Abstract
Although in vitro studies suggest that neutralization by monoclonal antibodies (mAbs) against SARS CoV2 Omicron sub lineages is reduced, in vivo virological response data are lacking. MONET (EudraCT: 2021–004188-28) was multi-centric phase 4 open-label parallel randomized clinical trial, conducted in Italy over 2022–2023, to assess the efficacy of sotrovimab (SOT), tixagevimab/cilgavimab (TIX/CIL) and Nirmatrelvir/ritonavir (NMV/r), in outpatients at high risk for severe COVID-19. The outcome (secondary in the trial protocol) was SARS-CoV-2 variation in cycle threshold (CT) values over the first 7 days (D1-D7) of the trial. CT variation was compared by trial arms using unadjusted linear regression and after controlling for age. We included 346 individuals: 116 (34%) received SOT, 113 (33%) TIX/CIL, 117 (34%) NMV/r. Main characteristics were balanced across arms. Most of the participants were infected with BA.2 (52%) or BA.4/5 (35.5%). The data carried strong evidence that the mean CT change over D1-D7 was larger in subjects receiving NMV/r vs. the other arms (p < 0.001). We found no evidence that viral variant was an effect measure modifier for the contrasts of interest (p = 0.14). Our analysis provides strong evidence that NMV/r exerts a greater in vivo antiviral effect than anti-Spike mAbs against Omicron sub lineages, confirming previous in vitro data.
Similar content being viewed by others
Introduction
In January 2022, in the Omicron era, both monoclonal antibodies (mAbs) and antiviral agents were available for the early treatment of mild-to-moderate COVID-19 for outpatients at high risk for progression to severe disease. Most of the data supporting their use come from placebo-controlled phase-3 randomized clinical trials (RCTs) that demonstrated a reduced risk of developing severe COVID-19 or death in subjects receiving mAbs such as sotrovimab (SOT) and tixagevimab plus cilgavimab (TIX/CIL), or antivirals such as nirmatrelvir plus ritonavir (NMV/r)1,2,3. However, these studies were conducted in the pre-Omicron phase, and it is likely that the efficacy of treatment against these earlier circulating variants was different. Indeed, SARS-CoV-2 evolution and the specific mutations in the spike protein (the binding target for mAbs) harboured by Omicron sublineages resulted in an evolving escape to in vitro neutralizing activity by mAbs4,5,6,7, with an unclear impact on in vivo treatment response. Indeed, all commercialised antiSARS-CoV-2 spike mAbs have been deauthorized by the Food and Drug Administration (FDA) to prevent or treat COVID-19 because of this viral evolution8 Conversely, laboratory data show that the protease inhibitor nirmatrelvir, enhanced with ritonavir, seemed to have retained its antiviral activity against various Omicron sublineages4,5,9.
For this reason, randomized clinical trials aimed to compare the virological response to available treatments in individuals infected with Omicron strains remain strategically important. In the outpatient setting, because of the low risk of severe outcomes during the Omicron phase of the pandemic, candidate surrogate markers, such as the viral load (VL) reduction in nasopharyngeal swab (NPS) samples, have been typically used in phase-3 studies as a measure of in vivo neutralizing or antiviral activity10,11.
Here we show the results of the analysis of the secondary outcome of the MONET trial, which was designed to compare the efficacy and safety of early treatment with SOT, TIX/CIL, or NMV/r in the setting of a high-risk outpatient population with mild to moderate COVID-19 enrolled in several clinical sites in Italy.
Materials and methods
Trial design
The MONET trial (registration number EudraCT: 2021–004,188-28, 13 September 2021) is a multicentric, phase 4, three-arm, superiority, open-label RCT conducted over March 2022-February 2023, to assess the efficacy and safety of 500 mg intravenous SOT (Arm 1), 300/300 mg intramuscular TIX/CIL (Arm 2) and oral 5-days course of NMV/r 300/100 mg (or 150/100 mg for those with a creatinine clearance of 30–60 mL/min) twice daily (Arm 3), randomly assigned in a 1:1:1 ratio, in non-hospitalized adults with early COVID-19 at high-risk of progression to severe disease.
