Abstract
This study focuses on the synthesis of a novel magnetic interphase palladium catalyst immobilized on pyromellitic dianhydride (PM)-coated magnetic SnFe2O4 nanoparticles. Such surface functionalization of magnetic particles represents a promising strategy to bridge the gap between heterogeneous and homogeneous catalysis methods. The structure, morphology, and physicochemical properties of these particles were thoroughly examined using various analytical techniques, including FT-IR, SEM, XRD, VSM, ICP, and EDS. The resulting SnFe2O4/SiO2/PM-Pd nanocatalyst exhibited excellent catalytic performance as a recyclable catalyst in Suzuki-Miyaura cross-coupling reactions at room temperature. Additionally, the catalyst demonstrated high reusability, showing minimal palladium leaching and no significant loss in activity across multiple cycles.
Similar content being viewed by others
Introduction
The Suzuki-Miyaura reaction plays a pivotal role in organic synthesis for forming carbon-carbon bonds in organic compounds1,2,3,4. This reaction employs aryl halides and boronic acids in the presence of a palladium catalyst. While homogeneous palladium catalysts are commonly used, they often pose challenges related to separation and recycling, leading to increased costs and environmental concerns5,6. To address these issues, heterogeneous palladium catalysts have been proposed as a more sustainable and economical alternative7,8,9. These catalysts, owing to their ease of separation and recyclability, enhance the reaction’s efficiency, reduce costs, and mitigate the environmental impact of catalytic materials10,11,12,13,14,15,16. Research in this area focuses on developing highly active, chemically and physically stable, and reusable heterogeneous palladium catalysts17,18,19. On an industrial scale, adopting heterogeneous catalysts can significantly optimize organic production by increasing efficiency and reducing costs20,21,22. Nanomaterials with magnetic separation capabilities have emerged as an essential class of materials, greatly appreciated for their unique physicochemical properties and receiving significant research attention23,24. Spinel ferrite compounds stand out as particularly promising materials for catalytic support, with their strong potential in various industrial and technological applications25,26. Their environmentally friendly catalytic behavior across diverse organic transformations highlights their utility, while their robust framework provides excellent support for catalyst performance27,28. Within this context, SnFe2O4 spinel has been identified as an effective nanomagnetic catalytic support for designing a new generation of catalysts29,30,31. Spinel ferrites, particularly tin ferrite (SnFe2O4/SiO2/PM-Pd), have garnered attention due to their magnetic properties, chemical and thermal stability, straightforward synthesis, and surface modifiability32,33. The spinel structure accommodates and immobilizes active metal species efficiently, while the magnetic properties facilitate simple separation from the reaction medium, making them valuable tools in catalysis and beyond34,35,36.
The SnFe2O4/SiO2/PM-Pd catalyst features a unique structure with a stabilized complex that enhances the active surface area, boosts palladium dispersion, and ultimately increases the efficiency of the Suzuki reaction. Furthermore, this design enhances the catalyst’s stability and makes it easier to separate and recycle. The research aims to assess the synthesized catalyst’s performance in the Suzuki reaction and compare it with similar catalysts found in existing literature. Additionally, the study examines how reaction parameters such as temperature, reaction time, and catalyst concentration affect the catalyst’s performance and conversion rate. The findings could contribute to developing stable and efficient methods for executing cross-coupling reactions in organic synthesis.
Experimental
Preparation of SnFe2O4/SiO2/PM-Pd
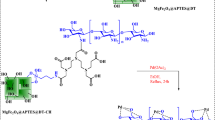
The synthesis of SnFe2O4@SiO2 began with the production of SnFe2O4 following well-established protocols. Subsequently, 1 g of SnFe2O4@SiO2 was dispersed in 50 mL of toluene through 30 min of sonication. To this dispersion, 3 mL of 3-aminopropyltrimethoxysilane (APTES) was added, and the mixture underwent reflux for 24 h. The resulting SnFe2O4/SiO2/PM nanoparticles were purified by washing four times with ethanol, separated via magnetic decantation, and dried at 50 °C. In the next phase, 2 g of SnFe2O4/SiO2/PM was dispersed in 40 mL of toluene using a similar 30-minute sonication process, followed by the addition of 3 mmol of dicyclohexylcarbodiimide (PM). The mixture was then stirred under reflux conditions for another 24 h. The obtained nanoparticles were similarly washed multiple times with ethanol, magnetically separated, and dried at 60 °C. To graft Pd onto the heterogenized ligand, 1 g of SnFe2O4/SiO2/PM was reacted with 1.5 mmol of Pd(OAc)2 in CH3CN at room temperature for 24 h. The SnFe2O4/SiO2/PM-Pd nanoparticles were isolated by filtration, thoroughly cleaned with hot water and hot ethanol to remove any residual Pd, and subsequently dried at 60 °C. The overall procedure is represented schematically in Fig. 1.
