Abstract
Cerebral blood flow (CBF) is crucial for supplying the brain with the oxygen and nutrients necessary for its proper development and metabolism. The poor autoregulation of CBF is believed to play a role in the development of brain injury in preterm infants. This study aimed to investigate the characteristics of CBF in perinatal brain injury. In this retrospective study, 108 healthy preterm infants (HP), 26 infants with intracranial hemorrhage (IVH), and 16 infants with periventricular leukomalacia (PVL) were included. CBF was assessed using arterial spin labeling imaging in the frontal, temporal, parietal, and occipital cortex, as well as the basal ganglia and thalamus. After adjusting for gestational age, postmenstrual age (PMA) at MRI scan, and birth weight, the PVL hemispheres exhibited consistently reduced CBF in various gray matter regions compared to the HP and IVH hemispheres, including the frontal, temporal, parietal, and occipital cortex, as well as the basal ganglia and thalamus. The comparison of HP and IVH hemispheres revealed decreased CBF in IVH hemispheres in the frontal, parietal, occipital cortex, as well as in the basal ganglia and thalamus. In multifactor linear regression models, the PMA at MRI scan, 5 min Apgar score, brain injury, and neonatal necrotizing stage ≥ 2 are independent factors affecting cerebral perfusion.
Similar content being viewed by others
Introduction
Advances in perinatal medicine have significantly enhanced the survival rate of extremely preterm infants. Nevertheless, infants that survive remain at high risk of brain injuries. In preterm infants, intracranial hemorrhage (IVH) and periventricular leukomalacia (PVL) are the most prevalent forms of brain injuries1,2. The third trimester of gestation is a crucial period for the maturation of the cerebral cortex, wherein the formation, enhancement, and development of neural connectivity significantly influence subsequent neurodevelopmental outcomes3. The extra-uterine environment may trigger hemodynamic instabilities, including ischemia-reperfusion, hypoperfusion, and hypoxia-ischemia, which can rupture fragile germinal matrix vessels or induce periventricular white matter injury4,5. Severe brain injuries frequently result in adverse neurological consequences, including cognitive and motor delays, cerebral palsy, neurosensory deficits, and behavioral disorders such as attention deficit hyperactivity disorder and learning disabilities6,7. However, the relationship between low-grade IVH/PVL and neurological outcomes remains controversial. Certain studies indicate that low-grade IVH/PVL may increase the risk of motor and cognitive deficits in preterm infants2,8,9. These neurodevelopmental disorders may require years to manifest, necessitating neuroimaging techniques to guide medical and rehabilitative interventions aimed at reducing risks. Nonetheless, contemporary neuroimaging methods are inadequate substitutes for prolonged neurodevelopmental assessment. Therefore, it is crucial to identify new neuroimaging techniques for assessing brain development in preterm infants to support early intervention strategies for preterm brain injury.
Although the precise mechanisms of brain injury in preterm infants remain incompletely understood, growing evidence has shed light on key pathological processes involved10. However, cerebral blood flow (CBF) may have been less researched. Consequently, CBF, associated with brain metabolism and the delivery of oxygen and nutrients via microcirculation, is considered a vital measure for assessing brain function development. Arterial Spin Labeling (ASL) imaging is a secure and non-invasive method for quantifying CBF in various brain regions. It is important for evaluating brain growth, damage, and repair11,12,13. Bouyssi-Kobar et al.10used ASL to show that preterm infants with parenchymal brain injury have lower global CBF and reduced CBF in specific brain regions, including the sensory-motor cortex, auditory cortex, visual cortex, anterior cingulate cortex, and meninges, in comparison to preterm infants without parenchymal brain injury. However, the study did not investigate the impacts of various types of brain injuries on the CBF of preterm infants. In spite of their distinct mechanisms and time of onset, cerebral perfusion can be altered by both IVH and PVL.
Nevertheless, there is limited focus on contrasting the impacts of IVH and PVL on the CBF of preterm infants. We conducted a prospective observational study to analyze the CBF in the cerebral cortex (frontal, temporal, parietal, occipital cortex) and metabolically active deep gray matter during the neonatal period (basal ganglia and thalamus) using ASL imaging technology with optimized post-labeling delay times in healthy preterm infants, preterm infants with IVH, and preterm infants with PVL. Additionally, we investigated the impact of clinical factors on the CBF of preterm infants.
