Abstract
This study aims to explore the risk associated with the Tumour Necrosis Factor-Alpha (TNFα)-308 G/A (rs1800629) gene polymorphism in relation to Childhood Nephrotic Syndrome (NS). The primary goal of the meta-analysis was to investigate the relationship between Nephrotic Syndrome and the TNFα 308 G/A [rs1800629] polymorphism of the cytokine gene. We conducted a systematic search across electronic databases like PubMed and Google Scholar to collect data from five distinct case-control studies focused on the TNFα 308 G/A gene variant, covering the period from 2010 to 2022. By aggregating allele and genotype frequencies from these studies, we computed the 95% confidence interval of odds ratio [OR] to assess the strength of the association. To evaluate heterogeneity and potential publication bias in the selected studies, Stats Direct software was employed. The sample size encompassed 1,560 individuals, including 628 cases and 932 controls from five separate case-control studies. The TNFα allele-A displayed significant heterogeneity [I²=80%, 95% CI] when compared to the G allele, and a statistically significant pooled OR of 2.32 [P = 0.0056*] was observed. In the overall analysis, no significance association was found in the dominant model [P = 0.068], but a significant association was detected in the recessive model [P = 0.0096*]. Consistent findings were observed in the co-dominant model, where both AA vs. GG [P = 0.0075*] and GA vs. GG [P = 0.0219*] showed significant associations. These results suggest a potential increase in the risk of the disease associated with the polymorphism of TNFα 308 G/A. The small number of studies, high heterogeneity (I² = 80%), and limited ethnic diversity may affect the robustness and generalizability of findings. Additionally, language bias and lack of confounder adjustment limit interpretability. The TNFα 308 G/A polymorphism was identified as being associated with the risk of developing childhood NS.
Registration: Registered in PROSPERO with ID: CRD420251083425.
Similar content being viewed by others
Introduction
Nephrotic syndrome (NS) is characterized by marked proteinuria, low levels of albumin in the blood, and the development of edema. It is estimated that NS affects roughly 1–3 per 100,000 children who are below 16 years old. Remarkably, about 85% of individuals with NS experience a full resolution of proteinuria when treated with standard doses of daily oral prednisolone1,2. The assessment of the complexity and prognosis of this syndrome is determined by a combination of how well it responds to steroids and the accompanying histological findings. This specific category is referred to as steroid-sensitive nephrotic syndrome (SSNS). If individuals fail to attain remission following 4–6 weeks of steroid treatment, they are typically to be at a heightened risk of developing steroid-resistant nephrotic syndrome (SRNS). The predominant histopathological patterns observed in cases of SRNS primarily involve focal and segmental glomerulosclerosis (FSGS), with a smaller proportion showing minimal change disease (MCD) and diffuse mesangial sclerosis3. New research has indicated that the development of nephrotic syndrome is quite intricate. There is some evidence to indicate that NS may be a primary immunological disorder in which the primary malfunction of Thymus-cells [T-Cell] is linked to an immuno-regulatory Disparity in T helper subtype 1 & 2 (Th1,Th2). The precise mechanisms behind this imbalance remain unclear. The pathophysiology of NS still lacks clarity, and it appears that a range of inflammatory cytokines, chemotactic substances, and transcription factors play a role in the disease’s progression and its related symptoms4,5. Th1 and Th2 cells secrete cytokines, which encompass Interleukin (IL)- 2, IL-4, IL-5, IL-6, IL-10 and IL-136. It has been documented that the origin and progression of the disease are affected by substances released into the bloodstream by these activated T-cells. Th1 cytokines are pivotal in the advancement of Focal Segmental Glomerulosclerosis (FSGS), whereas cytokines are significant factors in Minimal Change Nephropathy (MCN). These conditions represent the leading culprits behind the development of NS in both children and adults7. One of these elements, TNF-alpha, a powerful immune regulator and proinflammatory cytokine, has been associated with the development of several medical conditions8. TNF-alpha has exhibited connections with various inflammatory disorders, such as glomerulonephritis, ankylosing spondylitis, and multiple sclerosis9,10,11. Functional single nucleotide polymorphisms (SNPs) of TNF-α cytokine genes located in the promoter region have been discovered to impact both the function of the gene promoter and the levels of gene product production12,13. A variant at position 308 G/A in the TNF-α promoter linked to increased transcription of TNF. An earlier study has shown an increase in the production and expression of TNF-α in individuals with primary NS14. The choice of these particular genes is based on the potential for single nucleotide variant within these genes to influence an individual’s vulnerability to the disease or modify the clinical course of the disease. The SNP TNFα -308 G/A (rs1800629) is located on chromosome 6 at position 6p21.3, within the promoter region of the TNF gene.The current research focuses on analyzing genetic variants in the TNFα cytokine gene at position 308 G/A in both children with NS and a control group of healthy individuals. Many previous studies have examine the connection between TNFα 308 G/A polymorphisms and childhood Nephrotic syndrome in various populations7,15,16,17,18. Hence, the indispensability of this meta-analysis is to validate the correlation between the TNFα 308 G/A [rs1800629] genetic polymorphism in the cytokine gene and Nephrotic Syndrome in children. This analysis is aimed at providing a improved insight of the involvement of inflammatory marker in NS.