General procedure for the suzuki reactions
A combination of aryl halide (1.0 mmol), phenylboronic acid (1.0 mmol), K2CO3 (1.5 mmol), and 30 mg of SnFe2O4/SiO2/PM-Pd catalyst was stirred in 3 mL of PEG-400 at 100 °C. The reaction progress was monitored using TLC, and upon completion of the cross-coupling reaction, hot distilled water was added to the mixture. The catalyst was then separated by filtration, and the product was further purified. Extraction of the desired product was carried out using a 1:1 mixture of water and ethyl acetate (Fig. 2).
Selected NMR data
4-methyl-1,1’-biphenyl (Table 2, Entry 3) (Figure S1)1:H NMR (400 MHz, DMSO): δH = 7.4 (m, 4 H), 7.1 (m, 5 H), 2.0 (s, 3 H), ppm.
3-methyl-1,1’-biphenyl (Table 2, Entry 9) (Figure S2)1:H NMR (400 MHz, DMSO): δH = 7.7 (m, 3 H), 7.5 (m, 2 H), 7.4 (m, 4 H), 1.9 (s, 3 H), ppm.
4-nitro-1,1’-biphenyl (Table 2, Entry 11) (Figure S3)1:H NMR (400 MHz, DMSO): δH = 8.2 (s, 1 H), 7.5 (s, 1 H), 7.3 (s, 3 H), 7.1 (s, 3 H), 7.0 (s, 1 H), ppm.
4-nitro-1,1’-biphenyl (Table 2, Entry 5) (Figure S4)1:H NMR (400 MHz, DMSO): δH = 8.1 (s, 2 H), 7.3 (m, 7 H), ppm.
1,1’-biphenyl (Table 2, Entry 12) (Figure S5)1:H NMR (400 MHz, DMSO): δH = 7.7 (s, 3 H), 7.4 (s, 4 H), 7.2 (s, 3 H), ppm.
1,1’-biphenyl (Table 2, Entry 10) (Figure S6)1:H NMR (400 MHz, DMSO): δH = 7.2 (m, 7 H), 6.9 (s, 3 H), ppm.
4-methoxy-1,1’-biphenyl (Table 2, Entry 13) (Figure S7)1:H NMR (400 MHz, DMSO): δH = 7.0 (m, 1 H), 6.9 (s, 3 H), 6.5 (m, 5 H), 4.0 (s, 3 H), ppm.
4-methoxy-1,1’-biphenyl (Table 2, Entry 4) (Figure S8)1:H NMR (400 MHz, DMSO): δH = 8.3 (m, 1 H), 7.9 (m, 1 H), 7.6 (s, 3 H), 7.1 (m, 4 H), 4.3 (s, 3 H), ppm.
3-methoxy-1,1’-biphenyl (Table 2, Entry 6) (Figure S9)1:H NMR (400 MHz, DMSO): δH = 8.2 (m, 2 H), 7.8 (m, 1 H), 7.6 (m, 3 H), 7.3 (m, 3 H), 3.7 (s, 3 H), ppm.
1,1’-Biphenyl (Table 2, Entry 1) (Figure S10)1:H NMR (400 MHz, DMSO): δH = 7.4 (m, 4 H), 7.0 (m, 6 H) ppm.
3-Nitro-1,1’-biphenyl (Table 2, Entry 2) (Figure S11)1:H NMR (400 MHz, DMSO): δH = 7.5 (m, 1 H), 7.4 (m, 2 H), 7.2 (m, 3 H), 7.1 (m, 3 H), ppm.
1-Phenylanthracene (Table 2, Entry 7) (Figure S12)1:H NMR (400 MHz, DMSO): δH = 7.6 (m, 2 H), 7.4 (m, 2 H), 7.0 (m, 3 H), 6.8 (m, 7 H), ppm.
1-([1,1’-Biphenyl]−4-yl)ethan-1-one (Table 2, Entry 8) (Figure S13)1:H NMR (400 MHz, DMSO): δH = 7.0 (m, 5 H), 6.7 (m, 4 H), ppm.
