.6, respectively. The 3rd tertiles of METS-IR were positively correlated with the odds of three types of HL compared to the lowest tertile in the crude model. In the partially adjusted model (Model 1), the 3rd tertile of METS-IR was also positively related to the odds of different types of HL compared to the 1st tertile. In the fully adjusted model (Model 2), the 3rd tertile of METS-IR was only positively associated with the risk of SFHL. However, all P values for the association between any tertile of METS-IR and LFHL as well as HFHL were over 0.05 in Model 2. These results suggest that higher METS-IR increased the incidence of SFHL whether METS-IR is a continuity variable or a categorical variable. Poisson regression results indicated that the 3rd tertile of METS-IR was also positively associated with the incident rate of different types of HL compared to the 1st tertile (Table S4) (Fig. 1).
Nonlinear relationships detection and subgroup analysis
The RCS regression model was utilized to explore any nonlinear association between METS-IR and three types of HL (Fig. 2). The results suggested that no nonlinear trends were observed for LFHL, SFHL, or HFHL. The impact of different variables on the associations between METS-IR and SFHL was further detected by subgroup analysis based on diabetes, alcohol user, and hypercholesterolemia (Fig. 3). Subgroup analysis results demonstrated that the relationships between METS-IR and SFHL were markedly stronger in participants without diabetes or hypercholesterolemia.
Restricted cubic spline (weighted) of the relationship between METS-IR and the risk of hearing loss. The model was adjusted for age, gender, race, alcohol user, smoker, hypertension, diabetes, total cholesterol, ototoxic medication use, and noise exposure (firearm, occupational and off-work noise). The solid red lines and shaded areas represent the METS-IR OR and 95% CI, respectively.
Sensitivity analysis
The stability of the results in this study was validated by sensitivity analysis. We first explored the relationships between METS-IR and sensorineural HL in imputed data, the results of which indicated similar trend to our previous findings (Table S5). Then, we categorized METS-IR into dichotomous groups and quartiles. Multivariate logistic regression analysis was performed for sensorineural HL. With dichotomous groups, compared to low METS-IR group, the participants in high METS-IR group had a 45.2% increase in the likelihood of SFHL (Table S6). Within the quartile of METS-IR, compared with the reference of lowest quartile, the highest quartile had a 69.7% increase in the likelihood of SFHL (Table S7). These findings were largely consistent with the primary analysis and underscored the robust stability of our results.
Discussion
This cross-sectional study revealed a positive relationship between METS-IR and sensorineural SFHL among 3,921 individuals (weighted population: 43,438,988) from U.S. communities. This effect was especially significant in participants without diabetes or hypercholesterolemia. METS-IR had no significant effect on the risks of LFHL and HFHL after adjusting for covariates.
Existing studies on the association between IR and HL mainly focus on TyG, which is a surrogate marker of IR calculated according to TG and FPG9,11,12,13. The results from two studies have suggested that high TyG index has a positive association with HFHL but LFHL among Americans and Koreans, respectively11,12. A research performed by Wang et al. also has reported that high TyG is related to the increased risk for SFHL9, . These results are consistent with parts of those obtained in this study. The effects of metabolic disorders and obesity on HL have received much attention14,15. A study based on the Chinese population over 45s found that higher BMI and TyG-BMI were related to the elevated HL risk instead of TyG13. These findings highlight the importance of metabolic disorders and obesity in HL. Several studies have indicated that visceral and abdominal fat are correlated with the average hearing threshold among Asian women16,17,18. Therefore, it is necessary to consider metabolic disorders and obesity simultaneously when using IR to assess the risk of HL. METS-IR, as a simple and indirect IR detection method, not only has a similar IR prediction ability to TyG-BMI but also has a significant correlation with visceral, intrahepatic, and intrapancreatic fat content. METS-IR might be a relatively simple and reliable surrogate marker of IR for predicting HL.
IR is one of the pathophysiological abnormalities of T2DM. When tissues including muscle, fat, and liver begin to exhibit IR, they will not react properly to insulin19. Then, the pancreas will produce more insulin to eliminate glucose from the blood to the cells, which may contribute to T2DM eventually. Diabetes is commonly considered a risk factor for sensorineural HL20. The incidence of HL in people with diabetes is twice that of people without diabetes, while individuals with prediabetes also have a 30% higher rate of HL compared to those without diabetes20. This result suggested that patients already have risk factors for HL before they develop diabetes. In the present work, high METS-IR elevated risk of sensorineural SFHL among the participants without diabetes. Among the participants with diabetes, no longer has an impact on the risk of sensorineural SFHL. Thus, assessing IR may be useful in developing hearing protection interventions for HL, especially in the pre-diabetic stage.
