Abstract
The Heidelberg Study on Diabetes and Complications (HEIST-DiC) is a prospective longitudinal study focused on the development and progression of diabetes-associated complications. Participants with/without diabetes mellitus undergo annual phenotyping of diabetes-associated complications over 11 years. Assessments include: albuminuria, estimated glomerular filtration rate for chronic kidney disease; clinical neuropathy scores, Purdue Pegboard test, electrophysiological examination, transcutaneous electrical nerve fiber stimulation, quantitative sensory testing and high-resolution magnetic resonance neurography for distal sensorimotor polyneuropathy; heart rate variability for cardiovascular autonomic neuropathy; funduscopic examination of undilated pupils for retinopathy; the 6-minute walk test, spirometry, body plethysmography, and carbon monoxide-based diffusing capacity measurements for respiratory lung disease; non-invasive scores, transient elastography and hepatic ultrasound for metabolic dysfunction-associated steatotic liver disease; ankle-brachial index and carotid intima-media thickness for peripheral atherosclerosis; hand grip strength for muscle function; bioelectrical impedance analysis for body composition; skin autofluorescence for measurement of advanced glycation end products. Beta-cell function and tissue-specific insulin sensitivity are evaluated using oral glucose tolerance test or euglycemic hyperinsulinemic clamp. The biobank stores specimens of blood, urine, skeletal muscle, subcutaneous adipose tissue, and skin. Health-related quality of life, physical health, and somatic and depression symptoms are measured via standardized questionnaires. HEIST-DiC explores diabetes onset in high-risk individuals, disease progression and the development of complications, aiming to design personalized strategies to prevent, mitigate, or reverse diabetes-related complications.
Trial registration: The study was retrospectively registered at Clinicaltrials.gov (NCT03022721, date of registration 20170112).
Similar content being viewed by others
Introduction
Why was the cohort set up?
Diabetes mellitus (DM) remains a leading cause of blindness, kidney failure, lower limb amputation, heart attack, and stroke. Its global prevalence has surged, with over half a billion people currently affected1,2. Despite advances in DM treatment, particularly with the use of sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide 1 (GLP-1) agonists for type 2 diabetes (T2D), glucose-centered therapies moderately reduce cardiovascular risk while at the same time increasing the risk for hypoglycemia3. The aim of this cohort is to identify DM subtypes with distinct courses of disease related to complications. Additionally, this cohort seeks to investigate new associations between DM subtypes and diseases previously not attributed to DM. This will aid clinical decision-making and enable the development of personalized treatments to prevent, or potentially reverse diabetes-associated complications.
Complications of DM
The traditional classification of chronic diabetes-associated complications is based on the consequences of vascular damage and divides them into two main groups: microvascular and macrovascular complications4. While this classification is convenient in clinical practice, emerging evidence suggests it is limited, as diabetes-related damage extends beyond the vascular system, impacting nearly all organs, tissues and cells5. Microvascular complications are not solely due to microvascular changes, but other changes beyond the vascular bed, such as retinal neurodegeneration in retinopathy6, tubulointerstitial changes in nephropathy7 and neuronal deficits in neuropathy8, also play key roles. Additionally, impaired angiogenesis of large artery microvessels, a form of microangiopathy, contributes to the development of the diabetic macroangiopathic disease9,10,11. There is also increasing recognition of nonclassical diabetes-associated complications, including abnormal pulmonary function, metabolic dysfunction-associated steatotic liver disease (MASLD), cardiomyopathy, increased risk of carcinogenesis5, increased diabetes-related distress and impairment of psychological stress response12,13. By using a holistic approach, the HEIST-DiC cohort has the potential to investigate both classical and nonclassical diabetes-associated complications.
Diabetes subtypes and risk of complications
Efforts to better understand the heterogeneity of DM have led to a paradigm shift, classifying individuals with newly diagnosed adult-onset DM into five clusters based on six simple clinical variables14,15. This new classification is significant as it stratifies individuals with recent-onset DM (diagnosis of DM within the past 12 months) into subgroups with varying risks for diabetes-associated complications. Particularly, individuals with severe insulin-resistant diabetes (SIRD) are at higher risk for cardiovascular, kidney and fatty liver disease, while those with severe insulin-deficient diabetes (SIDD) are more prone to diabetic neuropathy, often already present at disease onset14,15. Another retrospective cohort study found an increased risk of diabetic retinopathy (DR) in the SIDD cluster and a higher risk of nephropathy in the SIRD cluster16. The concept of new diabetes classifications provides deeper insights into disease heterogeneity but raises several unresolved questions, including: (i) the statistical methods used to define clusters, (ii) the stability of clusters over time, (iii) the use of genotypic versus phenotypic datasets, (iv) generalizability across different ethnic groups, (v) clinical relevance of in some cases only marginal absolute differences between subtypes and (vi) applications in clinical care17.
Early studies used k-means clustering at diabetes onset, assigning individuals to fixed clusters14,15,18,19. This approach broadly validated novel diabetes subgroups across Chinese, US, and non-white Emirati populations, suggesting potential generalizability across ethnicities20,21. However, rigid clusters at diagnosis fail to account for dynamic, longitudinal changes driven by initial pathological mechanisms. A German study found that 23% of individuals switched clusters within five years after diabetes onset, raising concerns about the practical utility of this rigid partitioning in managing ongoing diabetes15.
A novel soft-clustering method for patient stratification in ongoing diabetes identified a subgroup with obesity, insulin resistance, dyslipidemia, impaired β-cell sensitivity, rapid disease progression, and higher need for anti-diabetic therapy22. Another soft-clustering approach using genotypic data identified five robust clusters of T2D pointing to disease mechanisms reflected by clinical traits23. Advanced methods, such as a tree-like graph structure using reversed graph-embedded dimensionality reduction, enable stratification of pathophysiological components and diabetes-related complications throughout the course of diabetes24.
A clustering approach in individuals with prediabetes (PRED), who have an increased risk for developing T2D, identified six subtypes based on pathophysiological parameters. Of these, only two had a high imminent risk for developing T2D, despite impaired glucose metabolism25. Notably, one subtype exhibited an increased risk for kidney disease and all-cause mortality, despite only moderate risk for T2D25. Furthermore, diabetes-associated complications are already present in some individuals with PRED. Similar to findings in T2D, clustering analysis for PRED based on genotypic dataset identified six subtypes with distinct genetic score patterns and metabolic traits. Two of these subtypes exhibited a high risk of progressing to T2D26. These findings suggest that pathophysiological heterogeneity emerges before T2D onset, shaping in this way the course of the disease early on. An important open question for future research is the role of PRED cluster identification in predicting progression to diabetes and development of complications independent of diabetes progression. Other studies have examined disease progression in low- and high-risk PRED subgroups during the disease’s natural course and lifestyle intervention27. In contrast, the HEIST-DiC takes a holistic approach, providing a comprehensive clinical and metabolic assessment of diabetes-related complications.
The HEIST-DiC cohort provides a unique opportunity to assess individuals as usually seen in clinical practice. This study enables early identification of high-risk subgroups before complications manifest and investigates whether mechanisms driving (pre)diabetes onset contribute to the progression and development of complications. Most importantly, the cohort’s inclusion of individuals with long-term diabetes allows for the identification of factors distinguishing those with specific diabetes-associated complications from those without, beyond the presence and duration of diabetes. Leveraging the study’s intensive clinical-experimental characterization, HEIST-DiC enables the investigation of pathophysiological cellular mechanisms underlying different diabetes subgroups.
Personalized intervention strategies for DM and its complications
Previous attempts to prevent or slow the progression of diabetes-associated complications have achieved only moderate success in reducing absolute risk for diabetes-associated complications in both type 1 diabetes (T1D)28,29,30 and T2D31. This limited success is likely due, in part, to the glucose-centered focus of these interventions, as well as the lack of consideration of DM subtypes. A post hoc analysis of longitudinal data from intensive lifestyle interventions in T2D, utilizing age at diabetes diagnosis and k-means clustering, identified a cluster with poor glucose control associated with increased cardiovascular risk after the intervention32. In a randomized controlled trial using a fasting-mimicking diet in a T2D subgroup from HEIST-DiC, assessing vulnerability to fasting required deep phenotyping and longitudinal observation to distinguish individuals who might benefit from the intervention from those at risk of adverse effects33. Data-driven cluster analysis demonstrated that individuals with SIRD benefit with better glycemic control from thiazolidinediones, while glycemic control in those with mild age-related diabetes is better controlled with sulfonylurea18. These findings emphasize that use of novel diabetes subtypes is justified only if they demonstrate clinical relevance, specifically through subtype-specific responses to stratified intervention strategies34. Focusing on pathophysiology-based therapeutic approaches is key to delivering personalized treatment in T2D, and subtype-specific randomized clinical trials will be critical in assessing the clinical relevance of these new DM subtypes17,34.