Catalyst characterizations
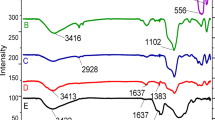
To verify the successful functionalization of SnFe2O4/SiO2/PM-Pd magnetic nanoparticles (MNPs), FT-IR spectroscopy was conducted using the KBr pellet method (Fig. 3). In Fig. 3(a), vibrational bands at 740 and 580 cm⁻¹, observed across all FT-IR spectra, correspond to the stretching vibrations of the Sn-O bond. Additionally, peaks near 3490 cm⁻¹ are attributed to hydroxyl groups located on the surface of the magnetite. Figures 3(b–c) reveal peaks at 1064 cm⁻¹ and within the range of 2919–3029 cm⁻¹, which can be attributed to the ν(Si─O) and ν(C─H) vibrational modes, respectively; these specific bands are absent in the magnetite spectrum. The presence of these vibrations indicates the successful incorporation of SiO2 and APTES onto the SnFe2O4 nanoparticles. The immobilization of the PM group onto SnFe2O4/SiO2 is confirmed by the appearance of a C═N vibrational band at approximately 1630 cm⁻¹, along with a C─H vibrational band near 1418 cm⁻¹ (Fig. 3d). Furthermore, in the spectrum of SnFe2O4/SiO2/PM-Pd (Fig. 3e), the downward shift of the C═N vibrational band to a lower frequency at approximately 1466 cm⁻¹ provides evidence for the successful formation of the Pd complex on the surface of the functionalized SnFe2O4 nanoparticles37,38,39,40.
The crystalline phase of SnFe2O4/SiO2/PM-Pd MNPs was analyzed using XRD. As depicted in Fig. 2, the material exhibited seven distinct and well-defined peaks at 2θ values of 18.3°, 30.4°, 36.7°, 37.4°, 43.1°, 54.8°, 56.4°, 63.5°, 72.5°, and 75.3°. These peaks corresponded to the (111), (220), (311), (222), (400), (422), (511), (200), (620), and (533) planes, respectively, and aligned closely with previously reported XRD patterns for SnFe2O4 MNPs. This confirms that the tubular structure of SnFe2O4 remains intact following its functionalization and stabilization within the silica sulfuric acid shell. Additionally, the analysis highlighted a noisy background caused by the amorphous dried PM-Pd shells, as illustrated in Fig. 441,42..
The TGA curve of SnFe2O4/SiO2/PM-Pd demonstrates three distinct stages of mass loss. The initial stage, involving a 7% mass reduction at temperatures below 200 °C, is attributed to the evaporation of adsorbed organic solvents. As illustrated in Fig. 5, the second stage occurs between 200 and 600 °C, during which the removal of organic components results in a 20% mass loss. Based on this data, it can be concluded that the synthesized catalyst remains thermally stable up to 600 °C without degradation. Lastly, a third stage of mass loss, approximately 3.27%, is observed at temperatures exceeding 600 °C. This is associated with the condensation of silanol groups, leading to the loss of OH groups. These findings confirm the successful integration of the PM-Pd complex into the SnFe2O4 framework.
To confirm the presence of palladium metal on the surface of functionalized boehmite, the EDS technique was employed. The resulting EDS spectrum of SnFe2O4/SiO2/PM-Pd nanoparticles is depicted in Fig. 6. The spectrum clearly indicates the presence of Sn, Si, C, N, O, and Fe, along with Pd species in the SnFe2O4/SiO2/PM-Pd composition. These results from the EDS analysis enhance the understanding of the catalyst’s structural makeup and offer potential insights into its catalytic performance. Further exploration of the relationship between elemental distribution and catalytic properties could provide critical guidance for refining catalyst design and improving performance in future research endeavors.
A scanning electron microscope (SEM) was employed to analyze the size and morphology of SnFe2O4/SiO2/PM-Pd particles (Fig. 7). The SEM analysis indicated that these particles possess uniform spherical shapes, with diameters ranging from 20 to 60 nm. Furthermore, the images revealed particle agglomeration, likely caused by the magnetic properties of the nanoparticles.
Figure 8 presents the XPS spectrum of the synthesized SnFe2O4/SiO2/PM-Pd catalyst, showcasing distinct peaks corresponding to Si, Sn, O, C, N, Fe, and Pd. These elemental contributions are further detailed in Fig. 8a. An in-depth XPS analysis of palladium is provided in Fig. 8b, which identifies its oxidation state through characteristic binding energy peaks at 331.2 eV and 346.4 eV, attributed to Pd 3d5/2 and Pd 3d3/2, respectively. This thorough examination confirms the structural composition of the SnFe2O4/SiO2/PM-Pd catalyst43,44,45,46,47,48..
