Abstract
Pimenta racemosa var. recemosa (Mill.) J. W. Moore is an aromatic plant belonging to the family Myrtaceae, native to Venezuela, Puerto Rico, and Jamaica and well-known for its traditional and medicinal uses. Our study was designated to explore the chemical composition of essential oil isolated from P. racemosa leaves growing in Egypt via Gas Chromatography/Mass Spectrometry (GC-MS) analysis, alongside investigation of its antioxidant properties and enzyme inhibitory activities against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, α-glucosidase, and α-amylase. The GC-MS analysis of the leaf oil revealed the presence of fourteen compounds (99.76%), predominated by eugenol (70.87%), β-myrcene (12.88%) and D-limonene (8.35%). The oil demonstrated the highest antioxidant capability in ferric ion-reducing antioxidant power (FRAP; 1506.62 mg TE/g), followed by 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS; 1346.85 mg TE/g), 1,1-diphenyl-2-picrylhydrazyl (DPPH; 1032.83 mg TE/g) and cupric-reducing antioxidant capacity (CUPRAC; 1001.03 mg TE/g). Further, it showed a metal chelating ability (MCA) of 25.63 mg EDTAE/, and phosphomolybdenum (PBD) activity of 209.59 mmol TE/g. The oil displayed significant AChE and BChE inhibitory activities, with values of 1.96 mg GALAE/g and 1.42 mg GALAE/g, respectively. Additionally, it exhibited a moderate level of tyrosinase inhibitory activity (38.83 mg KAE/g) and a significantly higher α-glucosidase inhibitory activity (2.38 mmol ACAE/g) than α-amylase (0.08 mmol ACAE/g). Consequently, the leaf oil of Pimenta racemosa could be used as adjuvant therapy for management of oxidative stress-related conditions and chronic diseases such as Alzheimer’s, diabetes mellitus, and skin pigmentation. However, further toxicological, in vivo and clinical studies are recommended.
Similar content being viewed by others
Introduction
In contemporary times, medicinal plants represent an essential therapy for some people especially in Western countries due to the undesirable effects of synthetic chemical drugs and the substantial financial benefits1,2. However, despite the difficulty of estimating the exact commercial value of plants and plant extracts, it is clear that folk medicine-related industries grow by more than 4% annually3,4. The standing of medicinal plants and non-conventional plants is attributed to the high multiplicity of bioactive components, like flavonoids, alkaloids, lignans, triterpenoids, essential oils, carotenoids, tocopherols, and vitamins5,6. Some of these bioactive molecules demonstrate remarkable antioxidant properties which are essentially searched for their valuable impact on various chronic diseases, like Alzheimer’s disease (AD), skin wrinkles, pigmentation, obesity, diabetes mellitus, and cancer7,8,9.
Natural products including essential oils have been recognized via scientific reported literature for their activities against AD. Tumeric (Curcuma longa)10, guava (Psidium guajava)11, and bitter orange (Citrus aurantium)12 showed potential against acetylcholinesterase and butyrylcholinesterase. Tyrosinase is a key enzyme in melanogenesis. The antityrosinase activity of many plants has also been studied for the development of functional cosmetics because the inhibition of this enzyme can attenuate melanin synthesis to induce a whitening effect. Several medicinal plants have been reported for tyrosinase inhibitory effects such as Milk thistle (Silybum marianum), lemongrass (Cymbopogon citrates), and licorice (Glycyrrhiza glabra)13.
Recently, there has been renewed interest in functional food products and plant-based therapies that modify the physiological properties for the management of diabetes mellitus and aging symptoms14,15. One of the targeted antidiabetic approaches is to inhibit carbohydrate-metabolizing enzymes like α-glucosidase and α-amylase3. Thus, hyperglycemia can be managed in the type-II diabetic patients and borderline patients3,16. Arrays of previous in vitro studies have shown the potential role of several plants as α-amylase and α-glucosidase inhibitors such as cranberry (Vaccinium macrocarpon)17, black plum (Syzygium cumini)18, turmeric (Curcuma longa)19, Black pepper (Piper nigrum)20, soybean (Glycine max)21 Fenugreek (Trigonella foenum-graecum ) and turmeric (Curcuma longa)22. Consequently, the plant-derived α-glucosidase and α-amylase inhibitors provide an attractive strategy to restrain postprandial hyperglycemia.
The Pimenta genus is one of the evergreen flowering trees that incorporates about fifteen species and belongs to the family Myrtaceae23,24. Pimenta plants are distributed in West Indies, Central America and Jamaica25. In traditional medicine, different Pimenta species were employed for treatment of common cold, viral infections, bronchitis, dental pain, muscle aches, rheumatic pains, and arthritis26. Moreover, Pimenta essential oils exert antimicrobial, antiseptic, anesthetic, and analgesic effects25. From the phytochemical aspect, the isolated essential oils of Pimenta plants are characterized by an abundance of eugenol, methyl eugenol, β-caryophyllene, and myrcene27. Whereas limonene, 1,8-cineole, terpinolene, β-selinene, and methyl eugenol were reported to be the predominant components in some studies28. P. racemosa (Mill.) J. W. Moore is one of the most characteristic aromatic plants of this genus, due to its high essential oil content and diverse medicinal targets29.P. racemosa is native to Venezuela, Puerto Rico, and Jamaica in the Caribbean30. It is characterized by the richness of essential oil which is commonly named “bay oil” or “Myrcia oil”30. Traditionally, P. racemosa was utilized for different diseases in the Dominican Republic of the Caribbean basin; the leaf oil is used for rheumatism and toothache due to its anti-inflammatory and analgesic effects31. The infusion of the bark is used against hypertension30. The leaf essential oil was reported to possess antimicrobial effects against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Aspergillus niger, Penicillium verrucosum and Cladosporium cladosporioides32. Several biological studies have demonstrated its anti-cancer33, anti-inflammatory34, nematicidal activity35, and insecticidal activities36.