Results
Population characteristics
During the study period, a total of 167 infants born at a gestational age (GA) of less than 32 weeks underwent cranial magnetic resonance imaging (MRI) and ASL. 17 infants were excluded (4 with genetic metabolic diseases, 2 with intracranial infection, 2 with cerebrovascular malformations, 1 with gray matter heterotopia, and 8 with poor ASL quality). Thus, a total of 150 infants were included in the final analyses. Among them, there were 108 healthy infants, 26 infants with IVH (14 with bilateral IVH and 12 with unilateral IVH), 16 infants with PVL (4 with bilateral PVL and 12 with unilateral PVL), and 4 infants with both IVH and PVL (4 with unilateral IVH and PVL) (Fig. 1). The characteristics of IVH and PVL were shown in Supplementary Table 1. All comprehensive neonatal characteristics, maternal characteristics, and neonatal morbidities were summarized in Table 1.
The three groups of preterm infants had similar perinatal characteristics, such as GA at birth, sex, small for gestational age, 5-min Apgar scores, fetal distress, gestational diabetes, pregnancy hypertension, acute chorioamnionitis, premature rupture of membranes, duration of mechanical ventilation, PMA at MRI scan, sepsis, neonatal necrotizing enterocolitis (NEC), and bronchopulmonary dysplasia (BPD). However, compared to healthy infants, those with PVL and IVH had higher birth weight and hematocrit (HCT) before cerebral MRI, as well as a greater incidence of hemodynamically significant patent ductus arteriosus (hs-PDA) (p = 0.028, 0.001, 0.005). Singleton births occurred more frequently among healthy infants and those with IVH (p = 0.037), while the incidence of cesarean section births was higher in healthy infants and those with PVL (p = 0.001) (Table 1).
Impact of brain injury on CBF in regions of interest (ROIs) of preterm infants
Accordingly, the analysis encompassed 216 healthy preterm hemispheres, 40 IVH hemispheres, and 20 PVL hemispheres. The unadjusted median CBF measured in mL/100 g/min ranged from 23.30 to 26.10 in the cortex of healthy preterm infants and from 34.60 to 41.40 in the deep gray matter. For infants with IVH, the unadjusted median CBF varied from 21.80 to 23.10 mL/100 g/min in the cortex and from 32.50 to 37.70 mL/100 g/min in the deep gray matter. The unadjusted median CBF in the cortex for infants with PVL ranged from 17.55 to 23.45 mL/100 g/min, while in the deep gray matter, it varied from 25.21 to 35.70 mL/100 g/min (Table 2).
The covariance analysis revealed statistically significant differences in CBF across the frontal, temporal, parietal, and occipital cortex, as well as in the basal ganglia and thalamus, among the three groups of brain hemispheres, after adjusting for GA at birth, PMA at MRI scan, and birth weight. Pairwise post hoc analysis indicated that PVL hemispheres consistently exhibited lower CBF in various gray matter regions compared to HP hemispheres and IVH hemispheres, including the frontal(P2 < 0.001, P3 = 0.046), temporal(P2 < 0.001, P3 = 0.002), parietal(P2 < 0.001), and occipital cortex (P2 = 0.026), as well as the basal ganglia (P2 < 0.001, P3 = 0.017), and basal ganglia (P2 < 0.001); Comparison between HP and IVH hemispheres revealed significantly lower CBF in IVH hemispheres in the frontal (P1 = 0.004), parietal (P1 = 0.004), occipital cortex (P1 = 0.004), as well as in the basal ganglia (P1 = 0.014) and thalamus (P1 = 0.019) (Table 2; Fig. 2).
Impact of clinical risk factors on CBF in ROI of preterm infants
Accordingly, all hemispheres (300 hemispheres) were analyzed. Among the clinical risk factors evaluated, the lower 5 min Apgar, longer mechanical ventilation, NEC stage ≥ 2, and brain injury were associated with reduced regional CBF in linear regression models controlling for GA at birth, PMA at MRI scan and birth weight (Supplementary Table 2). Multivariate regression analysis further demonstrated that PMA at MRI scan has a positive impact on CBF, while 5-minute Apgar score, brain injury and NEC stage ≥ 2 on have negative effects on CBF. (p < 0.05) (Table 3).