Figure 9 presents TEM images of the SnFe2O4/SiO2/PM-Pd magnetic nanoparticles (MNPs). The images reveal nearly spherical layers, with darker regions likely corresponding to the SnFe2O4 nanoparticles forming the core. Encasing these core nanoparticles, a brighter layer is visible, representing a distinct coating. This outer layer is likely evidence of the successful immobilization of the palladium (Pd) complex on the surface of the titanium ferrite nanoparticles. The contrast between the darker core and the brighter coating underscores the composite structure of the material, clearly delineating its core and functionalized outer layer as shown in Fig. 9.
The ICP method was employed to determine Palladium concentrations in the original catalyst and to evaluate Pd leaching following recycling. The findings revealed that the Pd contents in the fresh and recycled catalysts were 2.3 × 10⁻³ and 2.2 × 10⁻³ mol g⁻¹, respectively. This demonstrates negligible Pd leaching from the SnFe₂O₄/SiO₂/PM-Pd structure.
The magnetization of SnFe2O4 and SnFe2O4/SiO2/PM-Pd was examined at room temperature using a VSM, as illustrated in Fig. 10. The resulting magnetization curves indicated saturation magnetization values of 59 emu/g for SnFe2O4 and 41 emu/g for SnFe2O4/SiO2/PM-Pd. The decrease in saturation magnetization observed in the prepared catalyst is linked to the stabilization of the PM-Pd complex on the surface of SnFe2O4.
Catalytic study
After characterizing SnFe2O4/SiO2/PM-Pd, its catalytic efficiency for synthesizing biphenyl byproducts was analyzed using the Suzuki–Miyaura coupling reaction. Reaction parameters were initially optimized by varying the solvent, base, and catalyst concentrations in a model reaction between phenylboronic acid and iodobenzene, as outlined in Table 1. A blank experiment conducted without the catalyst in PEG solvent at 100 °C showed no reaction progress, even after an extended duration (Table 1, entry 1). Additionally, when the catalyst was removed midway through the reaction at 50% conversion, no further transformation occurred, reinforcing its critical role in driving the reaction forward. This underscores the importance of the SnFe2O4/SiO2/PM-Pd catalyst in facilitating the Suzuki–Miyaura coupling process. Table 1 (entry 4) demonstrates that 30 mg of SnFe2O4/SiO2/PM-Pd is sufficient to complete the reaction within 20 min with 1 mmol of iodobenzene in PEG at 100 °C. The next phase involved evaluating the effect of various solvents, including H2O, EtOH, PEG-400, DMF, and toluene, under identical conditions with 30 mg of catalyst. These tests revealed that environmentally friendly PEG was superior to all other solvents. To determine the impact of different bases on the reaction rate, accessible inorganic bases such as K2CO3, NaOH, and KOH were tested. Among these, K2CO3 emerged as the most effective base (Table 1), likely due to its higher solubility in PEG-400 medium. From both economic and reaction optimization perspectives, K2CO3 was validated as the most suitable choice. Further optimization involved varying the molar ratio of K2CO3. It was observed that reducing the amount to 1 mmol led to a slower reaction rate, whereas increasing it to 1.7 mmol provided no additional benefit (Table 1). These findings highlight the importance of fine-tuning reaction parameters for optimal efficiency.
After optimizing all reaction conditions, the coupling of phenylboronic acid with various aryl halides (I, Br, and Cl) was carried out using 30 mg of SnFe2O4/SiO2/PM-Pd under ultrasonication at the established optimal conditions. The results, summarized in Table 2, highlight the efficiency of the process in facilitating reactions between phenyl chlorides, bromides, and iodides with phenylboronic acid to produce the desired products. As shown in Table 2, the catalyzed reactions involving aryl halides with either electron-donating or electron-withdrawing functional groups consistently achieved high yields of the corresponding products. It is worth noting that while the process delivers high yields across all evaluated reactions, the coupling of aryl chlorides with phenylboronic acid requires a longer reaction time to achieve moderate product yields compared to the coupling reactions of aryl bromides and iodides.
A proposed mechanism for the anthrax reaction involves a series of key steps. First, aryl halides engage in oxidative addition to the Pd complex, resulting in the formation of intermediate 1. This is followed by intermediate 2 undergoing transmetallation, which leads to the production of intermediate 3. Lastly, reductive elimination takes place, restoring the SnFe2O4/SiO2/PM-Pd species and yielding the target product as outlined in Fig. 11.
Hot filtration
The hot filtration and leaching tests were employed to verify the heterogeneous nature of the synthesized material, independent of whether any catalyst particles were present in the filtrate solution. During the Suzuki–Miyaura cross-coupling reaction, the nanocatalyst was utilized for 10 min under optimal conditions, after which the reaction mixture was split into two portions. The catalyst was extracted from one portion using a magnetic field, and both portions were then allowed to react for an additional 10 min. It was observed that no conversion occurred in the catalyst-free environment, while the reaction in the other portion proceeded to completion, as detailed in the Supporting Information. These results strongly indicate that minimal to no Pd leaching occurred within the reaction mixture, thus confirming its genuine heterogeneity.