The HEIST-DiC cohort stands out for its comprehensive inclusion of individuals across the full spectrum of glucose metabolism—from normal glucose tolerance to prediabetes, recent-onset diabetes, and long-standing diabetes with and without complications. This breadth provides an unparalleled opportunity to address critical unanswered questions in diabetes research, including the validation and refinement of clustering methods, the stability of subtypes over time, and the identification of novel biomarkers and high-risk individuals. By enabling the stratification of participants into subtypes and focusing on their pathophysiological mechanisms, the HEIST-DiC cohort bridges the gap between large-scale epidemiological studies and clinical-experimental research. This approach will not only validate existing clustering methodologies but also drive the development of new frameworks for understanding the dynamic and heterogeneous nature of diabetes. Ultimately, the cohort’s design supports the testing of tailored therapeutic strategies and interventions, advancing the clinical relevance of subtype-specific approaches and paving the way for precision medicine in diabetes care35.
Study design and methods
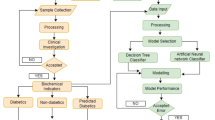
The Heist-DiC is an ongoing monocentric, prospective longitudinal observational study involving intensive metabolic and clinical phenotyping conducted annually for at least 11 years. Participants are recruited from the Clinic of Endocrinology, Diabetology, Metabolic Diseases and Clinical Chemistry (Internal Medicine 1) and are evaluated at the Study Center for Diabetes and Metabolism at the University Hospital of Heidelberg in Germany.
The aims of the HEIST-DiC cohort are to identify: (i) DM subtypes with different development patterns of diabetes-associated complications, (ii) predictors of diabetes-associated complications at early and late disease stages, (iii) pathophysiological mechanisms beyond hyperglycemia that contribute to the onset of diabetes-associated complications, (iv) novel nonclassical diabetes-associated complications, (v) personalized interventions for the prevention, improvement or remission of diabetes-associated complications.
The HEIST-DiC cohort is a hypothesis-generating study that will validate established clustering methods for diabetes classification, develop and apply new approaches – particularly for complications-specific clustering – and identify individuals from specific subtypes for targeted clinical-experimental studies.
Composition of the cohort
To capture the various stages of disease development and progression, the study imposes no restrictions on disease stage or DM duration. Therefore, the study includes normal glucose tolerant individuals (NGT) and individuals with PRED, defined by oral glucose tolerance test (oGTT) and/or glycated hemoglobin (HbA1c) according to current ADA guidelines36, as well as individuals already diagnosed with DM (T1D or T2D) aged 18 to 85 years. Inclusion of older individuals in the cohort allows for a more accurate representation and assessment of age-related variability, while enabling identification of short- to medium-term trends that remain clinically relevant, particularly with respect to the mild age-related diabetes subtype14,37. Individuals with an initial HbA1c ≥ 9.5% are excluded, to minimize the risk of acute glycemic deterioration during study visits and its potential impact on diabetes-related complications. This also ensures participant safety and the accuracy of metabolic phenotyping under stable medical conditions; however, later inclusion is possible after appropriate treatment lowers their HbA1c to < 9.5%. Individuals with type 3 (e.g. pancreatogenic) or type 4 (gestational) diabetes, as well as pregnant women, are also excluded. A complete list of inclusion and exclusion criteria is detailed in Table 1.
Study participants are recruited through advertisements in local newspapers, public events, and information shared via the institutional website or health practitioners. Potential participants undergo a prescreening telephone interview to assess inclusion and exclusion criteria. Suitable candidates are then invited to the first study visit, which includes medical history, physical examination, blood withdrawal, questionnaires, and assessment of diabetes-associated complications. Decisions regarding participation in advanced examinations, such as the euglycemic hyperinsulinemic clamp test and tissue biopsies, are made and scheduled afterward. All participants provide written informed consent to the study protocol.
As of October 2023, the study has enrolled 552 participants, including 68 individuals with NGT, 119 with PRED, 83 with T1D and 282 with T2D. DM diagnosis and type are confirmed according to current ADA guidelines36 only for participants diagnosed at their first study visit, including those with latent autoimmune diabetes of the adult (LADA). No genetic analysis is conducted to assess for maturity onset diabetes of the young (MODY).
Duration of the study
As of October 1 2023, all study participants reported here have completed the full study program at baseline and annually thereafter. Electrophysiological examination, by nerve conduction velocity (NCV), and quantitative sensory testing (QST) were not performed at year 3 and 5. Starting October 2023, participants undergo the complete study program at baseline and at years 4, 8, and 12, with a reduced study program in the years in between. A detailed list of examinations for each study visit is provided in Table 2. Recruitment is ongoing, making the precise dropout rate difficult to determine, particularly as participants may miss visits due to illness or scheduling problems. By beginning of October 2023, 19% of participants were lost to follow-up, with 11% of these being deceased (total mortality-rate 2%), while others cited reasons such as lack of time, transportation issues, or were simply inaccessible. Death cause was acute myocardial infarction (4 participants), hemorrhagic stroke (1 participant), respiratory failure due to fungal pneumonia (1 participant), urothelial carcinoma (1 participant), sarcoma (1 participant) and unknown (7 participants). 92% of the deceased participants were T2D individuals, predominantly male (69%) and 8 years older than the ongoing participants. The rest of the dropouts (non-deceased participants) were mostly females, 5 years older than the ongoing participants, with the group-distribution being comparable to the ongoing study participants. Nearly half (40%) of dropouts occurred in the first year following the baseline visit. Participants can pause their involvement for any reason and resume later. Most missed appointments were due to cancellations related to the Covid-19 pandemic, with visit 3 being the most affected (18% missed the visit, half due to Covid-19-related restrictions). Overall, the yearly follow-up visits (from visit 1 to visit 5), had an average response rate of 82%, including participants who attended or are still eligible to attend. As many participants are recruited through our university outpatient clinic, routine visits as part of standard care allow us to re-establish contact with those participants lost to follow-up, re-inform them about the study, and offer the opportunity to resume participation.
Measurements
Demographic data and diabetes-specific medical history, including current medication, are recorded at baseline and updated during each follow-up visit (Table 2). Clinical and metabolic variables are also documented at both baseline and follow-up visits (Tables 3 and 4).
To assess chronic kidney disease (CKD), the study utilizes albuminuria, estimated glomerular filtration rate (eGFR), and kidney ultrasound33,38. Distal sensorimotor polyneuropathy (DSPN) is evaluated using the neuropathy symptoms score (NSS), neuropathy disability score (NDS), Purdue pegboard test, NCV, transcutaneous electrical nerve fiber stimulation, QST in accordance with the DFNS (German Research Network on Neuropathic Pain) protocol, and high-resolution magnetic resonance neurography (MRN)39,40,41,42,43,44,45,46. Heart rate variability (HRV) is measured to assess cardiovascular autonomic neuropathy (CAN)12. Anamnestic assessment of CAN is performed using the SAS questionnaire47. Ophthalmological assessment include a funduscopic examination of the undilated pupil for DR48. Diabetes related restrictive lung diseases (RLD) is evaluated through the 6-minute walk test, spirometry, body plethysmography, and carbon monoxide-based diffusing capacity measurements (DLCO)49,50. Transient elastography and hepatic ultrasound are conducted to evaluate MASLD and liver fibrosis51. The ankle-brachial index and carotid intima-media thickness are used to examine peripheral atherosclerosis52. Hand grip strength and bioelectrical impedance analysis (BIA) are utilized to assess muscle strength and body composition52,53.
OGTT and euglycemic hyperinsulinemic clamp test are conducted to assess beta-cell function and insulin sensitivity54. Hepatic insulin sensitivity is measured through the co-infusion of [6,6-2H2]glucose55. Additionally, energy expenditure and substrate oxidation during fasting and hyperinsulinemia are evaluated using indirect calorimetry56.
At each visit, whole-blood, erythrocytes, leucocytes, plasma (EDTA), serum and urine samples are stored at −80 °C for biomarker analysis57,58. Beginning in 2022, skeletal muscle, subcutaneous adipose tissue, and skin samples can also be collected for future analyses. The collected biospecimens support a broad spectrum of molecular and cellular analyses, including biomarker discovery, tissue-specific investigations of insulin resistance, inter-tissue communication and metabolic dysregulation, as well as mechanistic studies using patient-derived specimens.
Health-related quality of life and physical health are assessed using the 12-item short-form health survey (SF-12), while somatic and depression symptoms are evaluated with the patient health questionnaire (PHQ)59,60. A detailed overview of the measurements is provided in Table 2, and the experimental protocols are described in Supplementary file 1.
Definition of diabetes-associated complications
CKD was defined by an increased urinary albumin-to-creatinine ratio (uACR ≥ 30 mg/g) and/or a decreased eGFR (< 60 ml/min/1.73 m2), following the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for DM management of CKD61.
