Abstract
Cardiac arrest (CA)-induced post-cardiac arrest brain injury (PCABI) represents a critical contributor to global mortality and neurological disability. While sleep deprivation (SD) is recognized to aggravate neurological outcomes, its role in PCABI pathogenesis remains underexplored. This study investigated the mechanisms by which SD exacerbates PCABI and evaluated the neuroprotective efficacy of electroacupuncture (EA). A CA model was established in SD rats, followed by RNA sequencing and molecular analyses to assess brain injury biomarkers, synaptic plasticity, and calcium signaling pathways. SD disrupted circadian rhythms, amplified neuronal apoptosis, and suppressed glutamate transporter Excitatory Amino Acid Transporter 2 (EAAT2) expression post-CA, correlating with worsened cognitive deficits. EA treatment significantly attenuated these effects, restoring EAAT2 levels, mitigating calcium overload, and enhancing synaptic integrity. Mechanistically, EA modulated the EAAT2/calcium signaling axis and rebalanced autonomic nervous activity, thereby reducing oxidative stress and neuronal excitotoxicity. These findings identify EAAT2 downregulation as a key mediator of SD-aggravated PCABI and establish EA as a dual-target intervention that rectifies glutamatergic dysregulation and autonomic dysfunction. The study provides translational insights into EA’s therapeutic potential for PCABI, particularly in populations with comorbid sleep disturbances.
Similar content being viewed by others
Introduction
Cardiac arrest (CA) and post-cardiac arrest brain injury (PCABI) are significant global health concerns, with a profound impact on morbidity and mortality. Global epidemiological studies reveal that out-of-hospital CA accounts for nearly half of all cardiovascular deaths, with an incidence rate ranging from 30 to 97 cases per 100,000 individuals1,2. A staggering 90% of patients experiencing out-of-hospital CA arrive at hospitals unconscious2, and approximately 80% of those who receive Cardiopulmonary resuscitation (CPR) after CA fall into a coma due to PCABI3. In the critical period of 48 to 72 h following the restoration of spontaneous circulation (ROSC), cardiovascular instability and multi-organ failure are the leading causes of death, with PCABI responsible for over two-thirds of these fatalities3. PCABI is also a primary contributor to acute phase mortality and post-resuscitation disability4. The long-term neurological consequences are equally concerning, with 60% of CA survivors exhibiting moderate to severe cognitive deficits three months post-hospital admission, and 40–50% suffering irreversible cognitive damage5. The urgency to address PCABI is thus paramount.
Among the multitude of factors precipitating CA and influencing CPR outcomes, sleep disorders stand out due to their high prevalence and underinvestigated status. Globally, approximately 1.3 billion individuals suffer from obstructive sleep apnea, a condition that disrupts normal sleep architecture6 and significantly elevates the incidence of CA, cardiovascular events, and all-cause mortality7,8. Furthermore, 26% of COVID-19 survivors report persistent sleep disturbances9. Accumulating evidence implicates sleep disorders—including sleep apnea, insomnia, and abnormal sleep duration—in increased risks of cardiovascular diseases and stroke10,11,12. Sleep deprivation (SD) exacerbates energy metabolism dysfunction and neuroinflammation13,14,15, both of which are established contributors to aggravated brain injury. Notably, experimental studies demonstrate that SD worsens traumatic brain injury16, underscoring the imperative to mitigate PCABI in populations with sleep disturbances. Our preliminary experiments corroborate this association, showing that SD-exposed rats exhibit significantly reduced probability of achieving ROSC following CA.
Despite the pressing need, there is a dearth of targeted clinical interventions to alleviate systemic organ dysfunction following CA, particularly in the context of PCABI. The modulation of autonomic imbalance represents a promising adjunctive therapy for various conditions, including tinnitus, epilepsy, arrhythmias, and neurological injuries. Electroacupuncture (EA) has demonstrated significant efficacy in regulating autonomic balance, offering the advantages of minimal invasiveness, reproducibility, and clinical applicability. Studies have shown that electrical stimulation of the ST36 acupoints in the hindlimb activates the vagal-adrenal anti-inflammatory axis17, and neurons labeled with PROKR2Cre play a pivotal role in mediating vagal efferent activity and catecholamine release17. These findings lay the anatomical groundwork for the use of EA in modulating autonomic homeostasis. Our previous research indicates that EA may reduce cerebral ischemia/reperfusion (I/R) injury by activating the vagus nerve18, and EA has been shown to normalize hemodynamics and reduce neurological impairments in a rat model of CA19. Collectively, these results suggest that EA could significantly improve PCABI by modulating autonomic nerves.
While previous studies have established EA’s neuroprotective effects in cerebral I/R injury18 and CA19, our study significantly advances this field by employing unbiased hippocampal transcriptome profiling to identify excitatory amino acid transporter 2 (EAAT2) as the pivotal target in SD-aggravated PCABI—a mechanism distinct from standard I/R injury. Through comprehensive pharmacological validation using both the EAAT2 activator LDN_212320 and inhibitor dihydrokainic acid, we establish causal relationships between EAAT2 modulation and neuroprotection. Importantly, we demonstrate for the first time that SD creates a unique pathological microenvironment in which EAAT2 dysfunction exacerbates calcium signaling collapse, specifically amplifying PCABI severity.
Materials and methods
The experimental procedures were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval No. SYDW2023-011), and the manuscript adheres to ARRIVE reporting guidelines. Adult male Sprague-Dawley rats (weight: 275 ± 25 g) were obtained from the same institution and maintained under standardized conditions with ad libitum access to food and water. To ensure adequate statistical power, we included n = 8 surviving animals per group for all endpoint analyses (CA, CA + EA, SD + CA, SD + CA + EA, and respective control groups), with mortality rates detailed in Supplementary Fig. 1.
Sleep deprivation protocol
We employed a novel cylindrical platform method for inducing SD20. The platform, with a diameter of 65 mm, was placed in a circular water reservoir with a diameter of 400 mm. Water was gradually added to submerge the platform to a height of 10 mm above the waterline. Rats were then placed on the platform, and a wire mesh enclosure was suspended 16 cm above, with food and water placed on top. The water in the reservoir was changed daily, and the SD period was maintained for 72 h prior to inducing CA. This modified platform method is a well-established SD paradigm, where the small platform size (65 mm diameter) prevents rats from adopting a stable sleeping posture, effectively suppressing rapid eye movement and non- rapid eye movement sleep. Successful SD was operationally defined by the completion of the 72-hour protocol, as this method reliably induces sleep deprivation by physical limitation.
Cardiac arrest induction
The CA model was established according to previously described methods21. Specifically, Rats were sedated using 3% sevoflurane in pure oxygen, which facilitated spontaneous respiration. Orotracheal intubation was performed with a 14-gauge cannula for mechanical ventilation. Arterial blood pressure was monitored continuously via a polyethylene PE-50 catheter inserted into the right femoral artery. Another PE-50 catheter was placed in the right femoral vein for the administration of sodium bicarbonate and adrenaline if necessary. CA was induced by clamping the tracheal intubation under general anesthesia, resulting in a mean arterial pressure (MAP) of less than 25 mmHg for 8.5 min. Post-CA, epinephrine (0.02 mg/kg) was administered via the femoral vein, and chest compressions were initiated at a rate of 200 compressions per minute using a mechanical chest compressor. Mechanical ventilation was then initiated at a rate of 70 breaths per minute with a tidal volume of 10 ml/kg.
