Abstract
Preterm birth (PTB) occurs in 10% of births worldwide and remains the leading cause of neonatal morbidity and mortality. Previously, we reported that N, N-dimethylacetamide (DMA) and N, N-dimethylformamide (DMF) prevent inflammation-induced PTB in a murine model and inhibit the NF-κB inflammatory pathway. Using in vitro and ex vivo models, we show here that two DMA analogs, N,N-diethylaceatmide (DEA) and N, N-dipropylacetamide (DPA), attenuate LPS-stimulated increased secretion of tumor necrosis factor (TNF)-α, IL-6, IL-1, GM-CSF, MCP-1 and IL-10 from RAW 264.7 cells; IL-6, IL-8 and MCP-1 from HTR-8/SVneo cells; and TNF-α, IL-6, GM-CSF, IL-8, MCP-1 and IL-10 from human placental explants. In addition, both analogs inhibited LPS induced up-regulation of nitric oxide (NO) secretion and inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. Further, both analogs, at 10 mM, inhibited LPS-induced degradation of IkB-⍺ in RAW 264.7 cells, leading to inhibition of the NF-kB pathway. We also found that both analogs inhibited LPS-stimulated NF-kB transcriptional activity but did not affect AP-1 or C/EBP activity. However, neither analog had any effect on the expression of native or phosphorylated forms of JNK1, ERK1/2 and p-38 MAPK. Finally, in a well-established in vivo model of preterm birth, DEA, at 750 mg/kg, prevented preterm birth for at least 24 h. DEA and DPA have potential as novel therapeutic agents for the prevention of inflammation-induced preterm birth and other inflammatory disorders.
Similar content being viewed by others
Introduction
Preterm birth (PTB), delivery before the completion of 37 weeks of gestation, occurs in approximately 10% of pregnancies worldwide and accounts for most perinatal morbidity, causing a significant economic burden to society1,2,3,4. Although several pathogenic pathways lead to PTB, inflammation5,6,7—and activation of the nuclear factor-kappa B (NF-kB) pathway specifically8,9,10,11 accounts for most cases of spontaneous preterm birth. Nuclear translocation of NF-kB triggers transcription of several inflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-812,13, which in turn leads to the synthesis of prostaglandins (PGs) and matrix metalloproteinases (MMPs)14. NF-kB activation also increases production of nitric oxide (NO), which increases the synthesis of PGs as well15,16,17. Production of these inflammatory mediators ultimately culminates in cervical ripening, preterm premature rupture of membranes and uterine contractions, resulting in the onset of PTB6,18,19,20,21.
We have previously reported that N, N-dimethylacetamide (DMA) and N, N-dimethylformamide (DMF), two readily available organic solvents, delay PTB in vivo by inhibiting NF-kB22,23,24,25. These discoveries establish that this family of aprotic solvents warrants investigation as a novel source of potential pharmacotherapy to delay or prevent PTB. Therefore, the aims of the present work were to test whether two DMA analogs, N,N-diethylacetamide (DEA) and N, N-dipropylacetamide (DPA), suppress cytokine release in vitro and ex vivo, whether the mechanism of action of these compounds is inhibition of NF-kB and whether lead compound DEA delays PTB in vivo.
Materials and methods
Cell culture and reagents
RAW 264.7 cells, a murine macrophage-like cell line, were purchased from American Type Culture Collection (ATCC TIB-71, Manassas, VA, USA). HTR-8/SVneo, an extravillous cytotrophoblast cell line isolated from first trimester human placental explant cultures and human embryonic kidney (HEK) 293 cells overexpressing toll-like receptor (TLR)4 genes were kindly donated by Dr. Charles Graham (Queens’s University, Ontario, Canada) and Dr. Vladimir Poltoratsky (St. John’s University, NY, USA), respectively. RAW 264.7 and HEK 293 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Cellgro, Corning, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologics, Lawrenceville, GA) and 1% penicillin-streptomycin (Cellgro, Corning, NY). HTR-8/SVneo cells were grown in Roswell Park Memorial Institute (RPMI) medium (Cellgro, Corning, NY) supplemented with 5% FBS and 1% penicillin-streptomycin. All cells were maintained in an incubator set at 37 °C and 5% CO2 and allowed to grow to 80–90% confluency before being subcultured or used in experiments.
Evaluation of cell viability
MTT (3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyl tetrazolium bromide) assay was performed to evaluate the effect of drug treatment on cell viability. HTR-8/SVneo (20,000 well), RAW 264.7 (20,000 well) and HEK 293 (25,000/well) cells were seeded in 96-well plates and incubated overnight. The next day, cells were pre-treated with or without different concentrations of DEA or DPA for 2 h and then treated with lipopolysaccharide (LPS, Escherichia coli 026:B6) (Sigma, St. Louis, MO, USA) at 1 µg/ml for 24 h. At the end of the 24-h treatment, MTT (Alfa Aesar, Ward Hill, MA, USA) was added to each well at a final concentration of 0.5 mg/ml. After incubation for 2 h, media was aspirated and dimethyl sulfoxide (DMSO) (100 µl/well) (BDH, Randor, PA) was added to dissolve the formed purple formazan crystals. The plates were shaken on an orbital microplate shaker for 10 min to ensure complete solubilization of the crystals and the absorbance of the resulting purple solution was measured at 570 nm, using an Opsys MR microplate reader (Dynex Technologies, Chantilly, VA, USA). Four independent experiments were performed in duplicate.
Nitric oxide (NO) secretion analysis by Griess assay
To determine the effect of DEA and DPA on NO secretion, HTR-8/SVneo (1.2 × 106) and RAW 264.7 (1.5 × 106) cells were seeded in T25 cm2 tissue culture flasks and incubated overnight, in their respective complete growth media. The following day, cells were pre-treated with or without DEA or DPA (0.1, 1 and 10 mM) or BAY 11-7082 at 5 µM (Enzo Life Sciences, Farmingdale, NY, USA) as a positive control for 2 h. LPS was then added to all flasks except for the untreated controls at a final concentration of 1 µg/ml. After 24 h, supernatants were collected by centrifuging at 4 °C and 1,000 x g for 10 min. The cell culture supernatants were evaluated for nitrite (NO− 2) levels by performing Griess assay as per the manufacturer’s protocol (Promega). Four independent experiments were performed in duplicate. A nitrite standard curve was generated according to the manufacturer’s instructions, which was used to calculate nitrite concentrations in the samples.
Analysis of in vitro cytokine and chemokine secretion
HTR-8/SVneo and RAW 264.7 cells were seeded at a density of 1.2 × 106 and 1.5 × 106 per T25 cm2 tissue culture flask, respectively, and incubated overnight. The next day, cells were pre-treated with or without DEA or DPA (0.1, 1 and 10 mM) or BAY 11-7082 (5 µM) for 2 h and then incubated with LPS (1 µg/ml) for another 24 h. At the end of 24 h treatment, cell culture supernatants were collected, centrifuged at 1,000 x g for 10 min. to remove cells and debris and stored at -80 °C for analysis. Four independent experiments were performed in duplicate.
Analysis of ex vivo cytokine secretion
Placentae associated with the NY Cord Blood Bank at the Albert Einstein College of Medicine, Bronx, NY were obtained from uncomplicated term elective cesarean deliveries after uneventful pregnancies (34–41 weeks’ gestation, n = 4). The protocol was approved and considered exempt by the Albert Einstein College of Medicine institutional review board and expedited by the St. John’s University’s institutional review board and performed with informed consent in accordance with accepted ethical, federal and professional standards for research. Sections of placental villous parenchyma were washed with cold sterile PBS to remove as much blood as possible and blood clots, blood vessels, connective tissue and membrane were removed using forceps and scissors. The tissue was then finely minced using an automated McIlwain Tissue Chopper (Ted Pella, Inc., Redding, CA, USA). The finely minced tissue was placed in cold complete DMEM media and centrifuged at 1,000 x g for 10 min. The supernatant was carefully decanted and aliquots of 200 mg (wet weight) of tissue were placed in 60 mm petri dishes. The finely minced explants were then dispersed in warmed DMEM in the absence or presence of different concentrations of DEA or DPA (0.1, 1 and 10 mM) or BAY 11-7082 (25 µM). Explants.
were pre-treated for 2 h and then LPS was added to all the explants at a final concentration of 1 µg/ml (except for the untreated controls), which were then incubated for another 20 h. Supernatants were collected, centrifuged at 1,000 x g for 10 min to remove cells and debris and stored at -80 °C for analysis. Four independent experiments were performed in duplicate.
Sandwich enzyme-linked immunosorbent assay (ELISA)
The cell culture and placental supernatants were evaluated to determine the levels of TNF-α, IL-6, IL-1β, IL-8, MCP-1, GM-CSF and IL-10 using Ready-SET-Go! sandwich ELISA kits (eBioscience, San Diego, CA) as per manufacturer’s protocol. First, optimal sample dilution for each target cytokine was determined to ensure that sample absorbance readings fall within the range of their respective standard curves. The concentration of each analyte was interpolated using a second order polynomial equation generated from a standard curve, using GraphPad Prism 6 software and then the value was multiplied by dilution factor used in the assay to calculate the concentration of the analyte in the original undiluted sample. ELISA for each experimental sample was performed in duplicate.