DSPN was defined by the presence of either at least moderate neuropathic deficits (NDS ≥ 6) or the combination of mild deficits with moderate neuropathic symptoms (NSS ≥ 5 together with NDS 3–5)62, or according to the Toronto consensus definition of confirmed DSPN: abnormal results of the nerve conduction study of the sural nerve and additional at least one abnormal parameter from nerve conduction studies of the common peroneal or tibial nerve (parameters below the 2.5 percentile values of the cohort) together with an NSS and/or NDS of ≥ 341,58,63,64. Cases with bilaterally non-recordable sural nerve were assessed as having abnormal sensory function and values were calculated as lowest value of the cohort.
CAN was defined by two pathological values for age-dependent beat-to-beat variation with deep breathing (E/I-quotient), 30/15 heart rate ratio with standing, or orthostatic hypotension (systolic or diastolic change below the 2.5 percentile values of the cohort)12,65,66.
DR was defined by criteria indicating at least stage I of retinopathy based on funduscopic examination of the undilated pupil, as previously reported67. In this study, we do not analyze non-proliferative or proliferative DR.
Diabetes related RLD was defined by reduced forced vital capacity (FVC < 80%), reduced total lung capacity measured with body plethysmography (TLC-B < 80%), or reduced single-breath diffusing capacity of the lung for carbon monoxide (DLCO < 80%), in the presence of a normal forced expiratory volume in 1 s adjusted to vital capacity (FEV1/VC > 70%), as previously reported49,68.
MASLD was defined by an increased controlled attenuation parameter (CAP) (M-probe: CAP ≥ 248 dB/m, XL-probe: CAP ≥ 302 dB/m) measured by transient elastography. Liver fibrosis was defined by increased liver stiffness (≥ 8 kPa) measured by transient elastography41.
Discussion
What has it found? Key findings and publications
Baseline characteristics
Baseline characteristics of the cohort recruited between September 2016 and October 2023 are presented in Tables 3 and 4. The percentage of individuals diagnosed with early-onset T2D (diagnosis at age < 45 years) in our cohort aligns with findings from previous studies based on U.S. registry data (Table 3)69. Additionally, the prevalence of PRED at an early age (< 45 years) is consistent with global prevalence rates of PRED (Table 3)70. In our cohort, the percentage of males with T2D is higher than that of males with T1D, reflecting trends seen in a previously reported German cohort of individuals with recent-onset T1D and T2D (Table 3)71. Overall, our cohort includes more male participants than female participants with PRED, T1D, and T2D. The increased proportion of males in the pathological DM groups aligns with findings from population-based studies72,73 and likely reflects the known underrepresentation of females in clinical research74. There is a higher percentage of female participants than male participants in the NGT group (Table 3), potentially attributed to the greater motivation of healthy women to participate in time-consuming examinations. To address this limitation and ensure a more balanced representation, we are planning to recruit a higher proportion of male participants in the future through public events, as well as through information shared on the institutional website and by healthcare practitioners. For the analysis of different subtypes, study participants will be matched for sex, age at diabetes diagnosis, BMI, glycemia, and homoeostasis model estimates calculated using c-peptide (HOMA-IR).
Family history of DM was more frequent in the T2D group (Table 3). The cohort has not been screened for genetic defects that could indicate MODY. Diagnosis of DM and its respective type is confirmed only for participants diagnosed with DM at the first study visit, which means individuals with MODY may still be included in the cohort.
Most participants with T1D (76%) had a DM duration exceeding 5 years (Table 3), and therefore a negligible residual beta-cell function (Table 4, Panel b). As expected, participants with T2D exhibited low insulin sensitivity and high whole-body and adipose tissue insulin resistance (Table 4, Panel b). In our cohort glycemic control at baseline appears to be slightly better in T2D participants compared to those with T1D (Table 4, Panel b). All but one individual with T1D were treated with insulin and only 5% with metformin, which was the most common medication in individuals with T2D (62%). Notably metformin was also taken by 2 participants with NGT (one with a history of polycystic ovary syndrome and one diagnosed with insulin resistance) and 1 with PRED (indication of metformin therapy unknown), Additionally, 2% of participants with T1D were treated with SGLT-2 inhibitors (Table 3).
While our cohort is not population-based and primarily focuses on diabetes-associated complications and distinct pathophysiological mechanisms, the mean age and body mass index (BMI) of participants align closely with other German and European cohorts (Table 4, Panel a and Panel b)71,75,76,77. As anticipated, participants with T1D are younger and leaner compared to those with T2D (Table 4, Panel b) and PRED. However, T1D participants exhibit similar age and BMI to those with NGT (Table 4, Panel a).
Participants in the T2D group exhibited a high prevalence of cardiovascular disease at baseline (Table 3). Hypertension was prevalent among T2D participants, with 77% reporting the condition, corresponding to a higher usage of antihypertensive therapy (69%) in this group compared to T1D (43%). Despite higher use of antihypertensive medication, the mean blood pressure values are higher in T2D participants than in those with T1D (Table 4, Panel b). Among PRED participants, 44% reported hypertension, with 40% receiving antihypertensive therapy. In the NGT group, 18% reported hypertension, and a similar percentage was on hypertensive therapy at baseline (Table 3). Statin use at baseline was reported by 6% of participants with NGT, 17% with PRED, 28% with T1D, and 45% with T2D (Table 3). While overall statin use was low across all groups, the undertreatment was particularly notable in T1D and T2D participants, with 73% of T1D participants and 86% of T2D participants classified as having high to very high cardiovascular risk according to the ESC-Score 2, independent of DM as a risk-factor (Table 3). Although low-density lipoprotein (LDL) levels for both T1D and T2D participants (Table 4, Panel b) are lower than in individuals with NGT and PRED (Table 4, Panel a), this poor utilization of statins is still reflected in the small proportion of participants achieving LDL-cholesterol goal levels, with only 17% of T1D and 30% of T2D participants meeting these targets.
Liver transaminases and lipoprotein (a) [Lp(a)] levels were comparable across all study groups (Table 4, Panel a and Panel b). However, levels of high-sensitivity C-reactive protein (hsCRP) were elevated in all groups except the NGT group, indicating a possible inflammatory response in participants with DM and PRED. N-terminal pro–B-type natriuretic peptide (proBNP) was elevated above the upper limit (125 ng/L < 71 year old; 450 ng/L for ≥ 71 year old) in 35% of T1D and 22% of T2D, and levels of high-sensitivity cardiac troponin T (hsTNT) were elevated above the upper limit (> 14 ng/L) in 14% of T1D and 27% of T2D (Table 4, Panel b), as markers indicating cardiac dysfunction and damage of heart muscle, respectively.
The primary focus of this cohort is the assessment of diabetes-associated complications, with findings summarized in Fig. 1; Table 5. At baseline, CKD was identified in 19% of individuals with T1D and 28% of those with T2D (Fig. 1). Additionally, uACR levels were elevated in both the T1D and T2D group compared to the NGT and PRED group, with T2D participants showing higher uACR levels than those of the T1D group (Table 5 Panel a and Panel b). The increased prevalence of CKD in T2D is supported by a greater usage of renin-angiotensin system blockers (Table 3). According to previous European cohort studies, CKD is more common in T1D than in T2D78,79. The higher prevalence of CKD in T2D within our cohort may be attributed to a greater number of T2D participants in older age groups despite longer diabetes duration, whereas the T1D group consists mainly of younger individuals (Table 2). This is partly due to the initial active recruitment of individuals with DM and established diabetic complications, such as increased albuminuria. A few participants from the non-DM groups also met criteria for diagnosing CKD at baseline, specifically four from the NGT group and seven from the PRED group (Fig. 1). This may be related to other underlying conditions contributing to nephropathy, such as hypertension or undiagnosed renal diseases. In terms of DSPN, baseline prevalence was found to be 11% in PRED participants, 23% in T1D, and 46% in T2D (Fig. 1). The higher prevalence of DSPN in T2D aligns with previous intervention and cohort studies, highlighting the increasing burden of DSPN in older populations80,81,82. A systematic review on DSPN prevalence in adults with PRED indicated that 72% of studies reported a prevalence of ≥ 10%, consistent with our findings83. CAN was identified in 11% of T1D and 17% of T2D participants (Fig. 1), with only 2% of PRED participants and 2% of NGT participants meeting the criteria for CAN at baseline. The observed prevalence of CAN in PRED in our cohort is notably lower than previously reported estimates65,84, likely due to variability in the definitions and methodologies used in earlier studies84. For DR, our cohort reported a higher prevalence in T1D (30%) compared to T2D (22%), consistent with findings from previous studies85,86, with only one case identified in the NGT group and three cases in the PRED group. Diabetes related RLD phenotype was found in 28% of NGT participants, 35% of PRED participants, 43% of T1D participants, and 44% of T2D participants (Fig. 1). These numbers exceed those previously reported49, indicating a potential underdiagnosis of diabetes related RLD that warrants further investigation in future studies, especially in terms of treatment responses compared to non-diabetic cases with RLD50. The prevalence of MASLD was reported as 43% in the NGT group, 56% in the PRED group, 23% in the T1D group, and 73% in the T2D group, whereas the prevalence for liver fibrosis was 6% in NGT, 13% in PRED, 5% in T1D and 23% in T2D (Fig. 1). The higher prevalence in PRED and T2D was also reflected by elevated liver enzymes and triglycerides in the PRED and T2D groups, with T2D expressing the highest levels (Table 4 Panel a and Panel b). While the fatty-liver-index (FLI), as an index of MASLD, follows the same pattern, the fibrosis indices - fibrosis-4 index (FIB-4) and metabolic dysfunction-associated steatotic liver disease fibrosis score (MASLDS) - were only elevated in T2D (Table 4 Panel a and Panel b). The high prevalence of MASLD in NGT, PRED and T2D is likely due to high BMI in our cohort (Table 4 Panel a and Panel b), since BMI was previously reported to be the strongest covariate in predicting MASLD when using transient elastography87. The prevalence of MASLD in T1D in our cohort is slightly lower than in previous reported studies88,89. The higher prevalence for liver fibrosis, measured by elastography, in individuals with PRED and T2D and the fibrosis indices indicating a higher risk for advanced liver fibrosis for T2D, highlight the important role of impaired glucose metabolism in progression to liver fibrosis.