Discussion
In our prospective observational study, we demonstrated using ASL imaging technology that different forms of brain injuries in preterm infants can result in reduced regional CBF. Our findings indicate that PVL significantly affects regional CBF. Furthermore, we noted that the CBF was affected by PMA at MRI scan, 5-minute Apgar score, and NEC stage ≥ 2.
IVH is a critical issue in preterm infants and a major factor contributing to negative neurodevelopmental outcomes. Tortora et al.14 indicated that compared to healthy preterm infants, those with low-grade germinal matrix hemorrhage exhibited reduced CBF in the parietal, temporal, occipital cortex, as well as in the caudate nucleus and thalamus at term-equivalent age. Li et al.15 found that following the resolution of hemorrhage, infants with IVH exhibited sustained decreases in CBF and cerebral oxygen metabolism in the cortex, as measured by frequency-domain near-infrared spectroscopy and diffuse correlation spectroscopy. We observed a widespread CBF reduction across all ROIs on the hemorrhage side in IVH infants. The impact of IVH on CBF is enduring, potentially due to compromised vascularisation in premature infants after hemorrhage. The germinal matrix serves as the source of neuronal precursor cells. Damage to this area may cause abnormal cell production and impede their migration to the cerebral cortex, resulting in cortical dysplasia. Neurons and astrocytes exhibit analogous morphologies and are functionally interconnected with smooth muscle cells and endothelial cells of cerebral arterioles, constituting the “neurovascular unit“16. IVH may hinder the proliferation of brain cells and increase neuronal cell mortality in the cortex, which can impact cerebral metabolism. This may reduce the release of vasoactive factors such as nitric oxide and adenosine by neurons, resulting in impaired cerebrovascular autoregulation and ultimately reducing cerebral perfusion17,18.
PVL is another typical MRI-detectable white matter injury in preterm infants. Currently, there are limited studies on the quantitative assessment of CBF in preterm infants with PVL, and the findings are inconsistent. Fukuda et al.19found that the global CBF in infants with PVL was significantly diminished compared to that of healthy preterm infants during the initial days post-birth and from day 21 to day 42 with neck ultrasound examination by evaluating 36 low-birth-weight infants born at 25–34 weeks. Tortora et al.20 demonstrated that there was significantly lower overall cerebral perfusion, as well as CBF in the frontal white and gray matter, parietal white and gray matter, and basal ganglia in PVL infants compared to healthy preterm infants, using ASL imaging technology. However, Piccirilli et al.13 reported that infants with PVL had increased CBF in the frontotemporal gray matter and decreased perfusion in the insular deep gray matter. The finding was obtained from the analysis of ASL perfusion maps using a combination of an atlas-based automated segmentation of structural MRI with spatial normalization and hierarchical clustering. Our observations indicate that the CBF in all ROIs of PVL preterm infants was generally lower than that in healthy preterm infants, which are consistent findings of Tortora et al. We also demonstrated that perfusion in the temporal, occipital cortex, and thalamus was diminished compared to that in healthy preterm infants. PVL is frequently accompanied by neuronal loss, gliosis in the deep gray matter and cortex, and diminished brain volume. This directly influences myelin formation or maturation/nutrition, which in turn affects changes in CBF1. The thalamus is the gray matter nucleus that exhibits the greatest susceptibility to neuronal loss and gliosis in PVL21,22. Adolescents born preterm who exhibit cognitive failure frequently have enduring cortical defects, primarily in the parieto-occipital, sensory-motor, premotor, and temporal regions23,24. The nutritional impacts of PVL and IVH on the cortex and subcortical gray matter are more pronounced in infants with PVL, especially in the frontotemporal gray matter and basal ganglia regions. PVL exerts a more significant and broad influence on the developing brain compared to IVH, maybe suggesting that PVL causes a stronger microglial response. Microglia, essential immune cells within the brain, worsen ischemia-reperfusion injury through the release of pro-inflammatory factors such as IL-6, IL-1β, IL-12, and IL-23. Furthermore, microglia constitute a crucial component of the neurovascular unit, and by regulating microglial activity, CBF or adaptation to reduced perfusion can be modified25. Nonetheless, when the developing brain is affected by ischemia and hypoxia, the regulatory function of microglia is impaired, resulting in vasospasm or the “no-reflow phenomenon” following cerebral ischemia26,27,28.
