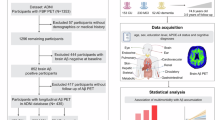
Abstract
Early, non-invasive assessment of Alzheimer’s disease (AD) progression remains a key challenge. This study evaluated whether nasal amyloid-β42 (Aβ42) levels reflect brain amyloid dynamics and cognitive decline. Nasal discharge from 161 individuals, ranging from cognitively unimpaired to AD dementia, was analyzed using ELISA, alongside neuropsychological assessments and amyloid PET imaging. Moderate nasal Aβ42 levels (9.53–11.10 pg/mL) were positively associated with PET amyloid burden and cognitive decline, identifying a critical transitional disease stage. Conversely, the highest Aβ42 levels showed weaker correlations, suggesting a non-linear progression. The pattern of nasal Aβ42 mirrored brain amyloid accumulation, which peaks and stabilizes in later disease stages. These findings highlight nasal Aβ42 as a promising, scalable biomarker for tracking AD pathology and offer the first evidence linking it with brain amyloid PET. This supports its potential use in both clinical and longitudinal research settings.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) poses growing societal and economic burdens, intensified by the rapid aging of the global population1. Despite substantial advances in understanding AD pathology, clinical trials aimed at halting or reversing disease progression have not yet yielded broadly effective treatments. This underscores the urgent need for accessible, non-invasive indicators capable of reflecting disease progression and underlying neuropathological mechanisms. Such indicators would significantly enhance our understanding of AD pathogenesis and potentially guide timely therapeutic interventions.
Currently, AD identification primarily involves familial medical history assessments, neuropsychological evaluations, advanced neuroimaging techniques—such as magnetic resonance imaging (MRI) or positron emission tomography (PET)—and analysis of cerebrospinal fluid (CSF) or blood biomarkers. Core biomarkers in CSF and blood have demonstrated strong correlations with central pathological features, notably amyloid deposition and tau pathology2,3. Nevertheless, these methods face practical limitations, including the invasive nature and risks of lumbar punctures, complexity and high costs of imaging modalities, and ongoing technical challenges in adopting blood-based assays for broader clinical use.
To overcome these challenges, alternative peripheral body fluids that are easily accessible and reflect central pathological changes should be explored4. Among these, nasal discharge fluid is particularly promising due to its anatomical proximity to the olfactory epithelium (OE). Olfactory dysfunction, such as anosmia or hyposmia, frequently precedes cognitive symptoms in AD5,6,7,8,9,10,11. This early dysfunction is closely associated with neurodegeneration within olfactory pathways and related brain regions, including the olfactory bulb (OB), entorhinal and transentorhinal cortices, and medial temporal lobes—regions known to overlap significantly with areas involved in early AD pathology5,12,13. Structural and functional abnormalities, including altered amyloid precursor protein (APP) processing and neuroinflammation, further indicate the presence of olfactory neuropathology14,15,16,17,18. Indeed, multiple postmortem and antemortem studies consistently identified classical AD hallmarks, such as intracellular neurofibrillary tau tangles and amyloid plaques, within central olfactory processing regions19,20,21,22,23.
Nasal discharge fluid bathes the OE, containing cellular debris derived from olfactory sensory neurons (OSNs), offering unique insights into neuronal and neuropathological processes closely linked to the brain. Given the high turnover and regenerative capacity of peripheral OSNs, nasal fluid may reflect ongoing neuronal changes associated with early stages of AD pathology24. Furthermore, its collection is straightforward, non-invasive, and cost-effective compared to established methods involving CSF sampling or advanced imaging modalities, thereby increasing its practical utility in both research and clinical settings.
Previous studies have identified Aβ peptides and APP within the olfactory mucosa samples obtained postmortem from AD patients20,25. Aggregated Aβ was detected in the olfactory mucosa of 71% of AD patients, compared to 22% of normal individuals and 14% of cases with other neurodegenerative diseases26. Biopsy examinations of the olfactory mucosa further confirmed the presence of these pathological proteins across the AD continuum, including mild cognitive impairment (MCI)27. Recent immunoassay-based studies revealed elevated oligomeric Aβ levels in nasal discharge fluid from AD patients compared to cognitively normal individuals, highlighting nasal fluid’s sensitivity to central AD-related neuropathological processes28,29.
Based on these observations, we hypothesized that nasal Aβ42 concentrations measured in nasal discharge fluid reflect neuropathological changes associated with AD progression, particularly amyloid deposition dynamics. To test this hypothesis, we quantitatively analyzed nasal Aβ42 levels across the entire AD continuum. Our findings suggest that nasal Aβ42 levels indeed mirror central neuropathological changes occurring in the brain, potentially clarifying the trajectory of AD proteinopathies and disease progression. Therefore, monitoring nasal Aβ42 presents a promising and non-invasive avenue for investigating the underlying pathological processes of AD, contributing to therapeutic research and advancing our understanding of disease pathogenesis.