Methods
Literature search
We utilized electronic databases such as PubMed and Google Scholar to conduct a thorough search for case-control studies that examined the connection between the TNFα 308 G/A gene polymorphism and the probability of childhood Nephrotic syndrome. The selection process for including studies in the meta-analysis was carried out meticulously using the database, and a summary of this process is outlined in Table 1. We collected research articles published between 2010 and 2022 for evaluation. To perform the literature search, we employed a combination of keywords, including terms like TNFα or Cytokine genes, inflammatory markers or genes, Tumour Necrosis Factor-alpha, gene or allele or polymorphism, interleukin gene, and childhood Nephrotic Syndrome. We scrutinized the title, abstract, and references of all relevant publications carefully. Whenever feasible, full-text articles from these relevant sources were chosen and integrated into the analysis. The statistical software Stats Direct [version 3.0] was employed to compute the pooled Odd’s Ratio(OR) with a 95% CI. The study was approved by the Institutional Ethics Committee of the Sri Ramachandra Institute of Higher Education and Research (SRIHER) in Porur, Chennai, India, under reference number IEC-NI/22/JUL/83/71.
The selection process for research articles in this meta-analysis involved clearly delineated requirements
The eligibility conditions were as follows:
-
- Case-control studies conducted between 2010 and 2022.
-
- Studies that evaluated the risk associated with TNFα -308 G/A gene polymorphisms in childhood Nephrotic syndrome.
-
- Availability of adequate data for calculating the OR along with a 95% CI.
-
-Genotype frequencies in the control groups of all included studies were assessed for conformity with Hardy-Weinberg Equilibrium (HWE) using the chi-square (χ²) test. and by a P-value > 0.05.
The specific elimination conditions are:
-
- Studies that did not have control groups.
-
- Exclusion of research involving experimental models involving animals, those absence of genotype information, summaries, case analyses and comprehensive reviews.
-
- Studies in which genotype distributions in the control group significantly deviated from HWE (P ≤ 0.05).
The extraction of data and evaluation of study quality for the selected research
The researcher, Yogalakshmi, conducted a comprehensive examination and data extraction, which included details like the author’s profile, article type, year of publication, study population, sample size for both cases and controls, type of polymorphism, and genotyping data. To evaluate the methodological attribute of the studies incorporated in the meta-analysis, the Newcastle-Ottawa Scale [NOS] was employed. The NOS employs a star-based rating system, encompassing three key categories: the selection of study participants [both cases and controls], the comparability of these participants, and the ascertainment of exposure. The highest possible score on the NOS scale is 9 points, with a score of ≥ 7 points indicating a study of high quality.
Data analysis
95% confidence intervals (CI) were used to assess the strength of the association between the TNFα − 308 G/A genetic polymorphism and childhood Nephrotic Syndrome. Statistical significance was defined as a P-value of less than 0.05. Cochran’s Q-test was used to assess whether there was variation among the combined studies with respect to the gene variant, and the I2 statistic was used to measure the degree of heterogeneity.
For every genetic comparison model, 95% Prediction Intervals (PIs) were computed in addition to I2 in order to estimate the anticipated range of effect sizes in subsequent research. The 95% PI takes into consideration between-study heterogeneity and gives a range that the true effect size of a new study is likely to fall within, in contrast to the 95% CI, which represents the precision of the pooled estimate.
Heterogeneity was classified into four categories: low, moderate, large, and extreme, based on the I2statistic, with ranges of 0–25%, 25–50%, 50–75%, and > 75%, respectively. In cases of low heterogeneity (I² ≤ 25%), the pooled odds ratio (OR) was computed using the Mantel-Haenszel method with a fixed-effects model, whereas in cases of moderate to high heterogeneity (I² > 25%), the DerSimonian-Laird method with a random-effects model was applied.