Placental explant viability - LDH assay
Placental explant viability was evaluated by measuring the amount of lactate dehydrogenase (LDH) released into the culture medium. Since placental explants consist of a heterogeneous population of cells and are therefore not suited for MTT assay, LDH, a cytoplasmic enzyme which is released into the culture media by damaged cells or tissues was measured to determine the viability of placental explants35,36. Pierce LDH assay kit (Thermo Fisher Scientific, Waltham, MA) was used to assess viability of placental explants according to the manufacturer’s instructions. Briefly, 50 µl of each placental supernatant sample was added into a 96-well tissue culture plate in duplicate. An equal volume (50 µl) of Reaction Mixture was added to each well, mixed by gentle tapping and the plate was incubated for 30 min at room temperature protected from light. Then, 50 µl of Stop Solution was added to each well. After mixing by gentle tapping, the absorbance was read at 490 nm using an Opsys MR microplate reader (Dynex Technologies, Chantilly, VA).
Intracellular protein expression experiments
The effects of both analogs on LPS-stimulated expression of various intracellular proteins such as inducible nitric oxide synthase (iNOS), inhibitor kappa B-α (IkB-α), native and phosphorylated forms of c-Jun NH2-terminal kinases (JNKs), extracellular-signal regulated protein kinase (ERKs) and p38 mitogen-activated protein kinases (MAPKs) were investigated in RAW 264.7 cells. Cells (4 × 106) were seeded per T25 cm2 tissue culture flask and incubated overnight using respective CGM. The next day, cells were pre-treated with DEA or DPA (0.1, 1 and 10 mM) or BAY 11-7082 (5 µM) or incubated in completed growth media alone for 2 h. Then, LPS was added to all flasks except for the untreated group at a final concentration of 1 µg/ml and incubated for another 6 h for iNOS or 15 min for IkBα, JNK, ERK and p38 MAPK protein expression analysis. At the end of the respective treatment periods, whole cell lysates were prepared using a modified protocol as described by Abcam. Briefly, cells were lysed in radioimmunoprecipitation assay (RIPA) lysis and extraction buffer (G-Biosciences, St. Louis, MO) freshly supplemented before use with phenylmethylsulfonylfluoride (PMSF) (1 mM) (Calbiochem, San Diego, CA) and ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor cocktail set III (Calbiochem, San Diego, CA). The cells were kept on ice with vortexing for 5 s every 10 min, for a total of 30 min and then the lysed cells were centrifuged at 14,000 x g for 20 min at 4 °C (Eppendorf 5424R, Hauppauge, NY). The whole cell lysate was collected, and the total protein concentration of each experimental sample was determined using Pierce bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA). At least four independent experiments were performed in duplicate.
Automated capillary Western blot analysis (WES simple Western)
Protein expression analysis was performed using WES, an automated capillary-based electrophoresis system (ProteinSimple, San Jose, CA) as per the manufacturer’s protocol. First, all standard pack reagents (DTT, 5X Fluorescent master mix and biotinylated ladder) were prepared. Then lysates were diluted using 0.1X sample buffer, mixed with 5X fluorescent master mix in a 4:1 ratio to have equal amounts of total protein (1 µg/µl) and heated at 95 °C for 5 min. The prepared lysates, blocking reagent, primary antibodies, HP-conjugated secondary antibodies, chemiluminescent substrate mix (Luminol: Peroxide mixture in a 1:1 ratio) and wash buffer were loaded into designated wells in an assay plate provided by the manufacturer. The assay plate and respective capillary cartridge was placed into the WES instrument (ProteinSimple, San Jose, CA), which carries out all further assay steps automatically using default settings. Proteins are separated in the individual capillaries as they migrate through a stacking and separation matrix, and then are immobilized in the capillary wall. Target proteins were identified using respective primary antibodies followed by subsequent immunodetection using HP-conjugated secondary antibodies and a chemiluminescent substrate mix. Primary antibodies used were iNOS, IkBα (Santa Cruz Biotechnologies, Dallas, TX), JNK1, ERK1/2, p38 MAPK, p-JNK1, p-ERK1/2 and p-p38 MAPK (Cell Signaling Technologies, Danvers, MA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technologies, Danvers, MA) was used as a loading control. All primary antibodies were diluted in an antibody diluent provided by the manufacturer and the optimal dilution for each primary antibody (Supplemental Table 1) was determined using Simple Western antibody database as a reference. In approximately 3 h, molecular weight and quantitate signal for target proteins are automatically reported by the Compass software (ProteinSimple, San Jose, CA). Protein expression was analyzed using ImageJ software (NIH, Bethesda, MD) and normalized to that of GAPDH.
Transfection with reporter plasmids and luciferase assay
The NF-kB-Luc, AP-1-Luc or C/EBP-Luc reporter plasmids and Renilla-Luc control plasmid were generously provided by Dr. Vladimir Poltoratsky (St. John’s University, NY, USA). HEK 293 cells (30,000 cells/well) were seeded in a 96-well white clear-bottom tissue culture plate (Greiner Bio-One, Monroe, NC) and incubated overnight at 37 °C and 5% CO2 using respective CGM. The following day, cells were co-transfected with NF-kB-Luc, AP-1-Luc or C/EBP-Luc reporter plasmids and Renilla-Luc control plasmid using Lipofectamine 3000 reagent (Invitrogen, Thermo Fisher, Waltham, MA) according to the manufacturer’s instructions. Each reporter and control plasmid solution was prepared in Opti-MEM I (Life technologies, Waltham, MA). The plate was incubated at 37 °C and 5% CO2 for 48 h and then transfected cells were pre-treated with DEA or DPA (0.1, 1 and 10 mM) or BAY 11-7082 (5 µM) or CGM alone for the untreated group for 2 h. Cells were stimulated with LPS (1 µg/ml) and incubated for another 24 h and luciferase assay was performed according to the manufacturer’s instructions (Promega, Madison, WI). The luminescence of firefly and renilla luciferase was measured using GloMax luminometer (Promega, Madison, WI) and the ratio of firefly/renilla luminescence readings for each sample was calculated. The effect of DEA or DPA treatment compared to the LPS control group is expressed in fold luciferase activity, as determined by calculating the ratio of each experimental sample firefly/renilla reading with that of the average ratio of firefly/renilla readings in the LPS control group, as shown below.
In-vivo study
Nine-week-old male C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All experimental protocols were approved by the St. John’s Institutional Animal Care and Use Committee (IACUC). All methods were carried out in accordance with ARRIVE guidelines (https://arriveguidelines.org). Mice were euthanized by carbon dioxide asphyxiation.
A total of 20 timed pregnant mice weighing between 27 and 36 g were administered 50 mg/kg LPS (serotype 026: B6; Sigma) intraperitoneally (i.p.) on E15.5 at T = 0, following our pre-established protocol22,23,24,25,26. The LPS only group (n = 6) were administered 100 µL of i.p. phosphate buffered saline (PBS) at T=-15 min and T = 10 h, while the two DEA treatment groups were administered 375 mg/kg DEA (n = 8) and 750 mg/kg DEA (n = 6) i.p. at T=-15 min and T = 10 h. Mice were randomly assigned to the different groups with mice assigned sequentially to the LPS only, the lower dose DEA and the higher dose DEA groups. By necessity, the investigator was aware of the group allocation.Sample sizes were based on predicted effect size and a prior significance level of P < 0.05. Mice were euthanized at T = 24 h to confirm pregnancy and evaluate gross congenital anomalies.
Statistical analysis
Data are represented as the mean ± SEM of at least four independent experiments and analyzed using GraphPad Prism 6 software (San Diego, CA, USA). Statistical significance among and between groups was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test for the cell and placental explant viability, nitric oxide and cytokine secretion, protein expression, and luciferase assays. For in vivo studies, the effect of the various DMA doses on the percentage of mice experiencing preterm delivery was determined with the Fisher exact test. Differences in rates of PTB over time were evaluated with the log-rank test. Differences between groups were considered significant for P values < 0.05 and noted based on the following hierarchy *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Results
Cell viability by MTT assay
Prior to testing the effect of DEA and DPA on levels of inflammatory mediators, the highest non-toxic concentrations of DEA and DPA in vitro were determined. Our MTT results show that up to 10 mM, the percent viability of DEA and DPA treated cells was not statistically significantly different when compared to the untreated control (Supplementary Fig. S1). At 20 mM, the percent viability of DEA treated cells was decreased significantly to approximately 70% in all cell lines (Supplementary Fig. S1A-S1C), whereas the percent viability of DPA treated cells was decreased significantly to approximately 70%, 20% and 50% in HTR-8/SVneo, RAW 264.7 and HEK 293/TLR4 cells, respectively (Supplementary Fig. S1D, S1E and S1F, respectively). Further increases in DEA and DPA concentrations up to 100 mM caused a sharp decrease in cell viability in all cell lines. Thus, based on these results, 10 mM was the highest concentration of DEA and DPA chosen in all further experiments in the present work.