The criteria for ROSC were sinus rhythm with a MAP of 60 mmHg or higher sustained for at least 10 min. If an independent cardiac rhythm was not resumed within 10 min, this was considered CPR failure. Following surgical procedures, local analgesia was administered via 2% lidocaine infiltration (0.1 ml/kg) at incision sites. Resuscitated rats were immediately placed on thermostatically controlled heating pads (37.0 ± 0.5 °C) for 6-hour thermal support. Postoperatively, all animals received ad libitum access to standardized rodent diet and hydration gel. At the conclusion of the experimental protocol, all animals were humanely euthanized to ensure minimal suffering and in compliance with ethical guidelines for animal research.
Electroacupuncture treatment
EA was initiated within 30 min following ROSC and was administered daily for 30 min over four consecutive days. The acupoints ST36 (Zusanli) and PC6 (Neiguan) were selected based on their established neuroprotective effects in previous studies and clinical relevance22,23. Four fine needles (0.16 mm × 7 mm) were inserted to a depth of 7 mm at ST36 and 1 mm at PC6, corresponding to their anatomical depths in rats while maintaining clinical translatability. The needles were connected to an acupoint stimulator (HANS-200E, Nanjing, China), with stimulation parameters set at 1.5 mA and a frequency of 2/15 Hz, which have been shown to be effective and safe in both experimental and clinical settings.
Cervical vagotomy procedure
Cervical vagotomy was performed 20 min following ROSC. Under aseptic conditions, a midline cervical incision was made to expose the sternocleidomastoid muscle, carotid artery, and vagal nerve. After isolating the left vagal nerve using microforceps, complete transection was achieved with ophthalmic scissors. To ensure postoperative analgesia, the surgical site received local infiltration of 2% lidocaine (0.1 ml/kg) prior to layered closure of muscular and cutaneous tissues.
RNA-seq
Hippocampal RNA-seq was performed by OE Biotech Co., Ltd (Shanghai, China) at 96 h post-CA. For comprehensive transcriptomic analysis, eight rats were randomly selected from each experimental group: CA group, CA + EA group, SD + CA group, and SD + CA + EA group. Total RNA was extracted with TRIzol (Invitrogen, CA, USA), quantified and purity-checked via NanoDrop 2000 (Thermo Scientific, USA), and integrity-assessed with Agilent 2100 Bioanalyzer (Agilent Tech, CA, USA). Libraries were prepared with VAHTS Universal V6 RNA-seq Kit and sequenced on Illumina Novaseq 6000. Raw fastq files were processed with fastq software, and alignment and DEG analysis were done using HISAT2 and DEGseq2. Differentially expressed genes (DEGs) were subjected to KEGG pathway enrichment analysis24,25,26 via hypergeometric distribution in R (v 3.2.0).
Nissl staining
Nissl staining was performed after 96 h post-CA. Rats were perfused intracardially with 4% cold paraformaldehyde, and brain samples were embedded in paraffin and sectioned into 20 μm coronal slices. For quantitative analysis, Nissl-stained sections were examined under a light microscope (×400 magnification) in three non-overlapping fields within the CA1 hippocampus (HP) and prefrontal cortex (PFC). Only neurons with intact morphology (clear nuclei/nucleoli, normal pyramidal/regional shape, and typical staining intensity) were counted, excluding hyperchromatic or shrunken cells. Data from bilateral measurements were analyzed blindly by two investigators.
Transmission electron microscopy (TEM)
Hippocampal CA1 region were pre-fixed in 2.5% glutaraldehyde and post-fixed in 0.1 M sodium cacodylate-buffered 1% OsO4 solution after 96 h post-CA. After dehydrated with a step-wise ethanol gradient solution, samples were incubated with propylene oxide, then impregnated with a mixture of propylene oxide/Spurr (1:1) and embedded in Spurr resin. Ultrathin sections were examined via TEM.
Immunofluorescence
Rats were perfused, and brain tissues were fixed in 4% paraformaldehyde after 96 h post-CA, followed by dehydration. The samples were sectioned into 30-mm-thick coronal slices. For immunostaining, free-floating sections were permeabilized with 1% Triton X-100 in PBS for 1 h and incubated in blocking buffer for 1 h. The sections were then incubated with the EAAT2 (ab205248, 1:200) on a shaking table overnight and followed by incubation with fluorescein-conjugated secondary antibodies (Alexa Fluor 488 Labeled Goat Anti-Rabbit IgG, 1:400); subsequent incubation of GFAP (ab7260, 1:5000), another secondary antibody (Alexa Fluor CY3 Labeled Goat Anti-Rabbit IgG, 1:300), and DAPI. Images were acquired and quantified by ImageJ.
Western blotting (WB)
The HP and PFC from rats were collected and extracted with RIPA lysis buffer after 96 h post-CA. Portions containing 30 µg protein were loaded on the gel, processed for electrophoresis, and transferred to PVDF membrane. The membranes were blocked with nonfat milk and incubated overnight at 4 °C with the following primary antibodies, ACTIN (ab179467, 1:2000), GAPDH (ab181602, 1:10000), CLOCK (A7265, 1:1000), PSD-95(ab238135, 1:2000), CAMKIV (ab75874, 1:2000), p-CREB (ab32096, 1:5000), BDNF (ab108319, 1:10000), and Caspase-3 (ab184787, 1:2000). The HRP-conjugated secondary antibodies were used at a 1:10000 dilution. The CLOCK antibody was purchased from Abclonal (Wuhan, China), the remaining antibodies were purchased from Abcam (Shanghai, China). Target protein expression was normalized to β-actin within each sample, and the normalized values of experimental groups were then expressed as fold change relative to the average of the corresponding control group.
Enzyme-linked immunosorbent assay (Elisa)
Fresh plasma was carefully isolated after 96 h post-CA. The Elisa kits including rat IL-1β (JM-01454R1), rat IL-6 (JM-01597R1), rat neurofilament light chain (NFL) (JM-11537R1), and rat Neuron-specific enolase (NSE) (JM-10829R1) were purchased from Jiangsu Jingmei Biotechnology Co., LTD (Jiangsu, China). Supernatants were used to measure cytokines according to the manufacturers’ protocols. Briefly, 10 µL samples and calibrators were added to the plate wells coated with an array of cytokine capture antibodies and incubated for 1 h in 37℃, followed by incubations with the primary antibody (for 1 h) and the second antibody (for 0.5 h). Added the terminating fluid and the plate was read immediately.
Assessment of cognitive function
To evaluate post-resuscitation cognitive outcomes, a dedicated cohort of rats was subjected to behavioral testing, including the Novel Object Recognition (NOR) test and the Morris Water Maze (MWM) test. The NOR test, administered on day 4, consisted of a 5-minute training session with two identical objects and a subsequent 5-minute test session where one object was replaced with a novel one. The discrimination index and ratio were calculated as follows: Discrimination index= (New object time − Familiar object time)/Total exploration time and Discrimination ratio = New object time/Total exploration time.
The MWM test, conducted from days 5 to 10, assessed spatial memory in a 160 cm pool at 22 °C, with a hidden 12 cm platform. Rats were trained for 5 days (four 60 s trials per day), and on day 6, a 60 s probe trial was conducted without the platform. Escape latency and platform crossings were recorded using a video tracking system.