Materials and methods
Participants and study design
All assessed participants were recruited from the Catholic University of Korea Yeouido St. Mary’s Hospital in Seoul and this study was approved by the Catholic University of Korea Institutional Review Boards (IRB) (IRB Number SC18TNSI0063). All clinical assessments and nasal discharge sample collections were conducted between October 2018 and October 2019. Participants underwent comprehensive clinical and neuropsychological assessment, including Clinical Dementia Rate (CDR) to stage the severity of dementia, the Global Deterioration Scale (GDS) to assess global cognitive decline, and the Korean version of Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery to evaluate multiple cognitive domains30,31,32. Korean version of Mini-Mental State Examination (MMSE) was also administered as a general cognitive screening tool. Following neuropsychological assessment, patients were classified as cognitively unimpaired (CU), mild cognition impaired (MCI), and AD dementia, following the criteria provided by the National Institute on Aging-Alzheimer’s Association (NIA-AA) and Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Out of 218 individuals initially screened for eligibility, 57 were excluded due to the presence of one or more of the following conditions: systemic illnesses known to affect cognition (such as thyroid disorders, severe anemia, or syphilis), significant sensory impairments (hearing or vision), neurological conditions (including brain tumors, encephalitis, or epilepsy), major cerebrovascular pathology, medications with cognitive side effects, or contraindications to undergoing MRI. As a result, 161 participants were included in the final analysis. All methods were performed in accordance with the relevant guidelines and regulations.
Nasal discharge fluid sampling and analysis
All subjects provided written informed consent before participating via self-referral or referral from their family. Samples were collected according to a protocol previously described and delivered to DGIST for analysis29. The whole nasal discharges were pooled (> 1.5 mL) in a microtube and immediately sonicated for 10–15 s, followed by centrifugation (10,000xg for 10 min at 4 °C) to remove cells and cellular debris. A Protease Inhibitor Cocktail was added to the supernatants (Roche, Mannheim, Germany). Samples were then rapidly frozen for permanent storage at − 80 °C. Nasal Aβ42 levels were quantified using enzyme-linked immunosorbent assay (ELISA; Thermo Fisher Scientific, #KHB3544) following the manufacturer’s instructions. All ELISA measurements were conducted between July and October 2020 after long-term frozen storage. This high-sensitivity assay has an intra-assay coefficient of variation of 8.6% and an analytical sensitivity below 1 pg/mL, with an assay range of 1.56–100 pg/mL. The corresponding standard deviation (~ 0.7–0.9 pg/mL in the measured range) supports the resolution of adjacent quartile boundaries (e.g., Q2: 7.63–9.53 pg/mL vs Q3: 9.53–11.10 pg/mL).
Aβ-PET imaging
All patients underwent Aβ-PET using [18F]flutemetamol and information regarding [18F]flutemetamol production, data collection, and analytical results were described previously33. We used T1 MRI images of each individual for co-registration, defining regions of interest (ROIs), and correction of partial volume effects associated with expansion of the cerebrospinal spaces due to cerebral atrophy. The standardized uptake value ratio (SUVR) measured 90 min post-injection was utilized for [18F]flutemetamol PET data analysis. To define global cerebral Aβ burden, SUVRs of the six cortical ROIs (frontal, superior parietal, lateral temporal, striatum, anterior cingulate cortex, and posterior cingulate cortex/ precuneus) were averaged. Two nuclear medicine radiologists separately confirmed amyloid positivity based on visual readings.
Statistical analysis
All statistical analyses were performed using GraphPad Prism and SPSS (version 23.0; IBM Corp, Armonk, New York, USA). Descriptive statistics for the subject characteristics were generated in which frequencies and percentages were calculated for categorical variables and means and standard deviations were calculated for continuous variables. Values are presented as mean ± standard deviation (SD) or standard error of the mean (SEM), and individual data points represent individual samples. Statistical significance was determined at a p-value below of 0.05, while p-values below or equal to 0.05, 0.01, 0.001, and 0.0001 were represented by *, **, ***, and ****, respectively.
The normality distribution of the nasal Aβ42 data was evaluated using the Kolmogorov–Smirnov test. Depending on the distribution characteristics of nasal Aβ42 data, participants were divided into quartiles. This quartile was used as an indicator to analyze the correlation between nasal Aβ42 levels and cognitive function measurements. Chi-square test and Fisher’s exact tests were applied for categorical variables, as appropriate. Student’s t-test was used to demonstrate statistical differences between two groups, and One-way ANOVA or two-way ANOVA with Dunnett’s or Turkey’s post hoc test was used when appropriate.
