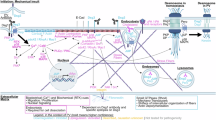
Abstract
In the context of Pemphigus Vulgaris (PV), an autoimmune skin disorder, our study explores the intricate relationship between dyslipidemia, oxidative stress, and mitochondrial-dependent apoptosis in PV. We investigated lipid profiles in PV patients, revealing significant dyslipidemia marked by elevated cholesterol, triglycerides, and apolipoprotein B, alongside decreased high-density lipoprotein and serum calcium levels. The imbalance was found to augment oxidative stress, evident from increased oxidative stress markers in lesion tissues and serum. Notably, oxidative stress correlated with classic PV biomarkers, anti-DSG1/DSG3 antibodies. Lipid-induced oxidative stress was further confirmed in vitro using HaCaT cells treated with PV patient serum, which led to STAT3 activation and mitochondrial-dependent apoptosis. This apoptosis was characterized by changes in mitochondrial membrane potential and activation of apoptotic proteins, implicating oxidative stress as a key player in PV pathogenesis. Our findings suggest the critical connections between lipid disturbances, oxidative stress, apoptosis, and STAT3 activation in PV, offering potential avenues for novel therapeutic interventions.
Similar content being viewed by others
Introduction
Pemphigus Vulgaris (PV), the most common and severe form of pemphigus, is a rare autoimmune blistering disease that poses a significant threat to human health and can be life-threatening1. PV typically leads to the loss of adhesion between keratinocytes or acantholysis2. Notably, pathogenic autoantibodies linked to PV specifically target cadherin-type adhesion molecules, specifically desmoglein 1 (Dsg1) and desmoglein 3 (Dsg3)3.
Oxidative stress, defined as an imbalance between oxidants such as reactive oxygen species (ROS) and the body’s antioxidant capacity leading to tissue damage, has been increasingly studied in relation to PV4. It is hypothesized that oxidative stress, through increased ROS production, may trigger apoptosis in PV epithelial cells, a critical dynamic equilibrium in the disease’s progression5. However, the precise mechanisms of oxidative stress and apoptosis in PV require further elucidation. This study discovered that oxidative stress induces mitochondria-dependent apoptosis and STAT3 activation in PV epithelial cells.
Methods
Patient samples
We collected blood biochemical data from the healthy individuals (n = 108) and PV patients (n = 108). All the patients enrolled in this study had no hypertension, diabetes, or hyperlipidemia and were from Sun Yat-sen Memorial Hospital. Among these, a subset of samples was selected for further histological and hematological analyses, and the detailed clinical information of these participants is provided in Supplementary Table S1. The study was conducted according to the standards of the Declaration of Helsinki and was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (approval number SYSKY-2024-240-01). Written informed consent was obtained from all subjects.
Histological analysis
The skin tissues were fixed in a 10% neutral formaldehyde solution for 24 h, followed by embedding, slicing, and hematoxylin-eosin (HE) staining. All samples were examined using an optical microscope (Olympus Optical Co., Tokyo, Japan).
The enzyme-linked immunosorbent assay (ELISA)
The levels of malondialdehyde (MDA), total thiols, superoxide dismutase (SOD), and catalase (CAT) were measured immediately after sample collection using the ELISA kits (MDA: Cat. #S0131S; Thiols: Cat. #S0138S; SOD: Cat. #S0101S; CAT: Cat. #S0051; all from Beyotime, Shanghai, China), following the manufacturer’s instructions.
Cell culture and treatment with PV serum
The human keratinocyte cell line HaCaT was obtained from American Tissue and Cell Collection (CLS Cat# 300493/p800_HaCaT, RRID: CVCL_0038) and was used to establish an in vitro model of PV. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM). Media was supplemented with 10% FBS (TransGen Biotech Co., Ltd, Beijing, China), 100 U/mL penicillin and streptomycin. Cells were grown at 37 °C in a 5% CO2 incubator. During the logarithmic growth phase, the medium was replaced with a mixture containing 20% PV serum and 80% DMEM supplemented with 10% FBS, and cells were cultured for 36 h.
