Abstract
Ionizing radiation exposure, whether from accidental incidents, radiation therapy, or radiological weapons, poses a significant risk to public health and military personnel. Survivors experience both acute and chronic physiological effects, including tissue dysfunction, fibrosis, and impaired organ function. Among affected tissues, skeletal muscle is particularly vulnerable, as radiation damages muscle precursor cells (MPCs), impairing their ability to regenerate and maintain muscle homeostasis. Extracellular vesicles (EVs), nano-sized lipid-bound vesicles released by cells, mediate intercellular communication by transferring bioactive molecules such as proteins, lipids, and microRNAs. EVs derived from MPCs have shown promise in promoting muscle regeneration, yet their role in radiation injury remains unclear. This study investigated whether EVs from healthy MPCs (NoRad-EVs) could improve cell function in irradiated cells compared to EVs from irradiated MPCs (Rad-EVs). Our findings demonstrated that viability and proliferation are improved in irradiated MPCs receiving NoRad-EVs whereas Rad-EVs fail to mitigate radiation-induced damage. Specifically, NoRad-EVs increased MPC viability from 52 ± 5.7% to 71 ± 4.9% and improved BrdU-measured proliferation by ~ 16% compared to untreated irradiated controls. NoRad-EVs also enhanced angiogenesis in human umbilical vein endothelial cells (HUVECs) and microvascular fragments (MVFs). HUVECs treated with NoRad-EVs showed significantly greater tube branching length (~ 1.5-fold increase) compared to cells exposed to Rad-EVs. Similarly, MVFs receiving NoRad-EVs treatment exhibited a ~ 3-fold increase in vessel density after 7 days as opposed to the group cultured with Rad-EVs. Differential miRNA expression analysis revealed significant alterations in Rad-EVs compared to NoRad-EVs, affecting key pathways related to muscle repair, angiogenesis, and oxidative stress response. Thirteen miRNAs were downregulated and seven were upregulated in Rad-EVs compared to NoRad-EVs (fold change ≥ 1.3, p < 0.05), including targets involved in VEGF, PI3K-Akt, and FoxO signaling. This research underscores the need for effective countermeasures against radiation-induced injuries, which have detrimental effects on the pro-angiogenic and proliferative functions of MPCs and their secreted EVs. Overall, these promising in vitro findings support the restorative impacts of NoRad-EVs as a potential therapeutic, including miRNA-enriched vesicles, in mitigating radiation-induced muscle injury, particularly in the context of military medicine and radiological emergencies.
Similar content being viewed by others
Introduction
Ionizing radiation exposure—whether accidental, therapeutic, or weaponized—poses serious risks to public health and military readiness. It is a growing concern for agencies like FEMA and the U.S. military1,2. Survivors may suffer acute and chronic effects, including fibrosis, tissue dysfunction, and damage to organs such as the digestive tract and salivary glands3,4. Radiation generates reactive oxygen species (ROS), which cause protein modifications and damage to DNA, RNA, and cell membranes, leading to disrupted cell signaling, senescence, or necrosis5,6. Over four million cancer patients receive radiation therapy annually, and even partial-body exposure can result in long-term side effects7,8. Dirty bombs and radiological weapons further elevate the risk of widespread exposure9. Therefore, there remains a critical need for effective, tissue-targeted strategies to mitigate both the immediate and delayed effects of radiation injury10,11.
Skeletal muscle is a highly organized tissue containing several bundles of post-mitotic muscle fibers or muscle cells that often get damaged due to normal wear and tear or injury and must be repaired or replaced12,13,14. Skeletal muscle regeneration or myogenesis is a highly orchestrated process involving the activation of various cellular and molecular responses15,16. Satellite cells or resident muscle stem cells play an essential role in the growth, maintenance, and repair of muscle tissue17,18. Under normal physiological conditions, skeletal muscle satellite cells are quiescent and in a low metabolic state but get activated upon damage to the muscle tissue. The progeny of activated satellite cells, sometimes referred to as myoblasts, muscle progenitor cells, or muscle precursor cells (referred to as MPCs hereafter) can proliferate and then either fuse with each other to form multinucleated myotubes, which can mature into new myofibers, or fuse with damaged segments of muscle fibers19. In addition to their myogenic potential, MPCs also promote angiogenesis—the formation of new blood vessels— by releasing paracrine and pro-angiogenic factors, which help establish a functional vasculature necessary for muscle tissue regeneration20,21. Although not fully understood, there is a growing body of evidence supporting the role of MPC-mediated angiogenesis in maintaining muscle homeostasis and promoting regeneration21,22. Since vascular remodeling improves oxygen and nutrient delivery, examining angiogenic responses alongside myogenesis provides a more comprehensive understanding of skeletal muscle repair following radiation injury.
Unlike muscle fibers, MPCs are sensitive to radiation and can lose their ability to self-renew and differentiate into muscle fibers upon irradiation23. Previous work has shown that even a single low to moderate dose of ionizing radiation can significantly diminish MPCs’ proliferation and differentiation potential, thereby limiting myogenesis24. Irradiation may reduce satellite cells’ ability to properly regulate angiogenesis and maintain a healthy capillary density, further reducing the growth and development of muscle tissue25,26.
Currently, a full understanding of the effects of irradiation on MPC homeostasis and the impact on cell-to-cell communication is lacking. Intercellular communication within the MPCs niche is essential for myogenesis, angiogenesis, and maintenance of skeletal muscle function, and this communication is mediated, at least in part, through extracellular vesicles (EVs)27.
Extracellular vesicles are a heterogeneous group of nano-sized lipid membrane-bound biovesicles. EV size ranges from 30 to 1000 nm in diameter. They originate from multivesicular endosomes and are released by all types of cells into the extracellular environment. Extracellular vesicles carry proteins, lipids, DNA, and RNA (including microRNA) from their parent cells and deliver them to the recipient cells, influencing the behavior and phenotype of the targeted cells28. EVs are the paracrine effectors and mimic important activities of their parental cells. For example, several studies have reported that mesenchymal stem cell (MSC)-derived EVs can promote wound healing and have a similar therapeutic effect as their parent MSCs29,30,31,32. The therapeutic properties of MSC-EVs are primarily attributed to their microRNAs (miRNAs) cargo which are capable of modulating gene expression at the post-transcriptional level33. Due to their low immunogenicity, slower clearance, and ease of storage through lyophilization, EVs have emerged as a promising candidate for cell-free therapeutics34. According to recent studies, extracellular vesicles derived from healthy MPCs can promote repair and growth in injured muscle tissue35,36,37. While EVs from other cell types such as mesenchymal stem cells have been studied in regenerative contexts, few investigations have directly compared EVs derived from irradiated versus non-irradiated MPCs. This study builds on earlier work38,39 by identifying how radiation alters the regenerative potential of MPC-EVs, which has not been previously characterized in detail.