The primary outcome of MONET was clinical failure within 30 days after randomization, defined as any-cause mortality, hospitalization, or progression to severe COVID-1912. Because MONET was underpowered for the clinical endpoint, the results of the primary outcome have been published only after pooling together the events recorded in another similar trial (MANTICO)12. Also, the comparisons were restricted to arms which were common to the 2 trials after a revision of the original MONET trial protocol which was also modified from a phase 3 to phase 4 (Sotrovimab, Casirivimab, and Imdevimab only). This paper reports the results of the analysis of the main secondary outcome which was the change in SARS-CoV-2 VL in NPS between enrolment (D1) and Visit 2 (D7) by PCR cycle threshold (CT) value conducted in 3/7 sites of MONET. We also analysed a binary outcome using a CT threshold of >35 to define a negative RT-PCR test result. Several other secondary outcomes have been evaluated during each visit (at D1, D7, D29): the variation of inflammatory markers [C-reactive protein (CRP), d-dimer, and neutrophils-to-lymphocytes ratio (NLR)] and antibody level (serum anti-S IgG and anti-N IgG).
All methods were performed in accordance with the relevant guidelines and regulations (see supplementary materials for further details on study design and participants, statistical analysis and methods to measure viral load).
Results
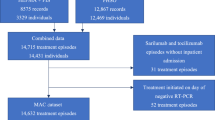
Out of a total of 466 participants enrolled in the trial, 346 (74.3%) for whom pairs of CT values at D1 and D7 were available were included: 116 (34%) received SOT, 113 (33%) TIX/CIL and 117 (34%) NMV/r. Four individuals (1%) were excluded because BMI was missing, 33 (7%) were enrolled in MONET sites that did not subscribe to the virological sub study, while the remaining 83 (18%) were excluded because either day7 sample had not been stored or CT value test was unsuccessful for technical problems or low-quality sampling (Fig. 1, the study flow chart). All MONET participants who developed the primary outcome of hospitalization due to COVID-19 or death are included also in this subset (4 events in TIX/CIL and 1 event in SOT).
Baseline characteristics of the study population, stratified by treatment arms, are reported in Table 1. Briefly, 49.4% (N = 171) of the subjects were female with an overall median age of 66 years [Interquartile Range (IQR) 55–76], mainly infected either with BA.2 (N = 180, 52%) or BA.4/5 (N = 123, 35.5%) and with median time from symptom onset to randomization of 3 days (IQR 2–4). The participants seemed balanced across study arms with respect to all variables examined possibly except for age (participants allocated to the SOT arm appeared to be slightly older than those allocated to the other trial arms) and for the prevalence of COPD which was higher in TIX/CIL (8%) vs. SOT (3%) (Table 1, Figs. S1–3 for love plots). Baseline CT values were also balanced: [mean (95% CI) SOT versus (vs.) NMV/r 0.02 (-0.07, 0.1), p = 0.875; TIX/CIL vs. NMV/r -0.01 (-0.10, 0.08), p = 0.931; TIX/CIL vs. SOT -0.03 (-0.12, 0.06) p = 0.680, Table 1, Fig. 2]. Of note, there was no evidence for a difference in main characteristics of the target population of MONET when compared to those included in this analysis with perhaps the only exception of history of vaccination (lower uptake in MONET, Supplementary Table S1).
Figure 2 also describes the distribution of the variation in raw CT values over D1-D7 by trial arm.