Catalyst recyclability
To evaluate the durability of the catalyst, the reaction between phenylboronic acid and iodobenzene was selected as a model system and carried out in ethanol using 30 mg of SnFe2O4/SiO2/PM-Pd. The catalyst was conveniently recovered with the help of a magnet, as described in the experimental section. Importantly, SnFe2O4/SiO2/PM-Pd maintained stable performance and was effectively reused across four successive cycles without any noticeable decline in catalytic activity (Table 3).
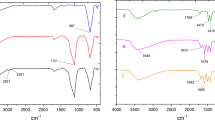
Figure 12 illustrates the FT-IR analysis of the recovered catalyst. A detailed assessment indicates the absence of notable changes in the spectrum, affirming that the catalyst retains its structural integrity and stability throughout the reaction mixture, with no evidence of degradation or major modifications to its chemical composition (Fig. 12).
The efficiency of the SnFe2O4/SiO2/PM-Pd catalyst was evaluated by comparing its performance with that of previously reported catalysts for the coupling reaction of iodobenzene and phenylboronic acid (as shown in Table 4). The nano SnFe2O4/SiO2/PM-Pd catalyst offers several advantages, including shorter reaction times, higher yields, simplified product purification, straightforward catalyst recovery, lower reaction temperatures, and the use of environmentally friendly solvents. These features highlight the effectiveness of SnFe2O4/SiO2/PM-Pd as a catalyst, making it a valuable tool for organic reactions and the synthesis of organic compounds (refer to Table 4).
Conclusion
This study highlights the synthesis of Pd immobilized on guanidine-conjugated SnFe2O4 nanoparticles (SnFe2O4/SiO2/PM-Pd) as a highly efficient, magnetically recoverable, and recyclable heterogeneous catalyst. This catalyst was specifically designed for the preparation of biaryl compounds via Suzuki–Miyaura coupling reactions. The SnFe2O4/SiO2/PM-Pd catalyst demonstrated excellent performance, achieving an impressive yield of approximately 98% using a small amount of catalyst (30 mg) in an aqueous medium, with a short reaction time of just 20 min and a straightforward work-up process for all Suzuki coupling reactions. Furthermore, reproducibility tests confirmed that the catalyst is a durable recoverable material, capable of being reused multiple times without any loss in activity during the Suzuki–Miyaura cross-coupling reactions. The practical reusability and ease of recovery of this developed catalyst make it a promising candidate for commercial and industrial applications. Additionally, all biphenyl derivatives exhibited high turnover numbers (TON) and turnover frequencies (TOF), reflecting the enhanced efficiency and selectivity of the SnFe2O4/SiO2/PM-Pd catalyst in Suzuki–Miyaura cross-coupling reactions as well as in the selective reduction of nitroarenes. It can thus be concluded that the application of these catalysts for reducing toxic dyes and chemicals offers a dual advantage by decreasing environmental pollution and lowering production costs for valuable compounds.
Data availability
The data produced or examined in this study is fully available within the published article and its supplementary information files.
Change history
10 December 2025
The original online version of this Article was revised: In the original version of this Article Suhas Ballal was incorrectly affiliated with ‘Department of Electronics and Communication Engineering, GLA University, Mathura, 281406, India’. The correct affiliation is listed here: Department of Chemistry and Biochemistry, School of Sciences, JAIN (Deemed to be University), Bangalore, Karnataka, India.
References
Tahmasbi, B., Darabi, M. & Nikoorazm, M. A new Schiff-base complex of palladium nanoparticles on modified boehmite with di(pyridin-2-yl)methanone as a robust, reusable, and selective nanocatalyst in the C-C coupling reaction. Appl. Organomet. Chem. 38, e7348 (2024).
Tahmasbi, B., Darabi, M., Moradi, P., Abbasi Tyula, Y. & Nikoorazm, M. Gadolinium Schiff-base complex on nanocomposite of graphene oxide magnetic nanoparticles as a robust, reusable and chemoselective nanocatalyst in the C–C coupling reactions. Polyhedron 258, 117038 (2024).
Gupta, P. et al. Amine grafted Fe3O4 immobilized graphene oxide as a recyclable and effectual nanocomposite for the regioselective ring opening reaction. Res. Chem. Intermed. https://doi.org/10.1007/s11164-021-04509-4 (2021).