Multivariate logistic regression was used to assess the predictive utility of nasal Aβ42 levels and cognitive measures (CDR, GDS, MMSE) for AD diagnosis. Both unadjusted and adjusted models were tested, with adjustment for age, sex, and years of education. Regression analyses included either clinical diagnoses or cognitive scores as covariates. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. Receiver operating characteristic (ROC) curve analysis was used to evaluate sensitivity, specificity and area under the curve (AUC) for nasal Aβ42 levels and cognitive function measurements to AD diagnosis. ROC curve analysis to evaluate the ability of nasal Aβ42 levels and cognitive function measurements to AD diagnosis, logistic regression models with the same covariates and confounding variables as above mentioned the regression model were calculated.
Results
Study population
We included in total 161 participants from the Catholic University of Korea Yeouido St. Mary’s Hospital in Seoul. Participants underwent neuropsychological testing, including Clinical Dementia Rate (CDR), Global Deterioration Scale (GDS), Mini-Mental State Examination (MMSE), and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). These participants were classified as cognitively unimpaired (CU), Preclinical, MCI, and AD (n = 32 CU, n = 29 with Preclinical, n = 73 with MCI, n = 27 with AD), according to the criteria provided by National Institute on Aging-Alzheimer’s Association (NIA-AA) and Diagnostic and Statistical Manual of Mental Disorders (DSM-V)34. Participants were selected based on the following inclusion criteria, adapted from a previous study35: age 55 years or older and absence of clinically significant psychiatric disorders, including depressive disorder, schizophrenia, and bipolar disorder. CU subjects were recruited from individuals undergoing brain examinations as part of routine health checkups at the outpatient clinic. Their cognitive function was assessed using the Korean version of CERAD. MCI was diagnosed based on the following criteria: (1) subjective memory complaints corroborated by an informant; (2) objective cognitive impairment in multiple domains (at least 1.0 standard deviation below age- and education-adjusted norms on Korean version of CERAD); (3) preserved activities of daily living; (4) CDR score of 0.5; and (5) absence of dementia according to DSM-V criteria. AD patients’ diagnosis adhered to the probable AD criteria established by the National Institute of Neurological and Communicative Disorders and Stroke and AD and Related Disorders Association (NINCDS-ADRDA), as well as the DSM-V criteria, with positive amyloid PET results36,37. Table 1 summarizes the demographic and clinical characteristics of this study.
Assessing the association between nasal Aβ42 levels and cognitive impairment
We first conducted immunoassays using enzyme-linked immunosorbent assay (ELISA) to identify if the nasal Aβ42 levels were measurable in human nasal discharge fluid. No significant difference was observed in mean nasal Aβ42 levels across the CU, Preclinical, MCI, and AD groups (p = 0.487). To investigate the association between nasal Aβ42 levels and cognitive function, we stratified nasal Aβ42 level into quartile (Q) groups38,39. The first quartile group (Q1) consisted of participants with lower than or equal to 7.63 pg/mL of nasal Aβ42 (n = 41), the second quartile group (Q2) with greater than 7.63 pg/mL and lower than or equal to 9.53 pg/mL of nasal Aβ42 (n = 40), the third quartile group (Q3) with greater than 9.53 pg/mL and lower than or equal to 11.10 pg/mL of nasal Aβ42 (n = 40), and the last quartile group (Q4) with greater than 11.10 pg/mL of nasal Aβ42 (n = 40).
When we analyzed the groups divided by the clinician’s diagnostic groups, we found that the second-highest quartile group (Q3) constituted the majority of patients with AD diagnosis (Fig. 1A; p = 0.036). When we examined the groups by different neuropsychological testing results, in all three tests, CDR, GDS, and MMSE, the Q3 groups constituted the majority of patients with most severe cognition impairment (Fig. 1B–D; p = 0.008, 0.037, 0.023, respectively).