In the current study, we sought to determine whether irradiation has an effect on the ability of MPC-derived EVs to impact myogenic cell proliferation and angiogenesis. Because EVs have been shown to mimic and promote the function of their parent cells, we hypothesized that EVs from healthy, non-irradiated MPCs (NoRad-EVs) will improve myogenesis in irradiated-MPCs and promote angiogenesis in vascular endothelial cells compared to EVs from irradiated-MPCs (Rad-EVs). Herein, we describe the effects of NoRad-EV and Rad-EV treatments on irradiated MPCs’ viability and proliferation capacity and human umbilical vein endothelial cell and microvascular angiogenesis. Furthermore, we describe the radiation-induced compositional changes in MPC-EV miRNA cargo and used the miRNA differential expression analysis to predict the genes and pathways that could be the targets on MPC-EV miRNA. Overall, our study contributes to the understanding of EV-mediated effects in skeletal muscle and also examines the possibility of employing an EV-mediated therapeutic strategy to mitigate the radiation damage in skeletal muscle tissue.
Materials and methods
This study has been conducted in compliance with the Animal Welfare Act and the Implementing Animal Welfare Regulations in accordance with the principles of the Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee at the US Army Institute of Surgical Research (Joint Base San Antonio, Texas) and the University of Texas at San Antonio. This study is also reported in accordance with the ARRIVE guidelines.
Muscle precursor cells (MPC) isolation and culture
Four to six month old male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used as the source of MPCs. Rat skeletal muscle tissues were obtained in accordance with approved institutional tissue-sharing agreements from another IACUC-approved study. Donor animals were euthanized using 2.5% isoflurane in 100% oxygen followed by exsanguination, in compliance with institutional ethical guidelines. MPCs were isolated from rat soleus muscle through a series of steps: tissue mincing, protease digestion, centrifugation, filtration, and selective pre-plating, following a protocol similar to that reported by Acosta et al.40. MPCs were cultured in a growth medium (GM) composed of 20% fetal bovine serum, 1% penicillin-streptomycin (15140-122; Gibco Inc., Billings, MT), 0.2% MycoZap (VZA-2031; Lonza. Walkersville, MD), and Dulbecco’s Modified Eagle Medium (DMEM) (11885-084, Gibco Inc., Billings, MT) containing 1 g/L D-glucose, L-glutamine, and 110 mg/L sodium pyruvate in a humidified incubator at 37 °C and 5% CO2 until ~ 80% confluency before passaging, with passage 2 or 3 cells being used for all experiments. MPCs were cultured on Geltrex (A1569601; Gibco Inc., Billings, MT) basement membrane matrix-coated tissue-culture plates or flasks. For each experimental group, EVs were collected from MPCs isolated from at least 4 individual rats. Biological replicates were not pooled; each replicate was independently processed and analyzed.
MPC irradiation
A CellRad benchtop X-ray irradiator (Precision X-Ray, Madison, CT) was used to irradiate MPCs using an automated dose control setting at 130 kV and 5 mA to deliver a preset total X-ray dose. Rad-Sure irradiation indicator labels (NC9149017; Ashland Specialty Ingredients LSC, Wilmington, DE) were used to verify MPC irradiation.
Preparation of MPC conditioned media and EV isolation
MPCs were cultured and expanded in T175 tissue-culture flasks in growth media to 80% confluency at passage 3 followed by 10 Gray (Gy) irradiation (Rad-MPCs) or no irradiation (NoRad- MPCs). EVs were isolated following a protocol similar to that reported by Chance et al.41. MPCs in flasks were rinsed twice with serum-free media (DMEM) to remove residual serum. Following these rinses, the cells were cultured in serum-free media for 48 h. The conditioned media from each MPC group (Rad or NoRad) was collected, centrifuged (Avanti J-25, Beckman Coulter, Brea, CA) for 20 min at 2,000 g at 4 °C to exclude large cell contaminants, and the supernatant was passed through a 0.8 μm pore-size filter (Thomas Scientific, Swedesboro, NJ). The filtrate was transferred to new 15-ml round bottom tubes, 5 mL/tube, and centrifuged for 90 min at 10,000 × g at 4 °C to concentrate EVs. The supernatant was removed to leave 200 µL of material in the tubes. The isolated EVs were either used immediately or frozen at − 80 °C in serum-free DMEM for up to two months. Where appropriate, EVs were thawed to room temperature and gently vortexed before use.
EV and miRNA characterization
The size distribution of particles was determined with the NanoSight NS300 system (Malvern Instruments Ltd., Worcestershire, UK); EV preparations with ≥ 95% of particles between 50 and 400 nm range were used for experiments. EVs size and morphology were visualized through Transmission Electron Microscopy (TEM) following a protocol similar to that reported by Jung and Mun42. 10 µL of 1:10 diluted EV suspension in filtered ultrapure water was loaded on a formvar/carbon film coated 300 mesh copper grid (Electron Microscopy Sciences, Hatfield, PA). Loaded EV samples were negative stained with 1% uranyl acetate for 2 min, followed by a quick rinse with a drop of water and allowed to air dry at room temperature for 10 min. Stained samples were observed under a transmission electron microscope (JEM-1400; JEOL, Tokyo, Japan) and imaged. EV purity was confirmed via detection of canonical exosomal markers flotillin-1 and CD9 (Supplemental Fig. 1). A subset of exosomes was lysed in radioimmunoprecipitation assay (RIPA) lysis and extraction buffer (Thermo Scientific, Waltham, MA), and their protein content was measured using the Bicinchoninic acid (BCA) assay. To assess miRNA levels within exosomes, miRNAs were isolated from EVs using the Qiagen exoRNeasy total RNA isolation kit (Qiagen, Germantown, MD). miRNA expression profiles were determined using the nCounter rat-miRNA Expression Assay Kit (Nanostring Technologies, Seattle, WA), following the manufacturer’s protocol.
Western blot using jess system
Flotillin-1 was analyzed using the Jess capillary-based western blot system (ProteinSimple, Bio-Techne brand, San Jose, CA). EV samples (1–2 µg) were prepared using fluorescent master mix and processed using the standard size-based separation program. Anti-flotillin-1 (BD Biosciences, Cat# 610821; 1:500 dilution) was used as the primary antibody. Detection and analysis were performed using Compass software.
