Abstract
Energy resource sustainability has been of critical concern as a result of unlimited energy demand worldwide. In this research work, extraction of the alternate fuel for diesel (i.e., biodiesel) from the source Dunaliella salina, which is a greenish microalga with higher lipid content comparatively, is being primarily addressed. Cultivation was provided under nitrogen starvation, f/2 trace-element supplemented, nutrient medium supplemented with vitamins and CO2. Ultrasonic extraction method at 50 Hz yielded 645 ml of Dunaliella salina bio-oil in repeated batches. One stage base catalysed process of transesterification with 1:8 mol ratio of methanol to oil, 0.6% w/w NaOH catalyst, reaction temperature of 50 °C and reaction time of 120 min yielded 612 ml Dunaliella salina biodiesel in 6 batches with 94.8% efficiency in transesterification. Physio-chemical properties of the produced biodiesel were determined to be as per ASTM specifications. Additional GCMS, NMR and FTIR spectroscopic study of the derived biodiesel established its suitability for CI engine. Dunaliella salina biodiesel blend at 20% volume ratio was compared with diesel on combustion, emission and performance characteristics of Kirloskar 240 PE test engine. Additional supplementation of Hydrogen by DuSaBD20 at 3 LPM, 6 LPM and 9 LPM had pronounced impact on the engine performance with remarkable CO reduction and UBHC and a slight increase in engine NOx was observed. Additionally, the study of cost of operation on H2 induction was also investigated.
Similar content being viewed by others
Introduction
The emphasis has come to advice in curbing CO2 emissions and further toxic emissions in an attempt to stop climatic chaos in current regulations. Still, the greatest cause of GHG emissions from automobiles is man-made, through fossil fuel combustion, which contains plenty of carbon and emits enormous quantities of hydrocarbons. One of the greatest human contributions to greenhouse gas emission is vehicle usage of traditional fuels. Because they contain more carbon, traditional fuels emit a substantial amount of CO2 when they are ignited. As such, efforts are presently being directed towards research and development of renewable fuel alternatives. Mixing renewable fuel with traditional fuel, may be a possibility in reducing emissions. Biodiesel-diesel blends are expected to be prevalent in the future and help reduce overall emissions1. Bio-oil production from vegetation plant seeds and animals through their Lipids were seen widely in the literature. Vegetable oils and animal fats are traditionally employed to create feedstock. It was more viscous and could not be heated as effectively. Thus, additional processing was necessary to receive biodiesel from it. It can be acknowledged from the literatures and experiments carried out by various research bodies that alternate biodiesel performed good than the bio-oil since it stands out in various aspects like energy content, toxic nature and being a renewable feature2.
Biodiesel made of vegetation possess several benefits over normal diesel, such as higher Cetane number and flash point, lower sulphur with improved properties of lubrication. Because biodiesel contains fatty acids that might impair cold flow characteristics and perhaps clog fuel filters, it has a higher viscosity than diesel. In general, biodiesel derived from vegetable oils has better cold flow characteristics than biodiesel derived from animal fats. Fuel structure is a critical component of engine performance since the molecular makeup of biodiesel influences emission levels and combustion efficiency. The performance of biodiesel made from Parinari polyandra oil in a diesel engine was investigated by Anish et al.3. According to their research, the fuel’s properties were very similar to those of regular diesel. In terms of power output and thermal efficiency, the B10 mix outperformed the other blends that were evaluated.
As such, studies on creating environmentally friendly alternatives to fossil fuels are gaining significance. In comparison to other typical feedstocks, algae have greater per growth site biodiesel production capacity since they have a large lipid content compared to other compounds (up to 70%). Algae’s carbon dioxide uptake capacity is approximately ten times larger compared to land crops, hence the greater environmental sustainability due to the application of algae. Algal biodiesel offers the added benefit of generating valuable by-products, conserving biodiversity, and requiring minimal resource input4. Biodiesel is produced through a stoichiometric reaction between oils and alcohol, resulting in the formation of methyl esters (biodiesel) and glycerol. It is favoured for its ability to provide smoother operation in diesel engines compared to petroleum-based fuels, even under varying environmental conditions5. Mixing it in proportion with purified diesel fuel can result in superior combustion over petroleum-derived diesel because of its high cetane value, oxygen and flash point. Over 300 oil crops have been pointed out as globally feasible raw materials for the production of biodiesel. Rubber seed oil and other vegetable oils with high free fatty acid (FFA) content were converted into biodiesel by Ramadhas et al.6 using both single-step and two-step transesterification techniques. The maximum biodiesel yield was obtained in the first step at a 6:1 molar ratio with potassium hydroxide acting as a catalyst for 30 min at about 45 °C. The highest efficient FFA reduction was accomplished with a catalyst of 5% sulfuric acid. In comparison to regular diesel, the resultant biodiesel was found to have a greater flash point, a lower energy content, and a higher viscosity.