Diabetes-associated complications in individuals with NGT, PRED, T1D and T2D. Percentage (x-axis) and total numbers (in bars) of individuals without (grey) and with (red) respective diabetes-associated complications. CKD: chronic kidney disease, DSPN: diabetic sensorimotor peripheral neuropathy, CAN: cardiovascular autonomic neuropathy, DR: diabetic retinopathy, RLD: diabetes related restrictive lung disease, MASLD: metabolic dysfunction-associated steatotic liver disease.
Summary of the results obtained so Far
By mid-2024, based on the HEIST-DiC study 44 articles were published in peer-reviewed journals, reflecting its ongoing contributions to the understanding of diabetes-associated complications. The distribution of these publications across the years is as follows: 1 article in 201790, 4 articles in 201849,91,92,93, 3 articles in 201994,95,96, 5 articles in 202097,98,99,100,101, 6 articles in 202150,57,102,103,104,105, 7 articles in 202212,33,46,106,107,108,109, 7 articles in 202338,44,45,58,110,111,112, and 11 articles by mid-202413,39,40,41,42,43,52,53,113,114,115.
Assessment of classical diabetes-associated complications
-
Using MRN, our previous studies have successfully identified fascicle lesions in the sciatic nerve trunk of individuals with T2D and DSPN116,117. These lesions were characterized by hyperintense mono- or multifocal patterns, predominantly located at the thigh level. In the HEIST-DiC cohort, we further established that the nerve lesion loads observed through MRN correlated with the severity of clinical symptoms associated with DSPN, as well as with impaired nerve conduction and sensory loss97 and with HbA1c118. Specifically, MRN-derived parameters, such as fractional anisotropy, which indicate nerve fiber integrity, demonstrated strong correlations with increased NDS and decreased nerve conduction velocity, independent of factors like age, sex, BMI, and HbA1c58. These findings suggest that MRN can serve as a valuable non-invasive diagnostic tool for evaluating nerve function in DSPN and DM. This method offers significant advantages over traditional assessment methods, such as standardized questionnaires and clinical examinations, which are often reliant on subjective evaluations and individuals’ cooperation.
-
Another significant finding from our research is the demonstration that QST can effectively identify early sensory deficits in individuals with and without T2D. These deficits were found to be associated with markers of insulin resistance, metabolic syndrome, and glycation end-products44. Based on QST data analysis, we confirmed four sensory phenotypes: healthy, thermal hyperalgesia, mechanical hyperalgesia, and sensory loss. Longitudinal analysis of these QST-based sensory phenotypes, provided valuable insights into the natural progression of DSPN, with the sensory loss phenotype being the most strongly correlated with DSPN43. Additionally, MRN of T2D individuals who exhibited the most severe sensory phenotypes (mechanical hyperalgesia and sensory loss) revealed diminished structural integrity of the sciatic nerve. This structural decline appears to precede the sensory loss observed in peripheral nerves, highlighting the potential of MRN as a tool for early detection and monitoring of nerve integrity in the context of diabetes-associated complications41.
-
Regarding CKD, our longitudinal analysis indicated a progression of CKD over a 4-year period. Specifically, we observed that 6% of individuals with PRED experienced CKD progression, while 12% of individuals with T2D without previously known CKD also showed similar progression. Notably, 46% of individuals with T2D who had existing CKD experienced a worsening of their condition38. Furthermore, we found that albuminuria could be temporarily improved through dietary interventions in individuals with T2D and known CKD. This improvement in albuminuria was associated with significant changes in parameters related to fatty acid oxidation and was not sustained after the end of dietary intervention. These findings indicate that dietary management could play a role in the treatment of renal complications in DM33.
-
Regarding MASLD, a 4-year longitudinal analysis showed that 24% of individuals with PRED experienced progression, indicated by worsening of liver stiffness. This was also observed in 19% of participants with T2D without increased liver stiffness at baseline and 15% of those with T2D and increased liver stiffness at baseline. Interestingly, 36% of the participants without known diabetes-associated complications also exhibited increased liver stiffness38.
Assessment of non-classical diabetes-associated complications
-
Our study identified a significant increase in breathlessness and a notable prevalence of RLD among individuals with long-term T2D (27%). In comparison, 21% of individuals with recent-onset T2D and 9% of individuals with PRED showed similar patterns of lung disease49. To confirm the presence of interstitial lung disease, we utilized multidetector computed tomography, and histological analysis revealed evidence of fibrosis in the lungs of individuals with T2D49. These findings align with previous studies that have linked a decline in pulmonary function with T2D119,120. Our results underscore the importance of recognizing diabetic RLD as a critical component in the standard care for managing diabetes-associated complications50.
-
We showed that that DSPN does not solely affect the lower limbs; it also significantly impacts the upper limbs, making them susceptible to diabetes-induced damage. The sensory phenotype observed in individuals with upper limb neuropathy closely resembles that of lower limb neuropathy, primarily characterized by loss of sensory function107.
-
We found that diabetes-related distress was significantly associated with lower glycemic control, higher insulin resistance, and longer DM duration in individuals with T2D13. Additionally, we demonstrated an impairment in the autonomic nervous system’s response to stress, which is partially reflected in the psychological stress response12. Importantly, we found a notable association between moderate to severe childhood neglect and an intensified psychological stress response in individuals with T2D. This connection highlights the interplay between psychological factors, physiological stress responses, and childhood experiences in the context of DM management, suggesting the need for comprehensive approaches addressing both emotional and physical health in T2D individuals105.
Identification of biomarkers for diabetes-associated complications
-
In our study, we identified circulating mRNA levels of myelin protein zero as a novel potential biomarker associated with DSPN, which together with the already reported biomarker for DSPN, neurofilament light chain protein, were found changed in our cohort; specifically, decreased levels of myelin protein zero were predictive of hypoalgesia, while increased levels of neurofilament light chain were linked to a hyperalgesia phenotype57. Furthermore, neurofilament light chain protein levels correlated with sensorimotor deficits in both the upper and lower limbs in individuals with T2D58. These findings suggest that these biomarkers could be valuable in assessing neuropathic pain and sensory function in diabetic individuals.
-
We demonstrated that the bioelectrical phase angle derived from BIA can serve as a straightforward tool for assessing cardiovascular risk and detecting DSPN in individuals with and without DM52,53. This finding highlights the phase angle’s potential utility in clinical settings for early identification of individuals at risk for cardiovascular complications and neuropathy, aiding in timely interventions and management strategies.
Novel cellular mechanisms linked to diabetes-associated complications and precise interventions
The traditional glucose-centric approach to diabetes management, which focused on lowering glycemia, has been increasingly challenged, leading to a shift toward a holistic, patient-centered approach aimed at reducing the risks of diabetes-related complications121. This framework acknowledges the complexity of diabetes as a chronic disease and emphasizes the importance of a pathogenesis-centric strategy, targeting the underlying pathophysiological mechanisms that both cause and complicate diabetes and its associated complications122.
-
Cross-sectional analysis of individuals with PRED and T2D showed a strong association between the presence of CKD, RLD phenotype, and increased liver stiffness with elevated markers for DNA damage, senescence, and senescence-associated secretory phenotype (SASP)38. Furthermore, longitudinal analysis over four years revealed that the progression of CKD was significantly predicted by these markers of DNA damage, senescence, and SASP. In contrast, the progression of RLD was primarily associated with increased DNA damage and elevated levels of interleukin-6 (IL-6)38. These findings suggest that cellular aging plays a critical role in the complications observed in individuals with diabetes.
