Abstract
Despite a correlative relationship between Hypertriglyceridemia-induced acute pancreatitis (HIAP) incidence and triglyceride (TG) levels, not all patients with very high TG levels in plasma develop HIAP, suggesting additional contributing factors. We used gas chromatography and thin layer chromatography for analysis of fatty acid (FA) composition in plasma, TGs, and red blood cells (RBC) in healthy volunteers and patients with severe hypertriglyceridemia with and without HIAP, providing a thorough understanding of recent and long-standing physiological states. We found that TGs of hypertriglyceridemic patients with current HIAP contain increased levels of oleic acid, linoleic acid, and the trans FA elaidic acid, all potentially toxic to pancreatic cells; and decreased levels of the pancreatic-protective FAs gondoic acid and docosahexaenoic acid. Moreover, we found a significant increase in relative assembly of the pro-inflammatory omega-6 PUFAs into RBC membrane phospholipids in HIAP patients. In conclusion, our data suggest a dietary FA composition characterized by higher consumption of ultra-processed foods and a metabolic milieu that increase HIAP susceptibility, and open avenues for further exploration for development of specific dietary or medical interventions to mitigate HIAP risk by altering the FA composition of plasma TGs.
Similar content being viewed by others
Introduction
Hypertriglyceridemia (hyper-TG) is an important etiology of acute pancreatitis (AP) worldwide, and may be the cause in more than 20% of cases of severe AP1,2. Relative to AP caused by other etiologies, hyper-TG-induced AP (HIAP) involves increased rates of morbidity and complications such as acute kidney failure, shock and infection. High levels of plasma triglycerides (TGs) are usually the result of a metabolic derangement secondary to diabetes, obesity, excessive alcohol consumption or pregnancy, often superimposed on a genetic predisposition. Hyper-TG is also closely linked to western diets rich in ultra-processed foods (UPFs)3. Fatty acids (FAs) assembled in TGs can be acquired in the diet or further metabolized by elongation or desaturation. Monounsaturated FAs (MUFAs) are either acquired in the diet or synthesized from saturated FAs (SFAs) by stearoyl-CoA desaturase (SCD1). Omega-6 and Omega-3 polyunsaturated FAs (PUFAs) are either acquired in the diet or synthesized from their respective precursors linoleic acid (LA) and α-linoleic acid by shared elongases and desaturases.
UPFs present significant content of trans FAs, known to increase the risk of developing obesity, diabetes and cardiovascular disease4. Elaidic acid (EA), the trans-isomer of the MUFA oleic acid (OA), is the most abundant artificial trans fatty acid in modern diets and UPFs, and is associated with metabolic derangements like obesity and insulin resistance5,6. UPFs are also characterized by a high omega-6 PUFAs to omega-3 PUFAs ratio, known to promote metabolic diseases like obesity and fatty liver7,8. Moreover, excessive UPF consumption was recently recognized to promote systemic inflammation, with a consequent predisposition to develop chronic diseases9.
The pathophysiology of HIAP involves breakdown of TGs by pancreatic lipases to free FAs (FFAs) that are toxic to pancreatic acinar cells. While HIAP incidence is correlative with plasma TG level, not all patients with severe hypertriglyceridemia (> 400 mg/dl) develop HIAP. The incidence of AP in hyper-TG is 5% at TG levels > 1000 mg/dl and 10–20% at TG levels > 2000 mg/dl10. This observation implies that other factors in hyper-TG patients beyond TG levels dictate propensity to develop HIAP, with a potential role for the specific FA composition of TGs.
Here, we reveal an aberrant FA composition of plasma TGs in HIAP patients - notably, increased levels of OA, LA and EA, and decreased levels of the long MUFA gondoic acid (GA) and the 3-omega PUFA docosahexaenoic acid (DHA). These data suggest for the first time that HIAP susceptibility may be increased by a dietary FA composition characterized by higher UPFs consumption and / or a metabolic milieu dictating a distinct TG composition, with a potential diagnostic and therapeutic relevance.
