Abstract
This study assessed the association between chronic kidney disease (CKD) and presbycusis and investigated whether inflammatory neutrophil percentage-to-albumin ratio (NPAR) indicators influence this relationship. This study used data from the National Health and Nutrition Examination Survey and employed methods such as restricted cubic spline analysis, multiple logistic regression analyses, and mediation analyses to explore these relationships. High-frequency hearing impairment (HI) was correlated with moderate CKD (OR = 3.25, 95% CI:1.25- 8.46, P = 0.02). HI grades were correlated with moderate CKD [for slight HI, (OR = 5.13, 95% CI:1.48- 17.86, P = 0.01); for moderate and severe HI, (OR = 4.68, 95% CI:1.29 -17.04, P = 0.02)]. Mediation analysis revealed that NPAR had a 17.85% mediating effect on the association between moderate CKD and presbycusis. Moderate CKD was associated with high-frequency HI in the elderly, and NPAR had a significant mediating effect on this relationship.
Similar content being viewed by others
Introduction
Presbycusis, a progressive and symmetrical form of age-related hearing impairment (HI), affects a significant proportion of older adults worldwide. It is one of the most prevalent public health issues globally, potentially leading to reduced communication abilities, diminished quality of life, social isolation, and heightened risks of depression, cognitive impairment, and dementia1. It is estimated that more than one-third of the world’s older adults experience some degree of HI, with prevalence rates rising to 80% among those aged 80 and older2,3.
Alongside presbycusis, chronic kidney disease (CKD) is a major public health challenge. CKD is associated with a range of complications, including hypertension, metabolic imbalances, electrolyte disturbances, and cardiovascular disease, which all contribute to reduced life expectancy and quality of life. The age-related decline in kidney function increases susceptibility to CKD, which in turn may lead to further systemic complications4.
Interestingly, both presbycusis and CKD share several physiological and structural similarities. Presbycusis is widely recognized as a degenerative disease primarily impacting the cochlea. Both the cochlea and kidneys share similarities in developmental origin, microcirculation, antigenicity, ultrastructure, and physiology, suggesting a potential correlation between presbycusis and CKD5. The nephron, the functional unit of the kidney, is structurally and functionally similar to the stria vascularis of the inner ear, which is crucial for maintaining the electrolyte stability of the internal and external lymph in the inner ear. The balance of the inner ear is inseparably linked to the regulation of human electrolytes by the normal kidney6,7. Aging of the cochlea can lead to degeneration of inner ear structures (such as the stria vascularis, spiral nerve cells, and sensory hair cells). Stria vascularis dysfunction can lead to inner ear fluid and electrolyte imbalances, reduced cochlear potential, weakened outer hair cell amplification, impaired sensory conduction, and high-frequency hearing loss8.Concurrently, during aging, the kidney experiences a gradual functional decline and both macroscopic and microscopic histological changes. Although aging itself does not directly cause kidney damage, age-related physiological changes may hinder the kidney’s repair capacity, making older individuals more susceptible to CKD9. Meanwhile, the age-related decline in kidney function may lead to electrolyte imbalances. These imbalances could affect the inner ear, potentially increasing the risk and progression of age-related hearing loss. However, evidence on the relationship between presbycusis and CKD remains scarce. Furthermore, the pathogenic factors and potential mechanisms of this association remain unclear.
To the best of our knowledge, inflammatory biomarkers such as the neutrophil-percentage-to-albumin ratio (NPAR) can reflect systemic inflammatory states and have been confirmed as prognostic predictors in several inflammatory diseases 10,11. The NPAR has a unique advantage as a relatively novel index that combines the neutrophil percentage and albumin levels, better reflecting the balance between inflammation and nutrition. In comparison, traditional inflammatory markers like C-reactive protein (CRP)12 and white blood cell count (WBC)13 are less specific and sensitive for chronic conditions such as presbycusis and CKD. Previous studies have identified systemic inflammatory markers as crucial for predicting survival rates and demonstrated strong correlations with all-cause mortality among CKD patients in the United States14. Moreover, the inflammatory response can accelerate aging, and inflammatory cells in the peripheral blood have been linked to presbycusis15. Nevertheless, there is currently no research on the role of NPAR in the relationship between moderate CKD and presbycusis. These previous studies led us to investigate whether the inflammation index NPAR plays a role in the association between moderate CKD and presbycusis.
Based on this premise, this study used data from 939 Americans aged 65 years and older from the National Health and Nutrition Examination Survey (NHANES) database spanning 2005 to 2012 and 2015 to 2018. eGFR served as an indicator of kidney function, with moderate CKD defined as eGFR < 60 mL/min/1.73 m2. This study aimed to investigate the association between moderate CKD and hearing impairment (HI) in older adults in the United States, examining whether NPAR played a mediating role in this relationship.