Statistics analysis
The analysis software involved in this study was Graphpad prism 9.5.1. All data were expressed as mean ± standard deviation. The normality of the data was assessed using the Shapiro-Wilk test, small samples default to conform to a normal distribution, and the homogeneity of variance was assessed by the Brown-Forsythe test (≥ three groups). We used ANVOA when the assumptions (equal variance and normal distribution) were met otherwise non-parametric tests were used to analyze the differences. For the MWM test, escape latency was analyzed using two-way ANOVA with time and group as factors. Multiple comparisons were performed using Tukey’s test (for parametric data) or Dunn’s test (for non-parametric data), with a statistical significance threshold set at p < 0.05.
Results
Neuroprotective effects of electroacupuncture against sleep Deprivation-Exacerbated Post-Cardiac arrest brain injury
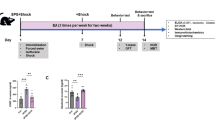
SD effectively induced circadian rhythm disturbances, as demonstrated by disrupted plasma melatonin oscillations and diminished CLOCK protein expression in the suprachiasmatic nucleus (Supplementary Fig. 1A-C). The animal screening process is detailed in Supplementary Fig. 2, where it can be seen that 82 of 242 rats (33.9%) failed to achieve ROSC and were excluded from further analysis. To evaluate the therapeutic potential of EA in PCABI, rats underwent daily EA treatment for four days following ROSC (Fig. 1A). CA triggered significant increases in plasma inflammatory cytokines (IL-1β, IL-6) and brain injury biomarkers (NSE, a marker of neuronal damage associated with hypoxia and cellular stress; NFL, a neurofilament protein reflecting axonal injury), with these elevations being exacerbated by SD. Notably, EA treatment effectively attenuated these pathological increases (Fig. 1B-E). Nissl staining revealed CA-induced neuronal loss and Nissl body depletion in the PFC and HP, effects that were amplified by SD. EA intervention partially preserved neuronal integrity, mitigating both structural and histochemical damage (Fig. 1F-H). TEM of hippocampal CA1 synapses demonstrated SD-aggravated synaptic impairment post-CA, manifesting as widened synaptic clefts and reduced postsynaptic density dimensions. EA treatment counteracted these ultrastructural deteriorations, preserving synaptic architecture (Fig. 1F). At the molecular level, CA-induced reductions in PSD95 expression and concurrent increases in caspase-3 activation were markedly intensified by SD. EA administration reversed these divergent trends, restoring synaptic protein levels while suppressing apoptotic signaling in both PFC and hippocampal regions (Fig. 1I-N).
In the NOR test, rats exhibited a reduced preference for novel objects post-CA, as evidenced by lower recognition index and ratios (Supplementary Fig. 3A-E), and demonstrated longer escape latencies and fewer platform crossings in the MWM (Supplementary Fig. 3 A, F-G). Across NOR and MWM, sleep-deprived rats showed poorer learning and memory capabilities, which were improved by EA treatment (Supplementary Fig. 3A-G).
Electroacupuncture alleviates post-cardiac arrest brain injury. (A) Flowchart of the experiment. (B-E) Plot of Elisa assays for IL-1β, IL-6, NSE and NFL in the plasma of rats on day 4 post-CA (n = 8). (F) Representative micrographs showing Nissl histochemical staining of hippocampus and prefrontal cortex neurons, alongside TEM of the CA1 hippocampal subfield at 4 days post-CA, black arrows indicate chromatolytic neurons with Nissl body disintegration, while green arrows indicate synaptic regions. (G-H) Nissl body number in hippocampus and prefrontal cortex on day 4 post-CA (n = 5). (I-N) Relative expression of PSD95, and Caspase-3 in hippocampus and prefrontal cortex on day 4 post-CA (n = 8).
Investigating sleep deprivation’s impact and electroacupuncture’s protective role on post-cardiac arrest brain injury
To systematically investigate SD-mediated molecular alterations and EA-induced neuroprotection in PCABI, we performed hippocampal RNA sequencing at 96 h post-CA. Differential gene expression analysis (|log2FC| > 0.58, p < 0.05) revealed distinct transcriptional profiles across experimental groups, visualized through comparative volcano plots (Fig. 2A-C). SD markedly amplified CA-induced hippocampal transcriptomic perturbations, while EA treatment substantially normalized these expression patterns. Enrichment analyses (KEGG/GO) identified calcium signaling as the predominant pathway associated with CA-induced transcriptional changes, SD-mediated exacerbation, and EA-driven normalization (Fig. 2D-F, Supplementary Fig. 4A-E). Cross-comparison of DEGs between resting and SD-exposed post-CA rats, combined with EA-modulated DEGs, revealed a conserved molecular network. Protein-protein interaction analysis prioritized three hub genes: Slc1a2 (EAAT2), Grin2a, and Nos1 (nNOS) (Fig. 2G).
Electroacupuncture corrects sleep deprivation-induced transcriptomic alterations in post-cardiac arrest hippocampus. (A-C) Volcano plots of differentially expressed RNAs (|log2FC| > 0.58, adjusted p < 0.05) between: (A) CA vs. SD + CA (309 upregulated, 274 downregulated); (B) CA vs. CA + EA (185 upregulated, 127 downregulated); (C) SD + CA vs. SD + CA + EA (220 upregulated, 553 downregulated). Dashed lines indicate significance thresholds. (D-F) KEGG pathway enrichment analysis of DEGs for: (D) CA vs. CA + EA; (E) SD + CA vs. CA; (F) SD + CA vs. SD + CA + EA. Bubble size represents gene count; color indicates -log10(q-value). (G) Protein-protein interaction network of consolidated DEGs (combined SD + CA vs. CA and SD + CA vs. SD + CA + EA comparisons). Hub genes identified by betweenness centrality: Slc1a2, Grin2a, Nos1.n = 8 biologically independent samples per group.
Electroacupuncture preserves EAAT2/Calcium signaling axis to counteract sleep Deprivation-Exacerbated Post-Cardiac arrest neurodegeneration
To elucidate the mechanistic role of the EAAT2/calcium signaling axis in SD-potentiated PCABI and EA-mediated neuroprotection, we analyzed HP and PFC expression patterns of EAAT2 and downstream calcium signaling mediators (p-CREB, BDNF, CAMKIV). Immunofluorescence imaging revealed CA-induced astroglial reactivity in the hippocampal CA1 subregion, evidenced by elevated GFAP expression. This astrocytic activation was markedly intensified by SD but attenuated following EA intervention. Parallel alterations in EAAT2 expression were observed, with CA triggering EAAT2 downregulation that was exacerbated by SD and subsequently normalized by EA treatment (Fig. 3A-D). Notably, analogous patterns of GFAP and EAAT2 modulation were identified in the PFC, demonstrating brain-wide SD effects and EA responsiveness (Fig. 3E-H). CA-induced dysregulation extended to calcium signaling components, with reduced levels of BDNF, p-CREB, and CAMKIV in both HP and PFC. SD amplified these reductions, while EA treatment effectively restored protein expression toward baseline levels (Fig. 3I-P).
The coordinated EA-mediated preservation of EAAT2 expression and calcium signaling integrity across hippocampal and cortical regions suggests a unified neuroprotective mechanism. These findings position EAAT2/calcium signaling regulation as a critical pathway through which SD exacerbates—and EA mitigates—post-CA neuronal injury.