To evaluate the likelihood of publication bias, we performed a Begg’s Funnel plot analysis. Any irregularities observed in the Begg’s funnel plot were further assessed using Egger’s regression test, where a P-value less than 0.05 would suggest the potential existence of statistically significant publication bias.
Literature selection and study subject characteristics
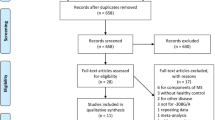
The process of selecting research articles for the meta-analysis on TNFα polymorphisms in childhood NS followed the principles laid out in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework19 represented in Fig. 1. At the outset, 36 studies were initially identified through a database search. After eliminating those without full-length articles and review records, 29 relevant studies remained. During the screening process, 20 studies were excluded for various reasons, including differences in study design, the focus on adult NS, studies examining the same gene but with different SNPs, and studies related to secondary NS.
Additionally, four publications were excluded because they either lacked genotyping data or investigated the association of different SNPs within the selected genes considered for this meta-analysis. As a result, a sum of five suitable studies that met the established inclusion and exclusion criteria were selected to be part of this current study. The particulars of these selected studies employed in the analysis are outlined in Table 1.
Altogether, the encompassed studies encompassed 628 instances and 932 individuals serving as controls. Upon scrutinizing the methodological quality of these five studies, it was observed that the NOS scores varied from 7 to 9, yielding an average score of 8.4 points7,15,16,17,18. These combined scores signify a high level of quality in the chosen literature. The NOS scores for each of the selected studies can be found summarized in Table 2.
Outcomes/result
A meta-analysis of cytokine gene polymorphism and childhood Nephrotic Syndrome was conducted, focusing on the TNFα − 308 G/A variant. A total of 628 cases and 932 controls were included across five case-control studies. The analysis revealed considerable heterogeneity (I² = 80%) between the TNFα allele-A and G allele. Specifically, allele-A showed a statistically significant pooled OR of 2.32 (95% CI: 1.27–4.20; P = 0.0056*), with a 95% PI of 1.00–5.37, indicating a potentially strong association.
Among genetic models, the dominant model (GA + AA vs. GG) showed a pooled OR of 2.57 (95% CI: 1.29–5.12; P = 0.0068*) with I² = 79.6% and a 95% PI of 0.97–6.81, suggesting possible variability. The recessive model (AA vs. GA + GG) showed a significant association with OR = 5.05 (95% CI: 1.48–17.23; P = 0.0096*), I² = 33%, and a 95% PI of 0.89–28.65.
For the co-dominant model, the GA vs. GG comparison yielded OR = 1.86 (95% CI: 1.09–3.17; P = 0.0219*), I² = 63.7%, and 95% PI: 0.88–3.95. The AA vs. GG comparison showed OR = 5.69 (95% CI: 1.59–20.36; P = 0.0075*), I² = 37.4%, and 95% PI: 0.94–34.52.
All pooled estimates were derived using the DerSimonian and Laird random-effects model, due to underlying between-study heterogeneity. These findings are illustrated in Fig. 2(a–e) and detailed in Table 3.
Publication bias
To measure the possibility of publication bias, we utilized Begg’s funnel plot analysis and conducted Egger’s bias test. For TNFα 308 G/A dominant GA + AA vs. GG, heterozygous [GA + GG], and Allele [A vs. G] models, no significant asymmetry was observed in the funnel plots, and Egger’s bias test Fig. 3(a-e) did not indicate substantial publication bias in these models. However, in the TNFα 308 G/A polymorphism recessive [AA vs. GA + GG] models and homozygous [AA vs. GG] comparison, notable publication bias was identified, as represented in Table 4.
Discussion
The exact pathogenesis of nephrotic syndrome remains unclear; however, immune system dysregulation is known to play a pivotal role.Current researches suggested the involvement of a circulating permeability factor derived from dysfunctional T-cells, with various cytokines particularly TNF-α proposed as potential contributors20. In this study we systematically reviewed 308 G/A SNP in the TNFα gene, thoroughly investigating their potential association with the risk of Childhood NS. Following this a meta-analysis carried out from data from five relavent studies, with a specific focus on the TNFα 308 G/A rs1800629 polymorphism. Our outcome unveil a noteworthy correlativity between the TNFα -308 G/A polymorphism and the susceptibility to Childhood NS across populations, encompassing individuals in India, Iran, and Egypt. Approximately 50–60% of children diagnosed with SRNS have an unknown cause, while the remaining patients exhibit glomerular podocyte impairment due to a single gene defect21.Cytokines pivotal in the development of NS and variants in a single nucleotide within the promoter region of a cytokine gene can impact its expression. This, in turn, can result in modified susceptibility to the disease and varied responses to therapeutic interventions22. A pilot study reported Weissbach et al.,23, persistently elevated TNFα levels in steroid-resistant nephrotic syndrome, suggesting its role in steroid non-responsiveness and disease pathogenesis.