Rate ratio of participants by nasal Aβ42 levels as quartiles. Proportions of participants in each nasal Aβ42 quartile group—Q1 (blue), Q2 (green), Q3 (orange), and Q4 (red)—are shown according to (A) clinical diagnosis, (B) Clinical Dementia Rating (CDR), (C) Global Deterioration Scale (GDS), and (D) Mini-Mental State Examination (MMSE) categories. Group sizes for clinical diagnosis (A) are: CU (n = 32), Preclinical (n = 29), MCI (n = 73), and AD (n = 27). Group sizes for CDR (B) are: CU (n = 42), MCI (n = 95), and AD (n = 24). Group sizes for GDS (C) are: CU (n = 6), MCI (n = 116), and AD (n = 39). Group sizes for MMSE (D) are: CU (n = 65), MCI (n = 40), and AD (n = 56). Aβ, amyloid beta; CDR, Clinical Dementia Rate; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CU, cognitively unimpaired; GDS, Global Deterioration Scale; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Assessing the association between each quartile group of nasal Aβ42 and cognitive function
Next, we analyzed the relation between cognitive function measurements and quartile groups. Q3 groups exhibited more impaired scores in all neuropsychological tests than any other group with statistical significance (Fig. 2A–D). Q3 group showed the most impaired results in CDR (p = 0.0157 with Q1, p = 0.0028 with Q2, and p = 0.0450 with Q4). Similar results were witnessed in GDS (p = 0.0071 with Q1 and p = 0.0033 with Q2), in MMSE (p = 0.0277 with Q1, p = 0.0230 with Q2, and p = 0.0230 with Q4), and in CERAD (p = 0.0427 with Q1, p = 0.0429 with Q2, and p = 0.0270 with Q4). These neuropsychological tests assess different domains in cognition, and this result demonstrates that quartile groups of nasal Aβ42 level may reflect diverse changes in cognition.
Clinical characteristics by quartiles of nasal Aβ42. Results of each quartile group’s cognitive function measured by CDR is shown in (A), GDS in (B), MMSE in (C), and CERAD in (D). Association with age is shown in (E) and years of education in (F). All data are presented as mean ± SEM. Statistical significance was tested by one-way ANOVA, followed by post-hoc using Dunnett. Statistical significance was determined at a p-value below of 0.05, while p-values below or equal to 0.05 and 0.01 were represented by * and **, respectively. Group sizes were as follows: Q1 (n = 41), Q2 (n = 40), Q3 (n = 40), Q4 (n = 40). Aβ, amyloid beta; ANOVA, analysis of variance; CDR, Clinical Dementia Rate; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination.
In contrast, age and education years did not differ between quartile groups, indicating the association between the nasal Aβ42 levels and cognition impairment was not affected by external factors.
Assessing the association between nasal Aβ42 and brain amyloid in PET
To address if nasal Aβ levels can reflect the brain amyloid load, we separated each quartile groups into Aβ PET positive and Aβ PET negative groups and compared the brain amyloid load across the groups (Fig. 3). The Aβ PET Positive group in Q3 had significantly higher SUVR values, indicating higher Aβ burden in the brain than any other quartile groups, including Q4 (p = 0.0433 with Q1 and p = 0.0068 with Q4).
Amyloid PET SUVR by quartiles of nasal Aβ42. Violin plots represent the SUVR values of Aβ PET negative (blue) and Aβ PET positive (red). Vertical lines indicate the interquartile range and medians are indicated by gray horizontal line. Statistical significance was tested by two-way ANOVA, followed by post-hoc using Dunnett. Statistical significance was determined at a p-value below of 0.05, while p-values below or equal to 0.05 and 0.01 were represented by * and **, respectively. Group sizes were as follows: Q1 (n = 41), Q2 (n = 40), Q3 (n = 40), Q4 (n = 40). Aβ, amyloid beta; ANOVA, analysis of variance; PET, positron emission tomography; SUVR, standard uptake value ratio.
Assessing the association between nasal Aβ42 and risk of developing AD
Next, in order to predict the odds ratio (OR) of being diagnosed with AD based on nasal Aβ42 levels, we performed multivariate logistic regression analysis. The first model was unadjusted, while the second model and was adjusted for age, sex, and years of education (Table 2). Having Q3 nasal Aβ42 level was associated with increased odds for developing AD by all neuropsychological tests and clinician’s diagnosis than having Q1 Aβ42 level.
Assessing the discriminative power of nasal Aβ42 as a biomarker
We performed ROC analysis to assess the ability of quartile nasal Aβ42 levels to discriminate AD from non-AD subjects (Fig. 4A–F). Within all participants, when a participant’s age, sex, and quartile nasal Aβ42 level information were provided, Q3 (AUC = 0.766) showed the highest performance for discriminating AD from non-AD subjects (Q1, AUC = 0.714; Q2, AUC = 0.683; Q4, AUC = 0.640). When a participant’s age, sex, education, and quartile nasal Aβ42 level information were provided, Q3 (AUC = 0.778) also reliably separated AD from non-AD (Q1, AUC = 0.741; Q2, AUC = 0.717; Q4, AUC = 0.679). When a participant’s neuropsychological test result was additionally provided, the Q3 showed the highest discriminating performance (AUC = 0.959 for MMSE; AUC = 0.885 for CDR; AUC = 0.892 for GDS; AUC = 0.904 for CERAD).
Receiver operating characteristic (ROC) curve analyses for nasal Aβ42 level biomarker performance in distinguishing AD from non-AD subjects in Q1 (blue), Q2 (green), Q3 (orange), and Q4 (red). Areas under the ROC curves (AUCs) are presented in legends. AUC, area under the curve; CDR, Clinical Dementia Rate; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; GDS, Global Deterioration Scale; MMSE, Mini-Mental State Examination; ROC, receiver operating characteristic.