Mitochondrial membrane potential (MMP) assay
Cells of interest were exposed to a 20% concentration of serum from PV patients before analysis of MMP by using the Mitochondrial Membrane Potential Assay Kit with TMRE (Cat. #C2001S, Beyotime) as instructed by the manufacturer. MMP was analyzed by fluorescence microscopy (Zeiss AxioVert A1).
Flow cytometry analysis
HaCaT cells were incubated with 20% patient-derived (PV) serum for 36 h as part of the experimental treatment protocol. A control serum was utilized as the vehicle control within the experiment. For flow cytometry detection, cells were collected, washed with PBS twice and stained using the annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s instructions (Cat. # C1062M, Beyotime). Stained cells were analyzed using a CytoFLEX S flow cytometer (Beckman Coulter, Brea, CA, USA).
Antibodies and reagents
Anti-caspase-3 (Cat# 19677-1-AP), anti-BAX (Cat# 50599-2-Ig), anti-Bcl2 (Cat# 60178-1-Ig), anti-PARP1 (Cat# 13371-1-AP), anti-STAT3 (Cat# 10253-2-AP), and anti-GAPDH (Cat# 10494-1-AP) were obtained from Proteintech Group. Anti-phospho-stat3 (Tyr705) (Cat# 9145 S) was from Cell Signaling Technology. EDTA solution (Cat# R21345) was purchased from Shanghai Yuanye Bio-Technology. Protease inhibitors (Cat# P1005), the enhanced chemiluminescence (ECL) reagent kit (Cat# P0018M), and Blotting Grade (Cat# P0216) were obtained from Beyotime Institute of Biotechnology. PVDF Transfer Membrane (Cat# INCP00010) was purchased from MERCK. Tris (Cat# T1010) was purchased from Solarbio Life Sciences. Sodium chloride (Cat# A501218) and SDS (Cat# A600485) were obtained from Sangon Biotech. Stattic (Cat# HY-13818) was purchased from MedChemExpress.
Immunoblotting
Cells and tissue samples were lysed immediately after collection in a buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.5% SDS, and protease inhibitors. After clarification at high speed for 10 min at 4 °C, an equal amount of proteins was fractionated in SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked with 5% skim milk for 1 h at room temperature. The membranes were then incubated with primary antibodies, typically diluted at 1:1,000 (dilution factors were based on the manufacturer’s instructions), overnight at 4 °C on a shaker. After washing with TBST, the membranes were incubated with HRP-conjugated secondary antibodies corresponding to the primary antibody species at a dilution of 1:5,000 for 60 min at room temperature on a shaker. Protein bands were visualized using an enhanced chemiluminescence detection method and captured using a Fully Automated Gel Imaging System (BLT, Model GelView 1500 Pro).
Statistics
Statistical assessments in our study were conducted using GraphPad Prism (GraphPad Software, Version 8.0). Student’s t-test was used for comparisons between two groups, and one-way ANOVA was performed for comparisons among multiple groups. P < 0.05 was considered statistically significant. All experiments were independently repeated at least three times to ensure reliability.
Results
Abnormal lipid profiles in PV patients
The study involved a healthy group of 108 individuals, comprising 53 males and 55 females, with an average age of (52.47 ± 11.62) years. The disease group included 108 patients, 49 males and 59 females, with an average age of (50.15 ± 14.84) years. These patients were all previously diagnosed with pemphigus vulgaris (PV), as detailed in Supplementary Table S2. No significant statistical differences were observed in terms of gender and age between the healthy and disease groups (P > 0.05). In the disease group, patients exhibited higher serum levels of cholesterol (CHOL), triglycerides (TG) and apolipoprotein B (ApoB) and lower levels of high-density lipoprotein cholesterol (HDL-C) and serum Ca2+, with these differences being statistically significant (P < 0.05), as indicated in Table 1.