Traditional western blot
EV samples (25 µg) were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were probed with anti-CD9 (BD Biosciences, Franklin Lakes, NJ; 1:500 dilution) diluted in TBST containing 5% non-fat dry milk. Detection was performed using HRP-conjugated secondary antibody and chemiluminescence.
Cell viability analysis
To determine the effect of EV treatment on the irradiated MPC viability, 20,000 MPCs were seeded in a Geltrex-coated 48 well plate to an 80% confluency, followed by 10 Gy irradiation. Immediately after irradiation, 100 ul of EVs (Rad or NoRad, 50 µg total protein) or vehicle control (serum-free DMEM) was added to each well. MPCs were collected at 48-hour time point after irradiation and stained with acridine orange (live cells) and propidium iodide (dead cells) and analyzed for viability using Celleca MX cell counter (Nexcelom Bioscience, Lawrence, MA).
Cell proliferation analysis
To determine the effect of EV treatment on cell proliferation in irradiated MPCs, MPCs were cultured in 96 well plate to ~ 80% confluency, followed by 10 Gy irradiation. Immediately after irradiation, 50 µl of EVs (Rad or NoRad, 25 µg total protein) or vehicle control (serum-free DMEM; 31053028, Gibco Inc., Billings, MT).) was added to each well. At 48 h after irradiation and EV addition, 20 µl of 1X BrdU reagent (supplied with BrdU cell proliferation ELISA kit, ab126556; Abcam, Cambridge, UK) was added to each well and incubated for 24 h as per manufacturer’s recommendations. At 24 h after BrdU addition (T = 72-hour post irradiation), 100 µL of Stop Solution was added to every well and the calorimetric absorbance was immediately read using SpectraMax® M5e Multi-Mode microplate reader (Molecular Devices, LLC, San Jose, CA) set at a dual wavelength of 450/550 nm.
Angiogenesis analysis (HUVEC tube formation)
Tube formation assays were performed on human umbilical vein endothelial cells; HUVECs (Lonza, Basel, Switzerland) at passages 4 to 5 and grown in endothelial cell growth medium; ECGM (Lonza, Basel, Switzerland). HUVECs were harvested in TrypLE media, washed, and resuspended in Endothelial cell basal medium; ECBM (Lonza, Basel, Switzerland). HUVECs were mixed with ECBM and 25 µg (low dose) or 75 µg (high dose) of total EV protein (100 µL EVs + 300 µL ECBM). EV doses of 25 µg and 75 µg total protein were selected based on previous literature demonstrating effective therapeutic ranges for EV dosing quantified by protein content29,43. Additionally, dose selection was practically limited by the experimental volume constraints to avoid dilution effects on cell culture media, which could influence experimental outcomes. A DMEM vehicle control treated similarly to the EVs was included. The HUVEC/media mixes were added to 24-well plates with 100 µL per well of basement membrane matrix Geltrex LDEV-free reduced growth factor matrix (per manufacturer’s recommendations (A1569601; Gibco Inc., Billings, MT). Final HUVEC cell concentration was 25,000 cells/cm2 in 400 µL of volume (47,500 cells/ well). Plates were incubated in a humidified incubator (37 °C, 5%CO2, ambient O2), and phase contrast images (five fields per well) were taken at 24 h (n = 4, biological replicates of two experiments run in duplicate). Time zero was used to denote the time at which the HUVECs and experimental groups were added to the matrix. Tube formation parameters were measured using the Angiogenesis Analyzer for ImageJ (Carpentier, Angiogenesis Analyzer, Image J version 1.52a; National Institute of Health, Bethesda, MD). Phase contrast images (five fields per well) were captured at 24 h post-seeding, a time point commonly used to assess peak HUVEC tube formation prior to structural regression43. Fields were randomly selected, and all imaging parameters (e.g., magnification, exposure, and focus) were held constant. Images were excluded from analysis only if they were out of focus, poorly illuminated, or lacked visible cellular structures. This ensured objective and consistent quantification using the Angiogenesis Analyzer plugin for ImageJ.
Angiogenesis analysis (microvascular growth and sprouting)
Microvascular fragments (MVFs) were isolated from the inguinal fat depots similar to that previously described by Acosta et al.44. Briefly, adipose tissue from inguinal subcutaneous fat from 8 to 12-month-old male Lewis rats (Envigo, Huntingdon, United Kingdom) were subjected to a 10 to 15 min collagenase type I digestion (Worthington Biochemical Corporation, Lakewood, NJ) at 37 °C with agitation. The digested material was centrifuged (400 g x 4 min) to obtain a pellet containing a heterogeneous mixture of cells and MVFs. The pellet was resuspended in phosphate-buffered saline containing 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and filtered through 500 and 37 μm filters (Carolina Biological Supply, Burlington, NC) to remove large debris and minimize cell contamination, respectively. MVFs were then counted, centrifuged, and resuspended in fibrinogen (Sigma-Aldrich) in Dulbecco’s modified Eagle’s medium (DMEM; 20 mg/ mL) at a concentration of 20,000 MVFs/mL. Fibrin hydrogels (5.7 mg/mL) were formed by combining MVFs containing fibrinogen (at a concentration of 20,000 MVFs/mL) and 10 U/mL thrombin (Millipore Sigma, St. Louis, MO) in 96-well culture plates. Hydrogels for polymerase chain reaction (PCR) were 100 µL in volume, whereas gels for all other analyses were 50 µL. Fibrin hydrogels containing MVFs was grown for a period of 7 days in growth media (GM). Sequential doses of 25 μg EV protein were added to MVF gels on days 1, 2, and 4. For all treatments the appropriate medium (100 µL in a 96-well plate) was replaced every other day throughout the study and cultures were maintained in a humidified incubator at 37 °C and 5% CO2. All media for hydrogels was supplemented with 1 mg/mL aminocaproic acid to inhibit fibrinolysis. On day 8, hydrogels were fixed in 4% formaldehyde for 2 h at room temperature, permeabilized using 0.5% Triton-X for 20 min, blocked using 10% goat serum for 2 h, then stained using Rhodamine-labeled Griffonia (Bandeiraea) Simplicifolia Lectin I (GS-1; 1:100; Vector Laboratories, Burlingame, CA). The distribution of both vessels and determined using a Leica TCS SP8 Confocal Microscope (Leica, Buffalo Grove, IL) using a rendering of 100 mm thickness/10 mm sections of the entire well coverage area. Quantification was performed using the Leica three-dimensional (3D) analysis toolkit using Otsu thresholding.