Sui et al.7 started their investigation by looking at changes in activation energy in order to determine how oxidation affected Jatropha methyl ester. Their findings demonstrated a significant reduction in the size of carbon smoke particles and a consistent drop in carbon monoxide emissions after the oxidation process. Supported by experimental data and numerical simulations, they studied biodiesel blends in diesel engines with an emphasis on performance metrics and emission characteristics. Their study supports the increasing interest in renewable fuels as possible near-term substitutes or additions for traditional diesel. This shift is anticipated to greatly reduce emissions and encourage the use of cleaner energy, according to Chen et al.8. The necessity of controlling CO2 and other unwanted emissions is highlighted by the pressing need to combat climate change. Study of Backiyaraj et al.9, reveal that when hydrogen is mixed with algae biodiesel, its higher thermal qualities improve combustion and engine performance. Significant decreases in CO, UHC, and smoke emissions are attained at optimal substitution rates (such as 43.2 g/h), however NOx increases. But when EGR is combined with hydrogen-enriched biodiesel, NOx is successfully reduced while maintaining better combustion and efficiency. In CI engines, hydrogen enrichment in algae biodiesel blends increases combustion efficiency and lowers emissions. According to experimental findings, when hydrogen volume increases, brake thermal efficiency and combustion characteristics improve. While NO emissions increase as a result of greater in-cylinder temperatures, CO and HC emissions drastically decrease. All things considered, hydrogen-algae biodiesel dual-fuel systems exhibit significant promise for cleaner and more effective engine operation10. The study emphasizes the possibility for cleaner and more effective energy generation from hydrogen–algal biodiesel dual-fuel engines. The best injection pressure and load circumstances were found using response surface methods in order to reduce emissions and increase brake thermal efficiency. High predicted accuracy was shown by the method, enabling efficient engine tuning for long-term performance11.
Dunaliella salina microalgae is a promising high lipid producing feedstock when maintained in phosphorus rich, nitrogen deficit condition. Its carbon neutrality property due to assimilation of CO2 during the photosynthesis process is an added advantage. Also, large scale cultivation of Dunaliella salina contributes to cleaner environment with enhanced sustainability. Algal biodiesel from Dunaliella saline has few challenges which are to be encountered like relatively higher operating and capital expenditure, higher energy inputs like bio-reactor and skilled manpower for harvesting and lipid extraction with energy intensiveness. Environmental implications also play a key role in algal biodiesel production which includes eutrophication, pathogenic contamination and quality of water and resources. Hydrogenated biodiesel (HB) holds the potential to mitigate urban air pollution, fossil fuel usage, and climate change. Because it mostly consists of trans-isomers rather than cis-isomers, it has a higher kinematic viscosity and a higher chance of crystallizing, which exacerbates problems with cold flow. Partial hydrogenation improves combustion stability and lessens engine vibrations, but it also raises the cloud point and pour point. The process is catalysed, which improves combustion properties and quality of biodiesel. Catalyst choice is based on the properties of biodiesel and the type of reactor. Higher concentrations of hydrogen support hydrogenation, while temperature plays little role, with longer processing affecting oxidation stability12,13. Herein, biodiesel was synthesized from microalgae Dunaliella salina, standardized, and thereafter used to study its combustion and emission properties by using diesel engines. To address the challenges of incomplete combustion and elevated NOx emissions associated with biodiesel, hydrogen was introduced into the combustion process to enhance combustion efficiency and reduce harmful emissions. The influence of hydrogen induction over emission patterns and combustion efficiency was extensively analysed.