Results
Characteristics of participants
As depicted in Tables 1 and 2, 936 participants were included in the data analysis, with an average age of 72.21 ± 5.40 years and a BMI of 29.91 ± 6.24 kg/m2. Overall, 62% of participants were female, and the majority were white (78%). Nearly half of the participants were recognized as smokers (43%). There were no statistically significant differences in race, educational level, hypertension, and coronary heart disease (CHD) between the groups for either low- or high-frequency pure tone audiometry (PTA). For low-frequency PTA, 52.61% of the participants exhibited normal hearing, 33.17% demonstrated slight HI, and 14.27% experienced moderate and severe HI.
In contrast, for high-frequency PTA, only 7.99% of the participants exhibited normal hearing, 26.09% experienced slight HI, and the majority (65.92%) demonstrated moderate and severe HI. The average concentrations of BUN and Scr were 17.94 ± 6.80 and 0.97 ± 0.51 mg/dL, respectively, which were statistically significant between the different subgroups of low- and high-frequency PTA. The average concentration of eGFR was 72.13 ± 19.28 mL/min/1.73 m2 (Table 2). According to the sampling density curve (Fig. 1), participants with an eGFR below the average concentration were more likely to have moderate and severe HI. In contrast, those with an eGFR above the average tended to have better hearing. Based on Table 1, the number of participants with eGFR ≥ 60 mL/min/1.73 m2 was 676 (73%), and 263(27%) participants were diagnosed with moderate CKD. The prevalence of moderate CKD among patients was 21% for individuals aged 65–69 years, 23% for those aged 70–74 years, and 22% for individuals aged 75–79 years, as illustrated in Fig. 2. Notably, prevalence significantly increased among individuals aged ≥ 80 years, reaching approximately 34%.
As depicted in Fig. 3A–B, among participants with eGFR ≥ 60 mL/min/1.73 m2, low-frequency PTA generally indicated normal hearing, except for those aged ≥ 80 years, who predominantly exhibited moderate and severe HI. Conversely, high-frequency PTA predominantly demonstrated moderate and severe HI, with a gradual decline in prevalence with age. In Fig. 3C–D, among patients with moderate CKD, the distribution of hearing classifications in low-frequency PTA closely resembled that observed in individuals with eGFR ≥ 60 (mL/min/1.73 m2).
For high-frequency PTA, moderate and severe HI predominated, displaying a gradual increase in prevalence with age. The mean ± SD of NPAR and albumin were 1,440.71 ± 263.86 and 4.13 ± 0.33, respectively, indicating statistical significance across the various subgroups of low-frequency PTA and high-frequency PTA. Alternatively, the mean ± SD of neutrophil percentage was 58.96 ± 8.67, indicating statistical significance only within the low-frequency PTA subgroup.
Power analysis
For the low-frequency PTA, with a moderate effect size (f = 0.25), the power was 0.996, indicating sufficient power to detect a moderate effect. For a small effect size (f = 0.20), the power was 0.956, suggesting that even small effects could be detected with high probability. Similarly, for the high-frequency PTA, the power for a moderate effect size (f = 0.25) was 0.926, indicating adequate power to detect moderate effects. For a small effect size (f = 0.20), the power was 0.765, which, although lower, still provides reasonable power for detecting small effects.
Association of eGFR with low- and high-frequency PTA
We used a 4-node RCS model to evaluate the nonlinear associations of eGFR with low- and high-frequency PTA, respectively. For participants with P for nonlinear > 0.05, a scatter plot was performed to assess the linear relationship. As depicted by Supplementary Fig. 1, there was no nonlinear correlation between eGFR and low-frequency HI (P for nonlinear > 0.05). Subsequently, we found a negative linear correlation between eGFR and low-frequency PTA (R = -− 0.21, P < 0.001) (Supplementary Fig. 1B). As demonstrated in Supplementary Fig. 1C–D, significant nonlinear correlations were found for the associations of eGFR with high-frequency HI and high-frequency PTA (P for nonlinear < 0.05). Table 3 illustrates the relationship between eGFR and the risk of low-frequency HI. In Model 1, for low-frequency HI, compared with those with eGFR ≥ 60 mL/min/1.73 m2, participants with moderate CKD exhibited a higher odds ratio (OR = 1.93, 95% CI = 1.35–2.77, and P < 0.001).
Nevertheless, following adjustments for age, gender, and race (Model 2) and subsequent adjustment for all covariates (Model 3), the association no longer remained statistically significant (P > 0.05). Table 4 demonstrates the association between eGFR and the risk of high-frequency HI. For high-frequency HI, in Model 1, participants with moderate CKD displayed a higher odds ratio (OR = 3.25, 95% CI = 1.56–6.78, and P = 0.002) compared with those with eGFR ≥ 60 mL/min/1.73 m2. Furthermore, after adjusting for age, gender, and race (Model 2) and after adjusting for all covariates (Model 3), this trend persisted and remained statistically significant.