Statistical analyses
Differences among the groups of interest were calculated using one-way analysis of variance. Significant differences were identified using Tukey’s post hoc test at p < 0.05. Formal normality testing was not performed due to small sample sizes (typically n = 3–4). Statistical tests were selected using default settings in GraphPad Prism Software 10 (GraphPad Software, Inc., La Jolla, CA). All results are presented as mean ± standard error of the mean. Graphs, volcano plots, and heat maps were generated using GraphPad Prism.
Software tools
Statistical analysis was performed using GraphPad Prism version 10.1.2 (GraphPad Software, Boston, MA, USA; https://www.graphpad.com); miRNA expression data were analyzed using nSolver Analysis Software version 4.0 (NanoString Technologies, Seattle, WA, USA; https://nanostring.com/products/ncounter-analysis-system/nsolver-advanced-analysis-software/); miRNA target prediction and network analysis were conducted using miRTargetLink 2.0 (https://ccb-compute.cs.uni-saarland.de/mirtargetlink2); Flotillin-1 protein expression was analyzed using Compass for Simple Western version 6.0.0 (Bio-Techne, Minneapolis, MN, USA; https://www.bio-techne.com/resources/instrument-software-download-center/compass-software-simple-western); Grammarly’s AI-powered writing assistant was used to check grammar, spelling, and sentence clarity during manuscript preparation. The tool did not contribute to the scientific content or interpretation of the results.
Results
EVs isolated from non-irradiated or irradiated MPCs maintain similar morphologies, size distributions, and total protein content
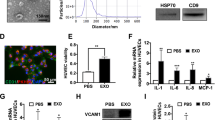
Conditioned media was collected from non-irradiated MPCs (NoRad-MPCs) or irradiated MPCs (Rad-MPCs) after 48 h of incubation in serum-free media, as illustrated in Fig. 1. Transmission electron microscopy (TEM) revealed no visually detectable differences between EVs from NoRad-MPCs (NoRad-EVs) and Rad-MPCs (Rad-EVs); both populations exhibited round or oval morphology ranging from 100 to 300 nm in diameter (Fig. 2a). While TEM confirmed the expected size and shape of EVs, the lack of observable differences may reflect the limited resolution of TEM. Subtle ultrastructural or compositional changes could require higher-resolution or quantitative approaches such as cryo-EM or proteomic profiling for detection45. Nanoparticle tracking analysis confirmed that the majority of secreted EVs fell within a size range of 50 to 300 nm across groups (Fig. 2b). The average size distribution of EV peaks and SDs of the isolated EVs were as follows: 194.3 ± 65.1 nm for NoRad-EVs and 166.0 ± 42.4 nm for Rad-EVs (Fig. 2c). There were no significant effects of irradiation on vesicle size (p > 0.05). Also, there was no significant difference between the total protein content of the two EV groups: 0.66 ± 0.03 µg/µL for NoRad-EVs and 0.69 ± 0.04 µg/µL for Rad-EVs (Fig. 2d).
Schematic illustration of the study design. Muscle precursor cells (MPCs) were isolated from rat skeletal muscle and expanded in vitro for three passages before being subjected to no irradiation or a 10 Gy X-ray irradiation, and then EVs from MPCs not subjected to radiation (NoRad-EVs) or those subjected to radiation (Rad-EVs) were isolated by ultracentrifugation and used for further EV-based treatment and analysis. Created with Biorender.com.
Extracellular vesicle characterization. Conditioned media was collected from non-irradiated MPCs or irradiated MPCs after 48-hour incubation in serum-free media. EVs from non-irradiated MPCs (NoRad-EVs) or EVs from irradiated MPCs (Rad-EVs) were isolated from the conditioned media using centrifugation and evaluated using (a) Transmission Electron Microscopy to assess size and morphology (Scale bar = 100 nm), (b) Nanoparticle Tracking Analysis to assess particle size distribution, (c) Vesicle mean size comparison, and (d) Protein concentration quantification using bicinchoninic acid protein assay. Results are reported as mean ± standard deviation. (n = 3)
NoRad-EV treatment improves cell viability and proliferation in irradiated MPCs
MPC viability 48 h after irradiation and EV treatment was measured through acridine orange/propidium iodide - live/dead staining assay. Compared to non-irradiated MPCs, cell viability at 48 h was decreased by 48% in the MPC cultures that received a single X-ray dose of 10 Gy (p < 0.0001); similar to that previously reported by Caiozzo et al. (Fig. 3a)23. NoRad-EVs treatment significantly increased the viability of irradiated MPCs from 52 ± 5.7% to 71 ± 4.9% (measured as % of non-irradiated control, p < 0.05). Rad-EVs or vehicle control (vehicle CTL) treatment had no significant effect on irradiated MPC viability (p > 0.05). Similarly, compared to non-irradiated MPCs, there was an 85% decrease in cell proliferation levels (BrdU incorporation assay) in irradiated cells at 72 h (p < 0.0001), consistent with previous reports by Jurdana et al. (Fig. 3b)46. NoRad-EVs treatment significantly improved (~ 16% increase) the proliferation of irradiated MPCs at 72 h (p < 0.05), while Rad-EVs or vehicle CTL treatment had no significant effect on irradiated MPC proliferation (p > 0.05). These findings demonstrate a substantial recovery in cell viability and proliferation in irradiated MPCs treated with NoRad-EVs. Given that MPC dysfunction contributes to long-term muscle degeneration following radiation exposure, this degree of functional rescue may have meaningful implications for tissue preservation in radiological emergencies or therapeutic settings.