Postnatal alterations in regional cerebral perfusion and metabolism may be associated with the development and maturation of cerebral structures. Our findings were consistent with previous studies that CBF in the deep gray matter was higher than that in the cerebral cortex and unaffected by perinatal brain injury10,13,29. The development of basal ganglia and thalamus, surrounded by abundant capillary networks, occurs prior to that of the cortex. Additionally, they have significant neuronal activity in reaction to sensory stimuli, resulting in increased cerebral perfusion. This study also found that in healthy preterm infants, the CBF in the occipital gray matter, corresponding to the visual cortex, is the highest. In contrast, the CBF in the temporal gray matter, which corresponds to the auditory cortex, is the lowest. The findings are consistent with the results reported by Smyser and Dubois30,31. This may be related to the thinner cortex in the frontal, temporal, and parietal association areas in preterm infants32,33. The reduced blood flow in the temporal cortex may be associated with extended exposure to noisy environments, suggesting that early exposure to atypical acoustic environments may alter the plasticity of auditory network development34,35.
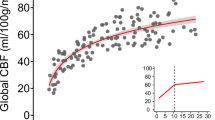
The CBF may be influenced by a range of perinatal factors. Several studies have investigated the relationship between PMA at MRI scan and CBF. Our findings support previous studies36,37,38,39showing that CBF in the cerebral cortex increases with PMA at MRI scan. With brain development, metabolism, and oxygen consumption increase with age, resulting in higher CBF. Furthermore, early exposure to the extrauterine environment facilitates experience-dependent cortical development, which enhances regional cerebral metabolic activity40. Our research aligns with prior findings, suggesting that NEC stage ≥ 2 may result in a decrease in CBF41. This event may be ascribed to the increased risk of compromised cerebrovascular autoregulation in these infants due to major surgery42. Moreover, the 5-minute Apgar score is a contributing factor influencing CBF. This may entail compromised autoregulation of CBF due to neurovascular coupling failure, hence elevating the risk of hemorrhagic brain injury43,44. Some studies have established that brain parenchymal injury can result in diminished CBF10,20indicating that such injury may lead to a loss in neuronal count and an increase in reactive glial cell proliferation in the cerebral cortex21,45. Despite GA at birth has been suggested to be a crucial predictor of CBF13,14we found no significant correlation between GA at birth and CBF in the multiple linear regression model. This finding may be related to the relatively small variation among individuals of GA at birth. Liu et al.46 discovered that premenopausal women had higher CBF than men, comparing premenopausal and postmenopausal women with men. However, our study did not find a significant impact of sex on CBF, which may be related to the inadequate release of sex hormones in infants.
It is important to note the limitations of our study. Firstly, as a preliminary exploratory study, our findings are limited by the small sample size and lack of longitudinal assessments, which restrict both the analysis of trends in post-injury cerebral perfusion dynamics and stratified evaluations based on IVH or PVL severity. Secondly, the study did not gather CBF during the acute stage of brain injury due to the necessity of respiratory support for infants. Additionally, there was no neurological follow-up, so the significance of changes in regional CBF for neurodevelopmental outcomes in preterm infants remains uncertain. Future studies should include long-term, dynamic, and comprehensive follow-ups to establish the relationship between CBF and long-term neurological outcomes.
We found that both IVH and PVL can decrease CBF in preterm infants, with PVL having a more significant impact on CBF. Additionally, PMA at MRI scan, asphyxia, and NEC stage ≥ 2 can adversely influence brain perfusion. Therefore, comprehensive management is necessary in clinical practice to prevent fluctuations in CBF in preterm infants. To address these challenges, further research is needed to investigate the underlying pathophysiological mechanisms between different forms of brain injuries and changes in CBF in preterm infants.
Methods
Study design
This prospective observational study enrolled preterm infants with a GA of less than 32 weeks, born at the Third Affiliated Hospital of Zhengzhou University between June 2022 and March 2024, who underwent cranial MRI and ASL imaging prior to discharge. Infants who presented with genetic or metabolic diseases and congenital brain malformations and experienced central nervous system infections were excluded from the study. This study was conducted in accordance with the principles embodied in the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (2022-126-01). The legal guardians of all participants signed informed consent.