Furthermore, we employed ordered logistic regression models to investigate the relationship between HI grades of low-frequency PTA, high-frequency PTA, and moderate CKD further. Upon adjusting for all confounders, it was noted that among participants with low-frequency PTA, the association between slight HI and moderate and severe HI with moderate CKD did not reach statistical significance compared with those with normal hearing (Fig. 4A, P > 0.05). However, among participants with high-frequency PTA, both slight HI and moderate and severe HI demonstrated significant associations with moderate CKD (Fig. 4B, P < 0.05). Moreover, among high-frequency PTA participants, moderate CKD increased the risk of slight HI (OR = 5.13, 95% CI = 1.48 -17.86, and P = 0.01) and moderate and severe HI (OR = 4.68,95% CI = 1.29 -17.04, and P = 0.02). In conclusion, moderate CKD was not significantly associated with the prevalence risk of low-frequency HI and HI grades. In contrast, it demonstrated a significant association with the prevalence risk of high-frequency HI and HI grades. Moreover, moderate CKD was found to increase the prevalence risk of high-frequency HI.
The correlation between moderate CKD and HI grades of low- and high-frequency PTA. (A)low-frequency PTA. (B)high-frequency PTA. OR, odds ratio; CI, confidence intervals; Model 1: no covariates were adjusted; Model 2: age, gender, and race were adjusted; Model 3: age, gender, race, educational level, PIR, BMI, smoke, hypertension, diabetes, and CHD were adjusted. Statistical significance was determined using ordered logistic regression models, with p-values ≤ 0.05 considered statistically significant. Results are expressed as odds ratios with 95% confidence intervals (CI).
Association of inflammatory biomarker NPAR with high-frequency PTA and moderate CKD
As the association between moderate CKD and low-frequency PTA was not significant, we performed further analyses of NPAR for high-frequency PTA and moderate CKD. As displayed in Supplementary Fig. 2A, there was no nonlinear correlation between NPAR and high-frequency HI (P for nonlinear > 0.05). We found a positive linear correlation between NPAR and high-frequency PTA (R = 0.19, P < 0.001) (Supplementary Fig. 2B). Among participants with high-frequency PTA (Fig. 5), after fully adjusting for covariates (Model 3), we discovered that the top NPAR quartile (Q4) had significantly higher high-frequency HI grades than the bottom quartile (Q1). Specifically, each 1-unit increase in the highest quartile of NPAR was linked to a 42% increase in the odds of a 1-level rise in HI grades (OR = 1.42, 95% CI = 1.06- 1.91, and P = 0.018). From Supplementary Fig. 3A, it can be observed that there was no nonlinear correlation between NPAR and moderate CKD. Following this (Supplementary Fig. 3B), we found a negative linear correlation between NPAR and eGFR of moderate CKD (R = –0.12, P = 0.044). In moderate CKD (Fig. 6), after fully adjusting for covariates (Model 3), we observed that the highest quartile of NPAR (Q4) was associated with a higher risk of moderate CKD compared with the lowest quartile (Q1) (OR = 2.73, 95% CI = 1.09–6.86, and P = 0.03).
Association between NPAR Quartile and high-frequency HI grades. OR, odds ratio; CI, confidence intervals; Model 1: no covariates were adjusted; Model 2: age, gender, and race were adjusted; Model 3: age, gender, race, educational level, PIR, BMI, smoke, hypertension, diabetes, and CHD were adjusted. Statistical significance was determined using ordered logistic regression models, with p-values ≤ 0.05 considered statistically significant. Results are expressed as odds ratios with 95% confidence intervals (CI).
OR, odds ratio; CI, confidence intervals; Model 1: no covariates were adjusted; Model 2: age, gender, and race were adjusted; Model 3: age, gender, race, educational level, PIR, BMI, smoke, hypertension, diabetes, CHD were adjusted. Statistical significance was determined using ordered logistic regression models, with p-values ≤ 0.05 considered statistically significant. Results are expressed as odds ratios with 95% confidence intervals (CI).
The mediating role of NPAR between moderate CKD and high-frequency HI
Mediation analysis demonstrated that NPAR exhibited a significant mediating effect on the association between moderate CKD and high-frequency HI, with a mediation ratio of 17.85% (see Fig. 7).
Discussion
It has been recognized that there is an association between the ear and the kidney16,17. However, most studies have concentrated on individuals with genetic abnormalities to explore this relationship6,17. Studies investigating the link between moderate CKD and presbycusis are notably scarce. This study primarily aimed to assess how moderate CKD correlates with low-frequency HI, high-frequency HI, and HI grades. Additionally, it aimed to investigate how the inflammatory biomarker NPAR mediates the link between moderate CKD and presbycusis for the first time.
We observed that out of 939 participants, 263 (27%) exhibited moderate CKD, while 676 (73%) demonstrated an eGFR ≥ 60 mL/min/1.73 m2. For low-frequency PTA, 52.61% of the participants exhibited normal hearing, 33.17% demonstrated slight HI, and 14.27% experienced moderate and severe HI. In contrast, for high-frequency PTA, only 7.99% of the participants exhibited normal hearing, 26.09% experienced slight HI, and the majority (65.92%) demonstrated moderate and severe HI. These findings were consistent with previous studies reporting that HI is generally more severe in the high-frequency range, especially in elderly populations18. This disparity further underscores the significance of high-frequency HI as a marker of presbycusis, especially in individuals with CKD.