Despite a lot of research on using biodiesel as a renewable alternative for conventional diesel, it also possesses unmatched challenges such as increased emissions of nitrogen oxides (NOx) and lower efficiency in combustion compared to fossil fuels. Due to these restrictions, biodiesel cannot be used in engines as a direct replacement for diesel without any changes. The potential synergy between hydrogen induction and biodiesel to lower emissions and improve combustion efficiency in diesel engines has received relatively little attention, despite a great deal of research on optimizing biodiesel production and properties using various feedstocks and transesterification techniques. There is still much to learn about the performance and yield of microalgae-based biodiesel, notably from Dunaliella salina, when paired with hydrogen fuel. Recent research has mostly focused on feedstock optimization, but it frequently ignores important factors like emissions reduction and engine performance in real-world combustion engine scenarios. By examining the dual function of hydrogen in improving combustion efficiency and lowering emissions, this work fills that knowledge gap and offers important insights into how to make biodiesel both ecologically friendly and practical for use in actual engine applications.
The present investigation mainly focusses on standardization and usage of derived Dunaliella salina biodiesel in compression ignition engine. The fuel standardization was carried out by characterizing the biodiesel using Gas Chromatography Mass Spectrometry, Fourier Transform Infra-Red analysis and Nuclear Magnetic Resonance studies. Additionally, its effect on CI engine with throttle body supplementation of Hydrogen at multiple flowrates with respect to the combustion and emission behaviour was also analysed.
Materials and methods
Microalgae can be the ideal alternative to fossil fuel in a sustainable economic way and can also make ways for minimized greenhouse gas emissions. Microalgae are the microorganisms possessing great photosynthetic potential. This proves to be useful not just for the development of lipids but also proves to be useful as an exceptionally good carbon sequestration method and oxygen generation. Algae can modulate the rate of growth and biological as well as biochemical content in different physio-chemical phases. Different genera of microalgae are cultivated on an industrial, pharmaceutical and biofuel scale at different stages. Dunaliella salina is one among them, which contains very high lipid content. Dunaliella salina possesses various competent traits that make it suitable for production of biodiesel. It is known there is no cellulose walls for Dunaliella group; also, other laboratory techniques employed to observe the intercellular processes are more inconvenient to carry out.
A unicellular, salt-tolerant microalga belonging to the Chlorophyta division, Dunaliella salina is well-known for flourishing in high salinity conditions. Because of its high fat content, rich biomass, and profusion of carotenoids, it has significant nutritional and biotechnological value. The algae used in this work to produce biodiesel is Dunaliella salina, which was obtained from the National Institute of Ocean Technology in Chennai. Research suggests that D. salina is unique among feedstocks due to its cost-effectiveness, environmental friendliness, and potential for producing biodiesel in a sustainable manner. It is a perfect option due to its high lipid content, ability to generate important co-products, minimal resource requirements, and resilience to environmental stress. Furthermore, it has been effectively grown on the f/2 nutrient medium in nitrogen-limited environments14.
Oil extraction from Dunaliella salina
The ultrasonic process involves transmission of sound waves with elevated frequencies between 40 and 60 Hz which results in heat and vibrations transfer. This process also removes the tiny liquid bubbles as a result of cavitation. This cavitational vibration disrupts the cell wall membrane of Dunaliella salina thereby rupturing its and separates the bio-oil from the cell. The ultrasonic bio-oil extraction technique was applied to harvested biomass of Dunaliella salina. The process is initiated through batch processes. In a single batch 100 g of Dunaliella salina biomass was thoroughly mixed with the extraction solvent. According to earlier research by Hu15 and Hariram et al.16, the extraction solvent was prepared using 30 ml of methanol, double-distilled water, and chloroform in a 1:0.7:2 ratio to efficiently break down the cell wall and separate the lipid content.
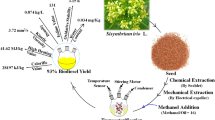
The biomass underwent centrifuged for 45 min using an ultrasonicator set at 60 Hz, which collected the bio-oil in a combined phase. The resultant mixture was transferred into a beaker and a settling period of 15 min yielded 45 ml of Dunaliella salina bio-oil in a single batch. Grade 1 Whatman’s filter paper with 11 μm pore size with was used to remove the sediment unwanted mixture. Further the remaining traces of H2O was removed by mixing 6 ml of Acetone at 45 °C for 10 min. The resultant Dunaliella salina biodiesel yield of 43 ml was obtained by this process. Repeating the batch process 15 times in the similar aseptic laboratory condition cohesively produced 645 ml of oil as shown in (Fig. 1).