Analysis of effects of EV treatment on irradiated muscle precursor cells. MPCs irradiated with a single 10 Gy X-ray dose (Rad-MPCs) received either no treatment, vehicle control, EVs from non-irradiated MPCs (NoRad-EVs), or EVs from irradiated MPCs (Rad-EVs). The effect of EV treatment on (a) Cell viability at 48 h post-irradiation and EV addition was measured by acridine orange and propidium iodide (AO/PI) live-dead staining. (b) Cell proliferation at 72 h post-irradiation and EV addition was measured through bromodeoxyuridine (BrdU) incorporation assay. Results are reported as mean ± standard error. (n = 4, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
NoRad-EV treatment improves human umbilical vein endothelial cells (HUVEC) tube formation at 24 hours
The effects of EV treatment on HUVEC tube formation were assessed at 24-hour time point after cell seeding and EV dosing (25 µg total EV protein – low dose and 75 µg total EV protein – high dose). Assessment at the 24-hour time point was determined as optimal based on a previously published study43. The following three HUVEC tube formation assay parameters were selected and quantified to compare the effect of EV treatment on 2d-angiogenesis at a 24-hour time point: total branching length, average number of branches, and average number of junctions. Representative images of HUVEC tube formation at 24 h post-treatment are shown in Fig. 4a. NoRad-EVs at a high dose significantly increased the total branching length compared to vehicle control (p < 0.05), NoRad-EVs at the low dose (p < 0.05), Rad- EVs at a low dose (p < 0.001) or Rad-EVs at a high dose (p < 0.01) (Fig. 4b). There was no significant difference between vehicle control, NoRad-EVs low dose, Rad-EVs low dose, or Rad-EVs high dose at 24 h. There was no significant difference (p < 0.05) between the treatment groups for the average number of branches and the average number of junctions at 24 h (Fig. 4b,c). However, NoRad-EVs high dose had increased persistence of the number of branches and junctions compared to all other treatment groups at 24 h.
Analysis of effects of EV-treatment on 2D-angiogenesis. Human umbilical vein endothelial cells (HUVECs) were cultured in basal media supplemented with vehicle control, EVs from non-irradiated MPCs (NoRad-EVs), or EVs from irradiated MPCs (Rad-EVs). Tube formation parameters were measured using the Angiogenesis Analyzer for ImageJ. (a) Representative images of HUVECs during angiogenesis at T = 0 h and at T = 24 h. (Scale bar = 100 μm) (b) Average total branching length at 24 h (c) Average number of branches at 24 h (d) Average number of junctions at 24 h. Results are reported as mean ± standard error. (n = 4, **p < 0.01)
NoRad-EV treatment promotes microvascular fragment growth and sprouting
The effect of EV treatment on microvascular fragment growth (in 3D) was assessed through immunohistochemistry. A predetermined number of freshly isolated adipose tissue-derived MVFs were seeded in a fibrin-based hydrogel and grown in growth media. An EV dose (25 µg total EV protein, NoRad-EVs or Rad- EVs) was added on days 1, 2, and 4. On day 8, the MVF gels were fixed and stained with Rhodamine-labeled GS Lectin I to visualize vessel formation. MVF growth and density were determined using confocal microscopy. Qualitative histological analysis of MVF gels (rhodamine visualization) shows a denser MVF network with more sprouting junctions among NoRad-EV treated MVFs than Rad-EVs treatment (Fig. 5a). Qualitative analysis was supported by quantitative analysis. To quantify the extent of angiogenesis and measure the vessel density, the percentage of the well coverage Lectin was quantified. The microvessel density with the NoRad-EV treatment was about three-fold higher than the Rad-EV treatment (p < 0.05) (Fig. 5b).
Analysis of effects of EV-treatment on 3D-angiogenesis. Microvascular fragments (MVFs) were cultured for 8 days in fibrin hydrogels and growth media supplemented with vehicle control, EVs from non-irradiated MPCs (NoRad-EVs), or EVs from irradiated MPCs (Rad-EVs). The effects of EV treatment on MVF growth and sprouting were assessed through histological analysis. (a) Qualitative histological analysis of vessel formation. Confocal images of MVFs grown in fibrin hydrogels stained with GS Lectin I. (Scale bar = 100 μm) (b) Quantitative histological analysis of vessel formation. Quantification was performed as the measurement of GS Lectin I percent well coverage. Results are reported as mean ± standard error. (n = 4, *p < 0.05)
Differentially expressed MiRNA in NoRad-EVs and Rad-EVs
The miRNA expression levels in NoRad-EVs and Rad-EVs were detected using NanoString nCounter system with nCounter® Rat v1.5 miRNA Expression Assay Kit. Total 423 miRNA were assessed for differential expression (Fig. 6a). The Rad-EV-miRNAs that exhibited at least a 1.3-fold increase or decrease (p < 0.05) compared to NoRad-EV-miRNAs were considered differentially expressed. Out of the top 20 differentially expressed (DE) miRNAs, 7 miRNAs were upregulated (miR-3065-3p, let-7c, miR-741-5p, miR-3547, mir-136, miR-181a, miR-409-5p) and 13 miRNAs (miR-105, mir-336, miR-568, mir-296, mir-770, miR-193, mir-499, mir-34a, let-7b, mir-132, miR-376b-3p, mir-200a, mir-206) were downregulated in Rad-EVs compared to NoRad-EVs (Fig. 6b; Table 1). Several top DE miRNAs play important roles in skeletal muscle regeneration and vascular homeostasis. For example, miR-206, which was downregulated in Rad‑EVs, promotes myogenic lineage commitment and muscle repair by regulating key transcriptional programs47,48. Let‑7b, also reduced in Rad‑EVs, has been shown to protect endothelial cells from oxidative stress and suppress reactive oxygen species (ROS) accumulation under injury conditions49. miR‑105, another strongly downregulated miRNA, has been linked to tissue repair and cell proliferation via modulation of the FAK/AKT signaling pathway50. In contrast, miR‑181a, which was upregulated in Rad‑EVs, is associated with inflammatory signaling and vascular stress responses51. The altered abundance of these regenerative and inflammatory miRNAs may contribute to the impaired myogenic and angiogenic response observed in irradiated tissues.
Analysis of irradiation-induced miRNA differential expression in MPC-derived EVs. Total RNA, including microRNA, was extracted from EVs from non-irradiated MPCs (NoRad-EVs) and EVs from irradiated MPCs (Rad-EVs). EV miRNA expression analysis was performed using NanoString nCounter rat miRNA panel, nSolver software, and Rosalind platform for nCounter data analysis. (a) Volcano plot depicting differentially expressed (DE) miRNAs in NoRad-EVs and Rad-EVs. (b) Heatmap showing the top 20 DE miRNA (n = 4). miRTargetLink 2.0 software was used to identify the potential gene targets and pathways that may be affected by one or more of the top 20 DE miRNAs. (c) DE miRNA gene targets. A total of 42 genes targeted by at least two or more miRNAs are identified; important myogenic or angiogenic genes are highlighted in pink color, and all other genes are labeled with green color. (d) DE miRNA pathway targets. Pathways regulated by at least four or more DE miRNAs are shown. Labels for major pathways involved in myogenesis or angiogenesis are magnified for better visualization.