Gupta, P. et al. Basic ionic liquid grafted on magnetic nanoparticles: an efficient and highly active recyclable catalyst for the synthesis of β-nitroalcohols and 4H-benzo[b]pyrans. J. Mol. Struct. 1274, 134351 (2023).
Lakshmidevi, J. et al. Pd(5%)-KIT-6, Pd(5%)-SBA-15 and Pd(5%)-SBA-16 catalysts in water extract of pomegranate ash: A case study in heterogenization of Suzuki-Miyaura reaction under external base and ligand free conditions. Sustain. Chem. Pharm. 19, 100371 (2021).
Patel, H. A., Patel, A. L. & Bedekar, A. V. Polyaniline-anchored palladium catalyst-mediated Mizoroki–Heck and Suzuki–Miyaura reactions and one-pot Wittig–Heck and Wittig–Suzuki reactions. Appl. Organomet. Chem. 29, 1–6 (2015).
Veisi, H. et al. Ultrasound assisted synthesis of Pd NPs decorated chitosan-starch functionalized Fe3O4 nanocomposite catalyst towards Suzuki-Miyaura coupling and reduction of 4-nitrophenol. Int. J. Biol. Macromol. 172, 104–113 (2021).
Moniriyan, F. & Sabounchei, S. J. A comparative study of catalytic activity on iron-based carbon nanostructured catalysts with Pd loading: using the Box–Behnken design (BBD) method in the Suzuki–Miyaura coupling. Appl. Organomet. Chem. https://doi.org/10.1002/aoc.6415 (2021).
Darabi, M., Nikoorazm, M. & Tahmasbi, B. Copper complex of bis(pyridin-3-ylmethyl)carbamimidothioate on mesoporous KIT-6 as a homoselective and stable nanocatalyst for the synthesis of tetrazoles. Inorg. Chem. Commun. 177, 114454 (2025).
Vibhute, S. P., Mhaldar, P. M., Shejwal, R. V. & Pore, D. M. Magnetic nanoparticles-supported palladium catalyzed Suzuki-Miyaura cross coupling. Tetrahedron Lett. 61, 151594 (2020).
Lakshmidevi, J., Naidu, B. R. & Venkateswarlu, K. CuI in biorenewable basic medium: three novel and low E-factor Suzuki-Miyaura cross-coupling reactions. Mol. Catal. 522, 112237 (2022).
Ramming, P. et al. Suppressed ion migration in powder-based perovskite Thick films using an ionic liquid. J. Mater. Chem. C. 9, 11827–11837 (2021).
Zhang, Y. et al. Porous, Tremella-like NiFe2O4 with Ultrathin Nanosheets for ppb-Level Toluene Detection. Crystals vol. 13 (2023).
Jia, Y., Lee, B. W. & Liu, C. Magnetic ZnFe2O4 nanocubes: synthesis and photocatalytic activity with visible Light/H2O2. IEEE Trans. Magn 53, 1-1 (2017).
Zeinivand-Lorestani, A., Taheri, A. & Tahmasbi, B. Chemoselective oxidation of sulfides and oxidative coupling of thiols by a new polyoxometalate and heterogeneous Cobalt and molybdenum hybrid nanocatalyst stabilized on modified mesoporous CMK-3. Appl. Organomet. Chem. 39, e7997 (2025).
Jabbari, A., Tahmasbi, B., Mohseni, E. & Darabi, M. Cycloaddition reaction of NaN3 with nitriles toward the synthesis of tetrazoles catalyzed by a copper complex on boehmite nanoparticles. Nanoscale Adv. https://doi.org/10.1039/d5na00081e (2025).
Yue, Q., Yang, J., Yuan, H. M. & Chen, J. S. A Two-coordinate Copper(I) complex constructed from cyanuric acid and 4,4′-Bipyridyl: synthesis, structure and photoluminescence. Chin. J. Chem. 24, 1045–1049 (2006).
Wang, Y. T. et al. Biodiesel production from esterification of oleic acid by a sulfonated magnetic solid acid catalyst. Renew. Energy. 139, 688–695 (2019).
Zhong, J., Huang, J., Chen, L. & Duan, J. Construction of a biocompatible MWCNTs–chitosan composite interface and its application to impedance cytosensing of osteoblastic MC3T3-E1 cells. RSC Adv. 12, 31663–31670 (2022).
Lhermitte, C. R. et al. Generalized Synthesis to Produce Transparent Thin Films of Ternary Metal Oxide Photoelectrodes. ChemSusChem 13, 3645–3653 (2020).