Materials and methods
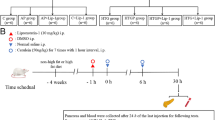
The study included a group of 10 healthy volunteers with normal to mildly elevated TG plasma levels, with an average TG level of 123 mg/dL (51–295 mg/dL); and two groups of participants with severe hypertriglyceridemia (≥ 400 mg/dl): a hyper-TG group with no current HIAP (n = 8), with an average TG level of 1846 mg/dl (447–4620 mg/dL); and a group with active HIAP, as evident from clinical, imaging and laboratory findings (n = 10), with an average TG level of 2466 mg/dl (882–4318 mg/dL). Age, TG levels, diagnosis of diabetes or obesity, or usage of statins, fibrates, omega-3 preparations or anti-diabetic drugs did not significantly differ between the hyper-TG and HIAP groups. Further characteristics of participants are listed in table S1. Blood samples were collected with informed consents between January 2020 and December 2023 at Sheba Medical Center. Samples of HIAP patients were taken in the Apheresis Unit during the first 48 h of admission prior to plasma apheresis treatment. Samples from the hyper-TG group were taken from ambulatory patients of the metabolic clinic. Samples were collected after an overnight fast (control and hyper-TG groups) or after a 24 h-fast in admitted HIAP patients. Plasma lipid samples were separated by thin-layer chromatography (TLC), and the TG component was analyzed for FA composition by gas chromatography with flame ionization detector (GC-FID) (Fig. 1A and supplementary methods). Red blood cell (RBC) lipids were similarly analyzed by GC-FID. More data on sample processing and analysis can be found in the supplementary methods section. Participants for EA analysis included healthy volunteers (n = 7, average: TG level: 108 mg/dL); a hyper-TG group with no current HIAP (n = 7, average TG level: 1220 mg/dl); and a group with active HIAP, as evident from clinical, imaging and laboratory findings (n = 7, average TG level: 2648 mg/dl). Other characteristics were similar to those described above.
Results
While fasted plasma and triglyceride FA composition reflect mainly recently-metabolized FAs in TG-rich lipoproteins, RBC membranes composition reflects mainly metabolic and nutritional lipid input into phospholipid FAs over 4 months11. All results are summarized in Table 1, and the main differences are presented in Fig. 1B-F. In plasma FAs assembled in TGs, we observed a significant increase in OA and LA levels and a sharp decrease in GA and DHA levels in the HIAP group as compared with the hyper-TG group. These patterns were consistent both in relative and in absolute levels. (Figure 1B and C), with no similar differences in whole-plasma FAs (Fig. 1D). Moreover, in whole plasma there was a significant increase in LA levels and a significant decrease in DHA levels in both hyper-TG and HIAP groups relative to the control group (Fig. 1D). We found a significant increase in omega-6 PUFAs relative assembly into RBC membrane phospholipids in HIAP patients, without a similar difference in omega-3 PUFAs or in whole plasma (Fig. 1E and F). To expand our analysis, we also analyzed the TG composition in three sets of samples from the control, hyper-TG and HIAP patients described above, using high-end GC column which is designed to identify trans FA isomers. In plasma TGs, there was significantly more EA in HIAP patients relative to the other groups (Fig. 1G and H), without significant differences in EA levels between groups in whole plasma (data not shown).
A. Schematic representation of blood samples analysis. Plasma, triglyceride (TG) fraction and red blood cells (RBCs) were isolated from blood samples of participants by centrifugation and TLC, and then analyzed for FA content by GC-FID. (CE – cholesterol ester; FFA – free fatty acids; C – cholesterol; PL – phospholipids). FAs concentrations were measured in TGs (B, C, G, H), plasma (D, F) and RBCs (E) of control, hyper-TG and HIAP groups, using GC-FID. FA levels are expressed as µmole of FA per 100 ml sample (B, D-G), or as absolute levels in mmol/L, corrected to plasma TG levels (C, H). Total omega-3 and omega-6 levels were calculated by summing concentrations of individual FAs within these classes. The results shown by taking mean ±SEM. Statistical analyses were carried out by two-sided student’s t test. * (p<0.05), ** (p<0.01), *** (p<0.001).
Discussion
OA and LA can cause pancreatic acinar cell damage in-vitro12 and in-vivo13, and their plasma levels are elevated in acute pancreatitis14. Significantly, enriching mice diet with GA decreased obesity-related metabolic dysfunction15, whereas providing omega-3 FAs to acute pancreatitis patients decreased complications and mortality16. Specifically, DHA was shown to protect pancreatic acinar cells from damage both in-vitro17 and in-vivo18. Together, these data imply that high ratios of OA to longer chain MUFAs and of omega-6 to omega-3 PUFAs in TGs may promote HIAP.
Some of our observed effects are likely explained by dietary habits. For example, the high relative plasma omega-6 PUFAs levels and low relative plasma omega-3 PUFAs levels in both the hyper-TG and the HIAP groups are probably the result of high-fat and high-energy food consumption characteristic of western diets and UPFs. Indeed, these diets typically contain high omega-6 PUFAs to omega-3 PUFAs ratios7,8.