Given the substantial differences in CHOL, TG, ApoB, HDL-C and serum Ca2+ between the two groups, a multifactorial logistic regression analysis was performed using PV as the dependent variable. The analysis identified elevated levels of CHOL (OR = 3.35), TG (OR = 1.46) and ApoB (OR = 1.59) as risk factors for PV, while higher levels of serum Ca2+ (OR = 0.29) and HDL-C (OR = 0.49) were found to be protective factors (Fig. 1). These statistically significant differences (P < 0.05) suggest the presence of lipid abnormalities in PV patients.
Oxidative stress in PV patients
The results indicated substantial differences abnormal lipid levels in patients with PV. Previous reports have linked oxidative stress to lipid level abnormalities. In this study, we collected samples from healthy individuals and PV patients for experimental analysis, as detailed in Supplementary Table S1. Compared to normal skin, perilesional tissue showed inflammatory cell infiltration in superficial dermal vessels, while lesional sites exhibited blisters and acantholysis (Fig. 2a). Upon examination of oxidative stress biomarkers within the tissues, it was found that Thiol levels in the lesional areas were lower than those in normal tissues (P < 0.05). In contrast, perilesional tissues did not show significant changes when compared to normal tissues (p > 0.05). This suggests that the reduction in Thiol levels within the lesional tissues may be due to their oxidation and consequent depletion. Both lesional and perilesional tissues exhibited higher MDA, SOD, and CAT levels than normal tissues (Fig. 2b), with lesional areas showing even higher levels compared to perilesional tissues (P < 0.05). Consistent with these findings, ELISA tests on serum samples revealed elevated MDA, SOD, and CAT levels but decreased Thiol levels in PV patients compared to healthy individuals (Fig. 2c). These results collectively indicate the presence of oxidative stress in PV patients.
Oxidative stress is observed in PV patients. (a) HE staining of normal, perilesional, and lesional sites in PV patients (sample from patient ID 9). Black arrows indicate inflammatory cell infiltration, red arrows indicate acantholysis, and asterisks (*) denote blister formation. Scale bar: 100 μm (overview images); 40 μm (magnified images). (b) The quantification of SOD, CAT, Thiols, and MDA levels in biopsies extracted from normal, perilesional, and lesional sites in PV patients (n = 8). Error bars represented standard error of the mean (s.e.m). Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test versus the normal group. ns: not significant; *P < 0.05, **P < 0.01, ***P < 0.001. (c) The levels of SOD, CAT, Thiols, and MDA in serum from the healthy control group (n = 7) and PV patients group (n = 8) were quantified. Results were representative of at least three independent experiments. Error bars represented standard error of the mean (s.e.m). Student’s t test, *P < 0.05, ***P < 0.001, against the control.
Lipid-induced oxidative stress in HaCaT cells
Given the increased oxidative stress observed in lesion tissues and serum of PV patients, the correlation between oxidative stress markers and classic PV biomarkers DSG1 and DSG3 autoantibodies6 was assessed. Heatmap results showed strong positive correlations between lipid oxidation marker MDA and both anti-DSG1/DSG3 antibodies(DSG1/DSG3 Abs), between Thiols and DSG1 Abs, and between SOD and DSG3 Abs (Fig. 3a), suggesting a significant relationship between oxidative stress markers and DSG1/DSG3 Abs.
Lipid exposure induces oxidative stress in HaCaT cells. (a) Heat map showing the Pearson correlation among variables. The positive values in red, negative in yellow. It ranges from − 0.5 to 1, − 0.5 means a perfect negative linear relationship between variables, 1 indicates a perfect positive linear relationship between variables and 0 indicates that there is no relationship between studied variables. DSG1 Abs, Desmoglein1 antibodies; DSG3 Abs, Desmoglein3 antibodies. (b) The cell culture media from HaCaT cells treated with healthy control serum and 20% PV serum were subjected to measurements of SOD, CAT, Thiols, and MDA release. Results were representative of at least three independent experiments. Error bars represented standard error of the mean (s.e.m). Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001.