Electroacupuncture restores SD-compromised EAAT2/calcium signaling axis in post-cardiac arrest neurodegeneration. (A-B) Representative immunofluorescent images of EAAT2 (green) and astroglial marker GFAP (red) in hippocampal CA1 and prefrontal cortex regions, Nuclei counterstained with DAPI (blue). (C-H) Quantitative analysis of EAAT2 and GFAP fluorescence intensity, and GFAP-positive area fraction in hippocampus and prefrontal cortex. (I, M) Western blot membranes showing calcium signaling proteins (CAMKIV, p-CREB, BDNF) with loading controls (GAPDH/β-actin) in hippocampus and prefrontal cortex lysates. (J-L, N-P) Densitometric quantification of CAMKIV, p-CREB, and BDNF expression levels in hippocampus and prefrontal cortex.
Autonomic nervous system integrity is required for Electroacupuncture-Mediated EAAT2/Calcium signaling neuroprotection in Post-Cardiac arrest brain injury
To investigate whether EA exerts neuroprotection through autonomic nervous system (ANS) regulation in SD-aggravated PCABI, we employed two complementary autonomic disruption models: (1) vagal inhibition via cervical vagotomy and atropine administration (5 mg/kg), and (2) sympathetic suppression using 6-hydroxydopamine (6-OHDA, 200 mg/kg) prior to EA intervention (Fig. 4A). Both autonomic perturbation strategies amplified PCABI manifestations, elevating plasma inflammatory cytokines (IL-1β, IL-6) and neural injury biomarkers (NFL, NSE) (Fig. 4B-E). This systemic dysregulation paralleled central neurochemical alterations, with vagal/sympathetic suppression reducing EAAT2 expression while increasing GFAP in PFC and hippocampal CA1 subfields (Fig. 4F-M). Autonomic inhibition attenuated EA’s therapeutic effects on calcium signaling mediators. Rats receiving vagotomy or 6-OHDA exhibited diminished p-CREB, BDNF, and CAMKIV levels in PFC and hippocampus, mirroring patterns observed in SD-potentiated injury (Fig. 5A-H). These findings establish autonomic integrity as prerequisite for EA-mediated neuroprotection. The coordinated suppression of EAAT2/calcium signaling and concomitant astrocyte activation under autonomic dysfunction demonstrates this pathway’s centrality in translating neural regulation to molecular neuroprotection.
Autonomic imbalance abrogates electroacupuncture-mediated EAAT2 restoration in post-cardiac arrest neurodegeneration (A) Experimental timeline for autonomic nerve inhibition (vagotomy/atropine vs. 6-OHDA) combined with EA intervention. (B-E) Plasma biomarker profiles showing elevated IL-1β, IL-6, NSE, and NFL under autonomic dysfunction, despite EA treatment (n = 8). (F-G) Representative confocal images of EAAT2 (green) and astroglial GFAP (red) in hippocampal CA1 and prefrontal cortex regions, nuclei stained with DAPI (blue). (H-J) Quantitative analysis of hippocampal EAAT2 and GFAP fluorescence intensity, and GFAP area fraction (n = 5). (K-M) Quantitative analysis of Prefrontal cortical EAAT2 and GFAP fluorescence intensity, and GFAP area fraction (n = 3).
Electroacupuncture enhances recovery from post-cardiac arrest brain injury by modulating autonomic homeostasis
Experimental disruption of autonomic equilibrium induced coordinated synaptic and cellular pathology in HP and PFC regions. Molecular analyses revealed reduced PSD95 expression coupled with heightened caspase-3 activation, indicative of synaptic destabilization and apoptotic signaling (Fig. 5I-M). Ultrastructural evaluation via TEM confirmed synaptic degeneration in hippocampal CA1 neurons, manifesting as attenuated postsynaptic density dimensions and widened synaptic clefts (Fig. 5N). Nissl staining demonstrated autonomic perturbation-induced neuronal compromise across both brain regions, characterized by diminished Nissl body integrity and elevated neuronal loss in PFC and hippocampal CA1 subfields (Fig. 5O-Q).
Additionally, Behavioral assessments, including the NOR test, showed a diminished preference for novel objects in rats that underwent these treatments (Supplementary Fig. 5A-E). In the MWM test, these rats exhibited increased escape latency and fewer platform crossings, suggesting a decline in spatial memory and learning abilities (Supplementary Fig. 5F-H). The convergence of synaptic deterioration, neuronal apoptosis, and cognitive impairment under autonomic suppression establishes neural regulatory balance as critical for maintaining EA-mediated neuroprotection. These findings position autonomic homeostasis as a prerequisite for effective electroacupuncture intervention in post-CA recovery.
Electroacupuncture fails to ameliorate severe nerve damage in the presence of autonomic imbalance. (A) WB bands of CAMKIV, p-CREB, BDNF, GAPDH and β-Actin in hippocampus. (B-D) Relative expression of CAMKIV, p-CREB, and BDNF in hippocampus (n = 8). (E) WB bands of CAMKIV, p-CREB, BDNF, GAPDH and β-Actin in prefrontal cortex. (F-H) Relative expression of PSD95, CAMKIV, p-CREB, BDNF and Caspase-3 in prefrontal cortex (n = 8). (I) WB bands of PSD95, Caspase-3 and β-Actin in hippocampus and prefrontal cortex. (J-M) Relative expression of PSD95, and Caspase-3 in hippocampus and prefrontal cortex (n = 8). (N) TEM of the CA1 region of the hippocampus, black arrows indicate synaptic regions. (O) Nissl histochemical stain for Nissl bodies in the neurons of hippocampus and prefrontal cortex in different rat groups, black arrows indicate chromatolytic neurons with Nissl body disintegration. (P-Q) Nissl body number in hippocampus and prefrontal cortex (n = 5).
Targeting EAAT2 amplifies electroacupuncture therapeutic outcomes in sleep Deprivation-Induced neurological dysfunction
To investigate the functional significance of EAAT2 in mediating the therapeutic effects of EA on PCABI, we conducted pharmacological interventions using LDN_212320 (EAAT2 agonist, 10 mg/kg, i.p.) and dihydrokainic acid (EAAT2 antagonist, 10 mg/kg, i.p.) in a SD rat model prior to EA administration (Fig. 6A). Pharmacological activation of EAAT2 substantially attenuated systemic inflammatory responses and neuronal injury in sleep-deprived rats, as evidenced by reduced plasma concentrations of pro-inflammatory cytokines (IL-1β, IL-6) and neural damage markers (NSE, NFL). Conversely, EAAT2 inhibition exacerbated these pathological alterations (Fig. 6B-E).
Immunohistochemical analyses revealed tissue-specific regulatory effects in key brain regions. EAAT2 activation enhanced EAAT2 expression while suppressing glial activation markers (GFAP) in both CA1 region of HP and PFC tissues, whereas antagonist administration produced opposing effects (Fig. 6F-M). At the molecular signaling level, EAAT2 potentiation upregulated critical components of the calcium signaling cascade (CAMKIV, p-CREB and BDNF) in these brain regions, while EAAT2 blockade significantly diminished their expression (Fig. 7A-H).
Under conditions of autonomic nerve imbalance, the regulatory effect of electroacupuncture on EAAT2 is ineffective. (A) The experimental flow chart. (B-E) Plot of Elisa assays for IL-1β, IL-6, NSE and NFL in the plasma of rats on day 4 post-CA (n = 8). (F-G) EAAT2 and GFAP immunofluorescence staining in hippocampus and prefrontal cortex. (H-J) Immunofluorescence semi-quantitative analysis of EAAT2 and GFAP in hippocampus (n = 5). (K-M) Immunofluorescence analysis of EAAT2 and GFAP in prefrontal cortex (n = 3).