Clinical data
We collected the following clinical data: GA at birth, birth weight, gender, cesarean section, singleton births, small for gestational age, 5 min Apgar score, pregnancy hypertension, gestational diabetes, fetal distress, premature rupture of membranes, duration of mechanical ventilation, HCT before MRI scan, PMA at MRI scan, NEC (stage ≥ 2), hs-PDA, sepsis, and BPD.
MRI acquisition and image processing
Cranial ultrasonography examinations were performed on infants within 3 days post-birth, at 7 days, and then weekly until discharge. Before discharge, MRI and ASL imaging with GE SIGNA Pioneer 3.0T MRI technology were conducted. Prior to acquisition, the infants were injected and sedated with a single dose of phenobarbital (5 mg/kg). Motion artifacts were reduced through the use of soundproof sponge pads, while a neonatologist consistently monitored oxygen saturation during the image acquisition process.
The scanning protocol included routine cranial MRI sequences (T1WI, T2WI, FLAIR, DWI) and ASL sequence. The ASL sequence is composed of Ax-T2CUBE brain structure image and pseudo-continuous ASL (pCASL). The Ax-T2CUBE brain structure image is used to examine brain morphology, and the following settings: TR = 5002ms, TE = 98.4ms, FOV = 240 mm×316 mm, slice thickness 2.0 mm, interslice gap 1 mm, matrix size 220 × 220, excitation times 2, slices 144, scan time 3 min 40s.The pCASL imaging was matched to the Ax-T2CUBE brain structure image, which was copied by a positioning line, and pure axial scanning was performed. The pCASL acquisition utilized a spin-echo spiral readout sequence and background suppression (TR = 4650ms, TE = 10.86ms, FOV = 240 mm×316 mm, slice thickness 4.0 mm, interslice gap 4.0 mm, matrix size 512 × 512, excitation times 3, slices 72). The pCASL protocol employed a labeling duration of 1800 ms and a post-labeling delay of 2000 ms, with the labeling plane positioned at the cervical region. The total scan time for the pCASL sequence was 4 min 10s. Image postprocessing was performed using an Advantage Workstation 4.7. Fusion images of the two aforementioned sequences were created using ReadyView software, generating a pseudocolor perfusion map with distinct color coding (Fig. 3). The ROIs on the fusion map were manually selected by an experienced pediatric neuroradiologist, including the cerebral cortex (frontal, temporal, parietal, occipital cortex) and the metabolically active deep gray matter (basal ganglia and thalamus). A circular measurement tool (10 ± 2 mm² in area) was used to delineate each ROI on a clear layer, ensuring symmetry of the ROIs in homologous regions of both hemispheres. To guarantee accuracy, the average value was calculated after three measurements.
MRI analysis
Brain injury was diagnosed based on a qualitative MRI assessment. IVH includes periventricular intraventricular hemorrhage (PIVH) and cerebellar hemorrhage (CBH). PVL and CBH were evaluated according to the Kidokoro score47 whereas the PIVH grade was based on Papile et al.48. Given that IVH and PVL predominantly manifest as unilateral cerebral hemisphere lesions, we employed a per-hemisphere analytical strategy concerning PVL and/or IVH in this study.
Statistical analysis
The analysis was conducted with SPSS 26.0 software and GraphPad Prism. For normally distributed quantitative data, differences were assessed using the one-way ANOVA test (with planned contrasts) and results were expressed as mean ± standard deviation (SD). Non-normally distributed quantitative data was expressed by median and interquartile range, then evaluated using the Kruskal-Wallis test. Count data was examined with the Chi-square test or Fisher’s exact test. The multivariate data analysis entailed applying covariance analysis to adjust for GA at birth, PMA at MRI scan, and birth weight on CBF. A pairwise post hoc analysis was performed using the LSD method. A two-sided p-value < 0.05 was considered statistically significant.
When evaluating the relationship between clinical factors and CBF, linear regression models were used to evaluate the relationship between regional CBF and each clinical risk factor with adjustments for GA at birth, PMA at MRI scan and birth weight. Multiple regression modeling was used to examine the combined influence of all clinical factors significantly (p < 0.05) associated with regional CBF variations.