Dunaliella salina bio-oil to biodiesel conversion
Titration procedure with Dunaliella salina bio-oil revealed the composition of Free Fatty Acid (FFA) as 1.92%. Therefore, single stage transesterification process was adopted to convert the triglycerides into its corresponding mono-alkyl fatty esters. The solvents used in the base catalysed esterification process are sodium hydroxide (as catalyst) and methanol (as base solvent) in a wide range of operational variables like methanol to oil molar ratio (molar ratio of 1:8, 1:7 and 1:6), catalyst concentration (0.5%, 1% and 1.5%) and reaction temperature (40 °C, 50 °C and 60 °C) were assessed to determine the optimal scenario to esterify the Dunaliella salina bio-oil. A 13 (3 × 3) factorial design was systematized to understand the behaviour of operating parameters. Initially, the conversation process started with the mixing the known quantity of methanol and sodium hydroxide to form sodium methoxide solution at molar ratio of 1:8, 1:7 and 1:6, catalyst concentration 0.5, 1 and 1.5% with reaction temperature between 40 and 60 °C as stated in (Table 1).
Design of experiment
Where Y is the biodiesel yield, \(\:{\beta\:}_{0}\), \(\:{\beta\:}_{1}\), \(\:{\beta\:}_{ii}\) and \(\:{\beta\:}_{iii}\) are co-efficient of regression analysis and Xi, Xii and Xiii are the independent variables.
The design of experiments uses a definitive screening technique (DST) to concentrate on the main variables operating parameters such as methanol to oil molar ratio, catalyst concentration and reaction temperature in the production of Dunaliella salina biodiesel. This technique reduces the number of iteration in identifying the optimal conditions. Generally, DST is employed in the beginning stage of experimental trials to narrow down the significant effective variables from a larger list of operating variable interaction. A major advantage of DST is the non-biasing of linear and quadratic terms to the orthogonal effect in predicting the biodiesel yield. In the present investigation, a 3 × 3 factorial design with 13 experiments were suggested by the DST as tabulated in (Table 2). The variables affect the biodiesel prediction with their levels are Xi – methanol to oil molar ratio (1:6 to 1:8), Xii – Catalyst concentration (0.5–1.5%) and Xiii – Reaction temperature (40 to 60 °C). Second order regression model based analysis was performed to evaluate the yield of Dunaliella salina biodiesel and the trial experimental runs were conducted in a random manner to reduce the uncertainties.
Further, Venu et al.17 reported similar outcome in the production of watermelon seed biodiesel with 60 °C reaction temperature, 55 minutes’ reaction duration, 20% molar ratio and 1.3% catalyst concentration.
Analysis of variance (ANOVA) was employed to understand the statistical importance of the developed model at the confidence level of 95%. The developed Definitive Screening Technique (DST) was found to have lower p-value of 0.0001 and F ratio of 119.55 which was very significant to predict the Dunaliella salina biodiesel yield. Further, the correlation co-efficient (R) was found as 0.993 showcasing more that 99.1% of the operating variables selected to has greater significance on the Dunaliella salina biodiesel yield. The statistical outcome finally outlines that the reaction temperature and catalyst concentration (quadratic terms) and methanol to oil molar ratio (linear term) has significantly higher and lower effect respectively on the yield of Dunaliella salina biodiesel.