-
In the HEIST-DiC cohort, we successfully validated a novel p21-dependent mechanism of tubular senescence previously identified in animal models, which contributes to hyperglycemic memory in the context of CKD and T2D109,115. We observed that tubular and urinary p21-levels from individuals with T2D were significantly associated with the severity of CKD. Notably, these p21 levels remained elevated even when blood glucose levels improved through treatment with SGLT-2 inhibitors or dietary interventions33,109,115. This persistence of p21 elevation, despite better glycemic control, underscores the potential for tubular senescence to play a pivotal role in the progression of CKD.
-
We discovered elevated hydroxyacetone levels and increased activity of aldo-keto-reductase in red blood cells of T2D individuals, as markers associated with compensatory mechanisms for methylglyoxal detoxification. These markers may serve as useful indicators to distinguish between T2D individuals with and without complications. Specifically, individuals with T2D who do not exhibit complications appear to retain protective alternative detoxification pathways for methylglyoxal, which likely contributes to their better health profile. Conversely, those with T2D complications show a loss of these protective mechanisms, suggesting that impaired detoxification processes may contribute to the progression of diabetes-associated complications91.
-
In a subgroup of individuals with T2D and CKD we conducted a randomized-controlled trial focusing on the effects of a diet intervention involving periodic fasting over 6 months. This study aimed to evaluate outcomes related to diabetes-associated complications, particularly the impact on microalbuminuria and somatosensory nerve function33,112. Our findings indicated that improvement of microalbuminuria under periodic fasting was linked to specific changes in acylcarnitine profile33, and had no effect on somatosensory nerve function112. Additionally, the effectiveness of periodic fasting on weight loss and maintenance appeared to be influenced by a genetic polymorphism of p53113. In a following proof-of-concept study we demonstrated that glucose intake during refeeding after periodic fasting led to an increased oxidative stress response in T2D individuals with complications114. T2D individuals without complications seemed to be unaffected by the glucose-induced changes, whereas individuals with NGT experienced enhanced cellular resistance to oxidative stress114. These results suggest that while periodic fasting may offer benefits for managing microalbuminuria in T2D individuals with CKD, the oxidative stress response post-refeeding could vary significantly based on the presence of diabetes-associated complications.
Taken together, our findings are significant as they shift the focus from the traditional glucose-centered hypothesis to a broader understanding of the various mechanisms influencing the development and progression of diabetes-associated complications. By identifying these novel mechanisms, we open avenues for designing targeted interventions aimed at preventing, improving, or even achieving remission of these complications.
What are the main strengths and limitations?
The HEIST-DiC cohort includes individuals across the full spectrum of glucose metabolism, offering a unique opportunity to address critical gaps in diabetes research, such as refining clustering methods, assessing subtype stability, and identifying biomarkers and high-risk individuals. By stratifying participants based on pathophysiological mechanisms, the cohort bridges epidemiological studies and clinical research. This approach will validate existing methods and drive new frameworks, supporting tailored therapeutic strategies and advancing precision medicine in diabetes care. The main strengths of this cohort include: (i) the inclusion of individuals exhibiting a broad diversity in metabolic and clinical stages, providing in this way a comprehensive representation of clinical trajectories of PRED, T1D and T2D; (ii) extensive metabolic and clinical phenotyping for classical and non-classical diabetes-associated complications; and (iii) an extended follow-up period of 11 years. Consequently, the HEIST-DiC cohort presents the opportunity to examine the natural course of DM while simultaneously investigating the onset and progression of diabetes-associated complications and differentiating between fast and slow progressors.
The comprehensive metabolic and clinical phenotyping of diabetes-associated complications has enabled us to implement novel methods by flexibly adapting the study protocol to current findings and refining the focus based on these insights. For instance, in December 2016, we incorporated the 6-minute walk test to assess emerging lung complications related to diabetes. Hand nerve conduction velocity was added in February 2021 in response to emerging evidence of impaired nerve function in the upper extremities. In June 2023, transcutaneous electrical nerve fiber stimulation was introduced to evaluate small nerve fibers (Table 2). The method we employ selectively assesses C-nociceptor excitability through slow, long-duration pulses at low frequency123,124. Alternative novel techniques utilizing short, high-frequency rectangular pulses, primarily assessing A-fiber function, should be considered in future protocol adaptations to allow for more comprehensive assessment of peripheral sensory function125.
This thorough characterization allows to design precise intervention studies aimed at prevention, improvement or ideally, remission of diabetes-associated complications.
Another notable strength is provision and explanation of detailed results to study participants, which they can share with their healthcare providers. This practice keeps participants informed about their health status and enhances their compliance, further supported by regular educational events organized by the study team on DM awareness and information. On the other hand, the communication of the detailed results to the healthcare providers may prompt adjustments in treatment strategy. Additionally, the annual study visits may increase personal motivation and adherence to therapeutic recommendations, thereby incorporating intervention elements compared to study participants.
HEIST-DiC provides more intensive assessments with annual clinical visits, compared to the German Diabetes Study (GDS), which conducts clinical evaluations every five years71. Unlike the Diabetes Prospective Follow-up Registry (DPV)—a registry collecting demographic, clinical, and treatment data for T1D and T2D since 1995—HEIST-DiC offers detailed insights into diabetes-related complications using advanced methods available only in specialized university research centers126. While the Verona Diabetes Study and the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) program focus on mortality and self-management, their follow-up periods are shorter, at five years and three years respectively127,128. While the current follow-up period is set at 11 years, future extensions will be implemented in response to emerging scientific questions or the integration of novel methodologies.
While predicting long-term disease trajectories in individuals aged 85 and older may have limited applicability, choosing a high upper age limit ensures inclusivity and better reflects the real-world population seen in clinical practice. Older adults are frequently underrepresented in clinical research, despite bearing a significant burden of chronic diseases such as T2D. Including them allows to capture age-related variability and potentially identify important trends within this population. Furthermore, with regard to diabetes subtypes, individuals with mild age-related diabetes are typically older and present with milder metabolic disturbances compared to other subtypes14,37. This approach opens the possibility of identifying a subgroup of individuals with diabetes who are less susceptible to complications and may achieve healthy aging despite the disease. Establishing and further characterizing this subgroup using advanced analytical tools represents an important goal for future research.
As the majority of participants are recruited from our outpatient university clinic, a potential source of bias may arise from the inclusion of individuals with a longer duration of diabetes and the presence of pre-existing diabetes-associated complications. However, these individuals provide valuable insights into the development of DM and the associated complications, without the beforementioned intervention elements inflicted by the monitoring during a study.
One limitation of the HEIST-DiC study is the insufficient representation of diverse ethnicities, as it exclusively includes individuals residing in Germany and does not record ethnicity data. Additionally, our cohort comprises more female participants in the NGT group, which is currently addressed by strategic recruitment of more male participants.
Given that the HEIST-DiC study is a hypothesis-generating cohort study, the initially conducted sample size calculations are based on a logistic regression model for a binary outcome with a single binary or continuous covariate. Typically, cohort studies incorporate additional co-variables that may act as confounders, which should be integrated into the regression model. This oversight reduces the statistical power of the analysis, necessitating adjustments to the initially calculated sample sizes using the variance inflation factor and a factor accounting for expected dropout rates129. As Heist-DiC adapts its study protocol in response to emerging findings, the inclusion of new target groups and outcomes will be continuously considered throughout the course of the study. Consequently, the final sample sizes may need to be larger than originally calculated. Heist-DiC was intentionally designed as an open-end hypothesis-generating cohort study, without a fixed participant target or end date, to maintain flexibility. This design also supports the potential inclusion of new target groups, correction of imbalances in existing groups, and replacement of drop-outs—ensuring an active cohort and supporting the study’s function as a recruitment platform for intervention studies.
Study investigators are not required to evaluate clinical findings beyond the parameters of the research question, an approach that is approved by the ethics committee and is clearly explained to participants prior to their inclusion in the study. Assessment of preclinical manifestation of diabetes-associated complications or other comorbidities may not result in immediate changes in clinical management. In case any relevant incidental abnormal finding is detected during magnetic resonance imaging, ultrasound or in blood chemistry, participants are informed and, if requested, the information is reported to their healthcare providers for further evaluation.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to national data protection laws but are available from the corresponding author on reasonable request. Researches may apply for data and/or biological samples by contacting the study coordinators via email (Stoffwechsel.Studien@med.uni-heidelberg.de). The steering committee of the study will evaluate the request. After approval, the requesting researcher and the principal investigator of HEIST-DiC sign a contract on data transfer and transmission of results.