Understanding the genetic factors underlying cytokine gene pathogenic mechanisms is valuable for categorization and the formulation of effective treatment strategies, even though these genes have a low prevalence rate. TNF-α, specifically, holds a central position in the initiation and advancement of various inflammatory and immunological disorders24. Among the Th1 cytokines [TNF-α] is a potent immunomodulatory and inflammation-promoting agent closely linked to various pathological disorders. Individuals with active primary Nephrotic Syndrome demonstrated elevated TNF-α production levels compared to controls, with notable polymorphism in TNF-α production observed during periods of remission5. Additionally, TNF-α and IL-1 were positively correlated with proteinuria levels, mesangial hypercellularity, and glomerulosclerosis, providing perceptions into the potential function of TNF-α in the pathophysiology of proteinuria and pathological alterations in NS25.
Individuals carrying the A allele are believed to exhibit increased TNF-α expression, thereby contributing to heightened inflammatory responses and renal injury26.TNF-α functions as a pro-inflammatory cytokine crucial for triggering the immune response by activating T and B cells. Transcription of TNF is linked to a polymorphism at position 308, involving a G to A base change in the TNF-α promoter27. Increased TNF-α levels have been observed in plasma and urine of Nephrotic Syndrome patients28. Our research also correlates with the above-stated findings, further substantiating the association between the TNF-α 308 G/A (rs1800629) polymorphism and increased susceptibility to childhood Nephrotic Syndrome. By conducting a meta-analysis that pooled data from five independent case-control studies, encompassing 628 NS patients and 932 healthy controls, we identified a significantly increased risk of NS among individuals carrying the A allele or AA genotype of the TNF-α variant. This elevated risk was consistently observed across multiple genetic models, including the allele model (A vs. G), dominant model (GA + AA vs. GG), recessive model (AA vs. GA + GG), heterozygous model (GA vs. GG), and homozygous model (AA vs. GG). The robustness of these associations was further supported by subgroup analysis, which confirmed the consistency of these findings in population-based studies. Collectively, our study reinforces the hypothesis that the A allele in the TNF-α − 308 polymorphism plays a contributory role in the development of childhood Nephrotic Syndrome, likely through enhanced cytokine expression and immune activation. These insights underscore the potential utility of TNF-α − 308 G/A as a genetic biomarker for disease susceptibility and provide a foundation for future research into its functional significance and clinical implications.
Conclusion
This meta-analysis, which included 1,560 participants (628 cases and 932 controls), shows a strong correlation between the TNFα -308 G/A (rs1800629) polymorphism and the risk of developing childhood nephrotic syndrome. The A allele showed a significant pooled odds ratio of 2.32 (P = 0.0056*), suggesting a possible elevated risk. The co-dominant models (AA vs. GG: P = 0.0075*; GA vs. GG: P = 0.0219*) and the recessive model (P = 0.0096*) both showed significant associations, but the dominant model did not reach statistical significance (P = 0.068). These results emphasise the clinical significance of TNFα promoter polymorphism as a genetic risk marker and suggest that it may play a part in the pathophysiology of childhood nephrotic syndrome.
In summary, a critical area of interest in pediatric Nephrotic Syndrome centers on understanding the genetic factors that impact the disease’s pathophysiology, progression, and response to treatment, all of which hold significant clinical relevance. In this study, emphasis has been placed on TNF-α as a crucial contributor to the development and progression of diverse inflammatory conditions linked to proteinuria in Nephrotic Syndrome. This research marks a initiative to explore the potential impact of TNF-α promoter gene polymorphisms, specifically the − 308 G/A variant and its related haplotype, on susceptibility to Nephrotic Syndrome.
In summary, the outcomes of our meta-analysis indicate that individuals harboring the at-risk allele of the TNFα 308 G/A polymorphism might face an exaggerated likelihood of developing Childhood Nephrotic Syndrome. Nevertheless, it is crucial to emphasize the importance of conducting more extensive case-control association studies encompassing diverse populations. Such endeavors are essential for gaining deeper insights into the involvement of these genes in the pathogenesis of Childhood Nephrotic Syndrome.