DEA and DPA inhibit NO− 2 secretion and iNOS expression in RAW 264.7 cells
RAW 264.7 and HTR-8/SVneo cell supernatants were evaluated for levels of nitrite.
(NO− 2) after treatment with DEA and DPA in the presence of LPS for 24 h. Cells were stimulated with LPS for 24 h because we have previously shown nitrite levels to peak at this timepoint27. LPS at 1 µg/ml significantly increased NO− 2 secretion (P < 0.0001) from RAW 264.7 cells when compared to untreated control (Fig. 1A and B). BAY 11-7082, used as a control for NF-kB inhibition, significantly reduced the levels of NO−2 as compared to LPS control. Both analogs, DEA (Fig. 1A) and DPA (Fig. 1B) significantly attenuated LPS-stimulated NO−2 secretion from RAW 264.7 cells at a concentration of 10 mM when compared to the LPS control group. The levels of NO−2 were also assessed in HTR-8/SVneo cells and human placental explants, but no detectable levels of NO−2 were found in supernatants of LPS-stimulated HTR-8/SVneo cells or human placental explants (data not shown).
DEA and DPA inhibit LPS-induced NO− 2 secretion and iNOS expression in RAW 264.7 cells. RAW 264.7 cells were treated with DEA, DPA or BAY 11-7082 (5 µM) in the presence of LPS for 24 h and then supernatants were evaluated for NO− 2 levels by Griess assay. (A and B) show the amount of NO− 2 secreted in cell culture supernatants after treatment with DEA (A) or DPA (B). RAW 264.7 whole cell lysates were prepared after the treatment with DEA, DPA or BAY 11-7082 in the presence of LPS for 6 h. The effect of DEA and DPA on iNOS expression was assessed by western blot analysis using WES. (C and E) show the western blot analysis of iNOS expression after treatment with DEA (C) and DPA (E). (D and F) show the quantification of iNOS bands after treatment with DEA (D) and DPA (F) DEA. The iNOS band intensities were normalized with GAPDH from each sample and analyzed using ImageJ software. The relative density was obtained by dividing normalized data of each sample with that of LPS control. Data are shown as mean ± SEM of four independent experiments performed in duplicate. The assay was likewise performed in triplicate. To test for significant effects of each concentration of each compound, ANOVA was performed followed by Tukey’s multiple comparison post hoc analysis using GraphPad Prism 6 software. ***P < 0.001 and ****P < 0.0001.
We then determined whether DEA and DPA affect LPS-induced iNOS expression in RAW 264.7 cells. Cells were incubated with LPS for 6 h. The length of the incubation period was based on our previously reported time profile of iNOS expression27. The expression of iNOS was significantly increased (P < 0.0001) by LPS (1 µg/ml) treatment for 6 h, and, as expected, the LPS induced rise in iNOS was significantly inhibited (P < 0.0001) by BAY 11-7082, an NF-kB inhibitor, (Fig. 1C and F). Both analogs, DEA (Fig. 1C and D) (P < 0.001) and DPA (Fig. 1E and F) (P < 0.0001), significantly attenuated the LPS-induced increase in iNOS expression at a concentration of 10 mM.
DEA and DPA suppress pro-inflammatory cytokine and chemokine secretion from HTR-8/SVneo and RAW 264.7 cells
LPS treatment (1 µg/ml) for 24 h significantly increased secretion of IL-6, IL-8 and MCP-1 from HTR-8/SVneo cells (Fig. 2A and E). This LPS-stimulated IL-6, IL-8 and MCP-1 secretion was significantly reduced by BAY 11-7082 by about 50% (P < 0.0001) (Fig. 2A and E). Both analogs, DEA and DPA, were able to suppress LPS-stimulated secretion of IL-6 (P < 0.0001). In fact, 10 mM DEA (Fig. 2A) and 10 mM DPA (Fig. 2D) were more effective in suppressing IL-6 secretion than BAY 11-7082. In addition, IL-8 secretion was also statistically significantly inhibited at all concentrations of both analogs. This inhibitory effect was greater (90% reduction) at 10 mM DEA (Fig. 2B) and DPA (Fig. 2E) than at 0.1 or 1 mM DEA and DPA and these compounds, at 10 mM, were more effective in suppressing IL-8 than BAY 11-7082. Similarly, inhibition of LPS-stimulated MCP-1 secretion was also observed at all concentrations of DEA and DPA, however, only 1 and 10 mM DEA (Fig. 2C) and DPA (Fig. 2F) were found to be statistically significant.
DEA and DPA suppresses LPS-induced cytokine and chemokine secretion from HTR-8/SVneo cells. HTR-8/SVneo cells were pre-treated with DEA, DPA or BAY 11-7082 (5 µM) for 2 h, then stimulated with LPS (1 µg/ml) for 24 h and sandwich ELISA was performed to determine the levels of cytokines and chemokines secreted in the supernatants. (A, B and C) show the levels of (A) IL-6, (B) IL-8 and (C) MCP-1 after treatment with DEA and (D, E and F) shows the levels of (D) IL-6, (E) IL-8 and (F) MCP-1 after treatment with DPA. Data shown are mean ± SEM of four independent experiments performed in duplicate. ELISA was likewise performed in duplicate. To test for significant effects of each concentration of each compound, ANOVA was performed followed by Tukey’s multiple comparison post hoc analysis using GraphPad Prism 6 software. **P < 0.01 and ***P < 0.001, ****P < 0.0001.
RAW 264.7 cell incubation with LPS significantly stimulated TNF-α, IL-6, IL-1β, granulocyte-macrophage colony stimulating factor (GM-CSF), monocyte-chemoattractant protein (MCP)-1 and IL-10 secretion, which was significantly reduced by BAY 11-7082, an NF-kB inhibitor (Figs. 3A and F and 4A and F). DEA was able to significantly reduce TNF-α secretion at a concentration of 10 mM (P < 0.0001) (Fig. 3A) whereas DPA (Fig. 4A) significantly decreased TNF-α secretion at 1 and 10 mM concentrations. The secretion of IL-6 as well as IL-1β was also significantly reduced by both analogs at 10 mM (Figs. 3B and C and 4B and C). It was noted that 10 mM DEA and DPA were more effective in reducing LPS triggered IL-1β secretion than BAY 11-7082 (Figs. 3C and 4C). Furthermore, LPS-stimulated GM-CSF secretion was statistically significantly suppressed by DEA (Fig. 3D) and DPA (Fig. 4D) at all concentrations. In fact, both analogs, at 10 mM, were found to suppress > 90% of GM-CSF secretion, reducing it to levels close to those in untreated controls. Similarly, MCP-1 and IL-10 secretion were effectively inhibited at all concentrations of both analogs. However, statistical significance was only achieved at 10 mM of DEA (Fig. 3E) and DPA (Fig. 4E) for MCP-1 secretion, whereas IL-10 secretion was significantly suppressed by DEA treatment at 10 mM (P < 0.0001) (Fig. 3F) and DPA treatment at 1 mM (P < 0.01) and 10 mM (P < 0.0001) (Fig. 4F).
DEA suppresses LPS-induced cytokine and chemokine secretion from RAW 264.7 cells. RAW 264.7 cells were pre-treated with DEA or BAY 11-7082 (5 µM) for 2 h and then stimulated with LPS (1 µg/ml) for 24 h. Post 24 h treatment, supernatants were collected and sandwich ELISA was performed to determine the levels of (A) TNF-α, (B) IL-6, (C) IL-1β, (D) GM-CSF, (E) MCP-1 and (F) IL-10 after the incubation with DEA. Data shown are mean ± SEM of four independent experiments performed in duplicate. ELISA was likewise performed in duplicate. To test for significant effects of each concentration, ANOVA was performed followed by Tukey’s multiple comparison post hoc analysis using GraphPad Prism 6 software. **P < 0.01 and ****P < 0.0001.
DPA suppresses LPS-induced cytokine and chemokine secretion from RAW 264.7 cells. RAW 264.7 cells were pre-treated with DPA or BAY 11-7082 (5 µM) for 2 h and then stimulated with LPS (1 µg/ml) for 24 h. Post 24 h treatment, supernatants were collected and sandwich ELISA was performed to determine the levels of (A) TNF-α, (B) IL-6, (C) IL-1β, (D) GM-CSF, (E) MCP-1 and (F) IL-10 after the incubation with DPA. Data shown are mean ± SEM of four independent experiments performed in duplicate. ELISA was likewise performed in duplicate. To test for significant effects of each concentration, ANOVA was performed followed by Tukey’s multiple comparison post hoc analysis using GraphPad Prism 6 software. **P < 0.01 and ****P < 0.0001.