Abbreviations
- ADA:
-
American Diabetes Association
- Adipo-IR:
-
Adipose tissue insulin resistance index
- ALAT:
-
Alanine aminotransferase
- ASAT:
-
Aspartate aminotransferase
- BIA:
-
Bioelectrical impedance analysis
- BP:
-
Blood pressure
- CAN:
-
Cardiovascular autonomic neuropathy
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- CNAP:
-
Compound nerve action potential
- Diast:
-
Diastolic
- DLCO :
-
Diffusing capacity of the lung for carbon monoxide
- DM:
-
Diabetes mellitus
- DML:
-
Distal motor latency
- DR:
-
Diabetic retinopathy
- DSPN:
-
Distal sensorimotor polyneuropathy
- eGFR:
-
Estimated glomerular filtration rate
- E/I:
-
Exhalation / inhalation
- ESC:
-
European Society of Cardiology
- FFA:
-
Free fatty acids
- FLI:
-
Fatty-liver-index
- FIB-4:
-
Fibrosis-4 index
- GGT:
-
Gamma glutamyl transferase
- GLP-1:
-
Glucagon-like peptide 1
- hsCRP:
-
High-sensitivity C-reactive protein
- hsTNT:
-
High-sensitivity troponin T
- HbA1c:
-
Glycated hemoglobin
- HDL:
-
High-density lipoprotein
- HIV:
-
Human immunodeficiency virus
- HRV:
-
Heart rate variability
- ins sens:
-
Insulin sensitivity
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- LADA:
-
Latent autoimmune diabetes of the adult
- LDL:
-
Low-density lipoprotein
- Lp(a):
-
Lipoprotein (a)
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MASLDS:
-
Metabolic dysfunction-associated steatotic liver disease score
- MODY:
-
Maturity onset diabetes of the young
- MNSI:
-
Michigan neuropathy screening instrument
- MRN:
-
High-resolution magnetic resonance neurography
- NCV:
-
Nerve conduction velocity
- NDS:
-
Neuropathy disability score
- NGT:
-
Normal glucose tolerance
- NSS:
-
Neuropathy symptom score
- NTproBNP:
-
N-terminal pro B-type natriuretic peptide
- NYHA:
-
New York Heart Association
- oGTT:
-
Oral glucose tolerance test
- PPAR:
-
Peroxisome proliferator-activated receptor
- PHQ:
-
Patient health questionnaire
- PRED:
-
Prediabetes
- QST:
-
Quantitative sensory testing
- RLD:
-
Restrictive lung disease
- SAS:
-
Symptom assessment score
- SASP:
-
Senescence-associated secretory phenotype
- SF-12:
-
12-item short-form health survey
- SGLT-2:
-
Sodium-glucose cotransporter-2
- SIRD:
-
Severe insulin-resistant diabetes
- SIDD:
-
Severe insulin-deficient diabetes
- SNAP:
-
Sensory nerve action potential
- Syst:
-
Systolic
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetes
- TLC:
-
Total lung capacity
- uACR:
-
Urinary albumin-to-creatinine ratio
References
Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, Dalton BE, Duprey J, Cruz JA, Hagins H. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet (2023)
Roth, G. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), The Lancet 2018; 392, 1736–1788 (2018).
Turnbull, F. et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52, 2288–2298 (2009).
Forbes, J. M. & Cooper, M. E. Mechanisms of diabetic complications. Physiol. Rev. 93, 137–188 (2013).
Mauricio, D., Alonso, N. & Gratacòs, M. Chronic diabetes complications: the need to move beyond classical concepts. Trends Endocrinol. Metabolism. 31, 287–295 (2020).
Solomon, S. D. et al. Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care. 40, 412 (2017).
Thomas, M. C. et al. Diabetic kidney disease. Nat. Reviews Disease Primers. 1, 1–20 (2015).
Vincent, A. M., Callaghan, B. C., Smith, A. L. & Feldman, E. L. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat. Reviews Neurol. 7, 573–583 (2011).
Moulton, K. S. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr. Opin. Lipidol. 17, 548–555 (2006).
Carter, A. et al. Intimal neovascularisation is a prominent feature of atherosclerotic plaques in diabetic patients with critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 33, 319–324 (2007).
Hayden, M. R. & Tyagi, S. C. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc. Diabetol. 3, 1–16 (2004).
Monzer, N. L. et al. The cardiac autonomic response to acute psychological stress in type 2 diabetes. Plos One. 17, e0265234 (2022).
Buckert, M. et al. Cross-sectional associations of self-perceived stress and hair cortisol with metabolic outcomes and microvascular complications in type 2 diabetes. Front. Public. Health. 12, 1289689 (2024).
Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6, 361–369 (2018).
Zaharia, O. P. et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 7, 684–694 (2019).
Tanabe, H. et al. Factors associated with risk of diabetic complications in novel cluster-based diabetes subgroups: a Japanese retrospective cohort study. J. Clin. Med. 9, 2083 (2020).
Herder, C. & Roden, M. A novel diabetes typology: towards precision diabetology from pathogenesis to treatment. Diabetologia 65, 1770–1781 (2022).
Dennis, J. M., Shields, B. M., Henley, W. E., Jones, A. G. & Hattersley, A. T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 7, 442–451 (2019).
Safai, N., Ali, A., Rossing, P. & Ridderstråle, M. Stratification of type 2 diabetes based on routine clinical markers. Diabetes Res. Clin. Pract. 141, 275–283 (2018).
Zou, X., Zhou, X., Zhu, Z. & Ji, L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 7, 9–11 (2019).
Bayoumi, R. et al. Etiologies underlying subtypes of long-standing type 2 diabetes. Plos One. 19, e0304036 (2024).
Wesolowska-Andersen, A. et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: an IMI DIRECT study. Cell. Rep. Med. 4;3(1):100477 (2022).
UdlerMS et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 15 (9), e1002654 (2018).
Schön, M. et al. Analysis of type 2 diabetes heterogeneity with a tree-like representation: insights from the prospective German diabetes study and the LURIC cohort. Lancet Diabetes Endocrinol. 12, 119–131 (2024).
Wagner, R. et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 27, 49–57 (2021).
Li, Y. et al. Genetic subtypes of prediabetes, healthy lifestyle, and risk of type 2 diabetes. Diabetes 1;73(7):1178-1187 (2024).
Fritsche, A. et al. Different effects of lifestyle intervention in high-and low-risk prediabetes: results of the randomized controlled prediabetes lifestyle intervention study (PLIS). Diabetes 70, 2785–2795 (2021).
Group, D. E. R. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N. Engl. J. Med. 365, 2366–2376 (2011).
Control, D., Interventions, C. T. E. D. & Group, C. S. R. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 39, 686–693 (2016).
Control, D., Interventions, C. T. E. D. & Group, C. R. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 342, 381–389 (2000).
Zoungas, S. et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 5, 431–437 (2017).
Bancks, M. P. et al. Type 2 diabetes subgroups, risk for complications, and differential effects due to an intensive lifestyle intervention. Diabetes Care. 44, 1203–1210 (2021).
Sulaj, A. et al. Six-month periodic fasting in patients with type 2 diabetes and diabetic nephropathy: a proof-of-concept study. J. Clin. Endocrinol. Metabolism. 107, 2167–2181 (2022).
Veelen, A., Erazo-Tapia, E., Oscarsson, J. & Schrauwen, P. Type 2 diabetes subgroups and potential medication strategies in relation to effects on insulin resistance and beta-cell function: A step toward personalised diabetes treatment? Mol. Metabolism. 46, 101158 (2021).
Seebauer, L. & Szendrödi, J. Die Neuen subtypen des (Prä–) diabetes auf dem Weg in die praxis. Die Diabetol. 19, 941–951 (2023).
Association, A. D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 37, S81–S90 (2014).
Zaharia, O. P. et al. Diabetes clusters and risk of diabetes-associated diseases-Authors’ reply. Lancet Diabetes Endocrinol. 7, 828–829 (2019).
Varun, K. et al. Elevated markers of DNA damage and senescence are associated with the progression of albuminuria and restrictive lung disease in patients with type 2 diabetes. EBioMedicine 90, (2023).
Foesleitner, O. et al. Diffusion tensor imaging in anisotropic tissues: application of reduced gradient vector schemes in peripheral nerves. Eur. Radiol. Experimental. 8, 37 (2024).
Mooshage, C. M. et al. Association of small fiber function with microvascular perfusion of peripheral nerves in patients with type 2 diabetes: study using quantitative sensory testing and magnetic resonance neurography. Clin. Neuroradiol. 34, 55–66 (2024).
Mooshage, C. M. et al. A diminished sciatic nerve structural integrity is associated with distinct peripheral sensory phenotypes in individuals with type 2 diabetes. Diabetologia 67, 275–289 (2024).
Mooshage, C. M. et al. Magnetization transfer ratio of the sciatic nerve differs between patients in type 1 and type 2 diabetes. Eur. Radiol. Experimental. 8, 6 (2024).
Tsilingiris, D. et al. Sensory phenotypes provide insight into the natural course of diabetic polyneuropathy. Diabetes 73, 135–146 (2024).
Tsilingiris, D. et al. Dysmetabolism-related early sensory deficits and their relationship with peripheral neuropathy development. J. Clin. Endocrinol. Metabolism. 108, e979–e988 (2023).