Limitations
Despite the important findings of this meta-analysis, several limitations must be acknowledged. Firstly, the relatively small number of included studies limits the statistical power and prevents reliable estimation of between-study variance (Tau²), thereby affecting the robustness of the conclusions. The generalizability of the findings is also restricted, as most studies were conducted in Middle Eastern populations, with only one from India, which may not reflect broader ethnic diversity. Moreover, the high level of between-study heterogeneity (I² = 80%) poses challenges to interpreting pooled results and compromises the accuracy of publication bias assessments using Egger’s test, which can yield false-positive outcomes under such conditions. The inability to perform subgroup analyses or meta-regression due to insufficient data further limits the exploration of sources of heterogeneity. Although meta-regression could have provided insight into potential sources of heterogeneity such as gender ratio, mean age, or minor allele frequency this was not feasible due to the small number of studies and inconsistent reporting of these variables. .Additionally, nephrotic syndrome is a multifactorial condition influenced by genetic, environmental, infectious, and psychosocial elements. Since this study is based on aggregated published data, it lacks the ability to adjust for baseline characteristics and potential confounders. The literature search was also restricted to English-language publications, which introduces language bias, and the asymmetry observed in funnel plots may reflect a higher likelihood of positive findings being published over negative results. While these limitations are inherent to retrospective meta-analyses, the consistency of the observed associations supports the relevance of TNFα -308 G/A as a genetic marker in NS, highlighting the need for larger, ethnically diverse studies to confirm these findings.
Data availability
No datasets were generated or analysed during the current study.
References
Dossier, C. et al. Epidemiology of idiopathic nephrotic syndrome in children: endemic or epidemic? Pediatricnephrology 31 (12), 2299–2308 (2016).
Bagga, A. Revised guidelines for management of steroid-sensitive nephrotic syndrome. Indian J. Nephrol. 18(1), 31 (2008). https://mc04.manuscriptcentral.com/jbn-scielo (Brazilian Journal of Nephrology).
Zhang, A. & Huang, S. Progress in pathogenesis of proteinuria. Int. J. Nephrol. 2012 (2012).
Zachwieja, J. et al. Intracellular cytokines of peripheral blood lymphocytes in nephrotic syndrome. Pediatr. Nephrol. 17 (9), 733–740 (2002).
Lama, G. et al. T lymphocyte populations and cytokines in childhood nephrotic syndrome. Am. J. Kidney Dis. 39(5), 958 – 65 (2002).
Fodor, P., Saitúa, M. T., Rodriguez, E., González, B. & Schlesinger, L. T-cell dysfunction in minimal-change nephrotic syndrome of childhood. Am. J. Dis. Child. 136(8), 713–717 (1982).
Madani, H. A., Bazaraa, H. M. & Rady, H. Association of cytokine genes polymorphisms and the response to corticosteroid therapy in children with idiopathic nephrotic syndrome: A pilot study in Egypt. Int. Res. J. Med. Med. Sci. 2[4], 84–90 (2014).
Wilson, A. G., Symons, J. A., McDowell, T. L., McDevitt, H. O. & Duff, G. W. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. U S A. https://doi.org/10.1073/pnas.94.7.3195 (1997). 94[7]:3195.
Shu, K. H., Lee, S. H., Cheng, C. H., Wu, M. J. & Lian, J. D. Impact of interleukin-1 receptor antagonist and tumor necrosis factor-alpha gene polymorphism on IgA nephropathy. Kidney Int. 58(2), 783-9 (2000). https://doi.org/10.1046/j.1523-1755.2000.00227.x.
Li, L. et al. Association of IL-1A and IL-1B polymorphisms with ankylosing spondylitis among the Chinese Han population: A case–control study. Oncotarget 8(17), 28278.11 (2017).
Mann, C. L. et al. Interleukin 1 genotypes in multiple sclerosis and relationship to disease severity. J. Neuroimmunol. 129(1–2), 197–204 (2002).
Jančić, I. et al. Influence of promoter polymorphisms of the TNF-α [-308G/A] and IL-6 [-174G/C] genes on therapeutic response to etanercept in rheumatoid arthritis. J. Med. Biochem. 34(4), 414 (2015).
Qidwai, T. & Khan, F. Tumour necrosis factor gene polymorphism and disease prevalence. Scand. J. Immunol. 74 (6), 522–547 (2011).