Discussion
This study demonstrates that nasal Aβ42 concentrations reflect dynamic changes in brain amyloid pathology across the AD continuum. Nasal discharge fluid, which bathes the olfactory epithelium, provides a unique window into neuronal changes occurring in regions among the earliest affected in AD, including the OB and adjacent medial temporal structures5,29,40. Notably, moderate nasal Aβ42 concentrations (9.53–11.10 pg/mL) were most strongly associated with cognitive impairment and amyloid PET positivity. Individuals in this range (Q3 group) accounted for the largest proportion of AD patients, suggesting a transition phase in which pathological burden becomes more clinically apparent. In contrast, those with the highest nasal Aβ42 levels exhibited weaker associations, indicating a potential non-linear relationship between nasal Aβ42 concentration and disease stage. These observations align with established brain amyloidosis trajectories, where amyloid accumulation accelerates early but eventually plateaus or declines in later stages41,42,43.
Importantly, our results show that nasal Aβ42 levels mirror the progression of cerebral amyloid pathology. Aβ exists in multiple forms—monomers, dimers, oligomers, fibrils, and plaques—and disruptions in its production and clearance are central to AD-related toxicity44,45,46,47,48. The pattern observed in our study—a rise in nasal Aβ42 concentrations followed by a plateau or modest decline—closely resembles trajectories documented in longitudinal PET imaging studies49,50,51,52,53. This parallel supports the utility of nasal Aβ42 as a reflective index of brain amyloid dynamics, offering a new avenue for non-invasive monitoring of disease processes.
A key strength of this study lies in the direct comparison of nasal Aβ42 concentrations with amyloid PET imaging data across preclinical, MCI, and AD stages. To our knowledge, this is the first report to demonstrate that nasal Aβ42 concentrations track brain amyloid burden in a stage-dependent manner. This mirroring effect highlights the potential of nasal discharge as a peripheral fluid that captures central pathological processes.
Although nasal discharge sampling does not substitute for established techniques such as CSF analysis or PET imaging, it offers notable practical advantages. Nasal sampling is non-invasive, low-cost, and logistically simple. It circumvents the risks associated with lumbar puncture and the high financial and infrastructural demands of neuroimaging. These attributes make it well suited for large-scale screening or longitudinal monitoring in resource-limited settings.
Our study also examined potential clinical relevance. While nasal Aβ42 levels alone demonstrated moderate discriminative ability in distinguishing AD from non-AD participants, performance improved when demographic factors such as education level were included. This finding is consistent with previous studies indicating that cognitive reserve may modulate the expression of AD symptoms54. Moreover, a recent large-scale community-based study reported that poor olfaction is significantly associated with plasma biomarkers of AD and neurodegeneration, reinforcing the value of olfactory-based markers as indicators of central pathology55. Incorporating neuropsychological testing further enhanced predictive accuracy, suggesting that nasal Aβ42 could complement cognitive assessments in settings where neuroimaging is unavailable or impractical56. While not intended for diagnostic purposes, these results support further investigation into its role as a stratification tool in research contexts.
While our findings are promising, several limitations must be considered. First, the study did not control for diurnal variation, which may fluctuate over the course of the day. Future studies should standardize sampling times to minimize this source of variability. Second, only a single nasal discharge sample was collected per participant, limiting insights into temporal dynamics. This decision was made to reduce participant burden and maintain compliance, particularly given the novelty and semi-invasive nature of the collection procedure. Longitudinal data will be essential for understanding how nasal Aβ42 evolves across disease progression. Third, while the study cohort spans the AD continuum, validation in larger and more diverse populations is needed. To support this, we analyzed an independent dataset from individuals across the cognitive spectrum. Although this secondary dataset lacks amyloid PET imaging, the similarity in Aβ42 concentration distributions reinforces the reproducibility of our measurement approach. This additional dataset offers an opportunity for future replication studies, particularly in longitudinal or imaging-limited settings. Fourth, as this study was conducted at a single clinical site, potential selection bias may limit generalizability. Moreover, unmeasured confounders—including comorbid conditions, medication use, and socioeconomic factors—were not fully controlled and may influence nasal Aβ42 levels. These factors should be addressed in future multicenter studies with broader demographic and clinical adjustment.