To address the issue of uneven sample sizes across different frequency HI categories, we conducted a power analysis for both low- and high-frequency HI categories. For low-frequency PTA, the power was 0.996 for a medium effect size (f = 0.25) and 0.956 for a small effect size (f = 0.20), indicating that even small effects can be detected with high probability. This suggests that the low-frequency analysis is robust in detecting differences across the HI categories. For high-frequency PTA, the power for a medium effect size (f = 0.25) was 0.926, which is sufficient to detect moderate effects. However, for a small effect size (f = 0.20), the power was 0.765. Although this is lower, it still provides reasonable power to detect small effects. It is important to note that the sample size for normal-hearing participants in the high-frequency PTA was considerably smaller, which may have contributed to the lower power for detecting small effects.
The relationship between kidney function and HI has been a subject of growing interest, particularly in understanding how CKD affects auditory health. When examining the relationship between kidney function and HI in the context of low-frequency PTA, we observed that participants with an eGFR ≥ 60 mL/min/1.73 m2 and moderate CKD predominantly exhibited normal hearing. Conversely, in the high-frequency PTA, individuals with eGFR ≥ 60 mL/min/1.73 m2 and moderate CKD tended to experience moderate and severe HI. Furthermore, the prevalence of moderate CKD patients with moderate and severe HI increased progressively with age. These findings suggest that high-frequency hearing may be more sensitive to the underlying pathophysiological processes associated with CKD, consistent with previous studies linking renal dysfunction to the auditory system19.
A key finding in our study was that the association between moderate CKD and low-frequency HI was confounded by age, gender, and race. In our initial model, a significant relationship between CKD and low-frequency HI was observed, but after adjusting for these demographic factors, the association lost significance. This implies that factors such as age and gender may play a more substantial role in low-frequency HI than previously understood. In contrast, the association between moderate CKD and high-frequency HI remained significant after adjusting for all covariates. Moreover, among high-frequency PTA participants, moderate CKD increased the risk of slight HI (OR = 5.13, 95% CI = 1.48–17.86, and P = 0.01) and moderate and severe HI (OR = 4.68, 95% CI = 1.29–17.04, and P = 0.02). This suggests that high-frequency HI is more directly and robustly related to the pathophysiology of moderate CKD. These findings were consistent with the results reported by Vilayur et al.19, where they found that after adjusting for all covariates, moderate CKD was significantly associated with moderate HI (OR = 1.46, 95% CI: 1.11–1.92, and P = 0.007) and increased the risk of severe HI (OR = 1.50, 95% CI: 1.04–2.18, and P = 0.03).
Furthermore, a longitudinal population-based study by Schubert et al.20 revealed a close association between eGFRCysC (an indicator of renal function) and HI in the elderly. Participants with eGFRCysC < 60 mL/min/1.73 m2 exhibited a potentially higher 20-year cumulative incidence of HI compared with those with eGFRCysC ≥ 60 mL/min/1.73 m2 (HR = 1.50 and 95% CI: 1.02–2.22). Although previous research has shown that CKD is associated with high-frequency HI, and its severity is closely related to age21, the relationship between eGFR and HI remains a topic of debate. A study on the Chinese population by Yang et al.22 found that higher eGFR levels increase the risk of HI and HI grades in individuals aged 45–65. However, no association was found between eGFR and HI in women and individuals aged ≥ 65, which contradicts our findings. Meanwhile, Gupta et al.23 also found no significant association between CKD and the risk of incident HI. This disparity may be attributed to factors such as race, dietary habits, geographical region, and others. Consequently, these differences suggest that the relationship between CKD and HI may vary across different populations or regions. Therefore, future studies should validate these hypotheses and take into account factors such as race, lifestyle, and geographical differences.
To our understanding, systemic inflammatory cells such as neutrophils and albumin have been linked not only to presbycusis15,24 but also to moderate CKD14. Elevated levels of neutrophils promote free radical aggregation, intensifying inflammatory responses and oxidative stress25, which exacerbate the progression of presbycusis. Conversely, albumin is believed to exhibit a negative correlation with inflammatory responses and oxidative stress, potentially slowing down the advancement of presbycusis24,26,27.
In addition, neutrophils can cause irreversible kidney damage by inducing the secretion of various inflammatory mediators, thereby further reducing and worsening kidney function. Hypoalbuminemia may accelerate renal endothelial cell damage by altering microcirculatory blood flow and increasing blood viscosity, potentially increasing morbidity and mortality in CKD patients14,28. Moreover, eGFR was found to decrease with increasing neutrophil levels and increase with increasing albumin levels29. NPAR is a new systemic inflammation marker, calculated from the neutrophil-to-albumin ratio, useful for predicting outcomes in various inflammation-related diseases. It has notably demonstrated a significant association with coronary atherosclerosis in older adults with CKD patients aged ≥ 65 years30.