Differentially expressed microRNA target prediction and pathway analysis
miRTargetLink 2.0 software52 was used to identify the potential gene targets and pathways that may be affected by one or more DE miRNAs. Since miRNA sequences and functions are highly conserved among rats and humans, and because human miRNA target prediction databases are more robust, we used human miRNA label prefixes (hsa instead of rno) to get a more comprehensive target analysis and prediction. To identify the genes targeted by the DE miRNAs, we set the analysis criterion to strongly validated gene targets only and excluded all weakly validated or predicted targets. A total of 42 genes were identified that were targeted by at least two or more DE miRNAs (Fig. 6c). Over half of these gene targets, including Sirtuin 1; SIRT1, Vascular Endothelial Growth Factor A; VEGFA, Interleukin 6; IL-6, and AKT Serine/Threonine Kinase 1; Akt1, play important roles in both myogenesis or angiogenesis53,54. For the miRNA target pathways analysis, validated pathways targeted by at least four or more DE miRNAs were included. (Fig. 6d). Besides several metabolic, transcription, and DNA damage response pathways, important pathways involved in myogenesis or angiogenesis, such as FoxO signaling, VEGF signaling, PI3K-Akt1 signaling, and growth factor response pathways, were identified.
Discussion
Several cases of irradiation-induced chronic muscle atrophy have been published55,56. Despite the ever-increasing threat of ionizing radiation exposure through terrorism, accidents, or radiation therapy, there is still no effective treatment plan to address radiation-induced myopathies. Ionizing radiation can cause DNA damage in MPCs and impair their activation, proliferation, and self-renewal potential, thereby limiting muscle growth during development and after injury57,58,59. A 5 Gy dose of ionizing radiation can reduce MPC proliferation by 70%, which may lead to a long-term deficit in the MPC pool23. The EV-mediated radiation-induced bystander effect further exacerbates the irradiation damage by transferring radiation-induced inflammatory and genotoxic signals from irradiated to non-irradiated cells60. Choi et al. and Murach et al. have reported a more favorable role of EVs and their miRNA in promoting skeletal muscle regeneration and reducing muscle fibrosis in the injury site37,61. However, the effects of EVs derived from healthy, non-irradiated MPCs on irradiated MPCs have not been studied. Accordingly, the experimental design of this study included a comparison of the ability of EVs derived from non-irradiated or irradiated skeletal muscle MPCs to affect key features of muscle repair and regeneration, to include proliferation and angiogenesis. Moreover, the radiation-induced changes in EV miRNA composition and identification of the genes and pathways that may be targeted were determined.
It was observed that NoRad-EVs were more effective in maintaining cell viability (Fig. 3a) and proliferation (Fig. 3b) in irradiated MPCs than Rad-EVs. These results could be attributed, at least in part, to the radiation-induced ROS accumulation in the irradiated MPCs, and the pro-apoptotic and pro-inflammatory signals of the Rad-EVs being counteracted or weakened by the pro-survival and anti-inflammatory properties of the NoRad-EVs. Our results align with findings by Schuler et al., showing that MPC-EVs can reverse mitochondrial dysfunction in oxidatively damaged myotubes36. Similarly, Ji et al. have shown that MPC-EVs can promote satellite cell proliferation and directly contribute to muscle fiber hypertrophy after mechanical loading62. It should be noted that these results differ from those where Rastogi et al. and Mrowczynski et al. reported that EVs released from irradiated cancer cells induce an increase in viability, proliferation, migration, and radio-resistance non-irradiated cancer cells63,64. In a 24-hour HUVEC tube formation assay, the NoRad-EV treatment increased the total branching length, number of branches, and number of branching junctions retained at the 24-hour time point compared to the Rad-EV treatment (Fig. 4). Our results are consistent with the previously reported findings that healthy myotube-derived EVs can improve endothelial cell functions and promote HUVEC proliferation, migration, and tube formation, while the EVs derived from MPCs undergoing stress-induced senescence decrease HUVEC proliferation and impair their angiogenic cell function65,66. Furthermore, NoRad-EVs had a similar pro-angiogenic effect on MVF growth and sprouting in 3D culture, where NoRad-EVs treatment increased the microvascular density by about three-fold compared to Rad- EVs treatment (Fig. 5). The relatively lower pro-angiogenic effects of Rad-EVs could be attributed to the RAD-EV-mediated propagation of radiation stress to healthy, non-irradiated cells.
miRNAs are considered among the most essential EV cargoes due to their ability to extensively regulate gene expression in critical biological processes, including myogenesis and angiogenesis67. As expected, MPC irradiation significantly affected the EV miRNA composition (Fig. 6a). These mechanisms are supported by recent studies. Shuler et al. showed that EVs from satellite cells can reverse oxidative mitochondrial damage in muscle cells36, while Di Mambro et al. highlighted EV involvement in ROS-related signaling68. EV-derived miRNAs also contribute to muscle regeneration and hypertrophy under stress39. Out of the top 20 differentially expressed miRNAs, 7 were upregulated, and 13 were downregulated in Rad-EVs compared to NoRad-EVs (Fig. 6b; Table 1). The DE miRNA target analysis revealed several critical myogenic and angiogenic pathways that may be targeted by EV miRNA in response to MPC irradiation and may play a role in the propagation of radiation injury in the muscle tissue in an EV-mediated manner (Fig. 6d). These include response to growth factor, cell cycle regulation, PI3K-Akt, VEGFA-VEGFR2, and SIRT1/FoxO signaling pathways. Besides their role in modulating MPC proliferation, muscle hypertrophy, and vascular homeostasis, the SIRT1/FoxO and PI3K-Akt signaling pathways have been shown to regulate nitric oxide (NO) mediated cellular responses during oxidative stress69,70,71,72,73,74. This is particularly interesting because a previous study by Cho-Lim et al. has shown that manipulating NO levels can affect the proliferation levels of irradiated MPCs and rescue them from the harmful effects of ionization radiation75. This warrants further investigation to identify the EV miRNAs that differentially target genes involved in SIRT1/FoxO and PI3K-Akt signaling pathways to alter cellular NO levels in response to irradiation. It may provide additional insight into the molecular mechanisms of radiation injury in the muscle tissue. Moreover, identifying “therapeutic miRNA cargoes” that can reduce the radiation-induced dysfunction of MPCs could be directly applied to developing futuristic therapeutics, such as synthetic EV-based miRNA delivery strategies for radiation injury treatment. Clinical translation of EV-based therapies will require addressing challenges such as scalable production, consistent dosing, and regulatory safety validation. Notably, EV-based approaches are being actively evaluated in preclinical and clinical settings, supporting their potential as cell-free therapeutics34,76.