In vitro antidiabetic assays are important to determine the activity of plant extract or compounds hence saving time, cost, and effort37. Interestingly, no previous data have been reported on the antioxidant or enzyme inhibition data of P. racemosa leaf oil. Thus, the current study was designated to explore the chemical composition of essential oil isolated from P. racemosa leaves growing in Egypt via GC-MS chemical analysis, alongside investigation of its antioxidant property and enzyme inhibitory activity against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, α-glucosidase, and α-amylase.
Materials and methods
Plant material and oil isolation
P. racemosa (Myrtaceae) were collected as fresh leaves in March 2023 from El-Zohrya Garden (30°02′45″N 31°13′28″E), Zamalek, Cairo Governorate, Egypt. The collection of plant material was permitted and established in compliance with the national guidelines. A voucher specimen under code PHG-P-PR-473 was put at the Herbarium of the Department of Pharmacognosy, at the Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. The leaves were accurately authenticated by Mrs. Treize Labib, a taxonomy expert at El-Orman Botanical Garden, Giza-Egypt. About 1 kg of the fresh leaves were finely cut, and hydrodistilled using a Clevenger apparatus for a period of 4 h. Afterwards, the essential oil isolation was accomplished and stored in a sealed glass tube at -5 °C waiting for GC-MS analysis and further biological exploration.
Chemicals
The chemicals were purchased from Sigma-Aldrich (Darmstadt, Germany). They were: 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), electric eel acetylcholinesterase (AChE) (type-VI-S, EC 3.1.1.7), horse serum butyrylcholinesterase (BChE) (EC 3.1.1.8), galantamine, acetylthiocholine iodide (ATChI), butyrylthiocholine chloride (BTChI) 5,5-dithio-bis(2-nitrobenzoic) acid (DTNB), tyrosinase (EC1.14.18.1, mushroom), glucosidase (EC. 3.2.1.20, from Saccharomyces cerevisiae), amylase (EC. 3.2.1.1, from the porcine pancreas), sodium molybdate, hydrochloric acid, sodium hydroxide, trolox, ethylenediaminetetraacetate (EDTA), neocuproine, cupric chloride, ammonium acetate, ferric chloride, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), ammonium molybdate, ferrozine, ferrous sulphate hexahydrate, kojic acid, and acarbose. All chemicals were of analytical grade.
Gas chromatography/mass spectrometry (GC-MS) analysis
At the pharmacognosy department of Ain Shams University, Cairo, Egypt, the isolated oil was analyzed with a Shimadzu GCMS-QP 2010 (Kyoto, Japan) and a TRACE GC Ultra Gas Chromatograph (THERMO Scientific Corp., USA), coupled to a thermo-mass detector as previously reported38,39,40. The column specifications (Restek, USA) include a TG-5MS capillary with an internal diameter of 30 m x 0.25 mm i.d. and film thickness of 0.25 μm, directly attached to a quadrupole mass spectrometer (SSQ7000; Thermo-Finnigan). The carrier gas is helium with a constant flow rate of 1.0 mL/min. The sample is diluted with a ratio 1% v/v, the injected volume = 1 µL, and a split ratio of 1:15. Initially, the oven temperature of 80 °C was maintained for 2 min (isothermal), and then 300 °C was reached by raising 5.0 °C/min and retaining it for 5 min (programmed). The temperatures of the injector and detector were set at 280 °C. Using an electron ionization (EI) mode of 70 eV, a scanned spectrum at m/z 35–500 was used to obtain mass spectra of the ions: interface temperature = 280 °C, ion source temperature = 200 °C. A peak area normalization technique was used to determine the relative proportions of the hexane extract constituents.
Percentage peak area method
The percentage peak area method uses the area of the target component peak as a proportion of the total area of all detected peaks to analyze quantity. This method is used to determine an approximate concentration of a sample mixture or changes in the concentration of a known sample mixture.
GC-MS identification of chemical components of the essential oil
To tentatively identify oil components, the GC-FID and GC-MS spectra were used, and fragmentation patterns, mass numbers, and Kovats retention indices were compared with those published by Wily, NIST, and literature sources18,41,42,43,44,45,46. Under the same conditions, homologous series of n-alkanes (C8-C28) were injected, and retention indices were calculated. In this method, peak areas were calculated as a ratio of the peak areas of each compound to the area of the entire FID chromatogram (100%).