However, no study has yet explored the relationship between the NPAR in moderate CKD and presbycusis. Based on previous studies, we investigated whether the inflammation index NPAR contributes to the association between moderate CKD and presbycusis. In this study, we confirmed a significant linear positive correlation between NPAR and high-frequency PTA (R = 0.19, P < 0.001), as well as a significant linear negative correlation between NPAR and eGFR of moderate CKD (R = –0.12, P < 0.044). When NPAR was divided into quartiles, participants in the highest quartile (Q4) exhibited a higher risk of high-frequency HI (OR = 1.42, 95% CI: 1.06–1.91, and P = 0.018) and moderate CKD (OR = 2.73, 95% CI: 1.09–6.86, and P = 0.03). We further evaluated the mediating role of NPAR between moderate CKD and high-frequency HI. Notably, through mediation analysis, NPAR was found to significantly mediate the association between moderate CKD and presbycusis, with a mediation ratio of 17.85%.
The strength of this study lies in its examination of the relationship between HI grades and both low-frequency PTA (0.5, 1, and 2 kHz) and high-frequency PTA (3, 4, 6, and 8 kHz). Unlike most previous studies that analyzed this relationship using only the average audiometric thresholds at 0.5, 1, 2, and 4 kHz19,20,22, this study provided a more comprehensive assessment. A key innovation of this study is its potential to be the first to assess how NPAR mediates the link between moderate CKD and presbycusis risk.
Our study has several limitations. First, the complex pathogenesis of presbycusis induced by moderate CKD involves multiple pathways, including inflammation, apoptosis, autophagy, and oxidative stress. This study exclusively focused on the role of the inflammation-related indicator NPAR in the correlation between moderate CKD and presbycusis. In the future, the specificity of NPAR should be validated through multiple biomarker analyses, such as simultaneous binding of NPAR, CRP, and fibrinogen, to enhance the understanding of its role and to refine the diagnostic and therapeutic implications of NPAR in this context. Meanwhile, future research should explore additional mechanisms linking moderate CKD to presbycusis. Second, potential confounding variables were not fully controlled for, and the generalizability of the findings may be affected by factors such as regional, lifestyle, or demographic differences. While we conducted rigorous statistical analyses to minimize the influence of known confounders, we acknowledge that unmeasured confounders may still be at play. In future research, a more robust multivariate model controlling for a wider range of confounders, including lifestyle factors and comorbidities, should be employed to better understand the generalizability of our findings across different populations. Finally, the retrospective and cross-sectional design of this study limits our ability to establish causality. Given that the data were collected at a single point in time, it is difficult to determine whether moderate CKD causes presbycusis or if there are other underlying factors contributing to both conditions. To overcome this limitation, we propose that future studies adopt longitudinal or prospective cohort designs. These designs would allow for the tracking of CKD progression and its association with changes in auditory function over time, providing stronger evidence for a causal relationship.
Despite these limitations, this study provides important insights into the relationship between moderate CKD and presbycusis. By identifying inflammation-related markers like NPAR, we highlight potential targets for early diagnostics and interventions to prevent or mitigate HI in CKD patients. Further validation in larger, diverse populations is needed, and prospective studies will be essential to confirm the clinical relevance of these findings.
Conclusion
In conclusion, our study suggested that reducing NPAR levels and improving renal function may be potential interventions for presbycusis. At a later date, we need to explore the potential mechanism of the role of NPAR in moderate CKD with presbycusis more comprehensively and deeply, and combining basic experiments with prospective or longitudinal clinical trial studies is essential.
Materials and methods
Study design and population. The NHANES database, a major initiative of the National Center for Health Statistics, is designed to assess the nutritional and health status of the non-institutionalized population in the United States. This study was approved by the Center for Research Ethics Review Board. All research participants provided written informed consent. Given the NHANES database’s sampling methodology—employing a complex, stratified, multi-stage probabilistic approach—we used sample weighting to adjust the results and minimize variations between individuals. Participants in this study were selected from six NHANES cycles (2005–2006,2007–2008,2009–2010,2011–2012,2015–2016,2017–2018).Information collected through interviews, questionnaires, laboratory tests, and physical examinations can be accessed via the official NHANES website (https://wwwn.cdc.gov/Nchs/Nhanes).
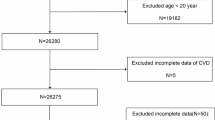
Inclusion and exclusion criteria. The inclusion criterion of participants aged 65 years and older was chosen to focus on the aging population, as they are more prone to hearing impairment and chronic kidney disease31. And 9,527 individuals met this age requirement. Our exclusion criteria were participants with severe ear pain, those unable to tolerate headphones, and individuals using fixed hearing aids or cochlear implants, as they are generally not enrolled in NHANES. Participants were excluded if their 1 kHz hearing test results differed by more than 10 dB on two occasions (n = 5157); those with missing hearing data (n = 55); those with conductive hearing loss due to external or middle ear causes or noise exposure (n = 821); those with abnormal otoscopic findings (n = 1166); those with hearing thresholds above 120 dB (n = 163); those with missing eGFR, blood urea nitrogen (BUN) and serum creatinine concentration (Scr) data (n = 115); and those with incomplete covariate data (n = 1111). Lastly, 939 participants were included (Fig. 8).