References
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Pascal, A. et al. The impact of intraventricular hemorrhage and periventricular leukomalacia on mortality and neurodevelopmental outcome in very preterm and very low birthweight infants: A prospective Population-based cohort study. J. Pediatr. 262, 113600 (2023).
Matthews, L. G. et al. Brain growth in the NICU – Critical periods of Tissue-Specific expansion. Pediatr. Res. 83, 976–981 (2018).
Kamei, A., Houdou, S., Mito, T., Konomi, H. & Takashima, S. Developmental change in type VI collagen in human cerebral vessels. Pediatr. Neurol. 8, 183–186 (1992).
Ballabh, P. & De Vries, L. S. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat. Rev. Neurol. 17, 199–214 (2021).
Song, J. et al. Clinical characteristics and long-term neurodevelopmental outcomes of leukomalacia in preterm infants and term infants: a cohort study. J. Neurodev Disord. 15, 24 (2023).
Bolisetty, S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133, 55–62 (2014).
Périsset, A. et al. Impact of low-grade intraventricular hemorrhage on neurodevelopmental outcome in very preterm infants at two years of age. Early Hum. Dev. 177–178, 105721 (2023).
Sakaue, S. et al. Low-grade IVH in preterm infants causes cerebellar damage, motor, and cognitive impairment. Pediatr. Int. 63, 1327–1333 (2021).
Bouyssi-Kobar, M. et al. Altered cerebral perfusion in preterm infants compared with Full-term infants. J. Pediatr. 193, 54–61e2 (2018).
Alsop, D. C. et al. Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. (2015).
Ouyang, M. et al. Heterogeneous increases of regional cerebral blood flow during preterm brain development: preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. NeuroImage 147, 233–242 (2017).
Piccirilli, E. et al. Cerebral blood flow patterns in preterm and term neonates assessed with pseudo-continuous arterial spin labeling perfusion MRI. Hum. Brain Mapp. 44, 3833–3844 (2023).
Tortora, D. et al. Regional impairment of cortical and deep Gray matter perfusion in preterm neonates with low-grade germinal matrix-intraventricular hemorrhage: an ASL study. Neuroradiology 62, 1689–1699 (2020).
Lin, P. Y., Hagan, K., Fenoglio, A., Grant, P. E. & Franceschini, M. A. Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix- intraventricular hemorrhage. Sci. Rep. 6, 25903 (2016).
Carlo, W. A. et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl. J. Med. 363, 1285–1286 (2010).
Sancho, M. et al. Adenosine signaling activates ATP-sensitive K + channels in endothelial cells and pericytes in CNS capillaries. Sci. Signal. 15, eabl5405 (2022).
Brew, N., Walker, D. & Wong, F. Y. Cerebral vascular regulation and brain injury in preterm infants. Am. J. Physiol. -Regul Integr. Comp. Physiol. 306, R773–R786 (2014).
Fukuda, S. et al. Late circulatory dysfunction and decreased cerebral blood flow volume in infants with periventricular leukomalacia. Brain Dev. 30, 589–594 (2008).
Tortora, D. et al. Prematurity and brain perfusion: arterial spin labeling MRI. NeuroImage Clin. 15, 401–407 (2017).
Pierson, C. R. et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. (Berl). 114, 619–631 (2007).
Ligam, P. et al. Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr. Res. 65, 524–529 (2009).
Nosarti, C. et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 131, 205–217 (2008).
Kesler, S. R. et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J. Pediatr. 152, 513–520e1 (2008).
Császár, E. et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 219, e20211071 (2022).
Eyo, U. & Dailey, M. E. Effects of Oxygen-Glucose deprivation on microglial motility and viability in developing mouse hippocampal tissues. Glia 60, 1747–1760 (2012).
Masuda, T., Croom, D., Hida, H. & Kirov, S. A. Capillary blood flow around microglial Somata determines dynamics of microglial processes in ischemic conditions. Glia 59, 1744–1753 (2011).
Kloner, R. A., King, K. S. & Harrington, M. G. No-reflow phenomenon in the heart and brain. Am. J. Physiol. -Heart Circ. Physiol. 315, H550–H562 (2018).