Mooshage, C. M. et al. Diametrical effects of glucose levels on microvascular permeability of peripheral nerves in patients with type 2 diabetes with and without diabetic neuropathy. Diabetes 72, 290–298 (2023).
Jende, J. M. et al. Sciatic nerve microvascular permeability in type 2 diabetes decreased in patients with neuropathy. Ann. Clin. Transl. Neurol. 9, 830–840 (2022).
Zilliox, L. et al. Assessing autonomic dysfunction in early diabetic neuropathy: the survey of autonomic symptoms. Neurology 76, 1099–1105 (2011).
Fong, D. S. et al. Diabetic retinopathy. Diabetes Care. 26, s99–s102 (2003).
Kopf, S. et al. Breathlessness and restrictive lung disease: an important diabetes-related feature in patients with type 2 diabetes. Respiration 96, 29–40 (2018).
Kopf, S. et al. Diabetic pneumopathy–a new diabetes-associated complication: mechanisms, consequences and treatment considerations. Front. Endocrinol. 12, 765201 (2021).
Ciardullo, S., Ballabeni, C., Trevisan, R. & Perseghin, G. Liver fibrosis assessed by transient elastography is independently associated with albuminuria in the general united States population. Dig. Liver Disease. 53, 866–872 (2021).
Tsilingiris, D. et al. Utility of bioelectrical phase angle for cardiovascular risk assessment among individuals with and without diabetes mellitus. Diabetes Vasc. Dis. Res. 21, 14791641231223701 (2024).
Schimpfle, L. et al. Phase angle of bioelectrical impedance analysis as an indicator for diabetic polyneuropathy in type 2 diabetes mellitus. J. Clin. Endocrinol. Metabolism 15;109(11):e2110-e2119. (2024).
DeFronzo, R. A., Tobin, J. D. & Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiology-Endocrinology Metabolism. 237, E214 (1979).
Bischof, M. G. et al. Hepatic glycogen metabolism in type 1 diabetes after long-term near normoglycemia. Diabetes 51, 49–54 (2002).
Schadewaldt, P., Nowotny, B., Straßburger, K., Kotzka, J. & Roden, M. Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. Am. J. Clin. Nutr. 97, 763–773 (2013).
Morgenstern, J. et al. Neuron-specific biomarkers predict hypo-and hyperalgesia in individuals with diabetic peripheral neuropathy. Diabetologia 64, 2843–2855 (2021).
Kender, Z. et al. Sciatic nerve fractional anisotropy and neurofilament light chain protein are related to sensorimotor deficit of the upper and lower limbs in patients with type 2 diabetes. Front. Endocrinol. 14, 1046690 (2023).
Ware, J. E., Kosinski, M. & Keller, S. D. A 12-Item Short-Form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 34, 220–233 (1996).
Gräfe, K., Zipfel, S., Herzog, W. & Löwe, B. Screening psychischer störungen Mit dem gesundheitsfragebogen für patienten (PHQ-D). Diagnostica 50, 171–181 (2004).
Rossing, P. et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 102, S1–S127 (2022).
Ziegler, D., Keller, J., Maier, C. & Pannek, J. Diabetic neuropathy. Exp. Clin. Endocrinol. Diabetes. 122, 406–415 (2014).
Dyck, P. J. et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and Estimation of severity. Diab./Metab. Res. Rev. 27, 620–628 (2011).
Tesfaye, S. et al. Diabetic neuropathies: update on definitions, diagnostic criteria, Estimation of severity, and treatments. Diabetes Care. 33, 2285 (2010).
Ziegler, D. et al. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia 58, 1118–1128 (2015).
Vinik, A. I. & Ziegler, D. Diabetic cardiovascular autonomic neuropathy. Circulation 115, 387–397 (2007).
Wilkinson, C. P. et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110, 1677–1682 (2003).
Pellegrino, R. et al. Interpretative strategies for lung function tests. Eur. Respir. J. 26, 948–968 (2005).
Hillier, T. A. & Pedula, K. L. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 24, 1522–1527 (2001).
Rooney, M. R. et al. Global prevalence of prediabetes. Diabetes Care. 46, 1388–1394 (2023).
Szendroedi, J. et al. Cohort profile: the German diabetes study (GDS). Cardiovasc. Diabetol. 15, 1–14 (2016).
Nordström*, A., Hadrévi, J., Olsson, T., Franks, P. W. & Nordström, P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J. Clin. Endocrinol. Metabolism. 101, 3740–3746 (2016).
Ali, J. et al. Overall clinical features of type 2 diabetes mellitus with respect to gender. Cureus 15, (2023).
Steinberg, J. et al. Analysis of female enrollment and participant sex by burden of disease in US clinical trials between 2000 and 2020. JAMA Netw. Open., Jun 1;4(6):e2113749 (2021).
Hartwig, S. et al. Anthropometric markers and their association with incident type 2 diabetes mellitus: which marker is best for prediction? Pooled analysis of four German population-based cohort studies and comparison with a nationwide cohort study. BMJ Open. 6 (1), e009266 (2016).
Kinmonth, A. L., Woodcock, A., Griffin, S., Spiegal, N. & Campbell, M. J. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. Bmj 317, 1202–1208 (1998).
Gatling, W., Guzder, R., Turnbull, J., Budd, S. & Mullee, M. The Poole diabetes study: how many cases of type 2 diabetes are diagnosed each year during normal health care in a defined community? Diabetes Res. Clin. Pract. 53, 107–112 (2001).
Kristófi, R. et al. Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: a two-country nationwide observational study. Diabetes Care. 44, 1211–1218 (2021).
Parving, H-H. et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy, and neuropathy in patients with insulin dependent diabetes. Br. Med. J. (Clin Res. Ed). 296, 156–160 (1988).
Ismail-Beigi, F. et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376, 419–430 (2010).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Pop-Busui, R., Lu, J., Lopes, N. & Jones, T. L. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J. Peripheral Nerv. Syst. 14, 1–13 (2009).
Kirthi, V. et al. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open. Diabetes Res. Care. 9, e002040 (2021).
Ziegler, D., Herder, C. & Papanas, N. Neuropathy in prediabetes. Diab./Metab. Res. Rev. 39, e3693 (2023).
Soumiya, B. et al. Prevalence and risk factors of retinopathy in type 1 diabetes: A Cross-Sectional study. Cureus 15, (2023).
Romero-Aroca, P. et al. Differences in incidence of diabetic retinopathy between type 1 and 2 diabetes mellitus: a nine-year follow-up study. Br. J. Ophthalmol. 101, 1346–1351 (2017).
Bhattacharyya M, Nickols-Richardson SM, Miller AL, Bhattacharyya R, Frankhauser F, Miller LE. Prevalence and determinants of undiagnosed liver steatosis and fibrosis in a nationally representative sample of US adults. Cureus 15, (2023).
Mertens J, Weyler J, Dirinck E, Vonghia L, Kwanten W, Mortelmans L, Peleman C, Chotkoe S, Spinhoven M, Vanhevel F. Prevalence, risk factors and diagnostic accuracy of non-invasive tests for NAFLD in people with type 1 diabetes. 5. https://doi org/101016/j jhepr 2023:100753 (2023).
De Vries, M., Westerink, J., Kaasjager, K. H. & De Valk, H. W. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. J. Clin. Endocrinol. Metabolism. 105, 3842–3853 (2020).
Hidmark, A. et al. Electrical muscle stimulation induces an increase of VEGFR2 on Circulating hematopoietic stem cells in patients with diabetes. Clin. Ther. 39, 1132–1144 (2017). e1132.
Schumacher, D. et al. Compensatory mechanisms for Methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metabolism. 18, 143–152 (2018).
Jende J, Gröner J, Oikonomou D, Heiland S, Kopf S, Pham M, Nawroth PP, Bendszus M, Kurz FT. Diabetic neuropathy differs between type 1 and type 2 diabetes. 20118
Kopf, S. et al. Deep phenotyping neuropathy: an underestimated complication in patients with pre-diabetes and type 2 diabetes associated with albuminuria. Diabetes Res. Clin. Pract. 146, 191–201 (2018).
Kender, Z., Groener, J. B., Reismann, P. & Kopf, S. A Metformin Hatása a vérzsírértékekre, illetve a Szív És érrendszeri Kockázatra Sztatinkezelésben Nem részesülő 2-es Típusú Cukorbetegekben. Orv. Hetil. 160, 1346–1352 (2019).
Groener, J. B. et al. Asprosin response in hypoglycemia is not related to hypoglycemia unawareness but rather to insulin resistance in type 1 diabetes. PloS One. 14, e0222771 (2019).
Jende, J. M. et al. Association of serum cholesterol levels with peripheral nerve damage in patients with type 2 diabetes. JAMA Netw. Open. 2, e194798–e194798 (2019).