The experiments demonstrated that using a methanol-to-oil molar ratio of 1:8, with 0.6% NaOH catalyst by weight, at a reaction temperature of 50 °C for 120 min, resulted in a significantly improved biodiesel yield from Dunaliella salina. The trial was initiated with 100 ml of methanol in around bottomed flask thoroughly mixed with 1.2 g of NaOH to form sodium methoxide on constant stirring at 400 rpm for 60 min. 40 ml of the sodium methoxide solution was separately mixed with 120 ml of Dunaliella salina bio-oil in a round bottomed flask equipped with a magnetic stirrer at 250 rpm for 45 min. Thereafter, the entire mixture was transferred into a separating funnel and a settling period of 60 min distinctly layered the glycerol as the lower portion and Dunaliella salina biodiesel as the upper portion with a ring structure in-between. The bottom knob tilting mechanism in the separating funnel parted the glycerol carefully and retained the biodiesel in the separating funnel. Further, the obtained biodiesel was washed with pellets of magnesium silicate (1.5 g) and n-hexane solvent to absorb moisture and impurities at 85 °C. Similar Solvent usage was also noticed in Rao at al.18. This process yielded 102 ml of Dunaliella salina biodiesel. The entire process was repeated six times in the similar laboratory condition which collectively yielded 612 ml of Dunaliella salina biodiesel. The transesterification efficiency of Dunaliella salina was found to be 94.8% using the Eq. (2).
Fuel properties and standardization
The diesel fuel employed in the engine was analysed for properties in order to confirm compatibility with operational standard values and it read values nearer to those presented in (Table 3). The extracted biodiesel was subjected to various spectroscopic to explore its suitability in CI engine as a fuel (blended with neat diesel in this study).
The physio-chemical properties including density, flash point, calorific value, fire point, cloud and pour point, aniline point, kinematic viscosity, specific gravity and free fatty acid content were estimated for Dunaliella salina bio-oil, its biodiesel and compared with straight diesel under ASTM standards. The density of Dunaliella salina bio-oil was found to be 865 kg/m3 whereas its biodiesel represented a lower value of 854 kg/m3 as a result of chemical rearrangement process due transesterification reaction. The calorific value of Dunaliella salina biodiesel was significantly elevated upto 41.45 MJ/kg from 35.78 MJ/kg. the cloud and pour point of Dunaliella salina was noticed as −19 °C and −16 °C respectively. The kinematic viscosity was found to be 4.75 mm2/s at 40 °C as identified by Anton 250 N viscometer. The other notable properties like Aniline gravity, carbon residue and energy content were also identified. The free fatty acid content of 2.01% in Dunaliella salina bio-oil was neutralized by the transesterification process as enlisted in the (Tables 1 and 2). The various instruments employed to estimate the physio-chemical properties and their ASTM standards are enlisted in (Table 3).
Experimental setup and experimentation
The test engine is a standard four stroke single cylinder Kirloskar 240PE without a catalytic converter as shown in the (Fig. 2). The stroke length and diameter of the cylinder of the engine are 110 mm and 87.5 mm respectively. The connecting rod length and orifice diameter of the engine are 234 mm and 20 mm. The arm length of the Dynamometer is 185 mm. The power and speed of diesel engines with which this experiment was carried out are 3.5 kW and 1500 rpm, respectively. The detailed specification of test engine is given in (Table 4). The experimentation initiated with a warm-up time period of 30 min before the start of each experimental trial. Initial experiments analysing combustion, performance, and emissions were conducted using pure diesel, followed by tests with the DuSaBD20 fuel blend across all load conditions. Subsequently, hydrogen was introduced at flow rates of 3, 6, and 9 LPM alongside the DuSaBD20 blend to evaluate its impact on engine performance.
Every experimental trials were repeated 3 times under the same laboratory condition and the average was plotted as the outcome in the “Results and Discussion” section to rule-out any ambiguities. The time taken for 10 cc consumption of fuel, eddy current dynamometer loading and speed of the engine were recorded to evaluate the performance parameters. The flow meter controlled the quantity of H2 gas supplemtation at a predetermined interval. Kristler pressure transduced connected with the NI USB Data Acquisition System recorded the Incylinder parameters to evaluate the Rate of pressure rise, heat release rate and mean gas temperature. AVL444 gas analyser was employed to determine the CO, UBHC, CO2 and NOx emission whereas AVL437 smoke meter was used to determine the smoke opacity.
Errors and uncertainties
Uncertainties and errors can arise due to multiple factors including environmental conditions, calibration and selection of instruments, observations, test data etc. Uncertainties are generally categorised as random and fixed errors. Random errors are emerged due to variation in analytical measuring instruments whereas fixed error are emerged due to variation in repeatability of the test conducted. In the present investigation, Gaussian distribution methodology was used to analyse the uncertainties and errors with a mean limit where 95% of measured data’s lie upon (i.e.) termed as ± 2σ. Table 5 shows the errors and uncertainty in the instruments used in the present study whereas Table 6 depicts the uncertainties in variables which are measured. The overall uncertainty (ΔRo) is estimated using root mean square (RMS) technique as in the Eqs. (3) and (4) and found to be 1.923%.