The regression model analysis using log-transformed values, carried strong statistical evidence that the mean change in CT was larger in subjects receiving NMV/r than in those receiving SOT or TIX/CIL [SOT vs. NMV/r -0.16 log2 (-0.25, -0.07), p < 0.001; TIX/CIL vs. NMV/r -0.20 log2 (-0.30, -0.11), p < 0.001; TIX/CIL vs. SOT -0.04 log2 (-0.13, 0.05), p = 0.48; Fig. 3], even after controlling for age [SOT vs. NMV/r -0.16 log2 (-0.25, -0.07), p < 0.001; TIX/CIL vs. NMV/r -0.20 log2 (-0.29, -0.11), p < 0.001; TIX/CIL vs. SOT -0.04 log2 (-0.13, 0.05), p = 0.50; Fig. 3]. Results were similar after further controlling for potential variability between participating sites and prevalence of COPD (Supplementary Table S2). In other words, by day 7 participants randomized to NMV/r showed a larger reduction in viral shedding than those allocated to other drugs. Figure S4 shows the post-hoc forest plot of the same contrasts after stratification for viral variants (BA.2 or BA.4/5). We found little evidence for effect measure modification (interaction p-value = 0.14) The proportion of participants who achieved a CT value > 35 at D7, was also higher in participants allocated to NMV/r compared to other arms but data were compatible with the null hypothesis of no difference (12% in SOT vs. 13% in TIX/CIL vs. 19% in NMV/r, p = 0.30). Results were similar after controlling for age: adjusted (a) OR 0.60 (95% CI:0.29–1.25) for SOT vs. NMV/r and 0.64 (0.31–1.31) for TIX/CIL vs NMV/r.
Regarding the other secondary outcomes, there was no evidence that the trajectories over D1-D29 of inflammatory markers varied by trial arm (p = 0.605 for CRP, p = 0.131 for d-dimer, p = 0.932 for NLR Fig. S5 and Table S3). CRP and NLR showed a clear reduction over time, regardless of the drug received, while d-dimer showed a more stable trend. Kinetics of antibody levels showed a rapid increase of serum anti-S IgG in both anti-Spike mAbs arms followed by a plateau vs. a steady linear increase in the NMV/r arm; the steady increase was seen in all arms for anti-N IgG values again with no evidence for a difference by study arm (Fig. S6 and Table S4).
Discussion
Our analysis provides in vivo evidence that, when used against Omicron sub lineages, NMV/r exerts a greater antiviral effect than SOT and TIX/CIL by day 7 from treatment initiation, regardless of the detected Omicron subvariant. These results confirm previous in vitro data suggesting that anti-Spike mAbs may not retain neutralizing activity against Omicron strains. In particular TIX/CIL appeared to show a progressive loss of efficacy against Omicron subvariants emerging over time4,5,6,7,9,13. Conversely, although for sotrovimab, there is some in vitro evidence that it may retain partial neutralizing activity against these variants5,6,7, including the most recent BQ.1.1 and XBB.1.5, in our analysis we found no evidence for a difference in virological potency when compared to TIX/CIL.
Of note, there are very few studies which compared the in vivo virological response to these compounds. Previously published placebo-controlled RCTs designed to assess the clinical efficacy of NMV/r3, SOT2, and TIX/CIL1,14 included the evaluation of virological outcomes, but a direct comparison between these drugs has not been performed within a single clinical trial. Several observational studies conducted in the setting of early treatment of COVID-19 for patients at high-risk of progression, have evaluated virological efficacy by comparing antivirals and mAbs15,16,17,18, and most of the results reported15,16,18 are in line with ours showing the superiority of NMV/r compared with mAbs, except for one retrospective study17 that failed to find any association between specific early therapies and time to achieve swab negativity. However, these are mainly small studies17, conducted during the first phase of the Omicron wave15,16, with not many participants treated with NMV/r16. To our knowledge, our trial is the first randomized study providing strong in vivo evidence of NMV/r antiviral superiority over both SOT and TIX/CIL in the Omicron era (including infections with BA.1/2 to BA.4/5 and BQ.1/BQ.1.1).