Antioxidant and enzyme inhibitory assays
The essential oil was subjected to six spectrophotometric tests to determine its antioxidant potential. The essential oil solution was carefully formulated in ethanol at a concentration of 2 mg/mL. All chemicals were freshly prepared, with special attention to keeping the enzymes and their corresponding substrates in an ice bath for upcoming. We prepared an ethanolic solution of the essential oil, and then transferred it to the reaction medium. To assess the antioxidants’ aptitude to neutralize free radicals, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assays were employed47. Also, the reduction capabilities of isolated essential oil were evaluated through ferric ion-reducing antioxidant power (FRAP) and cupric-reducing antioxidant capacity (CUPRAC) tests47. Furthermore, phosphomolybdenum (PBD) and ferrozine assays were used to measure the antioxidant activity and metal-chelating capability of the essential oil, respectively47,48. Apart from metal chelating ability (MCA), each of these assays was valued using a Trolox standard (mg TE/g). As for MCA, its comparison was estimated according to the EDTA equivalent per gram of essential oil (mg EDTAE/g). All procedures are detailed in our previous works47,48. To explore the inhibitory effects of the tested oil on various enzymes, we applied acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, and amylase, and glucosidase and the experimental procedures were described previously in our earlier work48,49. As a measure of AChE and BChE inhibition, we measured the inhibition using milligram galantamine equivalents per gm of essential oil (mg GALAE/g), the inhibition of tyrosinase expressed as mg kojic acid equivalents per gm essential oil (mg KAE/g), and the inhibition of amylase expressed in milligrams of acarbose equivalents per gm essential oil (mg ACAE/g). The measurements of these antioxidant and enzyme assays were carried out using a microplate reader ((Thermo Multiskan GO, Thermo, United States). Each assay was carried out with three technical replicates. The details of each essay were shown in the supplementary file. Further, the calibration curves of all used standards are illustrated in Table S1.
Results and discussion
GC-MS analysis of leaf essential oil from P. racemosa
The GC-MS analysis was employed to characterize the volatile components of essential oil isolated from P. racemosa leaves. It revealed a depiction of seventeen compounds constituting about 99.76% of the total oil components as illustrated in Table 1 and Fig 1. The Phenylpropanoids are the chief class of oil components representing 72.35%, among them eugenol (70.87%) and chavicol (1.48%). Hydrocarbon monoterpenes represent the second major class of oil components with a total percentage of 23.41% predominated by β-myrcene (12.88%), D-limonene (8.35%), and p-cymene (1.12%). The oxygenated monoterpenes accounted for about 2.33% of total oil including β-linalool (2.14%) and terpinene-4-ol (0.19%). Hydrocarbon sesquiterpene showed a minor composition percentage of oil (0.32%) represented by (E)-β-caryophyllene oxide (0.16%) and τ-cadinol (0.16%). The chemical structures of the identified major oil components are illustrated in Fig. 2. Certain Egyptian studies reported a predominance of monoterpene hydrocarbons (64.40%) predominated by β-myrcene (39.60%) and eugenol (31.00%) in P. racemosa leaf oils25. While the Cuban type of P. racemosa leaf oil demonstrated a high content of 1,8-cineole (14.69%)50. In Benin, the leaf essential oil was quantified with eugenol (45.20%), myrcene (25.10%), chavicol (7.10%), and limonene (3.0%) as major compounds30. Unlike that, another study in Santo Domingo demonstrated the presence of α-terpineol acetate (27%), α-terpineol (20%), and methoxy eugenol (12.6%)31. It is assumed that these differences in the chemical composition of the above-mentioned essential oils can be ascribed to differences in the cultivation environment, geographical resources, climate, genotype, and collection time51. Few literatures about the chemical nature of the essential oil of P. racemosa var. racemose were reported which matched our result. Contreras-Moreno et al., and Tucker et al., revealed the presence of eugenol as the major compound in the same chemotype (60.4–82.9%; 44.41–68.93% respectively)52,53. The main oil components previously identified in P. racemosa, their percentage, and the location are illustrated in Table 2.
The structures of the volatile components detected in the essential oil of P. racemosa leaves grown in Egypt [1: α-Pinene, 2: β-Myrcene, 3: α-Phellandrene, 4: p-Cymene, 5: D-Limonene, 6: (E)-β-Ocimene, 7: β-Linalool, 8: Terpinen-4-ol, 9: Chavicol, 10: Eugenol, 11: α-Copaene, 12: (E)-β-Caryophyllene, 13: α-Murolene, 14: 3-Cyclohexene-1-carboxaldehyde, 15: 4-(4-methyl-3-pentenyl)-, 16: δ-Cadinene, (E)-β-Caryophyllene oxide, 17: τ-Cadinol].
Assessment of antioxidant activity of the essential oil isolated from P. racemosa
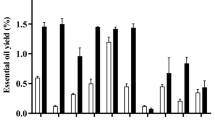
Reactive oxygen species are linked to various human diseases and aging. The elevated levels of free radicals, like reactive oxygen and nitrogen species, are central to oxidative damage in biomolecules (e.g., DNA, proteins, and lipids) and play a role in food and material degradation60,61,62. These factors are also associated with chronic diseases and aging63. Various assays are available to assess the antioxidant capacity of samples, and this study employs six different types including 2,2-diphenyl-1-picrylhydrazyl (DPPH), [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] (ABTS), cupric reducing antioxidant capacity (CUPRAC), ferric reducing ability of plasma (FRAP), and phosphomolybdenum (PBD) assay as illustrated in Table 3. The essential oil isolated from P. racemosa leaves demonstrated remarkable antioxidant capabilities in both anti-radical and radical scavenging assays. Specifically, it exhibited the highest FRAP activity with a reducing power of 1506.62 mg Trolox equivalent (TE)/g, followed by the ABTS scavenging test at 1346.85 mg TE/g. Additionally, the essential oil displayed 1032.83 mg TE/g DPPH scavenging activity and a reducing power of 1001.03 mg TE/g in the CUPRAC assay. These antioxidant properties can be attributed to its major phenolic component, eugenol, as well as other hydrocarbon monoterpenes constituents. Furthermore, the essential oil showed a metal chelation activity (MCA) of 25.63 mg EDTAE/, and (PBD) 209.59 mmol TE/g. These results support the use of P. racemosa leaf essential oil as a natural antioxidant, addressing concerns about the safety of synthetic antioxidants.