Hypercholesterolemia is demonstrated as a risk factor for HL21. The close relationship between METS-IR and SFHL was observed in individuals with normal total cholesterol. This means that when total cholesterol is normal, IR might become the main cause of SFHL. Pigs with destruction of oxosterol-binding protein-like 2, which mainly participated in maintaining cholesterol homeostasis through cholesterol transport, exhibit progressive HL and hypercholesterolaemia22. Drug center screening through whole cochlear single-cell sequencing revealed that statins are the preferred drugs for the treatment of noise-induced HL23. Thus, when the hypercholesterolemia itself has caused severe damage, the superimposed effect of IR might be masked or difficult to further intensify after reaching the damage threshold.
The exact mechanism of the link between IR and HL is still unclear. Researches have suggested that microangiopathy and peripheral neuropathy are the main bridges between IR and HL24,25. IR-induced microangiopathy can be accompanied by vascular atrophy and decreased capillary density in stria vascularis26. Endothelial dysfunction also appears to play a role in the initiation of microvascular pathology, including thickening of the basement membrane of the capillary striae within the cochlea and narrowing of the capillary lumen27. These alterations in the microcirculation of inner ear could have a profound influence on the blood supply of the inner ear and eventually contribute to HL.
Several limitations should still be acknowledged. Firstly, this work was a cross-sectional research and the data was from the database of NHANES. Thus, the results between exposure and outcomes could only infer correlation, not causation. The observed associations might also be reverse causation, shared confounding factors, or the possibility of spurious association. Secondly, all the participants were from U.S. communities, caution needs to be exercised in interpreting HL risk according to the current results. More studies from other regions are still necessary. Thirdly, it is plausible that other unknown covariates, including noise exposure levels and genetic factors, could affect the effectiveness of the conclusions despite adjusting for multiple covariates in the adjusted models. Fourthly, the determination of HL in this study was assessed primarily by PTA, which may lead to bias against the conductive components of HL. Finally, the cohort of this study only accounts for a small part of the original NHANE 1999–2018 cohort and cannot fully represent the entire cohort, the conclusion may underestimate the effect on the elderly population. Therefore, caution is needed when interpreting the results of this study.
In conclusion, positive association between METS-IR and the risk of sensorineural SFHL was observed. This relationship was markedly stronger in individuals without diabetes or hypercholesterolemia. Early management of IR might be beneficial for SFHL. Considering the limitations of cross-sectional studies, the evidence from longitudinal and follow-up studies are still needed.
Methods
Research participants
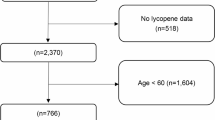
All data used in this investigation was obtained from the NHANES survey conducted between 1999 and 2018. As described previously, the NHANES database is a comprehensive, stratified, multistage, and cross-sectional survey program designed to evaluate the health and nutritional status of the noninstitutionalized U.S. population28. This cross-sectional study followed the criteria of the STROBE guidelines. The screening process of participants is shown in Fig. 1. From the initial cohort of 101,316 participants, 78,765 participants were excluded who had no audiometry data, and a further 2,625 participants were excluded who had no data to calculate METS-IR. Furthermore, 16,005 participants who lacked data on race, BMI, history of hypertension and diabetes, history of smoking and alcohol, or noise exposure data were also excluded. Following the above screening procedure, 3,921 eligible participants (weighted population: 43,438,988) were enrolled in this research eventually.
Audiometric measurement
As described in our previous work, hearing thresholds of both ears were measured at 0.5, 1, 2, 3, 4, 6, and 8 kHz according to standardized audiometry protocol29. Sensorineural HL was defined in accordance to the World Report on Hearing and was divided into low-frequency HL (LFHL) (pure-tone average (PTA) at 0.5, 1, and 2 kHz ≥ 20 dB), speech-frequency HL (SFHL) (PTA at 0.5, 1, 2 and 4 kHz ≥ 20 dB), and high-frequency HL (HFHL) (PTA at 4, 6, and 8 kHz ≥ 20 dB) in the better ear30.
METS-IR measurement
The calculation formula for METS-IR was shown as follow8: Ln[2 × FPG (mg/dL) + TG (mg/dL)] × BMI (kg/m²)/Ln[HDL-C(mg/dL)]. FPG (mg/dL), TG (mg/dL), and HDL-C (mg/dL) levels were strictly determined after fasting for at least 8.5 h. following the standard operating procedures. Meanwhile, BMI was calculated by dividing weight (kg) by the square of height (m) as a standardized weight index.
Covariate assessment
The covariates included age, gender, race, BMI, laboratory measurements, history of hypertension and diabetes, status of smoking and alcohol, noise exposure of firearm, occupational, and off-work. The information on lifestyle habits, complication history, and noise exposure history were obtained by self-reports.