Our findings demonstrated the presence of both dyslipidemia and oxidative stress in PV patients. To confirm lipid-induced oxidative stress, HaCaT cells were treated with PV patient serum. The treated cells showed higher levels of MDA, SOD and CAT, but Thiol levels were lower, consistent with findings in PV tissue and serum, indicating lipid-induced oxidative stress (Fig. 3b).
Oxidative stress triggers mitochondrial-dependent apoptosis and STAT3 activation in HaCaT cells
Considering that oxidative stress can trigger cell apoptosis, we next aimed to explore how oxidative stress affects apoptotic processes. HaCaT cells treated with PV serum were analyzed for mitochondrial membrane potential (MMP) changes using Rhodamine B staining7. Apoptotic cells failed to stain with Rhodamine B (Fig. 4a), indicating MMP loss and mitochondrial dysfunction due to lipid-induced oxidative stress.
Mitochondrial-dependent apoptosis and STAT3 activation in HaCaT cells are triggered by oxidative stress. (a) HaCaT cells were treated with control serum, 20% PV serum, or PV serum containing Stattic (10 µM) for 36 h, followed by TMRE staining. The MMP was visualized by fluorescence microscope, scale bar: 100 μm. (b) HaCaT cells were exposed to serum from control and PV patients at a final concentration of 20% for 36 h. For inhibitor experiments, cells were treated with PV serum containing Stattic (10 µM) under the same conditions. Cell lysates were then prepared for IB assays as indicated. Protein levels are expressed as mean ± SD (n = 3). Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test. **P < 0.01, *P < 0.001. (c) HaCaT cells were incubated for 36 h with 20% serum from control or PV serum, or with PV serum containing Stattic (10 µM), followed by Annexin V/PI staining and flow cytometric analysis. (d) Tissues from the PV patients were subjected to IB analyses as indicated. Protein levels are expressed as mean ± SD (n = 3). Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test versus the normal group. *P < 0.05, **P < 0.01, ***P < 0.001.
Mitochondrial membrane proteins, crucial indicators of mitochondrial integrity8, are predominantly regulated by the Bcl2 protein family, including Bcl2 and Bax, which play a pivotal role in initiating apoptosis9. In this context, apoptotic proteins were examined, and it was observed in HaCaT cells treated with PV serum that there was an increase in Bax protein and a decrease in Bcl2, along with cleavage of caspase3 and PARP, signifying the induction of apoptosis. Importantly, HaCaT cells co-treated with PV patient serum and the selective STAT3 inhibitor Stattic exhibited suppressed p-STAT3 expression and reduced levels of apoptotic markers, including Bax, cleaved caspase-3, and PARP, indicating that STAT3 activation is involved in PV serum-induced apoptosis (Fig. 4b). Flow cytometry further confirmed that co-treatment with the STAT3 inhibitor Stattic reversed PV serum-induced apoptosis in HaCaT cells (Fig. 4c). Concurrently, the analysis revealed that serum from PV patients with dyslipidemia triggered the activation of STAT3. Protein immunoblotting of tissues from PV patients showed more pronounced apoptosis in lesional areas compared to perilesional and normal tissues (Fig. 4d), indicating that oxidative stress is a contributing factor in the mitochondrial-dependent apoptotic process and activation of STAT3 in cells.
Discussion
In this study, we explored the intricate link between dyslipidemia and the initiation of oxidative stress-induced, mitochondrial-dependent apoptosis in patients with PV. We observed a pronounced disruption in lipid metabolism among PV patients, where excessive lipids augmented oxidative stress. This leads to the impairment of the mitochondrial electron transport chain, a decline in mitochondrial membrane potential, and the subsequent activation of the BAX/caspase-3/PARP pathway, culminating in cellular apoptosis.
Our investigation unveiled disparities in the lipid profiles between PV patients and healthy controls. Specifically, we identified elevated levels of CHOL, TG, and ApoB as risk factors for PV, while an increase in serum Ca2+ and HDL-C appeared to confer a protective effect. The crucial role of calcium ions in maintaining the stability of intercellular adhesion among keratinocytes is underscored10, as their depletion is linked to the loss of cellular adhesion. Furthermore, aberrant lipid metabolism leads to an increased production of free radicals11, inflicting direct damage to cellular membranes and internal organelles12. These findings demonstrate the presence of lipid abnormalities in PV patients.