Where R is the function of (\(\:{X}_{1}\), \(\:{X}_{2}\), ….\(\:{X}_{n}\))
Where \(\:{X}_{i}\) is the total number of readings and \(\:{\sigma\:}_{i}\) is the standard deviation.
Results and discussion
Optimizing the converted biodiesel
As detailed in Sect. 2.2, oil derived from Dunaliella salina was converted to biodiesel via a base-catalysed transesterification process. By adjusting important variables that impact conversion efficiency, such as catalyst type, methanol-to-oil ratio, reaction temperature and time, the procedure was verified. Based on previous research, the most widely used methanol-to-oil ratios for the manufacture of biodiesel are 1:8, 1:7, and 1:6. The typical catalyst concentrations are 0.5, 1, and 1.5% by weight, while the reaction temperatures are set at 40 °C, 50 °C, and 60 °C. The optimized variable parameters as discussed in Sect. 2.2 and 2.3 as suggested by DST, the methanol to oil ratio 1:8, catalyst concentration 0.6% weight, reaction temperature 50 °C and reaction duration 120 min yielded optimal biodiesel of 94.8% in 6 batch processes. Using a methanol-to-oil ratio of 1:7, a catalyst concentration of 1.5% by weight, a temperature of 60 °C, and a reaction time of 2 h resulted in a biodiesel yield of 92.95%. The other trials which yielded notably reduced yield of Dunaliella salina biodiesel as shown in (Table 3).
Biodiesel characterization
Gas chromatography - mass spectrometer (GCMS)
Methanol and sodium hydroxide were used in a single-step base-catalyzed transesterification process to transform bio-oil that was isolated from the marine microalga Dunaliella sp. into biodiesel. The biodiesel was examined using GC-MS to determine the range of fatty acid methyl esters (FAMEs) generated and assess the effectiveness of this conversion. Nine different FAME compounds with retention durations ranging from 19.93 to 30.48 min were identified by the chromatogram19.
Multiple fragmentation patterns with a base peak at m/z 74 were found in the study of fatty acid methyl esters (FAMEs), supporting the McLafferty rearrangement during transesterification. The carbo-methoxy ion was lost as a result of common fragmentation involving β-cleavage; certain patterns displayed several peaks as a result of atomic rearrangements and methoxy group displacement. The methyl ester of 14,17-Octadecadienoic acid, for example, showed structural alterations and shifts in hydrogen ions with a retention time of 22.36 min. Figure 3 depicted these fragmentation characteristics, and Table 7 provides a summary of the FAMEs found in the biodiesel sample. Similar work was also carried out in Hariram et al.16.
Fourier transform infrared spectrometer (FTIR)
The phenomenon of Attenuated Total Internal Reflectance (ATIR) is employed in Bruker-Alpha-Platinum spectrometer to understand the FTIR spectrum of Dunaliella salina biodiesel. The spectrometer has a range between 500 and 4000 cm-1 with 2 cm-1 resolutions. Rock-solid Michelson Interferometer with diamond brazed tungsten carbide crystal was used in this study with deurated triglycime sulphate as solvent. The spectrogram of FTIR reflects the vibration of organic compounds in bending and stretching signals of C–O, C–H, C=O etc. In the present investigation, FTIR analysis was employed to predict the presence of ester, ketones and alkyl groups based on its wave length. The image in Fig. 4 disseminates the correlation between wavenumber and transmittance in X and Y axis respectively.
Many vibrating signals with peaks formed between 547.17 cm-1 and 3008.32 cm-1 in the spectrum. A peak at 2853.91 cm-1 indicates the presence of O-H derivatives and carboxylic acid in the Dunaliella salina biodiesel. A characteristic peak at 1741.22 cm-1 confirmed the existence of saturated aldehydes (i.e.) C = O group. Cluster peak between 1460.99 cm-1 and 1360.64 cm-1 revealed the presence of ketones and aldehydes as it indicates α-CH2 bending. High dense peaks between 1243.60 cm-1 and 1169.21 cm-1 revealed the presence of carboxylic acid and it derivatives in prominent proportion. With an individual peak at 722.19 cm-1 shows the presence of alkenes in minor quantity. Similar outcomes of prominent biodiesel conversion as fatty acid methyl ester (FAME’s) were also reported by Pattanaik et al.20, Balasubramanian et al.21 and Hariram et al.22 in their studies. The detailed presence of organic compounds in Dunaliella salina is shown in Fig. 4 as a FTIR spectrum.