Given the anatomical proximity of the nasal cavity and olfactory system to brain regions affected early in AD, nasal Aβ42 may reflect central pathology more directly than distal fluids such as blood or saliva. Recent studies further demonstrate that olfactory dysfunction correlates with entorhinal cortex and hippocampal atrophy, particularly in individuals with subjective cognitive decline or MCI, reinforcing the idea that olfactory markers capture early medial temporal lobe degeneration57. In addition, recent longitudinal PET studies have shown that declining olfactory identification scores predict incident MCI and are associated with increasing regional amyloid and tau burden, especially in olfaction-relevant areas such as the orbitofrontal and entorhinal cortices58. Elucidating the biological mechanisms that underlie this relationship could open new opportunities for tracking disease progression or evaluating therapeutic response via the nasal route.
In conclusion, this study provides the first evidence that nasal Aβ42 concentrations, particularly in the moderate range, are significantly associated with AD diagnosis, cognitive decline, and brain amyloid deposition. These findings support the utility of nasal discharge as a research tool for tracking AD-related changes and improving our understanding of amyloid pathology across the disease continuum. With further validation and standardization, nasal Aβ42 monitoring may offer a practical and scalable means of investigating disease progression in both clinical and research settings.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to restrictions imposed by personal data protection regulations, but are available from the corresponding author on reasonable request.
References
2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 20, 3708–3821. https://doi.org/10.1002/alz.13809 (2024)
Blennow, K. & Zetterberg, H. Fluid biomarker-based molecular phenotyping of Alzheimer’s disease patients in research and clinical settings. Prog. Mol. Biol. Transl. Sci. 168, 3–23. https://doi.org/10.1016/bs.pmbts.2019.07.006 (2019).
Davda, N. & Corkill, R. Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. J. Neurol. 267, 2475–2477. https://doi.org/10.1007/s00415-020-10037-9 (2020).
Jung, D. H., Son, G., Kwon, O. H., Chang, K. A. & Moon, C. Non-invasive nasal discharge fluid and other body fluid biomarkers in Alzheimer’s disease. Pharmaceutics https://doi.org/10.3390/pharmaceutics14081532 (2022).
Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 15, 11–24. https://doi.org/10.1038/s41582-018-0097-5 (2019).
Waldton, S. Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatr. Scand. 50, 539–547. https://doi.org/10.1111/j.1600-0447.1974.tb09714.x (1974).
Doty, R. L., Reyes, P. F. & Gregor, T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res. Bull. 18, 597–600. https://doi.org/10.1016/0361-9230(87)90129-8 (1987).
Doty, R. L., Hawkes, C. H., Good, K. P. & Duda, J. E. in Handbook of Olfaction and Gustation, third ed. Wiley Online Books (ed Richard L. Doty) 403–452 (John Wiley & Sons, Inc., 2015).
Jobin, B., Zahal, R., Bussières, E.-L., Frasnelli, J. & Boller, B. Olfactory identification in subjective cognitive decline: A meta-analysis. J. Alzheimer’s Dis. 79, 1497–1507. https://doi.org/10.3233/JAD-201022 (2021).
Yan, Y. et al. A potential biomarker of preclinical Alzheimer’s disease: The olfactory dysfunction and its pathogenesis-based neural circuitry impairments. Neurosci. Biobehav. Rev. 132, 857–869. https://doi.org/10.1016/j.neubiorev.2021.11.009 (2022).
Knight, J. E. et al. Transitions between mild cognitive impairment, dementia, and mortality: The importance of olfaction. J. Gerontol. Ser. A 78, 1284–1291. https://doi.org/10.1093/gerona/glad001 (2023).
Braak, H. & Braak, E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1, 213–216. https://doi.org/10.1111/j.1750-3639.1991.tb00661.x (1991).
Masurkar, A. V. & Devanand, D. P. Olfactory dysfunction in the elderly: Basic circuitry and alterations with normal aging and Alzheimer’s disease. Curr. Geriatr. Rep. 3, 91–100. https://doi.org/10.1007/s13670-014-0080-y (2014).
Son, G. et al. Olfactory neuropathology in Alzheimer’s disease: A sign of ongoing neurodegeneration. BMB Rep. 54, 295–304. https://doi.org/10.5483/BMBRep.2021.54.6.055 (2021).
Kim, J. Y. et al. Distinct amyloid precursor protein processing machineries of the olfactory system. Biochem. Biophys. Res. Commun. 495, 533–538. https://doi.org/10.1016/j.bbrc.2017.10.153 (2018).
Wesson, D. W., Levy, E., Nixon, R. A. & Wilson, D. A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci. 30, 505–514. https://doi.org/10.1523/JNEUROSCI.4622-09.2010 (2010).
Yoo, S. J. et al. Differential spatial expression of peripheral olfactory neuron-derived BACE1 induces olfactory impairment by region-specific accumulation of beta-amyloid oligomer. Cell Death Dis. 8, e2977. https://doi.org/10.1038/cddis.2017.349 (2017).