Further analysis of lesion tissues and serum from PV patients confirmed the presence of oxidative stress in this group. The significant reduction in thiol levels in both lesion tissues and serum may be attributed to the oxidation of thiol groups, leading to a decrease in concentration. Thiols serve as markers of protein oxidation during oxidative stress, with oxidized proteins losing their normal structure and function, thereby disrupting physiological processes13. The altered conformation of oxidized proteins exposes hydrophobic groups, generating new phenotypes that promote autoantibody production. This also explains the binding between newly generated autoantibodies and autoantigens. Additionally, both lesion and perilesional tissues exhibit higher levels of oxidative stress markers, including MDA, SOD, and CAT, consistent with serum ELISA tests. Indeed, increased SOD and CAT activities may reflect a cellular response to the overproduction of free radicals by inflammatory cells in PV patients14. A previous report found elevated CAT levels in pemphigus patients’ tissues compared to healthy individuals, but no significant difference in SOD levels, possibly related to the selection of newly diagnosed pemphigus patients for the experiment15. The experiment further demonstrated that in PV patients, lesion tissues exhibited higher levels of oxidative stress markers such as MDA, SOD, and CAT, compared to perilesional tissues, along with a more intense inflammatory response. These results collectively indicated the presence of oxidative stress in PV patients, damaging the antioxidant defense system, weakening the ability to clear free radicals, and further exacerbating cell damage and inflammation in PV patients.
However, we acknowledge that these findings are derived from analyses with relatively small sample sizes, primarily due to practical constraints and ethical considerations associated with invasive skin biopsies in a rare disease like PV. Although the observed differences reached statistical significance, the limited sample size warrants caution regarding the generalizability of these results. Future studies with larger cohorts are required to validate and extend these findings.
DSG1 and DSG3 are located within the intercellular junction structures. In PV patients, an autoimmune response generates autoantibodies against these desmogleins, impairing cell adhesion and leading to blister formation16. We sought to elucidate the relevance of oxidative stress in this autoimmune context by evaluating the associations between oxidative stress biomarkers and the classical PV biomarkers DSG1 and DSG3 Abs. Through heatmap analysis, we found robust positive correlations between the lipid oxidation marker MDA and DSG1/3 Abs, between thiols and DSG1 Abs, and between SOD and DSG3 Abs antibodies. These findings suggest a significant linkage between oxidative stress and pivotal PV biomarkers. The oxidative modification of proteins, such as DSG1 and DSG3, in PV patients, is a direct consequence of heightened oxidative stress and lipid peroxidation. These modified proteins serve as targets for the immune system, triggering the production of autoantibodies and leading to acantholysis, a fundamental pathological mechanism in pemphigus. Similarly, oxidative stress-induced reactive oxygen species are implicated in modulating autoantibody levels in other autoimmune diseases, including rheumatoid arthritis and systemic lupus erythematosus17.
Further in vitro experiments using PV patient serum to treat HaCaT cells underscored the impact of lipid-induced oxidative stress. These cells demonstrated an elevation in oxidative stress markers such as MDA, SOD, and CAT, coupled with a reduction in thiol levels, aligning the serum findings in PV patients. This evidence emphasizes the contributory role of lipids in oxidative stress. Excessive lipid levels fuel free radical production, which in turn overwhelms the body’s antioxidative defenses, leading to cellular damage across multiple structural domains. The resulting imbalance between oxidative and antioxidative processes engenders a damaging cycle of oxidative stress, elucidating a key pathogenic pathway in PV. Notably, we treated HaCaT cells with whole serum from PV patients rather than anti-Dsg3 monoclonal antibodies, thereby exposing the cells to a complex mixture of disease-associated factors, including autoantibodies, cytokines, and complement proteins. These components may contribute to cellular responses through both synergistic and independent mechanisms. Although HaCaT keratinocytes are widely used in dermatological research, they differ from primary human keratinocytes in differentiation status and physiological responses. These inherent differences may influence cellular behavior and downstream signaling to some extent. Nevertheless, this model provides a reproducible and practical in vitro system for mechanistic exploration.