Our findings closely align with the results reported by Al-Gendy et al.58, indicating that the P. racemosa flower essential oil exhibits a remarkably high scavenging capacity of 85.96%. These findings align with prior research confirming the antioxidant properties of P. racemosa essential oils64. Phenolic compounds exert antioxidant activity via different mechanisms; transference of hydrogen atom or single electron, transition metal chelation, or sequential proton loss electron transfer65. The robust antioxidant activity observed in the essential oil of P. racemosa can be attributed to its significant eugenol content, a well-established phenolic compound renowned for its antioxidative properties, as substantiated by previous studies66,67,68. Eugenol’s effectiveness as an antioxidant is, in part, attributed to the presence of a methoxy group in the ortho position of the phenolic hydroxyl, facilitating the homolytic dissociation of the O–H bond69. Additionally, chavicol, another phenolic compound identified in P. racemosa essential oil in this study, is recognized for its antioxidant activity70. Notably, this essential oil also contains a substantial concentration of β-myrcene, which serves as a major oxidation product71. Moreover, it is important to mention that the synergy among various compounds, even at lower concentrations, may contribute to the overall antioxidant effect.
Assessment of enzyme inhibitory activities of the essential oil isolated from P. racemosa
The study of enzyme inhibition is extensively researched due to its pivotal role in comprehending enzyme mechanisms72 and its significance in both human and animal pharmaceuticals within the realm of pharmacological research73,74. For example, in the case of AD, which affects over 35 million individuals globally, inhibiting cholinesterase enzymes is a key therapeutic approach75. Enzyme’s acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) decrease acetylcholine levels, alleviating symptoms as the disease advances. Researchers are particularly interested in selective AChE and BChE inhibitors76. Plant bioactive compounds hold promise in effectively targeting acetylcholine-regulating enzymes for therapeutic purposes in diseases like AD77. Tyrosinase, another enzyme plays a central role in melanin synthesis and enzymatic browning in various contexts, including mammalian melanogenesis and fruit/fungi processes78. Inhibiting tyrosinase activity has broad applications in medicine and cosmetics, addressing hyperpigmentation and serving as a focal point in this study’s investigation79.
In the current study, in terms of enzyme inhibitory activity, the essential oil of P. racemosa displayed significant AChE and BChE inhibitory activities, with values of 1.96 mg GALAE/g and 1.42 mg GALAE/g, respectively (Table 4). Additionally, the essential oil exhibited a moderate level of tyrosinase inhibitory activity, measuring 38.83 mg KAE/g (Table 4). These findings underscore the potential of this essential oil in enzyme-related applications, with notable effects on acetylcholinesterase AChE and BChE inhibition, as well as a modest influence on tyrosinase inhibition.
Diabetes mellitus is a widespread epidemic, characterized by the inhibition of enzymes like α-amylase and α-glucosidase that convert dietary starch into glucose80. Plant extracts have shown promise in inhibiting these enzyme activities, offering potential health benefits, and serving as a focus in this study81. In the present study, for the 1st time, the essential oil extracted from the P. racemosa demonstrated a lower level of α-amylase inhibition activity, measuring at 0.08 mmol ACAE/g, while it exhibited a significantly higher α-glucosidase inhibitory activity, with a value of 2.38 mmol ACAE/g (Table 4).
In the current investigation, the plant under study exhibited a moderate inhibitory effect on the specified enzymes, as opposed to its antioxidant properties. No research has been conducted to date that demonstrates the inhibitory effects of P. racemosa extract or essential oil on AChE, BChE, and tyrosinase activities. Nevertheless, previous research by Adefegha et al., and Bilgicli et al., indicated that eugenol exhibited a more pronounced inhibitory effect on both AChE and BChE activities82]– [83. This compound possesses a diverse array of pharmacological properties, including neuromodulatory and antioxidant characteristics. Moreover, research conducted by Varela et al. and Marongiu et al. aligns with our findings, supporting the notion that eugenol exhibits a relatively less potent tyrosinase inhibition84]– [85. In our present study, we observed that P. racemosa essential oil displays a heightened level of α-glucosidase inhibitory activity when compared to its α-amylase inhibitory potential. In contrast to our current investigation, a previous study has validated that P. racemosa exhibits a greater inhibitory effect on α-glucosidase in comparison to α-amylase. To illustrate, the application of 2.00 mg of P. racemosa leaf essential oil led to a notable 63.90% increase in α-amylase inhibitory activity and a 53.00% increase in α-glucosidase inhibitory activity86. Similarly, several studies confirmed the pronounced α-glucosidase inhibitory properties of eugenol83,87]– [88. β-Myrcene is a monoterpene hydrocarbon that exerts antioxidant activity89 as well as neuroprotective activity where it could inhibit the acetylcholinesterase activity in different brain regions of AlCl3 and D - galactose-induced Alzheimer disease mice90. The essential oils of citrus have been reported by Matsuura et al. (2006) to possess strong antityrosinase activity due to the presence of β-myrcene91. Limonene, the 3rd predominant component of the Pimenta oil, is the most abundant compound in the peel oil of orange. The latter showed strong activity against α-amylase and α-glucosidase92, and anti-tyrosinase inhibitory activity. The presence of the major compounds as well as the synergistic effect with other minor compounds are responsible for the activity exhibited by the oil.