Other observations are better explained by metabolic differences between the groups. We identified an increase in the levels of OA and LA and a decrease in the levels of GA and DHA assembled in TGs in HIAP patients. This may be explained by a deranged selectivity for these FAs in TG assembly, or by a failed hepatic metabolism – for example, an increased SCD1 activity enhancing conversion of stearic acid (SA) into OA19, a decreased elongation of OA to GA, or an increased occupancy of shared elongases and desaturases in the omega-6 metabolic branch on the expense of the omega-3 branch.
In the HIAP group, the levels of several omega-6 PUFAs in RBCs were significantly increased, resulting in a relative high total omega-6 PUFA composition in RBC membranes, with no similar increase in total omega-3 PUFA composition. As RBCs lipid composition reflects lipids acquired and metabolized during the last 4 months, these patients likely accumulated omega-6 PUFAs before acute pancreatitis onset. This may reflect an additive effect of an increased dietary consumption of omega-6 PUFAs, combined with an enhanced potential to synthesize omega-6 PUFAs and to utilize them in activation of inflammatory pathways, as omega-6 PUFAs serve as precursor of the key pro-inflammatory signal molecules prostaglandins and leukotrienes20.
We cannot rule out that some of the differences identified in the HIAP group are consequences of pancreatitis rather than baseline characteristics. Nevertheless, several observations suggest that the FA patterns in the HIAP group have preceded the pancreatitis. While levels of OA, LA as FFAs can be elevated in acute pancreatitis14, there was no increase in relative levels of these FAs in whole plasma of HIAP patients as compared with the hyper-TG group. Moreover, elevated pancreatic lipase levels in pancreatitis can increase FFAs through hydrolysis of plasma TGs14. However, in HIAP patients we found an increase in plasma TG-integrated OA and LA rather than a decrease. Finally, although no data is available regarding dietary intake of participants, several of the observed FA levels in the HIAP groups are likely attributed to dietary habits. Specifically, EA is characteristic of UPFs and can be only acquired in the diet, so its increased levels in TGs of HIAP patients likely represents an increased UPFs consumption in this group.
Our study has three main limitations. First, sample sizes are small. Consequently, more studies characterizing TG composition of hyper-TG states are needed in larger patient cohorts. A second potential limitation of the study is that plasma FFAs levels were not measured. Nevertheless, the analysis of FAs within plasma TGs presented in this study is clinically important, as these are the source of FFAs directly involved in HIAP pathogenesis12. Third, in HIAP patients, including another time point after pancreatitis resolution would have provided potentially important additional data. Indeed, all HIAP patients included in this studies were instructed in their discharge notes to schedule follow-up tests in our metabolic clinic. However, not all patients adhered to these instructions, and were lost to follow-up, precluding acquisition of additional blood test. Future studies will have to be designed to overcome this limitation.
In conclusion, we reveal an altered FA profile in HIAP patients that may reflect dietary and metabolic aberrations decreasing the threshold to develop HIAP by changing the TG composition from a protective to a potentially cytotoxic one, and by creating a metabolic inflammation-predisposing milieu. Our findings may contribute to the development of targeted strategies to reduce the incidence of acute pancreatitis in patients with hypertriglyceridemia. These strategies may include dietary modifications, such as restricting UPFs and increasing the intake of potentially protective fatty acids like omega-3 PUFAs and long-chain MUFAs, as well as novel pharmacologic interventions aimed at reducing the risk of HIAP by altering plasma TG composition.
Data availability
Data is provided within the manuscript or supplementary information files. The raw data supporting the conclusions of this article will be made available by the authors on request from the corresponding author.
References
De Pretis, N., Amodio, A. & Frulloni, L. Hypertriglyceridemic pancreatitis: epidemiology, pathophysiology and clinical management. United Eur. Gastroenterol. J. 6 (5), 649–655 (2018).
Jin, M. et al. A 16-year trend of etiology in acute pancreatitis: the increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J. Clin. Lipidol. 13 (6), 947–953 (2019).
González-Palacios, S. et al. Increased ultra-processed food consumption is associated with worsening of cardiometabolic risk factors in adults with metabolic syndrome: longitudinal analysis from a randomized trial. Atherosclerosis 377, 12–23 (2023).
Menezes, C. A. et al. Ultra-Processed food consumption is related to higher trans fatty acids, sugar intake, and Micronutrient-Impaired status in schoolchildren of bahia, Brazil. Nutrients 15 (2), 381 (2023).