DEA and DPA suppress pro-inflammatory cytokine and chemokine secretion from human placental explants
The effects of both analogs on the secretion of various pro-inflammatory cytokines and chemokines were evaluated in human placental explants using sandwich ELISA. Prior to evaluating DEA and DPA effects on inflammatory mediators, the amount of lactate dehydrogenase (LDH) released into the placental supernatants was determined to test whether treatment conditions affected viability of tissue. Levels of LDH were found to be similar at all the concentrations of DEA (Supplementary Fig. 2A) and DPA (Supplementary Fig. 2B), regardless of the experimental conditions. LPS treatment significantly stimulated the secretion of TNF-α, IL-6, GM-CSF, IL-8, MCP-1 and IL-10 from placental explants (P < 0.0001) (Figs. 5A and F and 6A and F) when compared to untreated controls. As seen in the in vitro experiments, BAY 11-7082 also significantly inhibited the secretion of all above mentioned cytokines and chemokines in ex vivo experiments when compared to LPS controls (Figs. 5A and F and 6A and F). However, BAY 11-7082 was used at a higher concentration (25 µM) here compared to that used in in vitro experiments. Importantly, both analogs were found to inhibit the secretion of different cytokines and chemokines in human placental explants.
DEA suppresses LPS-induced cytokine and chemokine secretion from human placental explants. Human placental explants were processed as described previously and pre-treated with DEA or BAY 11-7082 (25 µM) for 2 h. Then LPS was added at a final concentration of 1 µg/ml and explants were incubated for 20 h. After 20 h treatment, supernatants were collected and evaluated for various cytokines and chemokines. The levels of (A) TNF-α, (B) IL-6, (C) GM-CSF, (D) IL-8, (E) MCP-1 and (F) IL-10 after the treatment with DEA were determined using sandwich ELISA. Data shown are mean ± SEM of four independent experiments performed in duplicate. Likewise, ELISA was performed in duplicate. To test for significant effects of each concentration, ANOVA was performed followed by Tukey’s multiple comparison post hoc analysis using GraphPad Prism 6 software. *P < 0.05, ***P < 0.001 and ****P < 0.0001.
DPA suppresses LPS-induced cytokine and chemokine secretion from human placental explants. Human placental explants were processed as described previously and pre-treated with DPA or BAY 11-7082 (25 µM) for 2 h. Then LPS was added at a final concentration of 1 µg/ml and explants were incubated for 20 h. After 20 h treatment, supernatants were collected and evaluated for various cytokines and chemokines. The levels of (A) TNF-α, (B) IL-6, (C) GM-CSF, (D) IL-8, (E) MCP-1 and (F) IL-10 after the treatment with DPA were determined using sandwich ELISA. Data shown are mean ± SEM of four independent experiments performed in duplicate. Likewise, ELISA was performed in duplicate. To test for significant effects of each concentration, ANOVA was performed followed by Tukey’s multiple comparison post hoc analysis using GraphPad Prism 6 software. *P < 0.05, **P < 0.01 and ****P < 0.0001.
LPS-stimulated secretion of TNF-α, IL-6 and GM-CSF was found to be significantly (P < 0.0001) attenuated by 10 mM DEA (Fig. 5A and C) and DPA (Fig. 6A and C) with DPA being more effective in inhibiting GM-CSF secretion than DEA (Fig. 6C). Also, TNF-α and IL-6 secretion were significantly decreased by 1 mM (P < 0.01) as well as 10 mM (P < 0.0001) DPA. In addition to their inhibitory effects on the secretion of cytokines, DEA and DPA significantly suppressed the secretion of chemokines such as IL-8 (Figs. 5D and 6D) and MCP-1 (Figs. 5E and 6E) at 10 mM. DPA was more effective (> 80%) in suppressing LPS induced IL-8 secretion (Fig. 6D) and able to inhibit LPS stimulated secretion of MCP-1 at 1 mM as well as at 10 mM (P < 0.01, Fig. 6E). Lastly, DEA treatment produced a significant reduction in IL-10 secretion at 1 mM (P < 0.05) and 10 mM (P < 0.0001) (Fig. 5F) concentrations whereas DPA was able to decrease secretion of this cytokine significantly at all concentrations tested (P < 0.05 at 0.1 mM, P < 0.0001 at 1 mM and 10 mM) (Fig. 6F).
DEA and DPA inhibit degradation of IkB-α in RAW 264.7 cells, but do not affect the MAPK pathway
To evaluate the mechanism by which DEA and DPA suppress cytokine and chemokine secretion, we studied both analogs for their specific effects on the main regulatory pathways that drive gene expression of inflammatory signaling molecules, namely the NF-kB and MAPK pathways. First, we tested whether DEA and DPA affect the NF-kB pathway by following IkB-α degradation in RAW 264.7 cells after incubation with LPS for 15 min. This incubation period was based on our previous study where we reported the time profile of IkB-α degradation in RAW 264.7 cells after exposure to LPS (1 µg/ml) and found that IkB-α is degraded 15 min after LPS treatment27. As expected, our western blot analysis (WES) showed that LPS treatment for 15 min significantly induced (P < 0.0001) IkB-α degradation when compared to the untreated control, as shown by the decrease in IkB-α expression (Fig. 7A, B, D and E). We then evaluated whether DEA and DPA affect this LPS-induced IkB-α degradation. Interestingly, we found that IkB-α expression was increased with DEA and DPA treatment. GAPDH was used to normalize the data and the statistical analysis of relative densities of IkB-α bands showed that DEA at a concentration of 10 mM (P < 0.01) (Fig. 7A and B) and DPA at 1 mM (P < 0.05) and 10 mM (P < 0.01) (Fig. 7D and E) concentrations significantly inhibited IkB-α LPS induced degradation.
DEA and DPA prevent LPS-induced IkB-α degradation but do not affect expression of MAPK proteins in RAW 264.7 cells. To determine the effect of DEA and DPA IkB-α degradation, RAW 264.7 cells were pre-treated with DEA or DPA for 2 h and then stimulated with LPS for 15 min. The whole cell lysates were prepared and the western blot analysis was performed to determine the expression of IkB-α and MAPK proteins using WES. (A and D) show the western blot analysis of IkB-α expression after treatment with DEA (A) and DPA (D). (B and E) show the quantification of IkB-α bands after treatment with DEA (B) and DPA (E). (C and F) show the immunoblots of native and phosphorylated forms of JNK1, ERK1/2 and p-38 MAPK after treatment with DEA (C) and DPA (F). The IkB-α band intensities were normalized with GAPDH from each sample and analyzed using ImageJ software. The relative density was obtained by dividing normalized data of each sample with that of the untreated control. The western blot shown is representative of four independent experiments performed in duplicate. To test for significant effects of each concentration of each compound, ANOVA was performed followed by Tukey’s post hoc test using GraphPad Prism 6. *P < 0.05, **P < 0.01 and ****P < 0.0001.
Next, the effect of DEA and DPA treatment on MAPK pathway was evaluated in LPS-stimulated RAW 264.7 cells by following expression of various upstream and downstream proteins involved in MAPK signaling cascades. After LPS treatment for 15 min, bands of phosphorylated JNK1, ERK1/2 and p38 MAPK (p-JNK1, p-ERK1/2 and p-p38 MAPK) were observed (Fig. 7C and F). On the other hand, as expected, bands of phosphorylated proteins were not seen in untreated controls (except for p-p38 MAPK). Treatment with different concentrations of DEA and DPA (0.1, 1 and 10 mM) did not affect the expression of LPS stimulated levels of p-JNK1, p-ERK1/2 and p-p38 MAPK. Similarly, the expression of native forms of these proteins was not affected by DEA and DPA treatment (Fig. 7C and F).
DEA and DPA inhibit the transcriptional activity of NF-kB, but not AP-1 and C/EBP
In a luciferase reporter assay, LPS (1 µg/ml) significantly increased NF-kB activity as compared to untreated controls (P < 0.0001, Fig. 8A and C), and this effect was significantly inhibited by 10 µM BAY 11-7082, an NF-kB inhibitor. DEA at a concentration of 10 mM was found to significantly suppress LPS-stimulated NF-kB activity (P < 0.0001, Fig. 8A), whereas DPA significantly suppressed LPS-stimulated NF-kB activity (P < 0.0001, Fig. 8D) at 1 and 10 mM concentrations. DEA and DPA were slightly more effective in inhibiting NF-kB activity than BAY 11-7082, but the difference may not be statistically significant. In contrast, both analogs, at all concentrations tested, did not affect the LPS-induced increase in AP-1 (Fig. 8B and E) or C/EBP activity (Fig. 8C and F). The 10 mM DEA concentration modestly increased C/EBP activity, but this effect was not statistically different (Fig. 8C), whereas 10 mM DPA significantly increased C/EBP activity when compared to LPS control (P < 0.01, Fig. 8F). DMSO was used as a solvent control for BAY 11-7082 in all the experiments with DEA and DPA and found to have no effect on the transcriptional activity of NF-kB, AP-1 and C/EBP (Fig. 8A and F).