Audiometric tests and determination of hearing impairment. Hearing tests were conducted in a standardized mobile testing facility, ensuring soundproof conditions and regularly calibrated audiometric equipment. The testing was carried out by licensed audiologists to ensure the accuracy and reliability of results. Each ear was tested for air conduction hearing thresholds in the frequency range from 0.5 to 8 kHz, with intensities ranging from -10 to 120 dB. We calculated the PTA hearing thresholds at low frequencies (0.5, 1, and 2 kHz) and high frequencies (3, 4, 6, and 8 kHz) concurrently. Categorization by hearing status was determined by the better of the two ears. Low-frequency PTA ≥ 20 dB was defined as low-frequency HI, and high-frequency PTA ≥ 20 dB was defined as high-frequency HI. The HI grades were classified as follows: < 20 dB for normal hearing, 20 dB to < 35 dB for slight HI, and ≥ 35 dB for moderate and severe HI31,32.
Criteria for determining presbycusis. Age ≥ 65 years; PTA ≥ 20 dB in the better ear across frequencies from 0.5 to 8 kHz; Exclusion of HI due to noise exposure, secretory otitis media, middle ear perforation, infections, or other identifiable causes.
Measurement of eGFR and moderate CKD definition. Scr was determined using the Jaffe rate method. The eGFR was estimated using the CKD-EPI equation, which is widely accepted for assessing kidney function in epidemiological studies33. This method accounts for variables such as age, sex, and serum creatinine levels, providing a more accurate reflection of kidney function across diverse populations. The eGFR was considered as both a continuous and a categorical variable. It was categorized as < 60 and ≥ 60 mL/min/1.73 m2, with the former defined as moderate CKD.
Measurement of neutrophil percentage, albumin, and NPAR. A complete blood count (CBC) was conducted using a Coulter® HMX. Hematological parameters, including neutrophil count and albumin, were obtained from the CBC profile in the NHANES database. The proportion of neutrophils in WBC counts was termed as the neutrophil percentage. NPAR = (neutrophil percentage (%) × 100)/albumin.
Covariates. Demographic data were collected through face-to-face interviews, including age, gender, race, body mass index (BMI), educational level, family income-poverty ratio (PIR), smoking status, hypertension, diabetes, and coronary heart disease (CHD). These factors were chosen for their well-known roles in influencing health, especially in older adults, where they contribute to the development and progression of chronic diseases22. Age was categorized into four groups: 65–69, 70–74, 75–79, and 80 years or older, based on the increased health risks with age. Gender was classified as either male or female, based on its known influence on disease prevalence. Race was categorized as White, Black, Mexican American, and Other, as race is known to impact disease rates. PIR was categorized as low (< 1.3), moderate (1.3–3.5), and high (≥ 3.5), due to it established association with health outcomes. BMI was categorized as normal (< 25), overweight (25, 30), and obese (≥ 30), to assess obesity-related health risks. Smoking was defined as having smoked at least 100 cigarettes in a lifetime, which is a well-known risk factor for numerous diseases. Hypertension was diagnosed in participants who had a systolic blood pressure of 140 mmHg or higher, a diastolic blood pressure of 90 mmHg or higher, or who had previously been diagnosed with hypertension by a physician. Diabetes was diagnosed in patients with a hemoglobin A1c concentration ≥ 6.5%, a fasting plasma glucose level ≥ 126 mg/dL, or who had previously been diagnosed with diabetes by a physician. CHD was diagnosed based on clinical criteria or a previous medical diagnosis by a physician.
Statistical analysis. Continuous variables are represented as weighted mean ± standard deviation (SD), whereas categorical variables are represented as weighted percentages. To compare baseline information between groups, the Wilcoxon rank sum test for complex samples was used for continuous variables, and the chi-squared test with Rao and Scott’s second-order correction for categorical information. Since the sampling method for the NHANES database is a complex, stratified multi-stage probability approach, we adjusted the sampling results using sample weighting to mitigate differences between individuals. R software survey package was used to make this adjustment.
Quartiles (Q1, Q2, Q3, and Q4) division of NPAR was chosen to examine its potential gradient effects on health outcomes, as this method enables the identification of dose–response relationships. The sample density curve was used to depict the distribution of eGFR among subjects under different HI grades at low and high frequencies, respectively. Restricted cubic splines (RCS) with four knots were used to explore potential non-linear relationships between variables. This method is preferred as it allows for flexible modeling of non-linear associations without imposing strict assumptions about the functional form of the relationship. If the nonlinear correlation did not fit, linear correlation analysis was conducted using scatterplots. Pearson’s correlation analyses were used to evaluate the relationships among eGFR, NPAR, low-frequency PTA, and high-frequency PTA.