Conclusion
Our findings proved that the essential oil of P. racemosa possesses potent antioxidant properties as confirmed in six in vitro assays, suggesting that it represents a highly promising resource for the development of therapeutic molecules and health-promoting agents. Our study, for the first time, indicates that the essential oil derived from P. racemosa substantially showed inhibitory activities against acetylcholinesterase, butyrylcholinesterase, tyrosinase, α-glucosidase, and α-amylase enzymes. This emphasizes the potential for exploiting P. racemosa-based preparations or adjuvant therapies for the management of chronic diseases such as Alzheimer disease, diabetes mellitus, and skin-related applications. However, further in vivo studies are recommended as an important phase in screening the plant oil for physiological, pharmacological, and toxicological properties.
Data availability statement
All data are described in the article and/or supplementary file.
References
Abdelghffar, E. A., El-Nashar, H. A. S., Al-Mohammadi, A. G. A. & Eldahshan, O. A. Orange fruit (Citrus sinensis) Peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 12 (19), 9443–9455 (2021).
Al-Madhagy, S. A. et al. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 10 (10), 6267–6275 (2019).
Tlili, N. & Sarikurkcu, C. Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind. Crop Prod. 151, 112448 (2020).
El-Nashar, H. A., Adel, M., El‐Shazly, M., Yahia, I. S. & Sheshtawy, E. Chemical composition, antiaging activities and molecular Docking studies of essential oils from Acca Sellowiana (Feijoa). Chem. Biodivers. 19 (9), e202200272 (2022).
Younis, M. M. et al. Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase activities of a Stenocarpus sinuatus leaves extract. Plants 11 (7), (2022).
El-Nashar, H., Eldahshan, O. & Singab, A. The tribe Caesalpinieae (Fabaceae): An updated review on Pharmacological aspects. Med. Aromat. Plants. 4 (215), 2167–0412 (2015).
Sferrazza, G. et al. Nature-derived compounds modulating wnt/ β-catenin pathway: A preventive and therapeutic opportunity in neoplastic diseases. Acta Pharm. Sin. B. 10 (10), 1814–1834 (2020).
Younis, T. et al. Antioxidant and pulmonary protective potential of Fraxinus xanthoxyloides bark extract against CCl4-Induced toxicity in rats. Chem. Biodivers. 20 (3), e202200755 (2023).
Ishaq, A. R. et al. Genus Lupinus (Fabaceae): A review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol. 74 (12), 1700–1717 (2022).
Fahmy, N. M. et al. Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into their Antioxidant and Enzyme (Inhibitory Activities Plants, 2023).
Aly, S. H., Eldahshan, O. A., Al-Rashood, S. T., Binjubair, F. A. & Hassab, E. Chemical constituents, antioxidant, and enzyme inhibitory activities supported by In-Silico study of n-Hexane extract and essential oil of guava leaves. Mol. (Basel Switz.). 27 (24), 8979 (2022).
Elhawary, E. A., Nilofar, N., Zengin, G. & Eldahshan, O. A. Variation of the essential oil components of Citrus aurantium leaves upon using different distillation techniques and evaluation of their antioxidant, antidiabetic, and neuroprotective effect against alzheimer’s disease. BMC Complement. Med. Th. 24 (1), 73 (2024).
El-Nashar, H. A. S., El-Din, M. I. G., Hritcu, L. & Eldahshan, O. A. Insights on the [online]nhibitory power of flavonoids on tyrosinase activity: A survey from 2016 to 2021 Mol. (Basel, Switz.), (2021).
Azab, S. S., Abdel-Daim, M. & Eldahshan, O. A. Phytochemical, cytotoxic, hepatoprotective and antioxidant properties of Delonix regia leaves extract. Med. Chem. Res. 22 (9), 4269–4277 (2013).
Younis, I. Y., El-Hawary, S. S., Eldahshan, O. A., Abdel-Aziz, M. M. & Ali, Z. Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 11 (1), 16868 (2021).
Abd El-Ghffar, E. A., Al-Sayed, E., Shehata, S. M., Eldahshan, O. A. & Efferth, T. The protective role of Ocimum Basilicum L. (Basil) against aspirin-induced gastric ulcer in mice: Impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 9 (8), 4457–4468 (2018).
Apostolidis, E., Kwon, Y. I. & Shetty, K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac. J. Clin. Nutr. 15 (3), 433–441 (2006).
El-Nashar, H. A. S. et al. GC/MS analysis of essential oil and enzyme inhibitory activities of Syzygium cumini (Pamposia) grown in Egypt: Chemical characterization and molecular Docking studies. Mol. (Basel Switz.) 26 (22), (2021).
Anis, E. et al. Alpha-glucosidase inhibitory constituents from Cuscuta reflexa. Chem. Pharm. Bull. 50 (1), 112–114 (2002).
Pullela, S. V. et al. HPLC assisted Chemobiological standardization of alpha-glucosidase-I enzyme inhibitory constituents from Piper longum Linn-An Indian medicinal plant. J. Ethnopharmacol. 108 (3), 445–449 (2006).
Georgetti, S. R., Casagrande, R., Moura-de-Carvalho Vicentini, F. T., Verri, W. A. Jr. & Fonseca, M. J. Evaluation of the antioxidant activity of soybean extract by different in vitro methods and investigation of this activity after its incorporation in topical formulations. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik E V. 64 (1), 99–106 (2006).