DEA and DPA inhibit NF-kB transcriptional activity but not AP-1 or C/EBP in HEK 293 cells. HEK 293 cells overexpressing TLR4 receptors were transiently transfected with NF-kB-Luc, AP-1-Luc, C/EBP-Luc reporter or Renilla-Luc control plasmids for 48 hs. Post 48 h transfection, cells were pre-treated with DEA or DPA for 2 h, then LPS for another 24 h and subsequently luciferase activity was measured using DualGlo luciferase reagent system. The luminescence of firefly luciferase from each reporter plasmid was normalized with that of renilla luciferase for each sample and fold of luciferase activity was calculated. (A, B and C) show luciferase activity of (A) NF-kB-Luc, (B) AP-1-Luc and (C) C/EBP-Luc after treatment with DEA and (D, E and F) shows luciferase activity of (D) NF-kB-Luc, (E) AP-1-Luc and (F) C/EBP-Luc after treatment with DPA. DMSO was used as a vehicle control. Data shown are mean ± SEM of four independent experiments performed in triplicate. To test for significant effects of each concentration of each compound, ANOVA was performed followed by Tukey’s post hoc test using GraphPad Prism 6 software. **P < 0.01, ***P < 0.001 and ****P < 0.0001.
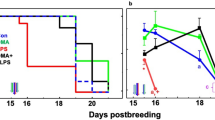
DEA delays LPS-induced PTB in a dose dependent fashion in a murine model
As expected, based on previous work22,23,24,25,26all the mice (6/6) in the LPS only group delivered within 21 h of LPS injection, by E16.5. In contrast, all the mice treated with 750 mg/kg DEA (6/6) had not delivered by the time the experiment was terminated, 25 h after the LPS injection, following the requirements of our IACUC (P < 0.01, Fig. 9). In the group treated with a low dose of DEA (375 mg/kg), 2/8 mice had not delivered by the end of the experiment and the mean delivery time was approximately two hours later than that in the LPS only group (Fig. 9).
Discussion
This work, for the first time, presents two novel anti-inflammatory agents, DEA and DPA, that control the pathologic inflammatory response associated with PTB. With the recent United States (US) Food and Drug Administration withdrawal of 17-alpha hydroxyprogesterone (aka, 17HP, Makena)28there is currently no approved drug to prevent or treat PTB in the US, despite progress made in understanding the pathogenic mechanism of PTB. Over the last several years, our laboratory has focused on identifying novel targets and therapeutic compounds, including DMA, for the prevention of inflammation-driven PTB22,23,24,25,26,29,30,−31.
The anti-inflammatory activity of experimental compounds is commonly investigated in vitro. Among different cell types involved in immune responses in pregnancy, trophoblasts, being the major cell type in the placenta, and macrophages, which are ubiquitous cells found both in the circulation and in tissues, play important roles in the innate immune response in the setting of infection and inflammation. Therefore, in the present study, HTR-8/SVneo (trophoblast cell line) and RAW 264.7 cells (macrophage cell line) were used to study the anti-inflammatory potential of DEA and DPA in in-vitro experiments. Supporting our choice of RAW cells, the role of decidual macrophages in murine models of inflammation-induced preterm birth—particularly LPS-induced preterm birth—has been established by other investigators37,38. Work by these prominent researchers has shown that decidual macrophages secrete various pro-inflammatory mediators, e.g., IL-1β, TNF-α, and IL-6, leading to cervical ripening, membrane rupture and increased uterine contractility. On the other hand, RAW 264.7 cells are known to exhibit both pro- and anti-inflammatory behavior depending on the stimulus and experimental conditions. As such, their response may not fully recapitulate the complexity of primary macrophages or in-vivo systems, and our use of these cells is one limitation in this manuscript.
HTR-8/SVneo cells, a human trophoblast cell line, naturally express high levels of toll-like receptors and are well suited for investigating the anti-inflammatory effects of the compounds.
First, cell viability was determined after treatment with DEA and DPA, two structural analogs of DMA. Our results show that neither analog, up to 10 mM, significantly affects cell viability in either cell line. Since NO plays an important role in mounting an innate immune response against invading pathogens by initiating the process of inflammation32we investigated the effects of DEA and DPA on the secretion of NO− 2, a stable metabolite of NO, in the presence of LPS (1 µg/ml). We found that LPS exposure stimulated the level of NO− 2 to its peak level (65 µM) in the cell culture supernatant 24 h post treatment, consistent with previous reports32,33.
Nitric oxide (NO) is an important biological mediator produced by a variety of cells, including macrophages, in response to bacterial products like LPS34. The production of NO is enzymatically regulated by a family of NOS enzymes: eNOS, nNOS, and iNOS. Various stimuli control the expression of these enzymes, however, iNOS has been implicated mostly in the setting of infection/inflammation and can be induced by various stimuli such as cytokines and bacterial products like LPS33,34. In the current study, we tested whether DEA and DPA affect iNOS expression in RAW 264.7 cells in the presence of LPS. Cells in this study were pre-treated with DEA or DPA for 2 h, while studies have shown inhibition of NO and iNOS after pre-treatment with experimental compounds for time incubation periods varying from 1 h to 6 h32,35. Therefore, direct comparisons of the efficacy of various compounds in affecting NO secretion and iNOS expression may be difficult across studies. We realize that pretreatment with DEA and DPA tests their prophylactic effects on NO secretion rather than their ability to rescue cells from the effects of increased NO levels. However, as this is the first preclinical study exploring the potential therapeutic application of DEA and DPA for preterm birth, it is appropriate to follow a stepwise approach and begin by pretreating the cells with the compounds. In addition, because LPS triggers a rapid cascade of molecular events, pretreatment helps isolate the mechanism of action of DEA and DPA and determine whether the compounds act on early molecular events leading to NO secretion.
We have previously reported that DMA, the parent compound from which DEA and DPA are derived, regulates the expression of pro-inflammatory cytokines and chemokines in mouse and human placental tissue as well as in JEG-3 and RAW 264.7 cells22,27. HTR-8/SVneo cells have been previously reported to secrete pro-inflammatory cytokines34 and chemokines3639. and to express TLR3, TLR4 and TLR540. Now, for the first time, we report the effect of DEA and DPA on regulating the secretion of pro-inflammatory cytokines and chemokines in HTR-8/SVneo and RAW 264.7 cells. Specifically, both analogs were found to significantly suppress TNF-α, IL-6, IL-1β, GM-CSF, MCP-1 and IL-10 secretion from LPS-stimulated RAW 264.7 cells and IL-6, IL-8 and MCP-1 from LPS-stimulated HTR-8/SVneo cells. Consistent with previous reports41we did not find any detectable levels of TNF-α, IL-1β, GM-CSF or IL-10 in HTR-8/SVneo cell supernatants. These findings, along with our data on DEA and DPA’s effect on NO secretion and iNOS expression, suggest that these analogs possess anti-inflammatory activity and may be considered novel cytokine suppressive anti-inflammatory compounds.
To further evaluate DEA and DPA’s anti-inflammatory activity and their potential application to the management of preterm parturition, we examined their effects on pro-inflammatory cytokine and chemokine secretion using human placentae. We used the placental explants as a platform for demonstrating the ability of DEA and DPA to attenuate LPS-induced inflammatory responses, based on the model of LPS-induced inflammatory cytokines in term human placental explants established by Duval et al.42. In addition, the placental explant, in contrast to an isolated fetal membrane culture, retains the three-dimensional architecture of the placenta, and thereby recapitulates the interactions between various cell types that may play a role in the inflammatory response, such as Hofbauer cells and trophoblast cells. Again, we realize that pretreating the explants with DEA and DPA does not test their ability to rescue the ex vivo cultures from the LPS-induced inflammatory cascade. On the other hand, pretreatment is appropriate for this first investigation of the compounds’ anti-inflammatory effects to isolate their mechanism of action on the initial steps in the inflammatory response.
The up-regulation of expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β), chemokines (IL-8 and MCP-1), eicosanoids (PGE2 and PGF2α) and various gestational hormones in placenta during labor has been well established39,43. To best control for this inflammatory response across our different human tissue samples, we collected normal term placentae from non-laboring women and incubated explants prepared from these specimens with LPS. The pro-inflammatory cytokines, such as TNF-α, IL-6 and GM-CSF, play a crucial role in parturition and the presence of these cytokines as a result of infection or resultant inflammation before term leads to increased production of PGs and MMPs, causing early activation of terminal events in the labor cascade, which ultimately results in premature delivery44,45,46,47. In addition to inhibiting cytokines, both analogs also inhibited the secretion of chemokines, such as IL-8 and MCP-1, in both HTR-8/SVneo cells and human placental explants. Chemokines are involved in the stimulation and chemotaxis of inflammatory cells to the site of injury and have been of particular interest in the last several years because of their likely involvement in the initiation of parturition48,49,50. Our previous study has also demonstrated an increase in the number of inflammatory cells in murine placental tissue in the presence of LPS, which was significantly reduced by DMA, the parent compound22. Increased concentrations of IL-8, macrophage inflammatory protein-1α (MIP-1α) and MCP-1 have been implicated in preterm birth associated with intra-amniotic infection51,52,53. Thus, the inhibitory effects of DEA and DPA on the secretion of cytokines and chemokines in our ex-vivo and in-vitro models of inflammation-induced PTB further strengthens their potential as anti-inflammatory agents for PTB.