Kilic, A. et al. The orthopalladation dinuclear [Pd(L1)(µ-OAc)]2, [Pd(L2)(µ-OAc)]2 and mononuclear [Pd(L3)2] complexes with [N, C, O] or [N, O] containing ligands: synthesis, spectral characterization, electrochemistry and catalytic properties. J. Organomet. Chem. 695, 697–706 (2010).
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 nobel prize. Angew Chemie - Int. Ed. 51, 5062–5085 (2012).
Pormazar, S. M. & Dalvand, A. Adsorption of reactive black 5 Azo dye from aqueous solution by using amine-functioned Fe 3 O 4 nanoparticles with L-arginine: process optimisation using RSM. Int. J. Environ. Anal. Chem. 00, 1–20 (2020).
Faroughi Niya, H., Hazeri, N., Kahkhaie, R., Maghsoodlou & M. & M. T. pharmaceutical pyrano[3,2-c]chromenes. Res. Chem. Intermed. 46, 1685–1704 (2020).
Ashraf, M. A., Liu, Z., Zhang, D. & Alimoradi, A. L-lysine‐Pd complex supported on Fe 3 O 4 mnps: a novel recoverable magnetic nanocatalyst for Suzuki C‐C Cross‐Coupling reaction. Appl. Organomet. Chem. 34, e5668 (2020).
Sahoo, J. K., Rath, J., Dash, P. & Sahoo, H. EDTA functionalized magnetic nanoparticle as a multifunctional adsorbent for congo red dye from contaminated water. AIP Conf. Proc. 1832, 1–4 (2017).
Salokhe, A. et al. Magneto-structural and induction heating properties of MFe2O4 (M = Co, mn, Zn) MNPs for magnetic particle hyperthermia application. SN Appl. Sci 2, 12 (2020).
Kurtan, U., Erdemi, H., Baykal, A. & Güngüneş, H. Synthesis and magneto-electrical properties of MFe2O4 (Co, Zn) nanoparticles by oleylamine route. Ceram. Int. 42, 13350–13358 (2016).
Sheikh, J. R., Gaikwad, V. M., Moon, V. C. & Acharya, S. A. Enhancement in dielectric behavior of (Ni, Zn)Fe2O4 ferrite. in AIP Conference Proceedings vol. 1728 (2016).
Ansari, L. et al. Comparing the two concentrations of Co0.6 Zn0.4 Fe2O4 nanoparticles coated with dimercaptosuccinic acid based on T2-and T2*- weighted MRI: an animal study. J. Radiat. Res. Appl. Sci. 15, 84–89 (2022).
Vahid, N. F., Marvi, M. R., Naimi-Jamal, M. R., Naghib, S. M. & Ghaffarinejad, A. X-Fe2O4-buckypaper-chitosan nanocomposites for nonenzymatic electrochemical glucose biosensing. Anal. Bioanal Electrochem. 11, 930–942 (2019).
Dippong, T., Levei, E. A. & Chapter, I. I. I. Synthesis and characterization of MFe 2 O 4 (M: zn, ca, Mg) photocatalysts. Nanomaterials, 4, 1–12 (2021).
Abbas, M., Zhang, J., Mansour, T. S. & Chen, J. Hierarchical porous spinel MFe2O4 (M = Fe, zn, Ni and Co) nanoparticles: facile synthesis approach and their Superb stability and catalytic performance in Fischer-Tropsch synthesis. Int. J. Hydrogen Energy. 45, 10754–10763 (2020).
Rafiq, M., Javed, A., Nasir, M., Khan, M. & Hussain, D. A. Understanding the structural, electronic, magnetic and optical properties of spinel MFe2O4 (M = Mn, co, Ni) ferrites. Ceram Int 46, (2019).
Phuruangrat, A., Kuntalue, B., Dumrongrojthanath, P., Thongtem, T. & Thongtem, S. Microwave-assisted solvothermal synthesis of cubic ferrite (MFe2O4, m = mn, zn, Cu and Ni) nanocrystals and their magnetic properties. Dig. J. Nanomater Biostructures. 13, 563–568 (2018).
Sabale, S. et al. Superparamagnetic MFe2O4 (M = Ni, co, zn, Mn) nanoparticles: synthesis, characterization, induction heating and cell viability studies for cancer hyperthermia applications. J Mater. Sci. Mater. Med 26, 3 (2015).
Sun, H. & Li, D. Recyclable MFe2O4(M = Mn, zn, cu, ni, Co) coupled micro-nano bubbles for simultaneous catalytic oxidation to remove X and SO2in flue gas. RSC Adv. 10, 25155–25164 (2020).
Xian, G. et al. Synthesis of spinel ferrite MFe2O4 (M = Co, cu, mn, and Zn) for persulfate activation to remove aqueous organics: effects of M-Site metal and synthetic method. Front Chem 8, (2020).