In this analysis, we used a CT threshold value of > 35 to define a negative RT-PCR result for SARS-CoV-2 on NPS samples, and although we observed that a higher proportion of participants treated with NMV/r achieved this goal after 1 week, our analysis was underpowered to show superiority vs. mAbs. However, while CT values have been hypothesized to be useful in guiding treatment decisions and influencing quarantine recommendations19, it should be noted that a specific CT threshold value to define the inability to transmit infectious viral particles is not yet known and some studies have even proposed a value of 3020, which is lower than the threshold used in our analysis. Indeed, a binary outcome using different thresholds is often used in the clinic but rarely for research purposes. Instead, treatment-induced acceleration of viral clearance in the first few days after therapy, rather than the proportion of individuals with CT above a certain threshold, has been proposed as a surrogate of clinical efficacy to prevent hospitalization with COVID-1910,11,21. Many studies, such as phase-2/3 randomized trials and observational cohorts, have compared the reduction in VL between treated and control groups (as a continuous measure) at different times after therapy as a surrogate marker of therapeutic effect. Analyses aiming to assess whether the CT reduction is a valid surrogate marker for clinical endpoints are still ongoing. A recent meta-analysis10 of 22 RCTs found a correlation between the virological effect of the different therapies measured during the first 7 days following initiation of treatment and the corresponding clinical efficacy in preventing severe forms of COVID-19. Of note, this meta-analysis included only studies conducted in unvaccinated individuals and underscored the need of validating these findings in other settings. The MONET trial was conducted in a population with a high proportion of vaccinated individuals (94% with at least two doses of vaccine), and the results reported here, along with those related to the primary clinical efficacy outcome12, further indicates CT reduction as a candidate surrogate marker for clinical efficacy.
Interestingly, in our analysis, the type of treatment did not seem to influence the development of the natural antibody response neither the level of inflammatory markers. We observed a more marked increase in serum anti-S IgG levels among participants receiving anti-Spike mAbs compared to those receiving NMV/r. This was somewhat expected as all the investigated anti-Spike mAbs targeted S antibodies; it is also possible that because infection was sub optimally controlled by mAbs, antigenic stimulation persisted for longer; in contrast, we found no evidence for a difference in the variation over time of anti-N IgG levels by intervention suggesting that mAb administration might have no impact on the endogenous immune response, an issue that had previously been raised22,23,24. Similarly, we found no evidence for a difference in the trajectories of inflammatory markers by trial arm, reflecting that the kinetics of these biomarkers are likely to be a consequence of the disease evolution regardless of the specific treatment used. Similar findings came from a recent analysis of a placebo-controlled RCTs on mAbs among hospitalized individuals24.
Our analysis has several limitations. First, 119 participants of the MONET trial with no measures for the secondary outcome had to be excluded from the analytic sample, and this may have led to selection bias25. However, missing CT values (either due to missing swabs or unsuccessful measurement, as a result of technical issues or low-quality sampling) seemed to have occurred randomly as treatment groups were still balanced for key predictors of outcome in the analytic sample (except perhaps for age), retaining internal validity. Main demographic characteristics of participants were also like those of individuals who were excluded because day7 CT values were missing. The clinical significance of the average difference of 0.2 log2 may appear modest but the primary analysis of the MONET/MANTICO trial suggests otherwise. Also, the analysis with outcome the CT negativity by day 7 was likely underpowered. In addition, although MONET was multi-centric, most of participants came from a single center and our analysis ruled out confounding due to differences in procedures at the various sites. However, the multicentric design of the trial, along with the underrepresentation of some high-risk groups, could limit the generalizability of our conclusions. Indeed, only a minority of the participants were classified as not immunocompetent, and these individuals are those who more urgently need new treatments, alternative to the FDA deauthorized compounds. Finally, the analysis was performed at the beginning of the advent of BQ.1.1, and it is unclear whether our results will be confirmed in the current epidemiological scenario of new circulating Omicron subvariants. Indeed, JN.1 (and similar variants under current monitoring by WHO) have evolved independently of BQ1.1, with a distinction mutation profile and potentially a different immune evasion strategy. One of the hallmark mutations in JN1 is the N-terminal domain (NTD) and receptor binding domain (RBD) of the spike protein, conferring a unique ability to escape neutralizing antibodies so we speculate that mAbs used in this study are even less effective with recent strains and CT values could be used as a marker to establish efficacy early.