Son, G., Steinbusch, H. W., López-Iglesias, C., Moon, C. & Jahanshahi, A. Severe histomorphological alterations in post-mortem olfactory glomeruli in Alzheimer’s disease. Brain Pathol. 32, e13033. https://doi.org/10.1111/bpa.13033 (2022).
Attems, J., Lintner, F. & Jellinger, K. A. Olfactory involvement in aging and Alzheimer’s disease: An autopsy study. J. Alzheimers Dis. 7, 149–157; discussion 173–180 https://doi.org/10.3233/jad-2005-7208 (2005).
Crino, P. B. et al. Beta-amyloid peptide and amyloid precursor proteins in olfactory mucosa of patients with Alzheimer’s disease, Parkinson’s disease, and down syndrome. Ann. Otol. Rhinol. Laryngol. 104, 655–661. https://doi.org/10.1177/000348949510400812 (1995).
Hock, C. et al. Histological markers in nasal mucosa of patients with Alzheimer’s disease. Eur. Neurol. 40, 31–36. https://doi.org/10.1159/000007953 (1998).
Attems, J., Walker, L. & Jellinger, K. A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 127, 459–475. https://doi.org/10.1007/s00401-014-1261-7 (2014).
Franks, K. H., Chuah, M. I., King, A. E. & Vickers, J. C. Connectivity of pathology: The olfactory system as a model for network-driven mechanisms of Alzheimer’s disease pathogenesis. Front. Aging Neurosci. 7, 234. https://doi.org/10.3389/fnagi.2015.00234 (2015).
Schwob, J. E. Neural regeneration and the peripheral olfactory system. Anat. Rec. 269, 33–49. https://doi.org/10.1002/ar.10047 (2002).
Struble, R. G. & Clark, H. B. Olfactory bulb lesions in Alzheimer’s disease. Neurobiol. Aging 13, 469–473. https://doi.org/10.1016/0197-4580(92)90074-8 (1992).
Arnold, S. E. et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann. Neurol. 67, 462–469. https://doi.org/10.1002/ana.21910 (2010).
Ayala-Grosso, C. A. et al. Amyloid-aβ peptide in olfactory mucosa and mesenchymal stromal cells of mild cognitive impairment and Alzheimer’s disease patients. Brain Pathol. 25, 136–145. https://doi.org/10.1111/bpa.12169 (2015).
Kim, Y. H. et al. Amyloid beta in nasal secretions may be a potential biomarker of Alzheimer’s disease. Sci. Rep. 9, 4966. https://doi.org/10.1038/s41598-019-41429-1 (2019).
Yoo, S. J. et al. Longitudinal profiling of oligomeric Aβ in human nasal discharge reflecting cognitive decline in probable Alzheimer’s disease. Sci. Rep. 10, 11234. https://doi.org/10.1038/s41598-020-68148-2 (2020).
Morris, J. C. The clinical dementia rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. https://doi.org/10.1212/wnl.43.11.2412-a (1993).
Reisberg, B., Ferris, S. H., de Leon, M. J. & Crook, T. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry 139, 1136–1139. https://doi.org/10.1176/ajp.139.9.1136 (1982).
Morris, J. C. et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39, 1159–1165. https://doi.org/10.1212/wnl.39.9.1159 (1989).
Wang, S. M. et al. Plasma oligomer β-amyloid and white matter microstructural integrity in cognitively normal older adults according to cerebral amyloid deposition. J Prev. Alzheimer’s Dis. 10, 837–846. https://doi.org/10.14283/jpad.2023.87 (2023).
Jack, C. R. Jr. et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Wang, S.-M. et al. Plasma oligomer beta-amyloid is associated with disease severity and cerebral amyloid deposition in Alzheimer’s disease spectrum. Alzheimer’s Res. Ther. 16, 55. https://doi.org/10.1186/s13195-024-01400-3 (2024).
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746. https://doi.org/10.1016/S1474-4422(07)70178-3 (2007).
Tay, L. et al. New DSM-V neurocognitive disorders criteria and their impact on diagnostic classifications of mild cognitive impairment and dementia in a memory clinic setting. Am. J. Geriatr. Psychiatry 23, 768–779. https://doi.org/10.1016/j.jagp.2015.01.004 (2015).
Chouraki, V. et al. Plasma amyloid-β and risk of Alzheimer’s disease in the Framingham Heart Study. Alzheimer’s Dement. J. Alzheimer’s Assoc. https://doi.org/10.1016/j.jalz.2014.07.001 (2014).
Montoliu-Gaya, L. et al. Mass spectrometric simultaneous quantification of tau species in plasma shows differential associations with amyloid and tau pathologies. Nat. Aging 3, 661–669. https://doi.org/10.1038/s43587-023-00405-1 (2023).