Synaptic integrity assessments demonstrated that EAAT2 activation synergistically enhanced EA’s neuroprotective effects, manifested through increased PSD95 expression and reduced caspase-3 activity in HP and PFC tissues. These effects were conversely diminished by EAAT2 inhibition (Fig. 7I-M). Ultrastructural analyses further confirmed that EAAT2 activation optimized synaptic architecture by enhancing postsynaptic density dimensions (length and thickness) while reducing synaptic cleft width, surpassing the effects observed with EA monotherapy (Fig. 7N).
Nissl staining provided complementary histological evidence, showing that EAAT2 potentiation amplified EA’s neurorestorative capacity through increased neuronal ribosome density and reduced cellular damage in CA1 and PFC regions. In contrast, EAAT2 antagonism significantly compromised neuronal survival (Fig. 7O-Q). These collective findings establish EAAT2 as a critical molecular mediator enhancing the therapeutic efficacy of EA in PCABI through multi-modal mechanisms involving inflammatory regulation, neurotrophic support, and synaptic preservation.
The effects of EAAT2 on cognitive performance were assessed using the NOR and MWM tests. Activating EAAT2 receptors improved cognitive function, as evidenced by a preference for new objects and better learning and memory. Blocking EAAT2 receptors led to poorer performance in similar tests (Supplementary Fig. 6A-H). Therefore, EAAT2 activation enhances the positive effects of EA on PCABI, while inhibition diminishes them, indicating that upregulating EAAT2 is pivotal to the benefits of EA on PCABI.
Under conditions of autonomic nerve imbalance, electroacupuncture cannot improve PCABI. (A) WB bands of CAMKIV, p-CREB, BDNF, GAPDH and β-Actin in hippocampus. (B-D) Relative expression of CAMKIV, p-CREB, and BDNF in hippocampus (n = 8). (E) WB bands of CAMKIV, p-CREB, BDNF, GAPDH and β-Actin in prefrontal cortex. (F-H) Relative expression of PSD95, CAMKIV, p-CREB, BDNF and Caspase-3 in prefrontal cortex (n = 8). (I) WB bands of PSD95, Caspase-3 and β-Actin in hippocampus and prefrontal cortex. (J-M) Relative expression of PSD95, and Caspase-3 in hippocampus and prefrontal cortex (n = 8). (N) TEM of the CA1 region of the hippocampus, black arrows indicate synaptic regions. (O) Nissl histochemical stain for Nissl bodies in the neurons of hippocampus and prefrontal cortex in different rat groups, black arrows indicate chromatolytic neurons with Nissl body disintegration. (P-Q) Nissl body number in hippocampus and prefrontal cortex (n = 5).
Discussion
The present study systematically elucidates the molecular and systemic mechanisms through which EA ameliorates SD-exacerbated PCABI. Our findings reveal that SD disrupts circadian homeostasis, and aggravates neuronal damage, while EA counteracts these pathological processes via dual regulation of the EAAT2/calcium signaling axis and ANS integrity. Critically, we demonstrate that EAAT2 serves as a central molecular mediator bridging synaptic preservation, and neurotrophic signaling, with ANS balance acting as a prerequisite for translating EA-induced neuromodulation into functional recovery. Below, we contextualize these findings within existing literature and discuss their implications for both mechanistic understanding and therapeutic development.
Sleep deprivation disrupts autonomic homeostasis to exacerbate Post-Cardiac arrest brain injury
SD exerts profound systemic and neurological consequences by destabilizing ANS equilibrium, a critical regulator of cardiovascular and cerebral homeostasis. Neuronal circuits governing sleep-wake cycles are anatomically and functionally intertwined with central autonomic networks, allowing SD to amplify sympathetic outflow while suppressing parasympathetic tone27. This autonomic imbalance manifests as elevated circulating catecholamines (norepinephrine/epinephrine) and heightened systemic inflammation—hallmarks of SD-induced pathophysiology28,29. In the context of PCABI, such autonomic dysregulation creates a hostile microenvironment for neuronal recovery. CA itself imposes a unique I/R insult, where rapid restoration of cerebral perfusion is paramount. However, even successful CPR maintains cerebral perfusion pressure at merely 20% of baseline levels, insufficient to meet the brain’s metabolic demands30. SD further compromises this fragile equilibrium through two synergistic mechanisms: (1) sympathetic hyperactivation, which exacerbates endothelial dysfunction and reduces vascular reactivity, and (2) systemic inflammation, driven by catecholamine-mediated cytokine release31,32,33.
Studies have demonstrated that acute SD significantly increases biomarkers of endothelial dysfunction, including von Willebrand factor, which precedes the development of hypertension31,32,33. These pathophysiological changes are likely to compromise cerebral perfusion through impaired vasodilatory capacity and enhanced microthrombotic activity. In resuscitated CA models, this endothelial dysfunction likely exacerbates cerebral hypoperfusion, creating a vicious cycle of ischemia, excitotoxicity, and neuronal apoptosis. Notably, SD-induced sympathetic overactivation may directly impair neurovascular coupling—a process essential for matching cerebral blood flow to neuronal activity34. This hypothesis is supported by studies linking norepinephrine surges to blood-brain barrier disruption and microglial activation, further amplifying neuroinflammation35. Collectively, these mechanisms position SD as a modifiable risk factor that amplifies PCABI severity by destabilizing ANS integrity and perpetuating cerebral metabolic crisis.
Electroacupuncture restores autonomic balance to mitigate SD-Aggravated PCABI
EA emerges as a potent modulator of ANS dysfunction in SD-aggravated PCABI, bridging neural regulation with molecular neuroprotection. Our experimental models demonstrate that EA’s therapeutic efficacy—marked by reduced inflammation, preserved synaptic integrity, and improved cognitive outcomes—is contingent upon vagal and sympathetic pathway integrity. Vagotomy or pharmacological vagal inhibition (via atropine) abolished EA’s anti-inflammatory effects, underscoring the indispensability of cholinergic signaling36,37. Our previous study also found that unilateral vagotomy combined with peripheral atropine administration weakens EA’s effects in cerebral I/R, including improved cerebral perfusion, reduced apoptosis, and oxidative stress18. Mechanistically, EA likely activates vagal afferents, triggering acetylcholine release that inhibits NF-κB-mediated cytokine production in macrophages through α7 nicotinic receptors37,38. This cholinergic anti-inflammatory pathway aligns with our observations of reduced IL-1β/IL-6 levels in EA-treated rats, an effect nullified by vagal disruption.
Paradoxically, sympathetic suppression via 6-OHDA exacerbated PCABI despite its therapeutic utility in other pathologies23,24,25. This divergence highlights the context-dependent role of sympathetic tone in post-CA recovery. Early post-resuscitation hemodynamic instability necessitates basal sympathetic activity to maintain cerebral perfusion pressure. Furthermore, while an elevation in MAP and HR following ROSC is observed, it is predominantly attributed to the temporary impact of catecholamines39, potentially concealing the actual deterioration of myocardial function post-CA. In animal-based studies, a notable reduction in ejection fraction coupled with an upsurge in central venous pressure is discernible within 30 min post-ROSC40. Excessive sympathetic inhibition may impair cerebral perfusion pressure through compromised cardiac function and systemic circulatory dysregulation, thereby accelerating ischemic penumbra expansion and potentiating secondary neuronal damage. Furthermore, EA’s neuroprotective effects may partially depend on spinal sympathetic pathways that integrate peripheral EA stimuli with central autonomic regulation41. Thus, indiscriminate sympathetic blockade disrupts EA’s capacity to fine-tune neuro-cardiac coupling, emphasizing the need for context-specific ANS modulation strategies.