The current study wherein the potentials of EVs from non-irradiated and irradiated MPCs to affect key components of muscle repair and regeneration add to the understanding of mechanisms underlying the maintenance of homeostasis in the face of irradiation. The differential expression of MPC-EV miRNA further adds to the understanding of the potential mechanisms. Further, the results suggest that EVs from healthy, non-irradiated MPCs may have a therapeutic application in mitigating ionizing radiation-induced muscle atrophy. These results are of great relevance to civilian and combat casualty care as we strive to develop cell-free medical countermeasures against muscle radiation injury, which can be efficiently administered and dispensed in hospitals as well as hostile environments. However, while our findings offer important insights into EV-mediated radioprotection, the study is limited by its reliance on in vitro models and short-term observations. Future research should focus on validating these findings in vivo and functionally testing the roles of specific EV miRNAs, such as miR-206 or let-7b, using knockout or gain-of-function approaches to better understand their therapeutic potential.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Boulton, F. & Dunn, T. Nuclear war and public health: preparedness, protection and the case for prevention. J. Public. Health (Oxf). 42 (3), e316–e322 (2020).
Grace, M. B. et al. The U.S. Government’s medical countermeasure portfolio management for nuclear and radiological emergencies: synergy from interagency Cooperation. Health Phys. 101 (3), 238–247 (2011).
Lindee, S. Survivors and scientists: hiroshima, fukushima, and the radiation effects research foundation, 1975–2014. Soc. Stud. Sci. 46 (2), 184–209 (2016).
Hasegawa, A. et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet 386 (9992), 479–488 (2015).
Akita, S. Treatment of radiation injury. Adv. Wound Care (New Rochelle). 3 (1), 1–11 (2014).
Kim, J. H., Jenrow, K. A. & Brown, S. L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 32 (3), 103–115 (2014).
Majeed, H. & Gupta, V. Adverse effects of radiation therapy. In StatPearls (Treasure Island (FL), 2022).
D’Souza, D. et al. The late effects of radiation therapy on skeletal muscle morphology and progenitor cell content are influenced by Diet-Induced obesity and exercise training in male mice. Sci. Rep. 9 (1), 6691 (2019).
Ring, J. P. Radiation risks and dirty bombs. Health Phys. 86 (2 Suppl), S42–S47 (2004).
Kiang, J. G. & Olabisi, A. O. Radiation: a poly-traumatic hit leading to multi-organ injury. Cell. Biosci. 9, 25 (2019).
Singh, V. K. et al. A review of radiation countermeasure work ongoing at the armed forces radiobiology research Institute. Int. J. Radiat. Biol. 88 (4), 296–310 (2012).
Frontera, W. R. & Ochala, J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 96 (3), 183–195 (2015).
Mukund, K. & Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip Rev. Syst. Biol. Med. 12 (1), e1462 (2020).
Cretoiu, D. et al. Myofibers Adv. Exp. Med. Biol., 1088: 23–46. (2018).
Bischoff, R. Regeneration of single skeletal muscle fibers in vitro. Anat. Rec. 182 (2), 215–235 (1975).
Feng, L. T., Chen, Z. N. & Bian, H. Skeletal muscle: molecular structure, myogenesis, biological functions, and diseases. MedComm 5 (7). (2024).
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Huo, F., Liu, Q. & Liu, H. Contribution of muscle satellite cells to sarcopenia. Front. Physiol. 13. (2022).
Hawke, T. J. & Garry, D. J. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91 (2), 534 – 51. (1985).
Rhoads, R. P. et al. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am. J. Physiol. Cell. Physiol. 296 (6), C1321–C1328 (2009).
Borselli, C. et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. U S A. 107 (8), 3287–3292 (2010).
Christov, C. et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 18 (4), 1397–1409 (2007).
Caiozzo, V. J. et al. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat. Res. 174 (5), 582–589 (2010).
Jurdana, M. Radiation effects on skeletal muscle. Radiol. Oncol. 42 (1), 15–22 (2008).
Olfert, I. M. et al. Advances and challenges in skeletal muscle angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 310 (3), H326–H336 (2016).
Choi, D. H. et al. Radiation induces acute and subacute vascular regression in a three-dimensional microvasculature model. Front. Oncol. 13. (2023).
Mytidou, C. et al. Muscle-derived exosomes encapsulate MyomiRs and are involved in local skeletal muscle tissue communication. FASEB J. 35 (2), e21279 (2021).
Schneider, A. & Simons, M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell. Tissue Res. 352 (1), 33–47 (2013).
Chance, T. C. et al. Extracellular vesicles derived from cardiosphere-derived cells as a potential antishock therapeutic. J. Trauma. Acute Care Surg. 91 (2S Suppl 2), S81–S88 (2021).
Byun, S. E. et al. Skeletal muscle regeneration by the exosomes of adipose Tissue-Derived mesenchymal stem cells. Curr. Issues Mol. Biol. 43 (3), 1473–1488 (2021).
Duan, A. et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell. Res. Ther. 12 (1), 427 (2021).
Wan, R. et al. The therapeutic potential of exosomes in soft tissue repair and regeneration. Int. J. Mol. Sci. 23 (7). (2022).
Collino, F. et al. AKI recovery induced by mesenchymal stromal Cell-Derived extracellular vesicles carrying MicroRNAs. J. Am. Soc. Nephrol. 26 (10), 2349–2360 (2015).
Kou, M. et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 13 (7). (2022).
Bittel, D. C. & Jaiswal, J. K. Contribution of extracellular vesicles in rebuilding injured muscles. Front. Physiol. 10, 828 (2019).
Shuler, K. T. et al. Muscle stem cell-derived extracellular vesicles reverse hydrogen peroxide-Induced mitochondrial dysfunction in mouse myotubes. Cells 9 (12). (2020).
Choi, J. S. et al. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control Release. 222, 107–115 (2016).
Rani, S. et al. Mesenchymal stem Cell-derived extracellular vesicles: toward Cell-free therapeutic applications. Mol. Ther. 23 (5), 812–823 (2015).
Yedigaryan, L. et al. Extracellular vesicle-derived MiRNAs improve stem cell-based therapeutic approaches in muscle wasting conditions. Front. Immunol. 13. (2022).
Acosta, F. M. et al. Divergent effects of myogenic differentiation and diabetes on the capacity for muscle precursor cell adipogenic differentiation in a fibrin matrix. Biochem. Biophys. Res. Commun. 526 (1), 21–28 (2020).
Chance, T. C. et al. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma. Acute Care Surg. 87 (1S Suppl 1), S74–S82 (2019).