Remarkably, both analogs resulted in more effective inhibition of cytokine and chemokine secretion than BAY 11-7082, an NF-kB inhibitor used here as a positive control. However, it is important to note that BAY 11-7082 was used at a concentration of 25 µM in our explant model, which is higher than the concentration used (5 µM) in our in-vitro experiments, because BAY 11-7082, at 5 µM, did not produce any inhibitory effect in placental explant experiments. This observation is consistent with our previously reported finding of no inhibitory effect of 5 and 10 µM concentrations of BAY 11-7082 in placental explants27. This might be explained by the fact that placental explants consist of a heterogeneous population of cells, where higher concentrations of compounds might be required to have some effect as compared to concentrations of the same compounds used in experiments involving a single cell population.
IL-10 is a predominantly anti-inflammatory cytokine primarily produced by macrophages and monocytes. It acts to terminate the inflammatory response by inhibiting the production of pro-inflammatory mediators such as TNF-α, IL-6, IL-1β and IL-8. In addition to immune cells, IL-10 is also produced by gestational tissues54,55,56 and has been shown to decrease the synthesis of TNF-α, IL-6 and PGs in human placental, chorionic and decidual cells54,55. However, IL-10 has also been demonstrated to exhibit immunostimulatory properties55. For example, treatment of CD4+ T cells, CD8+ T cells and/or NK cells with IL-10 increases their ability to produce cytokines after stimulation55. Also, IL-10 has been reported to exert pro-inflammatory activity in human amnion57. On the other hand, in the same study, the authors also reported anti-inflammatory actions of IL-10 in choriodecidua, suggesting a tissue specific dual role in inflammatory processes associated with labor. In the present study, we found that LPS increased IL-10 secretion in RAW 264.7 cells and human placental explants, which was then significantly decreased by treatment with DEA or DPA. We have previously reported similar observations with DMA treatment, although the effect was not statistically significant, suggesting that DEA and DPA might have more potent anti-inflammatory activity than DMA27. However, the IL-10 response to LPS we have observed in vitro is opposite to what we have observed in vivo, where DMA was shown to increase the levels of IL-10 in mouse placenta22.
In the current study, we have further explored the mechanism of anti-inflammatory activity of both analogs by looking at the NF-kB and MAPK pathways, because both are activated by LPS through its binding to TLR4 receptors, and both pathways mediate the production of all the inflammatory cytokines and chemokines investigated in this study. We have previously reported that DMA, the parent compound, inhibits LPS-induced IkB-α degradation and NF-kB nuclear translocation in RAW 264.7 cells27. Now, we are excited to report that both analogs also significantly attenuated LPS-induced IkB-α degradation in a concentration-dependent manner. Inhibition of IkB-α degradation prevents nuclear translocation of NF-kB dimers, preventing the activation of the NF-kB signaling cascade, which explains the inhibitory effects of both analogs on the secretion of pro-inflammatory cytokines and chemokines in both in-vitro and ex-vivo experiments as discussed above. Currently, many therapeutic approaches targeting this pathway, and inhibiting IkB-α and IKKs specifically, have been proposed for various disease conditions such as inflammatory bowel disease58cancer59,60metabolic diseases61arthitis62and most relevant to our work, PTB63. On the other hand, neither DEA nor DPA affected phosphorylation of various proteins in the MAPK pathway, such as JNK1, ERK1/2 and p-38 MAPK, which is similar to what we have reported previously about DMA27. This finding indicates that both analogs specifically affect the NF-kB pathway. However, the precise mechanism by which DEA, DPA and even DMA exert their inhibitory effects on IkB-α degradation remains to be elucidated.
To answer this question, future investigation should be aimed at testing whether these analogs affect IKK expression and/or activity, in order to determine whether the compounds inhibit IkB-α phosphorylation and thereby protect it from degradation by the proteasome, whether these compounds inhibit the proteasome directly or both. Also, how and why DEA and DPA selectively inhibit IkB-α degradation and do not affect the phosphorylation of JNK1, ERK1/2 and p-38 MAPK remains to be determined. An alternative mechanism of action is suggested by work performed by Ghayor et al. who, in their investigation focused on osteoporosis, also found that DMA, the parent, inhibits the NF-κB pathway64. This group has found that DMA acts at the epigenetic level and is a bromodomain ligand64. Taken together, this intriguing report coupled with our work suggest that DMA and its structural analogs may represent a novel group of anti-inflammatory molecules that suppress cytokine secretion via two completely disparate mechanisms and are thereby compounds that demonstrate so-called “polypharmacology.”
We further evaluated the involvement of NF-kB and MAPK pathways by looking at the transcriptional activity of NF-kB, AP-1 and C/EBP in HEK 293 cells overexpressing TLR4 receptors transiently transfected with NF-kB-Luc, AP-1-Luc and C/EBP-Luc reporter plasmids, respectively. The reporter gene assays have been extensively utilized in evaluating the effect of experimental compounds on the transcriptional activity of target genes65,66. Besides NF-kB, AP-1 and C/EBP transcription factors are also activated by inflammatory cytokines, regulating the expression of many inflammatory genes65,67,68,69. Interestingly, in the present study, both analogs were found to have no effect on the activity of AP-1 and C/EBP, which confirms our western blot data where both analogs did not affect the expression of phosphorylated JNK1, ERK1/2 and p-38 MAPK in LPS-stimulated HEK 293 cells. Because DEA and DPA specifically inhibit the NF-kB pathway and have no effect on the JNK1, ERK1/2 and p-38 MAPK, they are predicted to have limited and desirable anti-inflammatory effect and not act as major immune suppressors.
Finally, in a critical experiment, DEA delayed PTB in LPS-induced mice, with 100% of mice treated with 750 mg/kg DEA not delivering for 25 h. Unfortunately, the requirements of our IACUC prevented us from testing whether preterm delivery could be delayed until term and evaluating the condition of the pups after DEA treatment of the LPS stimulated mice. However, in very exciting recently published results of a study with the parent compound, DMA, we found that mice treated with LPS plus DMA delivered pups at term that were viable and able to nurse24.
In summary, we have shown for the first time that two DMA analogs, DEA and DPA, inhibit LPS-induced secretion of NO and pro-inflammatory cytokines and chemokines in both in-vitro and ex-vivo models. In addition, we are also excited to report for the first time that these inhibitory effects of DEA and DPA are attributed to their ability to suppress LPS-induced IkB-α degradation in RAW 264.7 cells. Furthermore, both analogs also attenuated LPS-induced NF-kB’s transcriptional activity, supporting their effects on IkB-α degradation. A pictorial summary of these findings is shown in Fig. 10. Although we observed that between the two analogs, DPA seems to be more effective in suppressing the inflammatory response produced by LPS, although additional studies comparing the two analogs plus the parent compound are required. Taken together, these results establish both DEA and DPA as novel compounds in the cytokine suppressive anti-inflammatory drug (CSAID) class. Importantly, DEA and/or DPA may be novel therapeutic agents useful not only for the treatment or prevention of inflammation-induced preterm birth, but also for the management of a wide array of other inflammatory disorders. In fact, we have shown that DMA attenuates inflammatory bowel disease in an in vivo model70 and neuroinflammation in in-vitro and ex-vivo models71. Further investigation of this family of compounds is warranted to determine their therapeutic potential.
Data availability
Original data are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ATCC:
-
American Type Culture Collection
- BCA:
-
bicinchoninic acid
- DEA:
-
N, N-Diethylacetamide
- DMA:
-
N, N-Dimethylacetamide
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- DMF:
-
N, N-Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- DPA:
-
N, N-Dipropylacetamide
- EDTA:
-
ethylenediaminetetraacetic acid
- ELISA:
-
Sandwich enzyme-linked immunosorbent assay
- ERK:
-
extracellular-signal regulated protein kinase
- FBS:
-
Fetal bovine serum
- HEK:
-
Human embryonic kidney
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- IkB-α:
-
inhibitor kappa B-α
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
c-Jun NH2-terminal kinase
- LDH:
-
Lactate dehydrogenase
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MCP:
-
Monocyte-chemoattractant protein
- MMP:
-
Matrix metalloproteinase
- MTT:
-
3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyl tetrazolium bromide
- NF-kB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- PG:
-
Prostaglandin
- PMSF:
-
phenylmethylsulfonylfluoride
- PTB:
-
Preterm birth
- RIPA:
-
Radioimmunoprecipitation assay
- RPMI:
-
Roswell Park Memorial Institute
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
References
Romero, R., Dey, S. K. & Fisher, S. J. Preterm labor: one syndrome, many causes. Science 345, 760–765. https://doi.org/10.1126/science.1251816 (2014).