SD-Induced EAAT2 downregulation: A nexus of excitotoxicity and cognitive decline
While EAAT2 emerged as the predominant hub in our transcriptomic analysis, we recognize that SD likely impacts PCABI through multiple parallel pathways. The hypothalamic-pituitary-adrenal (HPA) axis activation during SD may exacerbate neuronal vulnerability via glucocorticoid-mediated metabolic stress42,43, which could potentially worsen PCABI. Notably, EA has been shown to mitigate I/R injury through modulation of the HPA axis43. Additionally, SD-induced microglial activation and polarization shifts44 may further influence neurological outcomes. However, our study focused on EAAT2 due to its central role in glutamate/calcium homeostasis, strong correlation with cognitive outcomes in our experiments, and direct responsiveness to EA intervention.
Astrocytic EAAT2 (Slc1a2), a glutamate transporter critical for synaptic homeostasis45, emerges as a pivotal mediator of SD-aggravated PCABI. Our hippocampal RNA-seq data revealed SD-driven suppression of EAAT2 expression post-CA, correlating with synaptic degeneration and cognitive impairment. Previous evidence positions astrocytic EAAT2 as a critical regulator of calcium-dependent BDNF synthesis and synaptic glutamate clearance-mediated caspase-3 modulation23. This supports our hypothesis that SD exacerbates PCABI through EAAT2/calcium signaling axis disruption, while EA confers protection by restoring pathway homeostasis. Under physiological conditions, EAAT2 maintains glutamate clearance at tripartite synapses, preventing NMDA receptor overactivation and calcium overload46. SD disrupts this equilibrium, leading to glutamate accumulation, mitochondrial depolarization, and caspase-3-mediated apoptosis—a cascade recapitulated in Alzheimer’s and cerebral I/R models47,48,49,50. Notably, SD-induced GFAP upregulation—a marker of astrocyte reactivity51—coincided with EAAT2 downregulation, suggesting reactive astrocytes lose their neuroprotective glutamate buffering capacity52.
The functional consequences of EAAT2 suppression are profound. Glutamate excitotoxicity destabilizes hippocampal circuits essential for memory encoding, as evidenced by impaired NOR and MWM performance in SD-exposed rats. These findings resonate with cognitive dysfunction-related models where EAAT2 restoration rescues synaptic plasticity and cognitive deficits53,54. Importantly, our study establishes a direct link between SD, EAAT2 dysfunction, and calcium signaling collapse—a pathway previously undercharacterized in PCABI contexts.
EAAT2 as the linchpin of Electroacupuncture-Mediated neuroprotection
The therapeutic efficacy of EA in mitigating SD-aggravated PCABI is largely attributed to its ability to restore EAAT2 expression and calcium signaling homeostasis. Our pharmacological experiments provide compelling evidence that EAAT2 activation synergizes with EA to enhance synaptic preservation and cognitive recovery. This suggests that adjunctive EAAT2 agonists could significantly improve outcomes in populations vulnerable to SD. Conversely, the inhibition of EAAT2 abolishes the benefits of EA, a finding that aligns with observations in cognitive dysfunction-related models, where EAAT2 loss is associated with accelerated neurodegeneration55,56. These results underscore the importance of EAAT2 not merely as a biomarker of astrocytic function but as a viable therapeutic target for combinatorial treatment strategies.
Clinically, the ability of EA to restore EAAT2 expression offers significant promise for patients suffering from comorbid sleep disorders and PCABI—a population at heightened risk for delayed recovery. Given that SD both exacerbates and is exacerbated by PCABI, interventions targeting EAAT2 could disrupt this detrimental cycle. Furthermore, our RNA-seq data, which implicate EAAT2 in the normalization of calcium signaling, provide a mechanistic foundation for the development of small-molecule EAAT2 enhancers. Such advancements could potentially overcome the technical limitations associated with EA in critical care settings, offering a more accessible therapeutic option.
Pharmacological activation of EAAT2 using LDN_212320 replicated the neuroprotective effects of EA, attenuating neuroinflammation, preserving synaptic ultrastructure, and rescuing cognitive function57,58. In contrast, EAAT2 antagonism with dihydrokainic acid nullified these benefits59,60, confirming the central role of EAAT2 in EA-mediated recovery. Mechanistically, EA counteracts SD-induced EAAT2 suppression, thereby enhancing glutamate uptake and reactivating calcium-dependent neurotrophic pathways, including the CAMKIV/p-CREB/BDNF axis.
The CAMKIV/p-CREB/BDNF axis is critical for synaptic plasticity and neuronal survival61,62,63. p-CREB plays a pivotal role in orchestrating BDNF transcription, which in turn stabilizes synaptic architecture through TrkB receptor signaling64. By preserving this signaling cascade, EA effectively mitigates both excitotoxic damage (via enhanced glutamate clearance) and trophic support deprivation (via BDNF restoration). Our RNA-seq and WB analyses further highlight the role of EAAT2 in normalizing calcium signaling, providing a detailed molecular blueprint for EA’s dual-action mechanism. These findings build on previous research demonstrating EA’s neuroprotective role in cerebral I/R injury23,65,66, further solidifying EAAT2 as a key therapeutic target in SD-associated neurodegeneration.
In summary, EA’s ability to restore EAAT2 expression and calcium signaling homeostasis represents a critical mechanism underlying its therapeutic efficacy in SD-aggravated PCABI. The synergistic effects of EA and EAAT2 activation highlight the potential for combinatorial therapies to enhance synaptic preservation and cognitive recovery. Clinically, targeting EAAT2 could offer a promising strategy to break the vicious cycle of SD and PCABI, particularly in high-risk populations. The development of small-molecule EAAT2 enhancers, guided by our mechanistic insights, could further expand the therapeutic arsenal available for managing SD-related neurological disorders. These findings not only advance our understanding of EA’s neuroprotective mechanisms but also pave the way for innovative treatment approaches in critical care settings. While our findings suggest EAAT2 modulation as a potential therapeutic strategy for SD-aggravated PCABI, we acknowledge the fundamental physiological roles of EAAT2 in CNS homeostasis. Future studies should carefully evaluate the therapeutic window for EAAT2 activation, potential off-target effects on synaptic plasticity, and the risk of disrupting glutamate cycling in non-targeted brain regions.
Limitations
However, our study has several limitations. First, while we demonstrate that early sympathetic suppression exacerbates PCABI, the long-term consequences of sustained sympathoexcitation remain unexplored. Second, although EAAT2 emerges as a promising therapeutic target, we recognize the need for cautious interpretation given its fundamental roles in CNS physiology - future studies should systematically evaluate potential off-target effects of EAAT2 modulation on synaptic plasticity and metabolic coupling. Third, the pharmacological tools used (atropine and 6-OHDA) have widespread autonomic effects beyond our targeted pathways; while they provided initial mechanistic insights, future studies would benefit from more specific interventions such as optogenetic control of vagal nuclei or chemogenetic approaches to precisely dissect parasympathetic vs. sympathetic contributions. Fourth, our model focuses on acute post-CA phases, leaving open questions about chronic recovery phases. Future investigations should define optimal temporal windows for autonomic nervous system modulation, assess combinatorial therapies targeting EAAT2 alongside circadian rhythm stabilization, and incorporate conditional genetic models to evaluate the safety of sustained EAAT2 activation.