Nuclear magnetic resonance (NMR)
Avance III 500 NMR spectrometer with 11.7 tesla magnet, actively shielded, super conducting with cryo shims and 34 channel shim temperature cryo was employed in the present investigation to understand the nuclei behaviour. The RF console has a stable lock compatible with pulsed field gradient and deuterium lock. The split ratio of Avance III 500 follows the rule of n + 1 spin – spin splitting. 5 mm broadband gradient probe (BBO) with VT and auto tune for observing 1H decoupling and 5 mm quadrupole inverse probe with gradient (QXi) for observing 13 C decoupling was employed in Avance III 500 spectrometer.
Dunaliella salina biodiesel was prepared with methanol as solvent for 1H NMR analysis, using a 90-degree pulse and 10-second relaxation delay. The sample to solvent ratio employed in 1H NMR study was 4 mg of Dunaliella salina biodiesel with 0.8 mL of deurated methanol considering its solubility, signal intensity and molecular weight. Generally, deurated methanol is widely preferred due to its minimal interference in the output NMR signals. The spectrum revealed fatty acid esters as the main components, along with traces of steroids, alkaloids, and alkanes. Key signals included fatty acid esters at 3.669 ppm and a strong carbonyl peak at 5.387 ppm, with peaks between 3.33 and 2.11 ppm indicating unsaturated hydrocarbon chains and ester groups. 13C NMR, performed with deuterated methanol, showed peaks for epoxy esters, triglycerides, and monoglycerides between 0 and 200 ppm, including a terminal peak at 174.4 ppm confirming transesterification. Clusters at 127–130 ppm indicated unsaturated esters, while peaks between 26.7 and 33.5 ppm and at 16.4 ppm corresponded to carboxylic groups and monoglycerides, respectively23,24. The transmittance spectrum of 13C and 1H NMR are shown in Figs. 5 and 6 respectively.
Combustion characteristics
In-cylinder pressure
Under full load conditions, Fig. 7 shows how the in-cylinder pressure changes with crank angle for B20 (20% Dunaliella salina biodiesel), straight diesel, and B20 supplemented with hydrogen at flow rates of 3, 6, and 9 LPM. For straight diesel, the peak cylinder pressure was found close to top dead centre (TDC), showing a high-pressure, abrupt rise typical of conventional combustion. Because biodiesel has an increased oxygen content and a lower cetane number, which causes a longer ignition delay and more dispersed heat release, B20 showed a slightly lower PCP than diesel. Diesel fuel’s high energy density and auto-ignition feature will boost in-cylinder pressure25. The maximum pressure for D100 was noticed as 48.003 bar at 8 degrees after TDC and the biodiesel blend resulted in an in-cylinder pressure of 47.43 bar. When hydrogen was added to a biodiesel-diesel blend at 3 LPM, flame acceleration caused a faster pressure rise due to shorter ignition delay. Hydrogen’s low ignition energy and high diffusivity improve mixture uniformity, leading to rapid combustion, though pressure increases remain controlled with limited hydrogen addition. At 8° after top dead centre, pressure reached 47.68 bar; for 6 LPM and 9 LPM hydrogen additions, pressures were 47.79 bar and 49.003 bar, respectively. The reduced in-cylinder pressure for initial hydrogen addition may be due the the below mentioned reasons, when small amounts of hydrogen (around 3 to 6 L per minute) are added, it pushes out some of the intake air, which means there’s less oxygen available for burning the fuel. This leads to a weaker air-fuel mix that doesn’t burn as well. As a result, the fuel takes longer to ignite, and the combustion happens later than it should, which reduces the pressure build-up inside the engine.