Jung, M. K. & Mun, J. Y. Sample preparation and imaging of exosomes by transmission electron microscopy. J. Vis. Exp. (131). (2018).
Chance, T. C. et al. Human mesenchymal stromal cell source and culture conditions influence extracellular vesicle angiogenic and metabolic effects on human endothelial cells in vitro. J. Trauma. Acute Care Surg. 89 (2S Suppl 2), S100–S108 (2020).
Acosta, F. M. et al. A straightforward approach to engineer vascularized adipose tissue using microvascular fragments. Tissue Eng. Part. A. 26 (15–16), 905–914 (2020).
Parra, A. et al. Cryogenic electron microscopy reveals morphologically distinct subtypes of extracellular vesicles among Porcine ejaculate fractions. Sci. Rep. 14 (1). (2024).
Jurdana, M. et al. Effect of ionizing radiation on human skeletal muscle precursor cells. Radiol. Oncol. 47 (4), 376–381 (2013).
Chen, J. F. et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38 (2), 228–233 (2006).
Aránega, A. E. et al. MiRNAs and muscle regeneration: therapeutic targets in Duchenne muscular dystrophy. Int. J. Mol. Sci. 22 (8). (2021).
Bao, M. H. et al. Protective effects of Let-7a and Let-7b on oxidized Low-Density lipoprotein induced endothelial cell injuries. PLoS ONE. 9 (9), e106540 (2014).
He, B. et al. Extracellular Vesicle-Derived miR-105-5p promotes malignant phenotypes of esophageal squamous cell carcinoma by targeting SPARCL1 via FAK/AKT signaling pathway. Front. Genet. 13, 819699 (2022).
Su, Y. et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 10 (5). (2019).
Kern, F. et al. MiRTargetLink 2.0—interactive MiRNA target gene and target pathway networks. Nucleic Acids Res. 49 (W1), W409–W416 (2021).
Gibril, B. A. A. et al. Unlocking the nexus of sirtuins: A comprehensive review of their role in skeletal muscle metabolism, development, and disorders. Int. J. Biol. Sci. 20 (8), 3219–3235 (2024).
Verma, M. et al. Endothelial cell signature in muscle stem cells validated by VEGFA-FLT1-AKT1 axis promoting survival of muscle stem cell. eLife 13. (2024).
SO, M. and K. B, Parascapular muscle atrophy as a delayed effect of radiation treatment - PubMed. Pract. Neurol. 17 (2). (2017).
Furby, A. et al. Late-onset cervicoscapular muscle atrophy and weakness after radiotherapy for hodgkin disease: a case series. J. Neurol. Neurosurg. Psychiatry. 81 (1), 101–104 (2010).
Bachman, J. F. et al. Radiation-Induced damage to prepubertal Pax7 + Skeletal muscle stem cells drives lifelong deficits in myofiber size and nuclear number. iScience 23 (11), 101760 (2020).
Phelan, J. N. & Gonyea, W. J. Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat. Rec. 247 (2), 179–188 (1997).
Hur, W. & Yoon, S. K. Molecular pathogenesis of radiation-induced cell toxicity in stem cells. Int. J. Mol. Sci. 18 (12). (2017).
Smolarz, M. et al. Radiation-induced bystander effect mediated by exosomes involves the replication stress in recipient cells. Int. J. Mol. Sci. 23 (8). (2022).
Murach, K. A. et al. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function 1 (1). (2020).
Ji, S. et al. Myoblast-derived exosomes promote the repair and regeneration of injured skeletal muscle in mice. FEBS Open. Bio. 12 (12), 2213–2226 (2022).
Rastogi, S. et al. Extracellular vesicles transfer nuclear Abl-dependent and radiation-induced miR-34c into unirradiated cells to cause bystander effects. Mol. Biol. Cell. 29 (18), 2228–2242 (2018).
Mrowczynski, O. D. et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 9 (90), 36083–36101 (2018).
Nie, Y. et al. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-κB signalling. Exp. Physiol. 104 (8), 1262–1273 (2019).
Hettinger, Z. R. et al. Extracellular vesicles released from stress-induced prematurely senescent myoblasts impair endothelial function and proliferation. Exp. Physiol. 106 (10), 2083–2095 (2021).
Parashar, D. et al. MicroRNAs in extracellular vesicles: A potential role in cancer progression. Cell. Signal. 121, 111263 (2024).
Di Mambro, T. et al. The tricky connection between extracellular vesicles and mitochondria in Inflammatory-Related diseases. Int. J. Mol. Sci. 24 (9), 8181 (2023).
Rathbone, C. R., Booth, F. W. & Lees, S. J. Sirt1 increases skeletal muscle precursor cell proliferation. Eur. J. Cell. Biol. 88 (1), 35–44 (2009).
Potente, M. et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 21 (20), 2644–2658 (2007).
Rathbone, C. R., Booth, F. W. & Lees, S. J. FoxO3a preferentially induces p27Kip1 expression while impairing muscle precursor cell-cycle progression. Muscle Nerve. 37 (1), 84–89 (2008).
Glass, D. J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 346, 267–278 (2010).
Fang, J. et al. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell. Signal. 19 (12), 2487–2497 (2007).
Hughes, K. J. et al. FoxO1 and SIRT1 regulate β-Cell responses to nitric oxide. J. Biol. Chem. 286 (10), 8338–8348 (2011).
Cho-Lim, J. J. et al. Satellite cells say NO to radiation. Radiat. Res. 175 (5), 561–568 (2011).
Lener, T. et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J. Extracell. Vesicles. 4 (1), 30087 (2015).
Acknowledgements
The views expressed in this article are those of the authors and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government. This work was partially supported by the National Institutes of Health (5SC1DK122578, to C.R.R), the National Science Foundation (CBET–2144438, to C.R.R), the Department of Defense and Congressionally Directed Medical Research Programs (Grant #BA190083, Award #W81XWH-19-2-0016 to J.A.B.).
Author information
Authors and Affiliations
Contributions
U.S., S.P., J.A.B., and C.R.R. conceived and designed the study. U.S., S.P., J.A.B., and C.R.R. developed the methodology and conducted the experimental work. U.S. curated the data and performed the formal analysis. U.S. drafted the original manuscript. All authors contributed to the review and editing of the manuscript. C.R.R. and J.A.B. acquired funding for the project. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sharma, U., Pacelli, S., Meledeo, M.A. et al. Irradiation of muscle precursor cells impairs the proliferative and angiogenic functions of their extracellular vesicles. Sci Rep 15, 30563 (2025). https://doi.org/10.1038/s41598-025-15699-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15699-x