Gad, M. Z., El-Sawalhi, M. M., Ismail, M. F. & El-Tanbouly, N. D. Biochemical study of the anti-diabetic action of the Egyptian plants Fenugreek and balanites. Mol. Cell. Biochem. 281 (1–2), (2006). 173 – 83.
Chaverri, C. & Cicció, J. F. Leaf and fruit essential oil compositions of Pimenta guatemalensis (Myrtaceae) from Costa Rica. Rev. Biol. Trop. 63 (1), 303–311 (2015).
Abd-elaty, D. M. et al. Development of a novel allspice essential oil-loaded cream against skin wrinkles: GC-MS metabolic profiling, characterization and enzyme Inhibition assays. J. Essent. Oil Bear. Plants 1–14 (2025).
Ismail, M. M., Samir, R., Saber, F. R., Ahmed, S. R. & Farag, M. A. Pimenta oil as A potential treatment for Acinetobacter baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics (Basel Switz.) 9 (10), (2020).
Padmakumari, K. P., Sasidharan, I. & Sreekumar, M. M. Composition and antioxidant activity of essential oil of Pimento (Pimenta dioica (L) Merr.) from Jamaica. Nat. Prod. Res. 25 (2), 152–160 (2011).
Prasad, M. A., Zolnik, C. P. & Molina, J. Leveraging phytochemicals: The plant phylogeny predicts sources of novel antibacterial compounds. Future Sci. OA. 5 (7), Fso407 (2019).
Tucker, A. O., Maciarello, M. J. & Landrum, L. R. Volatile leaf oils of Caribbean myrtaceae. II. Pimenta dioica (L.) merr. Of Jamaica. J. Essent. Oil Res. 3 (3), 195–196 (1991).
Zabka, M., Pavela, R. & Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 30 (2), 250–253 (2009).
Alitonou, G. A. et al. Chemical composition and biological activities of essential oils of Pimenta racemosa (Mill.) JW moore. From Benin. Int. J. Biosci. 2 (9), 1–12 (2012).
Saenz, M. T., Tornos, M. P., Alvarez, A., Fernandez, M. A. & García, M. D. Antibacterial activity of essential oils of Pimenta racemosa var. Terebinthina and Pimenta racemosa var. Grisea. Fitoterapia 75 (6), 599–602 (2004).
Aurore, G. S., Abaul, J., Bourgeois, P. & Luc, J. Antibacterial and antifungal activities of the essential oils of Pimenta Racemosa var. Racemosa P. Miller (J.W. Moore) (Myrtaceae). J. Essent. Oil Res. 10 (2), 161–164 (1998).
Hartwell, J. L. Plants used against cancer. A survey.[Continued]. Lloydia 33, 97–194 (1970).
Fernández, A., Alvarez, A., García, M. D. & Sáenz, M. T. Anti-inflammatory effect of Pimenta racemosa var. ozua and isolation of the triterpene lupeol. Farmaco (Societa chimica italiana 56(4), 335-8 (2001). (1989).
Il-K, P. & Ju-Y, P. Nematicidal activity of plant essential oils and components from Garlic (Allium sativum) and cinnamon (Cinnamomum verm) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 7 (5), 767–774 (2005).
Noudogbessi, J. P., Kossou, D. & Sohounhloué, D. C. Effet insecticide, ovicide et larvicide des huiles essentielles de Pimenta racemosa (Miller) et de Chromolaena odorata (L. Robinson) Sur Le grand Capucin (Prostephanus truncatus (Horn)) du Maïs. J. Soc. Ouest-Afr. Chim. 26, 41–51 (2008).
Subramanian, R., Asmawi, M. Z. & Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and Andrographolide. Acta Biochim. Pol. 55 (2), 391–398 (2008).
El-Nashar, H. A., Eldahshan, O. A., Fattah, N. F. A., Loutfy, S. A. & Abdel-Salam, I. M. HPLC-ESI/MS-MS characterization of compounds in Dolomiaea costus extract and evaluation of cytotoxic and antiviral properties: Molecular mechanisms underlying apoptosis-inducing effect on breast cancer. BMC Complement. Med. Th. 23 (1), 354 (2023).
Rabie, O., El-Nashar, H. A., Majrashi, T. A., Al-Warhi, T. & Hassab, E. Chemical composition, seasonal variation and antiaging activities of essential oil from Callistemon subulatus leaves growing in Egypt. J. Enzyme Inhib. Med. Chem. 38 (1), 2224944 (2023).
Ashmawy, N. S., Gad, H. A. & El-Nashar, H. A. Comparative study of essential oils from different organs of Syzygium cumini (Pamposia) based on GC/MS chemical profiling and in vitro antiaging activity. Mol. (Basel Switz.). 28 (23), 7861 (2023).
Adams, R. Identification of essential oil components by gas chromatography/mass spectrometry. Identification Essent. Oil Compon. Gas Chromatogr./Mass Spectrometry (Ed. 4), (2007).
El-Nashar, H. A., Mostafa, N. M., El-Badry, M. A., Eldahshan, O. A. & Singab, A. N. B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from schinus terebinthifolius Raddi growing in Egypt. Int. J. Pharmacognosy Phytochemical Res. 11 (3), 235–239 (2019).
El-Nashar, H. A., Eldahshan, O. A., Elshawi, O. E. & Singab, A. N. B. Phytochemical investigation, antitumor activity, and hepatoprotective effects of Acrocarpus Fraxinifolius leaf extract. Drug Dev. Res. 78 (5), 210–226 (2017).