Statistical analysis
All the data analyses were weighted as recommended to represent the US population. Briefly, normality of the continuous variables was tested by Kolmogorov-Smirnov test28. Normally distributed continuous variables were evaluated by an independent sample Student’s t-test and presented as mean ± standard deviation (SD), while non-normally distributed continuous variables were analyzed by nonparametric Mann-Whitney U tests and presented as medians with interquartile ranges (IQRs). Categorical variables were assessed by the Chi-square test or Fisher’s exact test and presented as numbers and weighted percentages.
Relationships between METS-IR and several kinds of sensorineural LFHL, SFHL, and HFHL was presented as odd ratios (OR) with 95% confidence interval (CI) by univariate and multivariate logistic regression analysis, weighted by the survey package. Poisson regression analysis was also used to estimate incident rate ratio. No covariates were adjusted in the crude Model. The covariates of age, gender, and race were adjusted in Model 1. The covariates of age, gender, race, alcohol user, smoker, hypertension, diabetes, total cholesterol, ototoxic medication use, and the exposure of firearm, occupational, and off-work noise were adjusted in Model 2. Nonlinear associations between METS-IR and sensorineural HL were assessed by restricted cubic spline (RCS) regression models. All RCS regression models were adjusted for covariates consistent with Model 2. Subgroup analysis was conducted to explore potential factors influencing the associations between METS-IR and sensorineural SFHL. Interaction analyses were performed before proceeding with stratified analyses. Then, for subgroup analysis, sensorineural SFHL was stratified by the factors whose P for interaction < 0.05. Bonferroni correction was performed on the subgroup analysis results to decrease the risk of type I errors caused by multiple testing. The adjusted significance level was α’ = 0.05/n and n was the subgroups number (n = 3) in this research. Thus, we consider that the results were still statistically significant only when the P value < 0.017. Moreover, Bonferroni correction could also increase the type II error risk, cautious should be taken when these results were interpreted. We performed a sensitivity analysis to verify the stability of the results.
R environment (version 4.4.1) was used to analyse all the data in the present research. A two-tailed P < 0.05 was considered statistically significant. The original code used in this study was attached as Supplementary file 1. We would be most grateful if one could reference this article when using this original code.
Data availability
All data come from the information sharing platform (http://www.cdc.gov/nchs). All of them are available from the corresponding author on reasonable request.
References
Baumgartner, W. D. et al. Experts’ discussion: implications of the world health organization’s world report on hearing for the cochlear implant field. Braz. J. Otorhinolaryngol. 91, 101556. https://doi.org/10.1016/j.bjorl.2024.101556 (2025).
Babajanian, E. E. & Gurgel, R. K. Cognitive and behavioral effects of hearing loss. Curr. Opin. Otolaryngol. Head Neck Surg. 30, 339–343. https://doi.org/10.1097/moo.0000000000000825 (2022).
Cavallaro, G. et al. Endothelial dysfunction and metabolic disorders in patients with sudden sensorineural hearing loss. Medicina 59, 1718 (2023).
Martins, F. O. & Conde, S. V. Impact of diet composition on insulin resistance. Nutrients 14, 3716 (2022).
Lei, R., Chen, S. & Li, W. Advances in the study of the correlation between insulin resistance and infertility. Front. Endocrinol. 15, 1288326. https://doi.org/10.3389/fendo.2024.1288326 (2024).
Fahed, G. et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 23, 786 (2022).
Seo, M., Lee, Y. S. & Moon, S. S. Association of hearing impairment with insulin resistance, β-cell dysfunction and impaired fasting glucose before onset of diabetes. Diabet. Medicine: J. Br. Diabet. Association. 33, 1275–1282. https://doi.org/10.1111/dme.13096 (2016).
Bello-Chavolla, O. Y. et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 178, 533–544. https://doi.org/10.1530/eje-17-0883 (2018).
Wang, Y. et al. L-shaped association of triglyceride glucose index and sensorineural hearing loss: results from a cross-sectional study and Mendelian randomization analysis. Front. Endocrinol. 15, 1339731. https://doi.org/10.3389/fendo.2024.1339731 (2024).
Shirinezhad, A. et al. A systematic review of the association between insulin resistance surrogate indices and bone mineral density. Front. Endocrinol. 15, 1499479. https://doi.org/10.3389/fendo.2024.1499479 (2024).
Liu, L., Qin, M., Ji, J. & Wang, W. Correlation between hearing impairment and the triglyceride glucose index: based on a National cross-sectional study. Front. Endocrinol. 14, 1216718. https://doi.org/10.3389/fendo.2023.1216718 (2023).
Kim, D. O. et al. Correlation between hearing impairment and the triglyceride glucose index in Middle-Aged female based on a Korean National health and nutrition examination survey. Medicina 60, 1596 (2024).