In conclusion, our results provide high level of evidence for the superiority of NMV/r over anti-Spike mAbs (SOT and TIX/CIL), in reducing SARS-CoV-2 CT by day 7 in vaccinated non-hospitalized subjects at high-risk of progression to severe COVID-19, all infected with Omicron variants. In addition, these findings, together with the results of the analysis of the clinical outcome published elsewhere12, identify day 7 CT variation as a promising candidate surrogate marker for clinical efficacy. Given the currently inconsistent recommendations on the use of anti-Spike mAbs across countries in the Omicron era, robust data deriving from in vivo randomized studies are crucial to optimize and homogenize treatment guidelines for COVID-19. A few new mAbs have been recently developed following the FDA approach to deauthorize the old generation compounds based on immunobinding8. However, the mAbs pipeline has been relatively scarce compared to what was achieved in the early days of the pandemic and has proceeded slowly26. A new drug has been recently approved although available only for prophylaxis and not treatment27. New strategies are needed especially for immunocompromised patients, who often remain seronegative and unprotected after multiple vaccine boosts. Further research is warranted to verify whether the superior virologic potency of NMV/r over new generation anti-Spike mAbs is confirmed for newly emerging Omicron variants.
Data availability
Anonymized participant data will be made available upon reasonable requests directed to the corresponding author.
References
Montgomery, H. et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 10(10), 985–996. https://doi.org/10.1016/S2213-2600(22)00180-1 (2022).
Gupta, A., Gonzalez-Rojas, Y. & Juarez, E. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 385(21), 1941–1950. https://doi.org/10.1056/NEJMoa2107934 (2021).
Hammond, J. et al. Oral nirmatrelvir for high-risk, non-hospitalized adults with Covid-19. N. Engl. J. Med. 386(15), 1397–1408. https://doi.org/10.1056/NEJMoa2118542 (2022).
Takashita, E. et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA4, and BA5 subvariants. N. Engl. J. Med. 387(5), 468–470. https://doi.org/10.1056/NEJMc2207519 (2022).
Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602(7898), 657–663. https://doi.org/10.1038/s41586-021-04385-3 (2022).
Yamasoba, D. et al. Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect. Dis. 22(7), 942–943. https://doi.org/10.1016/S1473-3099(22)00365-6 (2022).
Touret, F. et al. Enhanced neutralization escape to therapeutic monoclonal antibodies by SARS-CoV-2 omicron sub-lineages. iScience 26(4), 106413. https://doi.org/10.1016/j.isci.2023.106413 (2023).
FDA updates Sotrovimab emergency use authorization. https://www.fda.gov/ drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-useauthorization. (Accessed 9 June 2023).
Imai, M. et al. Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N. Engl. J. Med. 388(1), 89–91. https://doi.org/10.1056/NEJMc2214302 (2023).
Elias, K. M. et al. Viral clearance as a surrogate of clinical efficacy for COVID-19 therapies in outpatients: a systematic review and meta-analysis. Lancet Microbe 5(5), e459–e467. https://doi.org/10.1016/S2666-5247(23)00398-1 (2024).
Schilling, W. H. K. et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect. Dis. 24(1), 36–45. https://doi.org/10.1016/S1473-3099(23)00493-0 (2023).
Mazzotta, V. et al. Pooled analysis of the MANTICO2 and MONET randomized controlled trials comparing drug efficacy for early treatment of COVID-19 during Omicron waves. J. Infect. 89(5), 106294. https://doi.org/10.1016/j.jinf.2024.106294 (2024).
Convertino, I. et al. Tixagevimab + cilgavimab against SARS-CoV-2: the preclinical and clinical development and real-world evidence. Expert Opin. Drug Discov. 18(3), 231–245. https://doi.org/10.1080/17460441.2023.2170348 (2023).
Bender Ignacio, R. A. et al. Safety and efficacy of combined tixagevimab and cilgavimab administered intramuscularly or intravenously in nonhospitalized patients with COVID-19: 2 randomized clinical trials. JAMA Netw. Open 6(4), e2310039. https://doi.org/10.1001/jamanetworkopen.2023.10039 (2023).
Mazzotta, V. et al. Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents. J. Med. Virol. https://doi.org/10.1002/jmv.28186 (2023).
Martin-Blondel, G. et al. Time to negative PCR conversion amongst high-risk patients with mild-to-moderate Omicron BA.1 and BA.2 COVID-19 treated with sotrovimab or nirmatrelvir. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2022.12.016 (2023).
Colaneri, M. et al. Exploring early COVID-19 therapies, variants, and viral clearance dynamics: Insights from a high-risk outpatients study. Diagn. Microbiol. Infect. Dis. 110(2), 116452. https://doi.org/10.1016/j.diagmicrobio.2024.116452 (2024).