El-Nashar, H. A. S., Mostafa, N. M., El-Badry, M. A., Eldahshan, O. A. & Singab, A. N. B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from schinus polygamus (Cav.) Cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 1–4 (2020).
Todirascu-Ciornea, E. et al. Schinus terebinthifolius essential oil attenuates scopolamine-induced memory deficits via cholinergic modulation and antioxidant properties in a Zebrafish model. Evid.-Based Complem. Altern. Med. eCAM., 5256781 (2019).
El-Nashar, H. A. S., Mostafa, N. M., El-Badry, M. A., Eldahshan, O. A. & Singab, A. N. B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) Cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 35 (23), 5369–5372 (2020).
Zengin, G. & Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline Anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. AJTCAM. 11 (2), 481–488 (2014).
Uysal, S. et al. Cytotoxic and enzyme inhibitory potential of two potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 8, 290 (2017).
Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: new sources of natural inhibitors for public health problems. Ind. Crops Prod. 83, 39–43 (2016).
Handschuh Briones, R. A., Silva Arcos, E. N., Urrutia, M. & Godoy-Martínez, P. Antifungal activity of mouthwashes against Candida albicans and Rhodotorula mucilaginosa: An in vitro study. Rev. Iberoam Micol. 37 (2), 47–52 (2020).
El-Nashar, H. A. S., Mostafa, N. M., El-Badry, M. A., Eldahshan, O. A. & Singab, A. N. B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) Cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 35 (23), 5369–5372 (2021).
Contreras-Moreno, B. Z., Velasco, J. J., Rojas, J. C., Méndez, L. C. & Celis, M. T. Antimicrobial activity of essential oil of Pimenta Racemosa var. Racemosa (Myrtaceae) leaves. J. Pharm. Pharmacogn Res. 4 (6), 224–230 (2016).
Tucker, A. O., Maciarello, M. J., Adams, R. P., Landrum, L. R. & Zanoni, T. A. Volatile leaf oils of Caribbean myrtaceae. I. Three varieties of Pimenta racemosa (Miller) J. Moore of the Dominican Republic and the commercial Bay oil. J. Essent. Oil Res. 3 (5), 323–329 (1991).
Gogoi, A. et al. Multifaceted in-vitro and in-silico evaluation of Pimenta racemosa (Mill.) essential oil: A potential alternative source of Eugenol. Ind. Crops Prod. 223, 120246 (2025).
Pragadheesh, V., Yadav, A., Singh, S., Gupta, N. & Chanotiya, C. Leaf essential oil of cultivated Pimenta racemosa (Mill.) JW Moore from North india: distribution of phenylpropanoids and chiral terpenoids. Med Aromat. Plants 2 (1), (2013).
Ayoub, I. M. et al. Valorization of Pimenta racemosa essential oils and extracts: GC-MS and LC-MS phytochemical profiling and evaluation of Helicobacter pylori inhibitory activity. Mol. (Basel Switz.). 27 (22), 7965 (2022).
Kim, J., Lee, Y. S., Lee, S. G., Shin, S. C. & Park, I. K. Fumigant antifungal activity of plant essential oils and components from West Indian Bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr. J. 23 (4), 272–277 (2008).
Al-Gendy, A. A., Moharram, F. A. & Zarka, M. A. Chemical composition, antioxidant, cytotoxic and antimicrobial activities of Pimenta racemosa (Mill.) JW Moore flower essential oil. J. Pharmacog Phytochem. 6 (2), 312–319 (2017).
Elshaarawy, F. S., Abdelhady, M. I., Hamdy, W. & Ibrahim, H. A. Investigation of the essential oil constituents of Pimenta racemosa aerial parts and evaluation of its antiviral activity against Hsv 1 and 2. Trends Adv. Sci. Technol. 1 (1), 2 (2024).
Wang, P., Gong, Q., Hu, J., Li, X. & Zhang, X. Reactive oxygen species (ROS)-Responsive prodrugs, probes, and theranostic prodrugs: Applications in the ROS-Related diseases. J. Med. Chem. 64 (1), 298–325 (2021).
Faure, A. M., Werder, J. & Nyström, L. Reactive oxygen species responsible for beta-glucan degradation. Food Chem. 141 (1), 589–596 (2013).
Raza, H. & John, A. Vitro protection of reactive oxygen species-induced degradation of lipids, proteins and 2-deoxyribose by tea catechins. Food Chem. Toxicol. Int. J. Published Br. Ind. Biol. Res. Assoc. 45 (10), 1814–1820 (2007).
Angelopoulou, R., Lavranos, G. & Manolakou, P. ROS in the aging male: model diseases with ROS-related pathophysiology. Reprod. Toxicol. (Elmsford N. Y.). 28 (2), 167–171 (2009).
Teissedre, P. L. & Waterhouse, A. L. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J. Agric. Food Chem. 48 (9), 3801–3805 (2000).
Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 44 (9), e13394 (2020).
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 46 (2), 446–475 (2008).
Pessoa, L. M., Morais, S. M., Bevilaqua, C. M. & Luciano, J. H. Anthelmintic activity of essential oil of Ocimum gratissimum linn. And Eugenol against Haemonchus contortus. Vet. Parasitol. 109 (1–2), 59–63 (2002).
Bortolomeazzi, R., Verardo, G., Liessi, A. & Callea, A. Formation of dehydrodiisoeugenol and dehydrodieugenol from the reaction of isoeugenol and Eugenol with DPPH radical and their role in the radical scavenging activity. Food Chem. 118 (2), 256–265 (2010).