Conclusion
SD exacerbates PCABI through ANS dysregulation, EAAT2 suppression, and calcium signaling collapse. Electroacupuncture counters these pathologies by restoring autonomic balance, upregulating EAAT2, and reactivating neurotrophic signaling. These findings not only elucidate the mechanistic interplay between SD and PCABI but also validate EA as a holistic intervention with translational potential. By bridging neural regulation, molecular repair, and clinical applicability, this work paves the way for innovative strategies to improve post-resuscitation outcomes in vulnerable populations.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- ANS:
-
Autonomic Nervous System
- CA:
-
Cardiac arrest
- CPR:
-
Cardiopulmonary Resuscitation
- DEGs:
-
Differentially Expressed Genes
- EA:
-
Electroacupuncture
- EAAT2:
-
Excitatory Amino Acid Transporter 2
- Elisa:
-
Enzyme-Linked Immunosorbent Assay
- HP:
-
Hippocampus
- HPA:
-
Hypothalamic-Pituitary-Adrenal
- I/R:
-
Ischemia/Reperfusion
- MAP:
-
Mean Arterial Pressure
- MWM:
-
Morris Water Maze
- NOR:
-
Novel Object Recognition
- NFL:
-
Neurofilament Light chain
- NSE:
-
Neuron-Specific Enolase
- PCABI:
-
Post-Cardiac Arrest Brain Injury
- PFC:
-
Prefrontal Cortex
- RNA-seq:
-
RNA Sequencing
- ROSC:
-
Restoration of Spontaneous Circulation
- SD:
-
Sleep Deprivation
- TEM:
-
Transmission Electron Microscopy
- WB:
-
Western blotting
References
Ramireddy, A. S. S. & Chugh Do peak times exist for sudden cardiac arrest? Trends Cardiovasc. Med. 31 (3), 172–176 (2021).
Perkins, G. D. et al. Brain injury after cardiac arrest. Lancet 398 (10307), 1269–1278 (2021).
Sekhon, M. S., Ainslie, P. N. & Griesdale, D. E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A two-hit model. Crit. Care. 21 (1). https://doi.org/10.1186/s13054-017-1670-9 (2017).
Sandroni, C., Cronberg, T. & Sekhon, M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 47 (12), 1393–1414 (2021).
Chen, C. et al. Rna-seq analysis of the key long noncoding Rnas and Mrnas related to cognitive impairment after cardiac arrest and cardiopulmonary resuscitation. Aging (Albany NY). 12 (14), 14490–14505 (2020).
Virani, S. S. et al. Heart disease and stroke statistics-2020 update: A report from the American heart association. Circulation 141 (9), e139–e596 (2020).
Yeboah, J. et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: Mesa. Atherosclerosis 219 (2), 963–968 (2011).
Qayoumi, P. et al. Sleep apnea, the risk of out-of-hospital cardiac arrest, and potential benefits of continuous positive airway pressure therapy: A nationwide study. Resuscitation 198, 110174 (2024).
Huang, C. et al. 6-month consequences of covid-19 in patients discharged from hospital: A cohort study. Lancet 397 (10270), 220–232 (2021).
Covassin, N. V. K. & Somers Sleep, melatonin, and cardiovascular disease. Lancet Neurol. 22 (11), 979–981 (2023).
Harris, E. How sleep apnea contributes to cardiovascular disease. Jama 330 (9), 798 (2023).
Del Pinto, R. et al. Diagnostic and Therapeutic Approach To Sleep Disorders, High Blood Pressure and Cardiovascular Diseases: A Consensus Document by the Italian Society of Hypertension (siia)28p. 85–102 (High Blood Press Cardiovasc Prev, 2021). 2.
Nguyen, A. D., Costa, P. C. & Raizen, D. M. A perfect storm: sleep loss causes systemic inflammation and death. Cell. Res. 34 (5), 341–342 (2024).
Sang, D. et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell 186 (25), 5500–5516e21 (2023).
Flemming, A. Sleep deprivation whips up cytokine storm. Nat. Rev. Immunol. 24 (1), 2 (2024).
Tapp, Z. M. et al. Sleep disruption exacerbates and prolongs the inflammatory response to traumatic brain injury. J. Neurotrauma. 37 (16), 1829–1843 (2020).
Liu, S. et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 598 (7882), 641–645 (2021).
Chi, L., Du, K., Liu, D., Bo, Y. & Li, W. Electroacupuncture brain protection during ischemic stroke: A role for the parasympathetic nervous system. J. Cereb. Blood Flow. Metab. 38 (3), 479–491 (2018).
Zeng, R. et al. Effect of electroacupuncture at Baihui ameliorated neurologic deficit and hemodynamic stability in rat model of post-cardiac arrest syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 34 (12), 1285–1290 (2022).
Menezes, L. et al. Chronic rem sleep restriction in young rats increases energy expenditure with no change in food intake. Exp. Physiol. 105 (8), 1339–1348 (2020).
Du, J. et al. Metabolically glycoengineered neural stem cells boost neural repair after cardiac arrest. Adv. Funct. Mater., 34(17). e2309866 (2024).
Zhang, X. H. et al. Electroacupuncture regulates microglial polarization via inhibiting nf-κb/cox2 pathway following traumatic brain injury. Brain Res. 1818, 148516 (2023).
Cai, L. et al. Mechanism of electroacupuncture against cerebral ischemia-reperfusion injury: reducing inflammatory response and cell pyroptosis by inhibiting nlrp3 and caspase-1. Front. Mol. Neurosci. 15, 822088 (2022).
Kanehisa, M. S. & Goto Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30 (2000).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28 (11), 1947–1951 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. Kegg: biological systems database as a model of the real world. Nucleic Acids Res. 53 (D1), D672–d677 (2025).
Cortelli, P. C. & Lombardi Sleep and Autonomic Nervous System Dysfunction, in Handbook of Clinical Neurophysiologyp. 343–353 (Elsevier, 2005).
Liu, H. A. & Chen Roles of sleep deprivation in cardiovascular dysfunctions. Life Sci. 219, 231–237 (2019).
Zhong, X. et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J. Appl. Physiol. 98 (6), 2024–2032 (2005).
Buunk, G., van der Hoeven, J. G. & Meinders, A. E. Cerebral blood flow after cardiac arrest. Neth. J. Med. 57 (3), 106–112 (2000).
Sauvet, F. et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. (1985). 108 (1), 68–75 (2010).
Mullington, J. M., Haack, M., Toth, M. & Serrador, J. M. Meier-Ewert, Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc. Dis. 51 (4), 294–302 (2009).
Al Haddad, N. et al. The association between sleep disturbances and blood pressure variability: A review of the literature. J. Clin. Sleep. Med. 19 (8), 1533–1544 (2023).
Rab-Bábel, K. S. et al. Sleep Deprivation Impairs Neurovascular Coupling Cereb. Vasomotor Reactivity Sci. Rep., 15(1): 9491. (2025).
Wang, X. X. & Ji Interactions between remote ischemic conditioning and post-stroke sleep regulation. Front. Med. 15 (6), 867–876 (2021).
Fu, P. et al. Vagal innervation limits brain injury by inhibiting gut-selective integrin-mediated intestinal immunocyte migration in intracerebral hemorrhage. Theranostics 14 (19), 7383–7404 (2024).
Yang, L. et al. Electroacupuncture promotes liver regeneration by activating Dmv acetylcholinergic neurons-vagus-macrophage axis in 70% partial hepatectomy of mice. Adv. Sci. (Weinh). 11 (32), e2402856 (2024).
Wu, Z. et al. Electroacupuncture at Neiguan (pc6) attenuates cardiac dysfunction caused by cecal ligation and puncture via the vagus nerve. Biomed. Pharmacother. 162, 114600 (2023).