Green, R. F. et al. Economic burden of preterm birth: updated estimates and implications for prevention. J. Perinatol. 41, 1101–1108. https://doi.org/10.1038/s41372-020-00863-1 (2021).
Hamilton, B. E., Martin, J. A. & Osterman, M. J. K. Births: provisional data for 2020. CDC Stacks. 12, 104993. https://doi.org/10.15620/cdc:104993 (2021).
Guo, X. et al. A birth population-based survey of preterm morbidity and mortality by gestational age. BMC Pregnancy Childbirth. 21, 291. https://doi.org/10.1186/s12884-021-03726-4 (2021).
Negishi, Y. et al. Inflammation in preterm birth: novel mechanism of preterm birth associated with innate and acquired immunity. J. Reprod. Immunol. 154, 103748. https://doi.org/10.1016/j.jri.2022.103748 (2022).
Chawanpaiboon, S. et al. Global, regional, and National estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Global Health. 7, e37–e46. https://doi.org/10.1016/S2214-109X(18)30451-0 (2019).
Beck, S. et al. Infection, inflammation, and the risk of preterm birth. Sem Fetal Neonatal Med. 27, 101145. https://doi.org/10.1016/j.siny.2021.101145 (2022).
Fitzgerald, K. A. & Kagan, J. C. Toll-like receptors and the control of immunity. Cell 180, 1044–1066. https://doi.org/10.1016/j.cell.2020.02.041 (2020).
Menon, R. & Papaconstantinou, J. Inflammation-induced preterm birth: new insights into the role of nuclear factor kappa B. Am. J. Reprod. Immunol. 84, e13265. https://doi.org/10.1111/aji.13265 (2020).
Paquette, A. G. & D’Alton, M. E. The role of NF-κB signaling in term and preterm labor. Am. J. Reprod. Immunol. 86, e13432. https://doi.org/10.1111/aji.13432 (2021).
Pineda, A., Verdin-Terán, S. L. & Romero, R. Toll-like receptor 4 and microbial-induced preterm birth: mechanisms and interventions. Reprod. Sci. 28, 639–649. https://doi.org/10.1007/s43032-020-00355-w (2021).
Gómez-Chávez, F. et al. NF-κB and its regulators during pregnancy. Front. Immunol. 12, 679106. https://doi.org/10.3389/fimmu.2021.679106 (2021).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Sig Transduct. Target. Ther. 2, 17023. https://doi.org/10.1038/sigtrans.2017.23 (2017).
Than, N. G. & Romero, R. Inflammation and labor: A double-edged sword. Placenta 99, 26–33. https://doi.org/10.1016/j.placenta.2020.08.007 (2020).
Oliveira, M. L. et al. Regulation of nitric oxide synthesis and function in pregnancy. J. Molec Med. 99, 1427–1438. https://doi.org/10.1007/s00109-021-02079-0 (2021).
Petroff, M. G. & Campbell, K. L. Dual regulation of prostaglandins by nitric oxide in human pregnancy. Reprod. Bio Endocrinol. 18, 101. https://doi.org/10.1186/s12958-020-00641-9 (2020).
Li, Y. et al. Nitric oxide synthase expression in the human cervix: role in labor and cervical ripening. Internat J. Molec Sci. 22, 2559. https://doi.org/10.3390/ijms22052559 (2021).
Morales-Hernandez, F. V. et al. Differential proMMP-2 and proMMP-9 secretion in human pre-implantation embryos at day 5 of development. Acta Biochim. Pol. 69, 683–689. https://doi.org/10.18388/abp.2020_5397 (2022).
El-Achi, V. et al. First-trimester prediction of preterm prelabour rupture of membranes. Fetal Diagn. Ther. 47, 624–629. https://doi.org/10.1159/000506541 (2020).
Olson, D. M. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 17, 717–730. https://doi.org/S1521693403000695 (2003).
Gomez-Lopez, N. et al. The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal–fetal homeostasis. Reproduction 164, R157–R181. https://doi.org/10.1530/REP-22-0046 (2022).
Sundaram, S. et al. N,N-dimethylacetamide regulates the Proinflammatory response associated with endotoxin and prevents preterm birth. Am. J. Path. 183, 422–430. https://doi.org/10.1016/j.ajpath.2013.05.006 (2013).
Wei, Z. et al. N,N-Dimethylformamide delays LPS-induced preterm birth in a murine model by suppressing the inflammatory response. Reprod. Sci. 29, 2894–2907. https://doi.org/10.1007/s43032-022-00924-z (2022).
Reznik, S. E., Kashou, A., Ward, D. & Yellon, S. M. N,N-dimethylacetamide blocks inflammation-induced preterm birth and remediates maternal systemic immune responses. Sci. Rep. 15, 8234. https://doi.org/10.1038/s41598-025-93282-0 (2025).
Mir, A. et al. Improving the safety of N,N-dimethylacetamide (DMA) as a potential treatment for preterm birth in a pregnant mouse model using a vaginal nanoformulation. BBA - Molec Basis Dis. 1871, 167822. https://doi.org/10.1016/j.bbadis.2025.167822 (2025).
Olgun, N. et al. Effect of the putative, novel selective ETA receptor antagonist HJP272, a 1,3,6-trisubstituted-2-carboxy-quinol-4-one, on infection mediated premature delivery. Can. J. Physiol. Pharmacol. 86, 571–575. https://doi.org/10.1139/Y08-057 (2008).
Pekson, R. et al. N,N-Dimethylacetamide significantly attenuates LPS- and TNFalpha-induced Proinflammatory responses via Inhibition of the nuclear factor kappa B pathway. Molec Med 22. https://doi.org/10.2119/molmed.2016.00017 (2016).
Blackwell, S. C. et al. 17-OHPC to prevent recurrent preterm birth in Singleton gestations (PROLONG study): A multicenter, international, randomized double-blind trial. Am. J. Perinatol. 37, 127–136. https://doi.org/10.1055/s-0039-3400227 (2020).
Olgun, N. S., Stephani, R. A., Patel, H. J. & Reznik, S. E. Blockade of endothelin-1 with a novel series of 1,3,6-trisubstituted-2-carboxy-quinol-4-one’s controls infection-associated preterm birth. Am. J. Path. 177, 1929–1935. https://doi.org/10.2353/ajpath.2010.100281 (2010).
Wang, W. et al. Endothelin-1 and matrix metalloproteinase-1 function in the same molecular pathway in infection-associated preterm birth. Molec Med. 16, 505–512. https://doi.org/10.1155/2010/657039 (2010).
Vyas, V. et al. Inhibition of sphingosine kinase prevents lipopolysaccharide induced preterm birth and suppresses pro-inflammatory responses in a murine model. Am. J. Path. 185, 862–869. https://doi.org/10.1016/j.ajpath.2014.10.026 (2015).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388. https://doi.org/10.1038/s41577-020-0285-6 (2020).
Lee, T. H., Jung, M., Bang, M. H., Chung, D. K. & Kim, J. Inhibitory effects of a Spinasterol glycoside on lipopolysaccharide-induced production of nitric oxide and Proinflammatory cytokines via down-regulating MAP kinase pathways and NF-kappaB activation in RAW264.7 macrophage cells. Internat Immunopharm. 13, 264–270. https://doi.org/10.1016/j.intimp.2012.05.005 (2012).
Solár, P., Sumbalová, Z. & Zeman, M. The role of inducible nitric oxide synthase in inflammation and cancer: an update. Physiol. Res. 69, S199–S212. https://doi.org/10.33549/physiolres.934374 (2020).
Wang, Y., Li, J., Zhang, Y., Zhang, X. & Liu, Y. MiR-335-5p regulates eNOS expression and trophoblast function in preeclampsia. Molec Med. Rep. 23, 1–9. https://doi.org/10.3892/mmr.2020.11733 (2021).
Xu, J., Zhou, Y., Wei, Y. & Fu, W. miR-155 contributes to preeclampsia pathogenesis by regulating SHH/GLI1/BCL2 signaling pathway in trophoblasts. Placenta 95, 17–25. https://doi.org/10.1016/j.placenta.2020.04.002 (2020).
Romero, R. et al. The role of inflammation and infection in preterm birth. Sem Reprod. Med. 25, 21–39. https://doi.org/10.1055/s-2006-956773 (2007).
Wang, H. & Hirsch, E. Bacterially induced preterm labor and regulation of Proinflammatory mediators in the murine uterus. Biol. Reprod. 69, 1957–1963. https://doi.org/10.1095/biolreprod.103.020230 (2003).