Chajès, V. et al. Plasma Elaidic acid level as biomarker of industrial trans fatty acids and risk of weight change: report from the EPIC study. PLoS One. 10 (2), e0118206 (2015).
Itcho, K. et al. Association between serum Elaidic acid concentration and insulin resistance in two Japanese cohorts with different lifestyles. J. Atheroscler Thromb. 24 (12), 1206–1214 (2017).
Hao, L., Chen, C. Y., Nie, Y. H., Kaliannan, K. & Kang, J. X. Differential interventional effects of Omega-6 and Omega-3 polyunsaturated fatty acids on high fat Diet-Induced obesity and hepatic pathology. Int. J. Mol. Sci. 24 (24), 17261 (2023).
Lopes, K. L. S. et al. The degree of food processing can influence serum fatty acid and lipid profiles in women with severe obesity. Front. Nutr. 10, 1046710 (2023).
Tristan Asensi, M., Napoletano, A., Sofi, F. & Dinu, M. Low-Grade inflammation and Ultra-Processed foods consumption: A review. Nutrients 15 (6), 1546 (2023).
Scherer, J., Singh, V. P., Pitchumoni, C. S. & Yadav, D. Issues in hypertriglyceridemic pancreatitis: an update. J. Clin. Gastroenterol. 48 (3), 195–203 (2014).
Ferreri, C. et al. Fatty acids in membranes as homeostatic, metabolic and nutritional biomarkers: Recent advancements in analytics and diagnostics. Diagnostics (Basel) 7(1), 1-14 (2016).
Chang, Y. T., Chang, M. C., Tung, C. C., Wei, S. C. & Wong, J. M. Distinctive roles of unsaturated and saturated fatty acids in hyperlipidemic pancreatitis. World J. Gastroenterol. 21 (32), 9534–9543 (2015).
Nishioka, T., Yamamoto, Y. & Yamamoto, Y. Studies on the influence of long-term doses of lipid peroxide generators on rat pancreas. Nihon Shokakibyo Gakkai Zasshi. 89 (9), 2037–2046 (1992).
Phillips, A. E. et al. Relationship of Circulating levels of long-chain fatty acids to persistent organ failure in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 325 (3), G279–G285 (2023).
Yang, Z. H., Miyahara, H., Iwasaki, Y., Takeo, J. & Katayama, M. Dietary supplementation with long-chain monounsaturated fatty acids attenuates obesity-related metabolic dysfunction and increases expression of PPAR gamma in adipose tissue in type 2 diabetic KK-Ay mice. Nutr. Metab. (Lond). 10 (1), 16 (2013).
Lei, Q. C. et al. The role of omega-3 fatty acids in acute pancreatitis: a meta-analysis of randomized controlled trials. Nutrients 7 (4), 2261–2273 (2015).
Song, E. A., Lim, J. W. & Kim, H. Docosahexaenoic acid inhibits IL-6 expression via PPAR-gamma mediated expression of catalase in cerulein-stimulated pancreatic acinar cells. Int. J. Biochem. Cell. Biol. 88, 60–68 (2017).
Jeong, Y. K., Lee, S., Lim, J. W. & Kim, H. Docosahexaenoic acid inhibits cerulein-induced acute pancreatitis in rats. Nutrients 9, 744 (2017).
Ntambi, J. M. & Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 43 (2), 91–104 (2004).
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294 (5548), 1871–1875 (2001).
Acknowledgements
The authors wish to acknowledge the support of the Kronitz Family Physician Scientist Program at Sheba Medical Center, Israel, and the kind donations of the Alrov Fund and the Neuhar Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization, D. H. and R. K.; methodology, R. K.; software, A. L.F and R. K.; validation, A. L.F and R. K.; formal analysis, A. LFF and R. K.; resources, R. M., T. L., A. S., H. C., Y. K., D. H. and M. M.; data curation, T. L. and R. K.; writing—original draft preparation, R. K.; writing—review and editing, A. L.F and R. K.; visualization, A. L. Fand R. K.; supervision, R. K.; project administration, M. M., D. H. and R. K.; funding acquisition, R. K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Sheba Medical Center (protocol code 6390-19-SMC, approved on 14 October 2019). Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Leikin-Frenkel, A., Mayorov, M., Luvish, T. et al. Fatty acid analysis identifies an aberrant circulating triglyceride composition in patients with hypertriglyceridemia-induced acute pancreatitis. Sci Rep 15, 29661 (2025). https://doi.org/10.1038/s41598-025-15391-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15391-0