Wang, C. et al. Relationship between TyG-related index and hearing loss in people over 45 s in China. Front. Public. Health. 13, 1506368. https://doi.org/10.3389/fpubh.2025.1506368 (2025).
Park, C. H. et al. Impact of low skeletal muscle mass and obesity on hearing loss in asymptomatic individuals: A Population-Based study. Healthcare 10, 2022 (2022).
Croll, P. H. et al. The association between obesity, diet quality and hearing loss in older adults. Aging 11, 48–62. https://doi.org/10.18632/aging.101717 (2019).
Kang, S. H. et al. Visceral fat area determined using bioimpedance analysis is associated with hearing loss. Int. J. Med. Sci. 12, 946–951. https://doi.org/10.7150/ijms.13184 (2015).
Lee, Y. & Park, M. Relationships among factors relevant to abdominal fat and Age-Related hearing loss. Clin. Exp. Otorhinolaryngol. 10, 309–314. https://doi.org/10.21053/ceo.2017.00017 (2017).
Kim, T. S. et al. Visceral adipose tissue is significantly associated with hearing thresholds in adult women. Clin. Endocrinol. 80, 368–375. https://doi.org/10.1111/cen.12184 (2014).
Sadeghi, A. et al. Crosstalk between autophagy and insulin resistance: evidence from different tissues. Eur. J. Med. Res. 28 https://doi.org/10.1186/s40001-023-01424-9 (2023).
Samocha-Bonet, D., Wu, B. & Ryugo, D. K. Diabetes mellitus and hearing loss: A review. Ageing Res. Rev. 71 https://doi.org/10.1016/j.arr.2021.101423 (2021).
Wu, J. et al. Impact of cholesterol homeostasis within cochlear cells on auditory development and hearing loss. Front. Cell. Neurosci. 17, 2023. https://doi.org/10.3389/fncel.2023.1308028 (2024).
Yao, J. et al. OSBPL2-disrupted pigs recapitulate dual features of human hearing loss and hypercholesterolaemia. J. Genet. genomics = Yi Chuan Xue Bao. 46, 379–387. https://doi.org/10.1016/j.jgg.2019.06.006 (2019).
Rodrigo, L., Campos-Asensio, C., Rodríguez, M., Crespo, I. & Olmedillas, H. Role of nutrition in the development and prevention of age-related hearing loss: A scoping review. J. Formos. Med. Association = Taiwan. Yi Zhi. 120, 107–120. https://doi.org/10.1016/j.jfma.2020.05.011 (2021).
Muniyappa, R. & Sowers, J. R. Role of insulin resistance in endothelial dysfunction. Reviews Endocr. Metabolic Disorders. 14, 5–12. https://doi.org/10.1007/s11154-012-9229-1 (2013).
Agrawal, Y., Carey, J. P., Della Santina, C. C., Schubert, M. C. & Minor, L. B. Diabetes, vestibular dysfunction, and falls: analyses from the National health and nutrition examination survey. Otology & neurotology: official publication of the American otological society. Am. Neurotology Soc. [and] Eur. Acad. Otology Neurotology. 31, 1445–1450. https://doi.org/10.1097/MAO.0b013e3181f2f035 (2010).
Lee, Y. Y. et al. Type 1 diabetes induces hearing loss: functional and histological findings in an Akita mouse model. Biomedicines 8, 343 (2020).
Gioacchini, F. M. et al. Diabetes mellitus and hearing loss: A complex relationship. Medicina 59, 269 (2023).
Wang, Y., Yang, H. & Huang, C. Association of serum lycopene with low-frequency hearing loss in adults in their 70s based on NHANES database. Sci. Rep. 15, 600. https://doi.org/10.1038/s41598-024-84341-z (2025).
Yang, H. et al. The association of sex steroid hormone concentrations with hearing loss: a cross-sectional study. Acta Otolaryngol. 143, 582–588. https://doi.org/10.1080/00016489.2023.2224398 (2023).
Chadha, S., Kamenov, K. & Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 99, 242–242a. https://doi.org/10.2471/blt.21.285643 (2021).
Funding
This project was supported by the General Project of Science and Technology Development Fund of Nanjing Medical University (Grant No. NMUB20240116).
Author information
Authors and Affiliations
Contributions
Q.H. and L.W. designed this study. L.W. and H.Y. analyzed the data; Z.D. prepared tables and figures; Q.H. interpreted the results of this study; L.W. wrote the original manuscript; H.Y. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, L., Yang, H., Ding, Z. et al. Association between hearing loss and insulin resistance as measured by metabolic score for insulin resistance in NHANES 1999 to 2018. Sci Rep 15, 29328 (2025). https://doi.org/10.1038/s41598-025-15059-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15059-9