Groener, J. B. et al. Understanding diabetic neuropathy—from subclinical nerve lesions to severe nerve fiber deficits: a cross-sectional study in patients with type 2 diabetes and healthy control subjects. Diabetes 69, 436–447 (2020).
Jende, J. M. et al. Diabetic polyneuropathy is associated with pathomorphological changes in human dorsal root ganglia: a study using 3T MR neurography. Front. NeuroSci. 14, 570744 (2020).
Jende, J. M. et al. Structural nerve remodeling at 3-T MR neurography differs between painful and painless diabetic polyneuropathy in type 1 or 2 diabetes. Radiology 294, 405–414 (2020).
Jende, J. M. et al. Troponin T parallels structural nerve damage in type 2 diabetes: a cross-sectional study using magnetic resonance neurography. Diabetes 69, 713–723 (2020).
Lou, B. et al. Elevated 4-hydroxynonenal induces hyperglycaemia via Aldh3a1 loss in zebrafish and associates with diabetes progression in humans. Redox Biol. 37, 101723 (2020).
Jende, J. M. et al. Diffusion tensor imaging of the sciatic nerve as a surrogate marker for nerve functionality of the upper and lower limb in patients with diabetes and prediabetes. Front. NeuroSci. 15, 642589 (2021).
Jende, J. M. et al. Fractional anisotropy and troponin T parallel structural nerve damage at the upper extremities in a group of patients with prediabetes and type 2 Diabetes–A study using 3t magnetic resonance neurography. Front. NeuroSci. 15, 741494 (2022).
Jende, J. M. et al. Magnetic resonance neurography reveals smoking-associated decrease in sciatic nerve structural integrity in type 2 diabetes. Front. NeuroSci. 15, 811085 (2022).
Monzer, N. et al. Associations of childhood neglect with the ACTH and plasma cortisol stress response in patients with type 2 diabetes. Front. Psychiatry. 12, 679693 (2021).
Jende, J. M. et al. Troponin T is negatively associated with 3 Tesla magnetic resonance peripheral nerve perfusion in type 2 diabetes. Front. Endocrinol. 13, 839774 (2022).
Kender, Z. et al. Diabetic neuropathy is a generalized phenomenon with impact on hand functional performance and quality of life. Eur. J. Neurol. 29, 3081–3091 (2022).
Loft, A. et al. A macrophage-hepatocyte glucocorticoid receptor axis coordinates fasting ketogenesis. Cell Metabol. 34, 473–486 (2022). e479.
Al-Dabet, M. M. et al. Reversal of the renal hyperglycemic memory in diabetic kidney disease by targeting sustained tubular p21 expression. Nat. Commun. 13, 5062 (2022).
Mooshage, C. M. et al. Insulin resistance is associated with reduced capillary permeability of thigh muscles in patients with type 2 diabetes. J. Clin. Endocrinol. Metabolism. 109, e137–e144 (2024).
Joshi, P. et al. Impact of the-1T > C single-nucleotide polymorphism of the CD40 gene on the development of endothelial dysfunction in a pro-diabetic microenvironment. Atherosclerosis 394, 117386 (2024).
Kender, Z. et al. Six-month periodic fasting does not affect somatosensory nerve function in type 2 diabetes patients. Front. Endocrinol. 14, 1143799 (2023).
Reinisch, I. et al. Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape. Nat. Commun. 15, 1391 (2024).
von Rauchhaupt E, Rodemer C, Kliemank E, Bulkescher R, Campos M, Kopf S, Fleming T, Herzig S, Nawroth PP, Szendroedi J. Glucose load following prolonged fasting increases oxidative stress-linked response in individuals with diabetic complications. Diabetes Care . Sep 1;47(9):1584-1592. (2024).
Elwakiel, A. et al. Factor XII signaling via uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease. Nat. Commun. 15, 7963 (2024).
Pham, M. et al. Magnetic resonance neurography detects diabetic neuropathy early and with proximal predominance. Ann. Neurol. 78, 939–948 (2015).
Pham, M. et al. Proximal neuropathic lesions in distal symmetric diabetic polyneuropathy: findings of high-resolution magnetic resonance neurography. Diabetes Care. 34, 721–723 (2011).
Jende, J. M. et al. Diabetic neuropathy differs between type 1 and type 2 diabetes: insights from magnetic resonance neurography. Ann. Neurol. 83, 588–598 (2018).
Davis, W. A., Knuiman, M., Kendall, P., Grange, V. & Davis, T. M. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle diabetes study. Diabetes Care. 27, 752–757 (2004).
Lange, P. et al. Diabetes mellitus and ventilatory capacity: a five year follow-up study. Eur. Respir. J. 3, 288–292 (1990).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 45, 2753–2786 (2022).
Galindo, R. J., Trujillo, J. M., Wang, C. C. L. & McCoy, R. G. Advances in the management of type 2 diabetes in adults. BMJ Med. Sep 4;2(1):e000372 (2023).
Landmann, G. et al. Local hyperexcitability of C-nociceptors May predict responsiveness to topical Lidocaine in neuropathic pain. Plos One. 17, e0271327 (2022).
Jonas, R. et al. Tuning in C-nociceptors to reveal mechanisms in chronic neuropathic pain. Ann. Neurol. 83, 945–957 (2018).
Røikjer, J. et al. Perception threshold tracking: validating a novel method for assessing function of large and small sensory nerve fibers in diabetic peripheral neuropathy with and without pain. Pain 164, 886–894 (2023).
Flechtner-Mors M, Schwab K, Fröhlich-Reiterer E, Kapellen T, Meissner T, Rosenbauer J, Stachow R, Holl R. Overweight and obesity based on four reference systems in 18,382 paediatric patients with type 1 diabetes from Germany and Austria. Journal of diabetes research 2015, 370753 (2015).
Muggeo, M. et al. Long-term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non–insulin-dependent diabetes mellitus: the Verona diabetes study. Circulation 96, 1750–1754 (1997).
Khunti, K. et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. Bmj 344, e2333 (2012).
Hsieh, F. Y., Bloch, D. A. & Larsen, M. D. A simple method of sample size calculation for linear and logistic regression. Stat. Med. 17, 1623–1634 (1998).
Acknowledgements
The authors thank all study participants and staff members of the Study Center for Diabetes and Metabolism Research at the Clinic of Endocrinology, Diabetology, Metabolic Diseases and Clinical Chemistry (Internal Medicine I) of the University Hospital Heidelberg in Heidelberg Germany. A special thank you goes to our study nurses, study coordinators, laboratory technicians and data entry personnel who have contributed to this work, including those currently involved in the study (in alphabetical order): Anita Pflästerer, Anna Liu, Axel Erhardt, Jennifer Laycock, Kerstin Zachert, Kevin Lange, Luis Gorfer, Nadine Hefner, Madlin Telcher, Silke Brunholz, Silvia Geiger-Eul, Waltraud Hampel-Schütte, and those who have previously contributed: Anja Buhl, Antje Hagedorn-Dambuk; Christa Kannengießer, Irina Kochetkova; Sandra Bischoff and Sandra Lupaschko. We thank all current and previous study physicians: Aikaterini Valkanou, Athanasia Ziagaki, Jan Gröner, Janik Dreesen, Jimmy Masjkur, Klea Ricku, Laila Terzic, Lucia Alvares Ramos, Ronalds Prikulis and Ruan Cheko, as well as our medical students: Christian Mudrack, Hannah Gottlieb, Julia Nowak; Lea Henke, Omar Eldesouky and Wiebke Neibig. For the publication fee we acknowledge financial support by Heidelberg University.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is funded by the German Federal Ministry of Education and Research (BMBF) through the German Center for Diabetes Research (DZD e.V.) and the German Research Foundation (DFG). Third-party funding supports sub-projects within HEIST-DiC.
Author information
Authors and Affiliations
Contributions
E.K., J.S. and A.S. wrote the manuscript and researched the data. E.K. and A.S. performed the statistical analysis. E.R., L.See, M.R., M.A., V.F., L.Sch, D.T., H.B., T.F., Z.K., J.J., C.M., D.S., M.B., P.S., S.K. and A.S. contributed to acquire the data. Z.K. and T.F. contributed to the discussion and reviewed/edited the manuscript. S.K., J.S. and A.S. designed the study, contributed to discussion and reviewed/edited the manuscript. All authors critically reviewed, read and approved the final manuscript. A.S. is the guarantor of this work and, as such, had full access to the data presented in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study is performed according to the Declaration of Helsinki and approved by the ethics committee of the medical faculty at the University of Heidelberg (ethic number S-383/2016). All study participants give written informed consent to the study protocol.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kliemank, E., von Rauchhaupt, E., Seebauer, L. et al. Cohort profile of the Heidelberg study on diabetes and complications HEIST-DiC. Sci Rep 15, 29580 (2025). https://doi.org/10.1038/s41598-025-15343-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15343-8