Neumar, R. W. et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the international liaison committee on resuscitation (american heart association, australian and new zealand council on resuscitation, european resuscitation council, heart and stroke foundation of canada, interamerican heart foundation, resuscitation council of asia, and the resuscitation council of southern africa); the american heart association emergency cardiovascular care committee; the council on cardiovascular surgery and anesthesia; the council on cardiopulmonary, perioperative, and critical care; the council on clinical cardiology; and the stroke council. Circulation, 118(23): pp. 2452-83. (2008).
Kern, K. B. et al. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with Dobutamine. Circulation 95 (12), 2610–2613 (1997).
Liu, S. et al. Somatotopic organization and intensity dependence in driving distinct npy-expressing sympathetic pathways by electroacupuncture. Neuron 108 (3), 436–450e7 (2020).
Hirotsu, C., Tufik, S. & Andersen, M. L. Interactions between sleep, stress, and metabolism: from physiological to pathological conditions. Sleep. Sci. 8 (3), 143–152 (2015).
Cai, Y. Y. et al. [influence of electroacupuncture of meridian acupoints on the related hormones of the hypothalamus-pituitary-adrenal axis in rats with cerebral ischemia reperfusion injury]. Zhen Ci Yan Jiu, 34(5): pp. 297–303. (2009).
Parhizkar, S. et al. Sleep deprivation exacerbates microglial reactivity and aβ deposition in a trem2-dependent manner in mice. Sci. Transl Med. 15 (693), eade6285 (2023).
Schousboe, A. H. S. & Waagepetersen Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox. Res. 8 (3–4), 221–225 (2005).
Das, S., McCloskey, K., Nepal, B. & Kortagere, S. Eaat2 activation regulates glutamate excitotoxicity and reduces impulsivity in a rodent model of parkinson’s disease. Mol. Neurobiol., 62(5):5787-5803 (2024).
Bishir, M. et al. Sleep deprivation and neurological disorders. Biomed. Res. Int. 2020, p5764017 (2020).
Yang, X. et al. Impact of comorbid sleep-disordered breathing and atrial fibrillation on the long-term outcome after ischemic stroke. Stroke 55 (3), 586–594 (2024).
Sehar, U. et al. Effects of sleep deprivation on brain atrophy in individuals with mild cognitive impairment and alzheimer’s disease. Ageing Res. Rev. 99, 102397 (2024).
Mukherjee, U., Sehar, U., Brownell, M. & Reddy, P. H. Mechanisms, consequences and role of interventions for sleep deprivation: focus on mild cognitive impairment and alzheimer’s disease in elderly. Ageing Res. Rev. 100, 102457 (2024).
An, J. R. et al. Effects of liraglutide on astrocyte polarization and neuroinflammation in db/db mice: focus on iron overload and oxidative stress. Front. Cell. Neurosci. 17, 1136070 (2023).
Luo, Y., Xu, N. G., Yi, W., Yu, T. & Yang, Z. H. Study on the correlation between synaptic reconstruction and astrocyte after ischemia and the influence of electroacupuncture on rats. Chin. J. Integr. Med. 17 (10), 750–757 (2011).
Carter, S. K. R. et al. Glutamate stress in the caudal nucleus tractus solitarii (nts): impact on respiratory function and synaptic signaling in an alzheimer’s disease model. Exp. Neurol. 387, 115190 (2025).
Fontana, I. C. et al. A medicinal chemistry perspective on excitatory amino acid transporter 2 dysfunction in neurodegenerative diseases. J. Med. Chem. 66 (4), 2330–2346 (2023).
Li, S. et al. Misprogramming of glucose metabolism impairs recovery of hippocampal slices from neuronal glt-1 knockout mice and contributes to excitotoxic injury through mitochondrial superoxide production. J. Neurochem. 169 (1), e16205 (2025).
Salcedo, C. et al. Increased glucose metabolism and impaired glutamate transport in human astrocytes are potential early triggers of abnormal extracellular glutamate accumulation in hipsc-derived models of alzheimer’s disease. J. Neurochem. 168 (5), 822–840 (2024).
Takahashi, K. et al. Restored glial glutamate transporter eaat2 function as a potential therapeutic approach for alzheimer’s disease. J. Exp. Med. 212 (3), 319–332 (2015).
Takahashi, K., Foster, J. B. & Lin, C. L. Glutamate transporter eaat2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell. Mol. Life Sci. 72 (18), 3489–3506 (2015).
Fan, S. et al. Ceftriaxone suppresses group Ii metabotropic glutamate receptor expression contributing to reversal of recognition memory deficits of amyloid precursor protein/presenilin 1 ad mice. Front. Neurosci. 16, 905403 (2022).
Mei, B. et al. Activating astrocytic α2a adrenoceptors in hippocampus reduces glutamate toxicity to attenuate sepsis-associated encephalopathy in mice. Brain Behav. Immun. 117, 376–398 (2024).
Zhang, H., Liu, Q. Q., Ding, S. K., Li, H. & Shang, Y. Z. Flavonoids from stems and leaves of scutellaria baicalensis Georgi improve composited aβ-induced alzheimer’s disease model rats’ memory and neuroplasticity disorders. Comb. Chem. High. Throughput Screen. 26 (8), 1519–1532 (2023).
Kong, L. et al. Research advances on camks-mediated neurodevelopmental injury. Arch. Toxicol. 98 (12), 3933–3947 (2024).
Mousa, H. H., Sharawy, M. H. & Nader, M. A. Empagliflozin enhances neuroplasticity in rotenone-induced parkinsonism: role of bdnf, Creb and npas4. Life Sci. 312, 121258 (2023).
Shu, Y. et al. Exposure to malathion impairs learning and memory of zebrafish by disrupting cholinergic signal transmission, undermining synaptic plasticity, and aggravating neuronal apoptosis. J. Hazard. Mater. 488, 137391 (2025).
Zhang, Q. et al. Electroacupuncture and human ipsc-derived small extracellular vesicles regulate the gut microbiota in ischemic stroke via the brain-gut axis. Front. Immunol. 14, 1107559 (2023).
Li, S. S. et al. Electroacupuncture treatment improves motor function and neurological outcomes after cerebral ischemia/reperfusion injury. Neural Regen Res. 17 (7), 1545–1555 (2022).
Acknowledgements
RNA-seq data associated with this study have been deposited in the Gene Expression Omnibus (GEO) database under the accession number GSE295500. The dataset is accessible through the following link using the token"gbcxscimtrqvlsd”:Access URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE295500 Access Token: gbcxscimtrqvlsd.
Funding
This work was supported by the National Natural Science Foundation of China (No.81871025).
Author information
Authors and Affiliations
Contributions
WL and WF conceived and designed the study. WF, CH, CM, SL, XZ, TL, JC, XH, XT and XL performed the experiment. WF and CH were responsible for data analysis and checking. WF wrote the original draft of the manuscript, which was reviewed and revised by WL and XZ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Compliance with ethics requirements
All animal experiment protocols were performed in accordance with the guidelines of Animal Care and Use Committees at the Harbin Medical University (Harbin, Heilongjiang, China) and were in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85 − 23, revised 2011).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, W., Hu, C., Ma, C. et al. Harnessing electroacupuncture: a promising strategy against sleep deprivation-exacerbated post-cardiac arrest brain injury. Sci Rep 15, 29277 (2025). https://doi.org/10.1038/s41598-025-15563-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15563-y