Three statistical models were established to assess the risk associations of NPAR with moderate CKD and hearing impairment (HI) or HI grades. Model 1 was unadjusted, providing a rough estimate of the association without considering confounding factors. Model 2 was adjusted for age, gender, and race to analyze the impact of demographic differences. Model 3 was further adjusted for age, gender, race, educational level, PIR, BMI, smoking, hypertension, diabetes, and CHD, adding more covariates to control for additional confounding factors closely related to health34,35.
Multiple logistic regression analyses, including binary and ordinal logistic regression analyses, were established. Binary logistic regression was applied when the variables were binary (normal hearing and HI; eGFR ≥ 60 and < 60 mL/min/1.73 m2). Furthermore, ordinal logistic regression was selected for the HI grades (normal hearing, slight HI, moderate, and severe HI). The odds ratio (OR) with a 95% confidence interval (CI) for the relationship between HI and eGFR or NPAR. Mediation analysis was performed using the mediation package in R software. Power analysis was conducted using the pwr package in R to assess the adequacy of the sample size for both low-frequency and high-frequency conditions. The significance level was set to α = 0.05. All data analyses were conducted using R software (version 4.3.3). A bilateral P-value < 0.05 was considered statistically significant.
Data availability
This study applied the public NHANES database.All data of this study were free of charge and can be accessed at https://www.cdc.gov/nchs/nhanes/.
References
Rivas-Chacon, L. D. M. et al. Role of oxidative stress in the senescence pattern of auditory cells in age-related hearing loss. Antioxidants Basel https://doi.org/10.3390/antiox10091497 (2021).
Li, W. et al. The prevalence and global burden of hearing loss in 204 countries and territories, 1990–2019. Environ. Sci. Pollut. Res. Int. 29, 12009–12016. https://doi.org/10.1007/s11356-021-16582-8 (2022).
Collaborators, G. B. D. H. L. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet 397, 996–1009. https://doi.org/10.1016/S0140-6736(21)00516-X (2021).
Collaboration, G. B. D. C. K. D. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733. https://doi.org/10.1016/S0140-6736(20)30045-3 (2020).
Torban, E. & Goodyer, P. The kidney and ear: Emerging parallel functions. Annu. Rev. Med. 60, 339–353. https://doi.org/10.1146/annurev.med.60.052307.120752 (2009).
Cuna, V. et al. Hypoacusia and chronic renal dysfunction: New etiopathogenetic prospective. Ther. Apher. Dial. 19, 111–118. https://doi.org/10.1111/1744-9987.12232 (2015).
Lang, F., Vallon, V., Knipper, M. & Wangemann, P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am. J. Physiol. Cell. Physiol. 293, C1187-1208. https://doi.org/10.1152/ajpcell.00024.2007 (2007).
Keithley, E. M. Pathology and mechanisms of cochlear aging. J Neurosci Res 98, 1674–1684. https://doi.org/10.1002/jnr.24439 (2020).
Fang, Y. et al. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 63, 101151. https://doi.org/10.1016/j.arr.2020.101151 (2020).
Chen, L. et al. Systemic immune inflammation index and peripheral blood carbon dioxide concentration at admission predict poor prognosis in patients with severe traumatic brain injury. Front. Immunol. 13, 1034916. https://doi.org/10.3389/fimmu.2022.1034916 (2022).
Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 122, 474–488. https://doi.org/10.4149/BLL_2021_078 (2021).
Sproston, N. R. & Ashworth, J. J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754. https://doi.org/10.3389/fimmu.2018.00754 (2018).
Dixon, L. R. The complete blood count: physiologic basis and clinical usage. J. Perinat. Neonatal. Nurs. 11, 1–18. https://doi.org/10.1097/00005237-199712000-00003 (1997).
Chen, Y. et al. Association between systemic inflammatory indicators with the survival of chronic kidney disease: a prospective study based on NHANES. Front. Immunol. https://doi.org/10.3389/fimmu.2024.1365591 (2024).
Lassale, C. et al. Association of inflammatory markers with hearing impairment: The english longitudinal study of ageing. Brain Behav. Immun. 83, 112–119. https://doi.org/10.1016/j.bbi.2019.09.020 (2020).
Izzedine, H., Tankere, F., Launay-Vacher, V. & Deray, G. Ear and kidney syndromes: Molecular versus clinical approach. Kidney Int. 65, 369–385. https://doi.org/10.1111/j.1523-1755.2004.00390.x (2004).
Greenberg, D., Rosenblum, N. D. & Tonelli, M. The multifaceted links between hearing loss and chronic kidney disease. Nat. Rev. Nephrol. 20, 295–312. https://doi.org/10.1038/s41581-024-00808-2 (2024).
Bainbridge, K. E. & Wallhagen, M. I. Hearing loss in an aging American population: extent, impact, and management. Annu. Rev. Public Health 35, 139–152. https://doi.org/10.1146/annurev-publhealth-032013-182510 (2014).