In this study, we investigated the effects of oxidative stress on apoptosis and the activation of the STAT3 pathway in HaCaT cells. We found that oxidative stress induces mitochondrial-dependent apoptosis, characterized by the loss of mitochondrial membrane potential (MMP) and the dysregulation of crucial apoptotic proteins, such as Bax, Bcl2, caspase3, and PARP. Notably, these effects were significantly attenuated by co-treatment with the STAT3 inhibitor Stattic, which suppressed STAT3 phosphorylation and reversed the PV serum-induced upregulation of pro-apoptotic proteins. These results suggest that STAT3 activation plays a critical role in mediating oxidative stress-induced mitochondrial dysfunction and apoptosis in keratinocytes. These findings were further substantiated by flow cytometry analysis. The intricate involvement of mitochondrial structures in various pathways offers an explanation for these phenomena. For example, in PV, mitochondrial antibodies binding to Complex I proteins can hinder electron transfer18, affecting the establishment of a chemiosmotic gradient essential for cellular energy production. Our research also demonstrated that dyslipidemia-induced oxidative stress prompts the activation of the STAT3 pathway. STAT3 is a transcription factor that mediates intercellular signal transduction, contributing to disease onset by inhibiting apoptosis and promoting inflammation. This is consistent with prior studies indicating that enhanced oxidative stress triggers abnormal STAT3 activation in conditions like primary Sjögren’s syndrome19 and psoriasis20. Moreover, our findings revealed that, in PV patients, apoptosis and STAT3 activation are markedly more evident in blistered areas than in normal or perilesional tissues, further implicating oxidative stress as a catalyst for mitochondrial-dependent apoptosis in PV. Interestingly, while oxidative stress in PV can lead to cell damage and apoptosis, it can also activate the STAT3 pathway, fostering cell proliferation and survival. This apparent paradox can be resolved by considering the varied cellular responses to increased reactive ROS levels. Low to moderate ROS levels might activate STAT3, promoting cell survival and proliferation, whereas high ROS levels can induce cell damage and apoptosis. In PV, this delicate equilibrium might be disrupted, contributing to the onset of inflammation and disease progression.
Despite a general understanding of PV’s pathophysiology and the established role of the interaction between desmoglein core glycoproteins as autoantigens and autoantibodies in PV onset, the specific roles of oxidative damage and mitochondria-dependent apoptosis in disease progression remain elusive. Furthermore, emerging evidence suggests the significance of signal activation in the blister-inducing capability of pemphigus autoantibodies. A notable previous study indicated that copper sulfate (CUSO4)-induced oxidative stress plays a role in activating p38 MAPK, leading to Dsg3 internalization, thereby triggering acantholysis in epidermal cells21.
To summarize, our investigation shed light on the complex interaction among lipid abnormalities, oxidative stress, and apoptotic pathways in PV. The present study demonstrates that lipid-induced oxidative stress plays a pivotal role in PV’s pathogenesis and can pave the way for novel therapeutic interventions. Future research is essential to fully understand the exact mechanisms through which oxidative stress influences PV and to explore targeted treatments that can alleviate its impact on disease progression.
Data availability
The data analyzed in this study are available from the corresponding author upon reasonable request.
References
Schmidt, E., Kasperkiewicz, M., Joly, P. & Pemphigus Lancet (London England) 394, 882–894 (2019).
Altman, E. M. Novel therapies for pemphigus vulgaris. Am. J. Clin. Dermatol. 21, 765–782 (2020).
Lee, J. et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Investig. 130, 6317–6324 (2020).
Huang, Y. et al. Oxidative Stress-Mediated YAP dysregulation contributes to the pathogenesis of pemphigus vulgaris. Front. Immunol. 12, 649502 (2021).