Mastelić, J. et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and Eugenol derivatives. J. Agric. Food Chem. 56 (11), 3989–3996 (2008).
Oliveira, S. D. D. S. et al. Radical scavenging activity of the essential oils from Croton grewioides Baill accessions and the major compounds Eugenol, Methyl Eugenol and Methyl chavicol. J. Essent. Oil Res. 33 (1), 94–103 (2021).
Bonamin, F. et al. The effect of a minor constituent of essential oil from Citrus aurantium: The role of β-myrcene in preventing peptic ulcer disease. Chem.-Biol. Interact., 212, 11– 9 (2014).
Boyer, P. D. & Krebs, E. G. The enzymes (Academic Press, 1986).
Zengin, G. et al. A comparative study on UHPLC-HRMS profiles and biological activities of Inula Sarana different extracts and its Beta-Cyclodextrin complex: Effective insights for novel applications. Antioxidants 12 (10), (2023).
Yazbeck, D. R., Martinez, C. A., Hu, S. & Tao, J. Challenges in the development of an efficient enzymatic process in the pharmaceutical industry. Tetrahedron 15 (18), 2757–2763 (2004).
Yao, Z. et al. Artificial intelligence-based diagnosis of alzheimer’s disease with brain MRI images. Eur. J. Radiol. 165, 110934 (2023).
Geula, C. & Mesulam, M. M. Cholinesterases and the pathology of alzheimer disease. Alzheimer Dis. Assoc. Disord. 9 Suppl 2, 23–28 (1995).
Acquaviva, A. et al. Chemical characterization of different extracts from Artemisia annua and their antioxidant, enzyme inhibitory and Anti-Inflammatory properties. Chem. Biodivers. 20 (8), e202300547 (2023).
Li, J. et al. Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 224, 113744 (2021).
Acquaviva, A. et al. Screening for chemical characterization and Pharmacological properties of different extracts from Nepeta Italica. Plants 12 (15), (2023).
Papoutsis, K. et al. Fruit, vegetables, and mushrooms for the Preparation of extracts with α-amylase and α-glucosidase Inhibition properties: A review. Food Chem. 338, 128119 (2021).
Chiavaroli, A. et al. Adding new scientific evidences on the pharmaceutical properties of Pelargonium Quercetorum Agnew extracts by using in vitro and in Silico approaches. Plants 12 (5), (2023).
Adefegha, S. A., Okeke, B. M. & Oboh, G. Antioxidant properties of eugenol, butylated hydroxylanisole, and butylated hydroxyl toluene with key biomolecules relevant to alzheimer’s diseases-In vitro. J. Food Biochem. 45 (3), e13276 (2021).
Genç Bilgiçli, H. et al. Novel Eugenol bearing oxypropanolamines: Synthesis, characterization, antibacterial, antidiabetic, and anticholinergic potentials. Bioorg. Chem. 88, 102931 (2019).
Varela, M. T. et al. Coumaric acid derivatives as tyrosinase inhibitors: Efficacy studies through in silico, in vitro and ex vivo approaches. Bioorgan. Chem. 103, 104108 (2020).
Marongiu, B. et al. Supercritical CO2 extract of Cinnamomum zeylanicum: Chemical characterization and antityrosinase activity. J. Agric. Food Chem. 55 (24), 10022–10027 (2007).
El-gizawy, H., Mosaad, Y. O., Gobba, N. & Hussein, M. A. Chemical composition of the essential oil of the leaves of Pimenta diocia (L.) merr. & Pimenta racemosa (Mill.) cultivated in Egypt and evaluation of their in-vitro antioxidant and antidiabetic activities. Int. J. Phytomed. 10, 226–234 (2018).
Salehi, P., Asghari, B., Esmaeili, M. A., Dehghan, H. & Ghazi, I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plants Res. 7 (6), 257–266 (2013).
Singh, P. et al. Potential dual role of Eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci. Rep. 6, 18798 (2016).
Ciftci, O., Ozdemir, I., Tanyildizi, S., Yildiz, S. & Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health. 27 (5), 447–453 (2011).
Kumar, R., Kumar, R., Sharma, N. & Khurana, N. Ameliorative effect of myrcene in mouse model of alzheimer’s disease. Eur. J. Pharmacol. 911, 174529 (2021).
Matsuura, R., Ukeda, H. & Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 54 (6), 2309–2313 (2006).
Oboh, G., Olasehinde, T. A. & Ademosun, A. O. Inhibition of enzymes linked to type-2 diabetes and hypertension by essential oils from peels of orange and lemon. Int. J. Food Prop. 20 (sup1), 586–594 (2017).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Omayma A. Eldahshan: Conceptualization, Validation, Visualization, and revising the whole manuscript. Nilofar Nilofar: Resources, Data analysis, writing–original draft.Gokhan Zengin: Conceptualization, resources, Data analysis, Validation, Visualization, and revising the whole manuscript. Heba A. S. El-Nashar: Conceptualization, methodology, resources, data analysis, investigation, software, writing–original draft, reviewing and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were performed following the relevant guidelines and regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldahshan, O.A., Nilofar, N., Zengin, G. et al. Chemical profile of essential oil from Pimenta racemosa leaves, antioxidant potential, and its enzyme inhibitory properties. Sci Rep 15, 30705 (2025). https://doi.org/10.1038/s41598-025-15542-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15542-3