Colaneri, M. et al. Early administration of nirmatrelvir/ritonavir leads to faster negative SARS-CoV-2 nasal swabs than monoclonal antibodies in COVID 19 patients at high-risk for severe disease. Virol. J. 21(1), 68. https://doi.org/10.1186/s12985-024-02333-x (2024).
Finks, S. W. et al. Clinical significance of quantitative viral load in patients positive for SARS-CoV-2. Am. J. Med. Open 10, 100050. https://doi.org/10.1016/j.ajmo.2023.100050 (2023).
Platten, M. et al. SARS-CoV-2, CT-values, and infectivity-conclusions to be drawn from side observations. Viruses 13(8), 1459. https://doi.org/10.3390/v13081459 (2021).
Parienti, J. J. & de Grooth, H. J. Clinical relevance of nasopharyngeal SARS-CoV-2 viral load reduction in outpatients with COVID-19. J. Antimicrob. Chemother. 77, 2038–2039 (2022).
Zhang, L. et al. Endogenous antibody responses to SARS-CoV-2 in patients with mild or moderate COVID-19 who received Bamlanivimab alone or Bamlanivimab and Etesevimab together. Front. Immunol. 12, 790469. https://doi.org/10.3389/fimmu.2021.790469 (2021).
Kim, P. S., Dimcheff, D. E., Siler, A., Schildhouse, R. J. & Chensue, S. W. Effect of monoclonal antibody therapy on the endogenous SARS-CoV-2 antibody response. Clin. Immunol. 236, 108959. https://doi.org/10.1016/j.clim.2022.108959 (2022).
Jensen, T. O. et al. Effect of neutralizing monoclonal antibody treatment on early trajectories of virologic and immunologic biomarkers in patients hospitalized with COVID-19. J. Infect. Dis. https://doi.org/10.1093/infdis/jiad446 (2024).
Lu, H., Cole, S. R., Howe, C. J. & Westreich, D. Toward a clearer definition of selection bias when estimating causal effects. Epidemiology 33(5), 699–706. https://doi.org/10.1097/EDE.0000000000001516 (2022).
Focosi, D., Franchini, M., Casadevall, A. & Maggi, F. An update on the anti-spike monoclonal antibody pipeline for SARS-CoV-2. Clin. Microbiol. Infect. 30(8), 999–1006 (2024).
Loubet, P. et al. Characteristics of the first immunocompromised patients to receive sipavibart as an early access treatment for COVID-19 pre-exposure prophylaxis in France. Hum. Vaccin. Immunother. 20(1), 2387221 (2024).
Acknowledgements
We acknowledge the MONET Clinical Trial Group, the nurse staff, and all the study participants.
Funding
The study has been funded by the Italian Drug Agency (AIFA) and by the Italian Ministry of Health (Ricerca Corrente Linea 1). Alessandro Cozzi-Lepri work is supported by EuCARE project funded by the EU under the HORIZON Europe programme, Grant agreement n. 101046016.
Author information
Authors and Affiliations
Consortia
Contributions
VM and AA conceived the study; IM and ACL wrote the first draft of the manuscript; GM, FC, MR, GB, and FM were responsible for the virological tests; VM, SL, IM, AO, AV, SR and EN enrolled the patients; JP was responsible for data entry; SL and ACL were responsible of data management and statistical analysis; VM, AA, EN, FM, EG reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All included individuals have signed a written informed consent to participate in the study. The study protocol and the informed consent were approved by the Scientific Committee of the Italian Medicines Agency (AIFA) and by the Ethical Committee of the National Institute for Infectious Diseases “Lazzaro Spallanzani” in Rome, Italy, as National Review Board for COVID-19 pandemic in Italy (approval number: n. 380, 30/09/2021. FAV del Registro delle Sperimentazioni 2020/2021).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mastrorosa, I., Cozzi-Lepri, A., Matusali, G. et al. CT changes in a randomized trial comparing early therapies in an outpatient population at high risk of severe COVID19 disease. Sci Rep 15, 30244 (2025). https://doi.org/10.1038/s41598-025-15641-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15641-1