Vilayur, E. et al. The association between reduced GFR and hearing loss: a cross-sectional population-based study. Am. J. Kidney Dis. 56, 661–669. https://doi.org/10.1053/j.ajkd.2010.05.015 (2010).
Schubert, C. R. et al. Association between cystatin C and 20-year cumulative incidence of hearing impairment in the epidemiology of hearing loss study. JAMA Otolaryngol. Head Neck Surg. 144, 469–474. https://doi.org/10.1001/jamaoto.2018.0041 (2018).
Sarin, V., Sharma, A. & Chopra, I. High frequency hearing loss in chronic renal disease: A cross-sectional study. Indian J. Otolaryngol. Head Neck Surg 74, 4046–4052. https://doi.org/10.1007/s12070-021-02811-6 (2022).
Yang, D. et al. Association between kidney function and hearing impairment among middle-aged and elderly individuals: a cross-sectional population-based study. Postgrad. Med. 133, 701–706. https://doi.org/10.1080/00325481.2021.1933554 (2021).
Gupta, S. et al. Chronic kidney disease and the risk of incident hearing loss. Laryngoscope 130, E213–E219. https://doi.org/10.1002/lary.28088 (2020).
Verschuur, C. A. et al. Markers of inflammatory status are associated with hearing threshold in older people: findings from the Hertfordshire ageing study. Age Ageing 41, 92–97. https://doi.org/10.1093/ageing/afr140 (2012).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102, 209–217. https://doi.org/10.1161/CIRCRESAHA.107.160697 (2008).
Verschuur, C., Agyemang-Prempeh, A. & Newman, T. A. Inflammation is associated with a worsening of presbycusis: evidence from the MRC national study of hearing. Int. J. Audiol. 53, 469–475. https://doi.org/10.3109/14992027.2014.891057 (2014).
Watson, N., Ding, B., Zhu, X. & Frisina, R. D. Chronic inflammation - inflammaging - in the ageing cochlea: A novel target for future presbycusis therapy. Ageing Res. Rev. 40, 142–148. https://doi.org/10.1016/j.arr.2017.10.002 (2017).
Andrade-Oliveira, V., Foresto-Neto, O., Watanabe, I. K. M., Zatz, R. & Camara, N. O. S. Inflammation in renal diseases: New and old players. Front. Pharmacol. 10, 1192. https://doi.org/10.3389/fphar.2019.01192 (2019).
Amdur, R. L. et al. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 11, 1546–1556. https://doi.org/10.2215/cjn.13121215 (2016).
Amdur, R. L. et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: Findings from the CRIC study. Am. J. Kidney Dis. 73, 344–353. https://doi.org/10.1053/j.ajkd.2018.09.012 (2019).
Lin, F. R. Age-related hearing loss. N. Engl. J. Med. 390, 1505–1512. https://doi.org/10.1056/NEJMcp2306778 (2024).
Humes, L. E. The World Health Organization’s hearing-impairment grading system: An evaluation for unaided communication in age-related hearing loss. Int. J. Audiol. 58, 12–20. https://doi.org/10.1080/14992027.2018.1518598 (2019).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Levy, R. V. et al. Analysis of active and passive tobacco exposures and blood pressure in US children and adolescents. JAMA Netw. Open 4, e2037936. https://doi.org/10.1001/jamanetworkopen.2020.37936 (2021).
Wen, Z. et al. Association of serum vitamin D with anxiety in US adults: a cross-sectional study. Front. Nutr. 11, 1371170. https://doi.org/10.3389/fnut.2024.1371170 (2024).
Acknowledgements
We thank the Home for Researchers editorial team (www.home-for-researchers.com) for language editing services. We sincerely thank Professor Jianping Sun, a nephrologist, for his valuable insights and guidance, especially in analyzing chronic kidney disease data. His expertise was crucial to this research, and we deeply appreciate his support.
Funding
The author declares that financial support was received for the research, authorship, and/or publication of this article. Youth Science Fund Project of National Natural Science Foundation of China (81403439); the Henan Provincial Science and Technology Research Project (24A360007).
Author information
Authors and Affiliations
Contributions
Qingling wang performed all data analysis,designed the research,drafted the manuscript and wrote the original manuscript. Ruizhe huang designed the research and analyzed the data. Shiduo guo collected,analyzed the data, and revised the whole manuscript. Zhenju xu, Yichuan huang and Dapeng liang revised the whole manuscript. Administrative, technical, or material support: Xiangdong guo and Liwei Jiang.The article’s submission was reviewed and approved by all of the writers.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The NHANES abided by the Declaration of Helsinki and was approved by the Center for Research Ethics Review Board. All research participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Q., Huang, R., Guo, S. et al. Inflammatory mediation by neutrophil percentage to albumin ratio in the association between chronic kidney disease and presbycusis. Sci Rep 15, 30064 (2025). https://doi.org/10.1038/s41598-025-15420-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15420-y