Tieranu, I. et al. Preliminary study regarding the association between tumor necrosis factor- alpha gene polymorphisms and childhood idiopathic nephrotic syndrome in Romanian pediatric patients. Maedica [Buchar]. 12, 164168 (2017).
Sadeghi-Bojd, S. et al. Lack of association between TNF-alpha rs1800629 [-308 G > A] polymorphism and nephrotic syndrome. Iran. J. Kidney Dis. 1 (2), 95 (2021).
Youssef, D. M., Amal, S., Hussein, S., Salah, K. & Abd El Rahman, E. A. Tumor necrosis factor alpha gene polymorphisms and haplotypes in Egyptian children with nephrotic syndrome. Cytokine 102, 76–82 (2018).
Jafar, T. et al. Cytokine gene polymorphism in idiopathic nephrotic syndrome children. Indian J. Clin. Biochem. 26[3], 296–302 (2011).
Midan, D. A., Elhelbawy, N. G., Ahmedy, I. A. & Noreldin, R. I. Cytokine gene polymorphism in children with idiopathic nephrotic syndrome. Iran. J. Kidney Dis. 11(6), 414 (2017).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting.
Pukajło-Marczyk, A. & Zwolińska, D. The role of TNF-α in the pathogenesis of idiopathic nephrotic syndrome and its usefulness as a marker of the disease course. J. Clin. Med. 13 (7), 1888. https://doi.org/10.3390/jcm13071888 (2024). Published 2024 Mar 25.
Lipska-Ziętkiewicz, B. S. & Genetic Steroid-resistant nephrotic syndrome overview. Aug 26. In GeneReviews® [Internet] (Adam, M.P., Feldman, J., Mirzaa, G.M. et al., eds.). 1993–2025. (University of Washington,2021). https://www.ncbi.nlm.nih.gov/books/NBK573219/
Tieranu, I. et al. Genetic variants of Interleukin-4 in Romanian patients with idiopathic nephrotic syndrome. Med. (Kaunas). 58 (2), 265. https://doi.org/10.3390/medicina58020265 (2022). Published 2022 Feb 10.
Weissbach, A., Garty, B. Z., Lagovsky, I., Krause, I. & Davidovits, M. Serum tumor necrosis Factor-Alpha levels in children with nephrotic syndrome: A pilot study. Isr. Med. Assoc. J. 19 (1), 30–33 (2017). PMID: 28457111.
Jang, D. I. et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 22 (5), 2719. https://doi.org/10.3390/ijms22052719 (2021). Published 2021 Mar 8.
Li, G. et al. Serum levels of tumor necrosis factor alpha in patients with IgA nephropathy are closely associated with disease severity. BMC Nephrol. 19(1), 326 (2018). https://doi.org/10.1186/s12882-018-1069-0.
Salazar-Camarena, D. C. et al. Association of TNF-alpha promoter polymorphisms with disease susceptibility, mRNA expression, and lupus nephritis in Mexican patients with systemic lupus erythematosus. J. Clin. Med. 14 (11), 3693. https://doi.org/10.3390/jcm14113693 (2025).
Elahi, M. M., Asotra, K., Matata, B. M. & Mastana, S. S. Tumor necrosis factor alpha – 308 gene locus promoter polymorphism: an analysis of association with health and disease. Biochim. Biophys. Acta. 1792(3), 163 – 72. 2009). https://doi.org/10.1016/j.bbadis.2009.01.007.
Bakr, A. et al. Tumor necrosis factor-alpha production from mononuclear cells in nephrotic syndrome. Pediatr. Nephrol. 18 (6), 516–520. https://doi.org/10.1007/s00467-003-1122-4 (2003). Epub 2003 Apr 18. PMID: 12707837.
Acknowledgements
We would like to acknowledge all the authors of the research articles used for the analysis, for providing the detailed genotyping data.
Author information
Authors and Affiliations
Contributions
Yogalakshmi.V, PraveenKumar.K.S -Conceived and designed the analysis; Collected the data; Contributed data or analysis tools; Performed the analysis; Indira Bhagam- Drafted the article; C.D.Mohanapriya -Drafted the article or revised it critically for important intellectual content; Thendral Hepsibha, Vettriselvi.V, Sangeetha.G -Approved the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Venkatachalapathy, Y., Suresh, P.K., Balraj, T.H. et al. Impact of tumor necrosis factor-alpha gene variant in pediatric nephrotic syndrome: a meta-analysis. Sci Rep 15, 29797 (2025). https://doi.org/10.1038/s41598-025-15387-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15387-w