Singh, P., Mishra, S., Sahoo, A. & Patra, S. A magnetically retrievable mixed-valent Fe3O4@SiO2/Pd0/PdII nanocomposite exhibiting facile tandem Suzuki coupling/transfer hydrogenation reaction. Sci. Rep. 11, 9305 (2021).
Nasrollahzadeh, M., Issaabadi, Z. & Safari, R. Synthesis, characterization and application of Fe3O4@SiO2 nanoparticles supported palladium(II) complex as a magnetically catalyst for the reduction of 2,4-dinitrophenylhydrazine, 4-nitrophenol and chromium(VI): A combined theoretical (DFT) and experiment. Sep. Purif. Technol. 209, 136–144 (2019).
Hussain, M. et al. Improvement of SnFe2O4 OER electrochemical property by Sm doping for water splitting. Ceram. Int. 50, 19525–19533 (2024).
Sargazi, S. et al. Assessment of SnFe2O4 nanoparticles for potential application in theranostics: synthesis, characterization, in vitro, and in vivo toxicity. Mater. (Basel). 14, 1–19 (2021).
Narayanan, V. & Mandal, B. Type-II ternary Bi 2 WO 6 /rGO/SnFe 2 O 4 heterojunction nanocomposites and their photocatalytic efficiency towards 4-nitrophenol reduction. RSC Adv. 13, 22616–22629 (2023).
Tahir, S., Zahid, M., Hanif, M. A. & Javed, M. Y. g-C3N4/graphene oxide/SnFe2O4 ternary composite for the effective sunlight-driven photocatalytic degradation of methylene blue. Environ. Sci. Pollut Res. 30, 125540–125558 (2023).
Jia, Y. et al. One-pot solvothermal synthesis of magnetic SnFe2O4 nanoparticles and their performance in the photocatalytic degradation of Chlortetracycline with visible light radiation. RSC Adv. 6, 76542–76550 (2016).
Hosseini, M., ghasem, Hosseinzadeh, F., Zardari, P. & Mermer, O. Pd-Ni nanoparticle supported on reduced graphene oxide and multi-walled carbon nanotubes as electrocatalyst for oxygen reduction reaction. Fullerenes Nanotub Carbon Nanostruct. 26, 675–687 (2018).
Yang, S. et al. One-Pot synthesis of Graphene-Supported monodisperse Pd nanoparticles as catalyst for formic acid Electro-oxidation. Sci. Rep. 4, 4501 (2014).
Guo, Z. et al. Carbon supported Oxide-Rich Pd-Cu bimetallic electrocatalysts for ethanol electrooxidation in alkaline media enhanced by cu/cuox. Catalysts 6, 62 (2016).
Le, X., Dong, Z., Jin, Z., Wang, Q. & Ma, J. Suzuki–Miyaura cross-coupling reactions catalyzed by efficient and recyclable Fe3O4@SiO2@mSiO2–Pd(II) catalyst. Catal. Commun. 53, 47–52 (2014).
Gogoi, N., Bora, U., Borah, G. & Gogoi, P. K. Nanosilica-anchored Pd(II)-Schiff base complex as efficient heterogeneous catalyst for activation of Aryl halides in Suzuki–Miyaura cross-coupling reaction in water. Appl. Organomet. Chem. 31, e3686 (2017).
Nutt, M. O., Heck, K. N., Alvarez, P. & Wong, M. S. Improved Pd-on-Au bimetallic nanoparticle catalysts for aqueous-phase Trichloroethene hydrodechlorination. Appl. Catal. B Environ. 69, 115–125 (2006).
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia for supporting this work through project number TU-DSPP-2024-19.
Funding
The research was funded by Taif University Saudi Arabia project number TU-DSPP-2024-19.
Author information
Authors and Affiliations
Contributions
Anjan Kumar. Magda H. Abdellattif. Chou-Yi Hsu. Jayanti Makasana. Suhas Ballal. Munther Kadheem. Abhayveer Singh. KRITHIGA. Swati Mishra. and Pushpa Negi Bhakuni. Funding acquisition, Supervision, Resources, Conceptualization, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of competing interest
The authors confirm that they have no relevant financial interests or personal relationships that might have influenced the work presented in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kumar, A., Abdellattif, M.H., Hsu, CY. et al. Palladium nanoparticles immobilized in SnFe2O4/SiO2/PM as efficient heterogeneous catalysts for the suzuki cross-coupling reaction. Sci Rep 15, 32015 (2025). https://doi.org/10.1038/s41598-025-16233-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16233-9