Liang, J. et al. Naringenin protects keratinocytes from oxidative stress injury via Inhibition of the NOD2-mediated NF-κB pathway in pemphigus vulgaris. Biomed. Pharmacotherapy = Biomedecine Pharmacotherapie. 92, 796–801 (2017).
Abida, O. et al. Catalase and lipid peroxidation values in serum of Tunisian patients with pemphigus vulgaris and foliaceus. Biol. Trace Elem. Res. 150, 74–80 (2012).
Liang, J. P. et al. Proteasomal inhibitors induce myeloma cell pyroptosis via the BAX/GSDME pathway. Acta Pharmacol. Sin. 44, 1464–1474 (2023).
Bock, F. J. & Tait, S. W. G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21, 85–100 (2020).
Czabotar, P. E. & Garcia-Saez, A. J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 24, 732–748 (2023).
Schmitt, T. et al. Ca(2+) signalling is critical for autoantibody-induced blistering of human epidermis in pemphigus. Br. J. Dermatol. 185, 595–604 (2021).
Nakai, K. & Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 22, 10799 (2021).
Xu, L., Liu, Y., Chen, X., Zhong, H. & Wang, Y. Ferroptosis in life: to be or not to be. Biomed. pharmacotherapy = Biomedecine Pharmacotherapie. 159, 114241 (2023).
Bourgonje, A. R. et al. Systemic oxidative stress associates with new-onset hypertension in the general population. Free Radic. Biol. Med. 187, 123–131 (2022).
Abida, O. et al. Biomarkers of oxidative stress in epidermis of Tunisian pemphigus foliaceus patients. J. Eur. Acad. Dermatol. Venereol.:JEADV. 27, e271–e275 (2013).
Javanbakht, M. H. et al. Evaluation of antioxidant enzyme activity and antioxidant capacity in patients with newly diagnosed pemphigus vulgaris. Clin. Exp. Dermatol. 40, 313–317 (2015).
Egu, D. T., Schmitt, T. & Waschke, J. Mechanisms causing acantholysis in Pemphigus-Lessons from human skin. Front. Immunol. 13, 884067 (2022).
Wójcik, P., Gęgotek, A., Žarković, N. & Skrzydlewska, E. Oxidative stress and lipid mediators modulate immune cell functions in autoimmune diseases. Int. J. Mol. Sci. 22, 723 (2021).
Agip, A. A., Blaza, J. N., Fedor, J. G. & Hirst, J. Mammalian respiratory complex I through the lens of Cryo-EM. Annual Rev. Biophys. 48, 165–184 (2019).
Charras, A. et al. JAK inhibitors and oxidative stress control. Front. Immunol. 10, 2814 (2019).
Xu, F., Xu, J., Xiong, X. & Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Report: Commun. Free Radical Res. 24, 70–74 (2019).
Boilan, E. et al. Role of p38MAPK and oxidative stress in copper-induced senescence. Age (Dordrecht Netherlands). 35, 2255–2271 (2013).
Acknowledgements
The authors extend their sincere appreciation to all individuals who contributed to the preparation of this manuscript. Special thanks are due to Professor Xinliang Mao from the Guangdong Provincial Key Laboratory of Protein Modification and Degradation, School of Basic Medical Sciences, Guangzhou Medical University, for his critical reading and editing of the manuscript. We would also like to express our gratitude to the Department of Pathology at Sun Yat-sen Memorial Hospital for their invaluable pathological technical assistance, which was instrumental to our research.
Author information
Authors and Affiliations
Contributions
JPL designed the study; JPL, JMX, JBC, YJX and SMW conducted experiments; JPL, JMX, YJO analyzed data; JMX provided key materials. JPL and JMX wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, Jp., Xu, Jm., Ou, Yj. et al. Mitochondrial-dependent apoptosis and STAT3 activation induced by oxidative stress in pemphigus vulgaris. Sci Rep 15, 29900 (2025). https://doi.org/10.1038/s41598-025-15534-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15534-3