Shaheen, S., Al-Maari, S., Alshaikh, M. & Ahmed, M. Decreased expression of placental endothelial nitric oxide synthase and increased oxidative stress in preeclampsia. Mol. Genet. Genomic Med. 8, e1019. https://doi.org/10.1002/mgg3.1019 (2020).
Shaamash, A. H., Elsonosy, E. D., Zakhari, M. M., Radwan, S. H. & El-Dien, H. M. Placental nitric oxide synthase (NOS) activity and nitric oxide (NO) production in normal pregnancy, pre-eclampsia and eclampsia. Internat J. Gynecol. Obstet. 72, 127–133. https://doi.org/10.1016/s0020-7292(00)00314-3 (2001).
Cho, W. S., Nam, C. W., Kim, M. Y. & Lee, J. S. Antioxidant and anti-inflammatory activities of Orostachys japonicus ethanol extract in LPS-stimulated RAW 264.7 cells. Arch. Pharm. Res. 43, 1023–1031. https://doi.org/10.1007/s12272-020-01284-w (2020).
Duval, C. et al. Differential effects of LPS and IL-1b in term placental explants. Placenta 75, 9–15. https://doi.org/10.1016/j.placenta.2018.11.006 (2019).
Xue, P. et al. Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PloS One. 10, e0124001. https://doi.org/10.1371/journal.pone.0124001 (2015).
Torres-Torres, M. C., Torres-Salazar, C. J. & Medina-Juárez, L. A. The role of inflammation in preterm and term parturition: an updated review. Hypertension 83, 681–692. https://doi.org/10.1161/HYPERTENSIONAHA.117.09321 (2024).
Umesalma, S. & Sudhandiran, G. Altered expression of endothelin-1 and endothelial nitric oxide synthase in placenta of normal and preeclamptic women: role of inflammation. Placenta 125, 1–8. https://doi.org/10.1016/j.placenta.2022.01.004 (2022).
Gierman, L. M. et al. Toll-like receptor profiling of seven trophoblast cell lines warrants caution for translation to primary trophoblasts. Placenta 36, 1246–1253. https://doi.org/10.1016/j.placenta.2015.09.004 (2015).
Leimert, K. B., Stelzer, I. A. & Gomez-Lopez, N. Setting a stage: inflammation during preeclampsia and postpartum. Front. Immuno. 12, 9995795. https://doi.org/10.3389/fimmu.2021.9995795NCBI (2021).
Ravanos, K. et al. Factors implicated in the initiation of human parturition in term and preterm labor: A review. Gynecol. Endocrinol. 31, 679–683. https://doi.org/10.3109/09513590.2015.1076783 (2015).
Romero, R. Intrauterine infection: Inducer of preterm labor induction. J. Labor. Childbirth. 6, 38–41. https://doi.org/10.37532/jlcb.2023.6(2).038-041 (2023).
Zhang, P. et al. Placental growth factor mediates pathological uterine angiogenesis by activating the NFAT5-SGK1 signaling axis in the endometrium: implications for preeclampsia development. Internat J. Molec Sci. 24, 524. https://doi.org/10.3390/ijms24010524PubMed (2023).
Kumar, D. et al. Decidual GM-CSF is a critical common intermediate necessary for thrombin and TNF induced in-vitro fetal membrane weakening. Placenta 35, 1049–1056. https://doi.org/10.1016/j.placenta.2014.10.001 (2014).
Laudanski, P. et al. Chemokine profiling of patients with preterm birth. Mediat Inflam. 185758 https://doi.org/10.1155/2014/185758 (2014).
Romero, R., Basso, R. M. & Hassan, S. S. Cytokines and preterm labor: a review of the literature. Am. J. Reprod. Immunol. 84, e13267. https://doi.org/10.1111/aji.13267 (2020).
Groux, H., Bigler, M., de Vries, J. E. & Roncarolo, M. G. Inhibitory and stimulatory effects of IL-10 on human CD8 + T cells. J. Immunol. 160, 3188–3193 (1998).
Ouyang, W., Rutz, S., Crellin, N. K., Valdez, P. A. & Hymowitz, S. G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Ann. Rev. Immunol. 39, 295–326. https://doi.org/10.1146/annurev-immunol-120419-023843 (2021).
Saraiva, M., Vieira, P. & O’Garra, A. Biology and therapeutic potential of interleukin-10. Nat. Rev. Immunol. 20, 680–694. https://doi.org/10.1038/s41577-020-0359-7 (2020).
Menon, R. et al. Inflammation and senescence in fetal membranes: potential therapeutic targets for adverse pregnancy outcomes. Placenta 104, 150–160. https://doi.org/10.1016/j.placenta.2020.11.014 (2021).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 7, 282. https://doi.org/10.1038/s41392-022-01107-154 (2022).
Tang, Z. et al. A novel NF-κB pathway inhibitor suppresses inflammation in vitro and in vivo. Bioorg. Med. Chem. Lett. 64, 129889. https://doi.org/10.1016/j.bmcl.2023.12988955 (2023).
Yang, X. et al. NF-κB and cancer: mechanisms and therapeutic targeting. Cells 10, 2293. https://doi.org/10.3390/cells10092293 (2021).
Zhang, Q., Lenardo, M. J. & Baltimore, D. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 43, 46–58. https://doi.org/10.1016/j.it.2022.07.004 (2022).
Zhou, Y. et al. NF-κB in biology and targeted therapy: new insights and clinical implications. Signal. Transduct. Target. Ther. 9, 1–18. https://doi.org/10.1038/s41392-024-01757-9 (2024).
Pavlidis, P. et al. Preterm birth therapies to target inflammation. J. Clin. Pharmacol. 62, 3–14. https://doi.org/10.1002/jcph.2107 (2022).
Ghayor, C. et al. E. N,N dimethylacetamide a drug excipient that acts as bromodomain ligand for osteoporosis treatment. Sci. Rep. 7, 42108. https://doi.org/10.1038/srep42108 (2017).
Wang, H. et al. The regulation of reporter transgene expression for diverse applications. Nat. Biomed. Eng. 9, 345–358. https://doi.org/10.1038/s44303-025-00070-6 (2025).
Roda, B. et al. Shining light on biosensors: chemiluminescence and bioluminescence in enabling technologies. TrAC Trends Anal. Chem. 180, 117975. https://doi.org/10.1016/j.trac.2024.11797562 (2024).
Kyriakis, J. M. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Exp. 7, 217–231 (1999).
Lee, A. K., Sung, S. H., Kim, Y. C. & Kim, S. G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by Sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation. Brit J. Pharm. 139, 11–20. https://doi.org/10.1038/sj.bjp.0705231 (2003).
Ren, Q. et al. C/EBPβ: the structure, regulation, and its roles in inflammation-related diseases. Biomed. Pharmacother. 169, 115938. https://doi.org/10.1016/j.biopha.2023.115938.65 (2023).
Koya, J. et al. FDA-approved excipient N,N-dimethylacetamide attenuates in vitro and in vivo inflammatory bowel disease. Fortune J. Health Sci. 5, 499–509. https://doi.org/10.26502/fjhs.076 (2022).
Wei, Z. et al. N-N-Dimethylacetamide attenuates neuroinflammation in Alzheimer’s disease in in-vitro and ex-vivo models. Sci. Rep. 13, 7077. (2023). https://doi.org/10.1038/s41598-023-34355-w
Acknowledgements
This work was supported by an AMAG Pharmaceuticals Lumara Health Research Grant awarded to JM and SER and St. John’s University Seed Grants awarded to SER. We are very grateful to Dr. Francine Hughes, Dr. Rodica Ciubotariu and the staff of the New York Cord Blood Bank at Albert Einstein College of Medicine for providing the placentas. We are also grateful to Dr. Charles H. Graham for providing the HTR-8/SVneo cells and to Dr. Vladimir Poltoratsky for providing the transfected HEK293 cells.
Funding
This work was supported by NIH grant 1R16GM145586 to SER, AMAG Pharmaceuticals Lumara Health Research Grant awarded to JM and SER and St. John’s University Seed Grants awarded to SER.
Author information
Authors and Affiliations
Contributions
SG performed the experiments and wrote the paper. JM assisted with the experiments and provided funding. SY and LB synthesized the compounds. RP assisted with the design of the experiments. CRA contributed to the original concept of the study. SER developed the concept of the study, contributed to the writing of the paper, and provided funding.
Corresponding author
Ethics declarations
Competing interests
CRA and SER have an allowed patent (US11717499B2) on the use of DMA, the parent compound, for the prevention of PTB. This work was funded by a grant from AMAG/Lumara Health.
Ethics approval and consent to participate
All animal care and experimental protocols were approved the St. John’s University Institutional Animal Care and Use Committee.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gorasiya, S., Mushi, J., Yoganathan, S. et al. N,N-Diethylacetamide and N,N-Dipropylacetamide inhibit the NF-kB pathway in in vitro, ex vivo and in vivo models of inflammation-induced preterm birth. Sci Rep 15, 29861 (2025). https://doi.org/10.1038/s41598-025-16076-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16076-4