Baek, M. S. et al. Effect of A/T/N imaging biomarkers on impaired odor identification in Alzheimer’s disease. Sci. Rep. 10, 11556. https://doi.org/10.1038/s41598-020-68504-2 (2020).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–356. https://doi.org/10.1126/science.1072994 (2002).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608. https://doi.org/10.1038/s44318-024-00057-w (2016).
Hampel, H. et al. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 26, 5481–5503. https://doi.org/10.1038/s41380-021-01249-0 (2021).
Hampel, H. et al. The β-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 89, 745–756. https://doi.org/10.1016/j.biopsych.2020.02.001 (2021).
Nilsberth, C. et al. The’Arctic’APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci. 4, 887–893. https://doi.org/10.1038/nn0901-887 (2001).
Tomiyama, T. et al. A new amyloid β variant favoring oligomerization in Alzheimer’s-type dementia. Ann. Neurol. 63, 377–387. https://doi.org/10.1002/ana.21321 (2008).
O’Nuallain, B. et al. Amyloid β-protein dimers rapidly form stable synaptotoxic protofibrils. J. Neurosci. 30, 14411–14419. https://doi.org/10.1523/JNEUROSCI.3537-10.2010 (2010).
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842. https://doi.org/10.1038/nm1782 (2008).
Jack, C. R. Jr. et al. Brain β-amyloid load approaches a plateau. Neurology 80, 890–896. https://doi.org/10.1212/WNL.0b013e3182840bbe (2013).
Kadir, A. et al. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer’s disease. Neurobiol. Aging 33(198), e191-198.e114. https://doi.org/10.1016/j.neurobiolaging.2010.06.015 (2012).
Villemagne, V. L. et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 69, 181–192. https://doi.org/10.1002/ana.22248 (2011).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804. https://doi.org/10.1056/NEJMoa1202753 (2012).
Rodriguez-Ayllon, M. et al. Physical activity and amyloid beta in middle-aged and older adults: A systematic review and meta-analysis. J. Sport Health Sci. 13, 133–144. https://doi.org/10.1016/j.jshs.2023.08.001 (2024).
Gatz, M. et al. Education and the risk of Alzheimer’s disease: Findings from the study of Dementia in Swedish Twins. J. Gerontol. Ser. B 56, P292–P300. https://doi.org/10.1093/geronb/56.5.P292 (2001).
Shrestha, S. et al. Olfaction and plasma biomarkers of alzheimer disease and neurodegeneration in the atherosclerosis risk in communities study. Neurology 104, e213706. https://doi.org/10.1212/WNL.0000000000213706 (2025).
Tombaugh, T. N. & McIntyre, N. J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 40, 922–935. https://doi.org/10.1111/j.1532-5415.1992.tb01992.x (1992).
Papadatos, Z. & Phillips, N. A. Olfactory function reflects episodic memory performance and atrophy in the medial temporal lobe in individuals at risk for Alzheimer’s disease. Neurobiol. Aging 128, 33–42. https://doi.org/10.1016/j.neurobiolaging.2023.04.001 (2023).
Tian, Q., Bilgel, M., Moghekar, A. R., Ferrucci, L. & Resnick, S. M. Olfaction, cognitive impairment, and PET biomarkers in community-dwelling older adults. J. Alzheimer’s Dis. 86, 1275–1285. https://doi.org/10.3233/JAD-210636 (2022).
Acknowledgements
This work was supported by the Korea Health Industry Development Institute (KHIDI) by the Korea government by the Ministry of Health & Welfare (HI18C0154), and the National Research Foundation of Korea (NRF) grants funded by the Korea government by the Ministry of Education (RS-2020-NR049577) and the Ministry of Science & ICT (RS-2023-00278057).
Author information
Authors and Affiliations
Contributions
D.H.J. conceived and designed the study, conducted data analysis and interpretation, and drafted the manuscript. G.S. contributed to sample processing, methodology development, and data interpretation. S.M.W. was responsible for clinical recruitment, neuroimaging data acquisition, and contributed to clinical interpretation. S.-J.Y. assisted with biochemical assays, data validation, and figure preparation. A.J. provided guidance on study design and critically revised the manuscript. H.K.L. oversaw clinical components of the study, contributed to funding acquisition, and supervised clinical data integration. C.M. supervised the overall project, secured funding, and contributed to study design, data interpretation, and manuscript revision. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
The study was approved by the Catholic University of Korea Institutional Review Boards. All participants provided the necessary informed consent to participate in this research study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jung, D.H., Son, G., Wang, S.M. et al. Nasal Aβ42 mirrors brain amyloid dynamics and cognitive decline across the Alzheimer’s disease continuum. Sci Rep 15, 30413 (2025). https://doi.org/10.1038/s41598-025-15230-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15230-2