Qin, C. et al. Evaluation of the effect of intraventricular haemorrhage on cerebral perfusion in preterm neonates using three-dimensional pseudo-continuous arterial spin labelling. Pediatr. Radiol. 54, 776–786 (2024).
Schwab, A. L. et al. Cerebral oxygenation in preterm infants developing cerebral lesions. Front. Pediatr. 10, 809248 (2022).
Dubois, M. et al. Multiparametric analysis of cerebral development in preterm infants using magnetic resonance imaging. Front. Neurosci. 15, 658002 (2021).
Zubiaurre-Elorza, L. et al. Cortical thickness and behavior abnormalities in children born preterm. PLoS ONE. 7, e42148 (2012).
Schmitz-Koep, B. et al. Decreased cortical thickness mediates the relationship between premature birth and cognitive performance in adulthood. Hum. Brain Mapp. 41, 4952–4963 (2020).
Chang, E. F. & Merzenich, M. M. Environmental noise retards auditory cortical development. Science 300, 498–502 (2003).
Sanes, D. H. & Bao, S. Tuning up the developing auditory CNS. Curr. Opin. Neurobiol. 19, 188–199 (2009).
Kim, H. G. et al. Multidelay arterial Spin-Labeling MRI in neonates and infants: cerebral perfusion changes during brain maturation. Am. J. Neuroradiol. 39, 1912–1918 (2018).
Kim, H. G., Choi, J. W., Lee, J. H., Jung, D. E. & Gho, S. M. Association of cerebral blood flow and brain tissue relaxation time with neurodevelopmental outcomes of preterm neonates. Invest Radiol 00, (2021).
De Vis, J. B. et al. Regional changes in brain perfusion during brain maturation measured non-invasively with arterial spin labeling MRI in neonates. Eur. J. Radiol. 82, 538–543 (2013).
Zun, Z. et al. Longitudinal trajectories of regional cerebral blood flow in very preterm infants during third trimester ex utero development assessed with MRI. Radiology 299, 691–702 (2021).
Jandó, G. et al. Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proc. Natl. Acad. Sci. 109, 11049–11052 (2012).
Howarth, C. et al. Cerebral oxygenation in preterm infants with necrotizing Enterocolitis. Pediatrics 146, e20200337 (2020).
Kuik, S. J. et al. Preterm infants undergoing laparotomy for necrotizing Enterocolitis or spontaneous intestinal perforation display evidence of impaired cerebrovascular autoregulation. Early Hum. Dev. 118, 25–31 (2018).
Parmentier, C. E. J. et al. Brain MRI injury patterns across gestational age among preterm infants with perinatal asphyxia. Neonatology 121, 616–626 (2024).
Badurdeen, S. et al. Haemodynamic instability and brain injury in neonates exposed to Hypoxia–Ischaemia. Brain Sci. 9, 49 (2019).
Sharma, D. R., Agyemang, A. & Ballabh, P. Cerebral Gray matter injuries in infants with intraventricular hemorrhage. Semin Perinatol. 46, 151595 (2022).
Liu, W., Lou, X. & Ma, L. Use of 3D pseudo-continuous arterial spin labeling to characterize sex and age differences in cerebral blood flow. Neuroradiology 58, 943–948 (2016).
Kidokoro, H. et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134, e444–e453 (2014).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 Gm. J. Pediatr. 92, 529–534 (1978).
Acknowledgements
We would like to thank all the infants and their parents who participated in this study and the pediatric neuroradiologist for performing the image processing and MRI analysis.
Funding
This study was supported by the Open project of the Clinical Medical Research Center of Pediatric Disease in Henan Province (KFKT2021103), a grant of the Henan Medical Science and Technique Foundation (LHGJ20190350).
Author information
Authors and Affiliations
Contributions
The study’s conception and design: C Z, and FL X. Data acquisition: C Z, HY F, WL L, H S, and Q Y. Data analysis: C Z, M N, and HF D. Drafting the manuscript: C Z and HY F. Revising the manuscript: HF D, WL L, and FL X. All authors contributed to the article and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Medical Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (2022-126-01). The legal guardians of all participants signed informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Li, W., Fan, H. et al. The impact of different types of brain injuries on cerebral perfusion in preterm infants: an arterial spin labeling. Sci Rep 15, 29700 (2025). https://doi.org/10.1038/s41598-025-15667-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15667-5