Also, at these lower hydrogen levels, the hydrogen doesn’t mix well with the biodiesel and air, so the burn isn’t very efficient. On the above, the small amount of hydrogen added doesn’t provide much extra energy to improve the combustion. All these factors together cause the pressure inside the engine cylinder to be lower compared to when more hydrogen is used26,27,28. Overall, hydrogen enhances combustion and fuel oxidation, supporting the conclusion that algae-derived biodiesel is carbon-neutral29.
Rate of pressure rise
Figure 8 shows how the rate of pressure rise (RoPR) changes with crank angle for B20 (20% Dunaliella salina biodiesel), straight diesel, and B20 with hydrogen enrichment (3, 6, and 9 LPM). Diesel fuel normally exhibits a steep pressure rise rate due to high energy content and fast auto-ignition by compression. Pressure rise, in diesel-only combustion, is mainly governed by the premixed combustion phase due to the rapid nature. The pressure rise rate value was found to be 1.78 bar at 3 degrees after TDC30. The biodiesel blended with algal diesel is marginally less in pressure rise ratio than when pure diesel is used. Biodiesel contains more oxygen and less calorific value, imparting very well-controlled nature of combustion. The combustion exhibited a gentler pressure rise with enhanced stability, registering 1.64 bar at 3 degrees before TDC. When hydrogen was introduced into the diesel-algal biodiesel blend at 3 LPM, the pressure increased more rapidly due to hydrogen’s high combustion rate and low ignition energy. Hydrogen promoted earlier and more uniform auto-ignition, boosting the pressure rise rate. Specifically, the rate of pressure rise (ROPR) was 1.76 bar at 2 degrees after TDC for 3 LPM, 1.68 bar at 3 degrees after TDC for 6 LPM, and 1.75 bar at 2 degrees after TDC for 9 LPM hydrogen addition31.
Net heat release rate
The net heat release rate (HRR) profile, plotted against crank angle for diesel, B20 (80% diesel with 20% Dunaliella salina biodiesel), and hydrogen-enriched B20 blends (3, 6, and 9 LPM), is presented in Fig. 9 which results in the multiple ignition behaviour, flame speeds, and combustion efficiency-cause HRR variability. A single-cylinder engine’s HRR is a crucial combustion parameter that represents the energy released during combustion and has a direct impact on the engine’s performance, emissions, and efficiency. A highly reactive premixed combustion phase was supported by the pure diesel HRR curve’s steep and narrow peak near TDC. Diesel’s high energy density and short ignition delay, which allow for quick and concentrated energy release right after ignition, are responsible for this characteristic. Recent research has shown that while such rapid burning increases thermal efficiency, it also raises NOx emissions and may cause combustion noise. D100 provided 20.94 joules at 1-degree post TDC. Diesel-algal biodiesel blend will help the burning process by supplying additional oxygen for complete combustion and results in a milder heat release pattern. The combustion process was smooth, with a consistent heat release rate (HRR) indicating complete burning, reaching 21.89 joules at 1 degree after TDC (Fig. 9). Introducing hydrogen at 3 LPM notably increased the HRR compared to diesel alone, thanks to hydrogen’s low ignition energy and high flame speed that facilitate faster ignition. This caused an earlier heat release phase and a higher peak HRR, enhancing combustion efficiency and heat output. At this concentration, the HRR remained stable without causing excessive pressure spikes, measuring 20.47 joules at 3 LPM, 19.83 joules at 6 LPM, and 20.47 joules at 9 LPM, all at 1 degree post-TDC. Overall, blending algae biodiesel with diesel provided a stable baseline HRR, while hydrogen addition significantly improved combustion by influencing the HRR profile32,33.
Cumulative heat release rate
In a four-stroke, single-cylinder diesel engine, the cumulative heat release rate (CHRR) is the rate at which energy is released during the whole combustion cycle. This measure is essential for determining how completely the fuel burns and how effectively its energy is used. Conventional diesel’s high calorific value and efficient combustion characteristics usually result in an ideal CHRR. The premixed phase of diesel combustion is characterized by a rapid increase in heat release, whereas the diffusion phase proceeds more slowly34. CHRR provides a trustworthy benchmark for comparing diesel to alternative fuel blends. In comparison to pure diesel, a 20% blend of diesel and algae-based biodiesel often results in a little increase in CHRR. This is mostly because biodiesel has less energy in it. Nonetheless, biodiesel’s naturally high oxygen content promotes more thorough burning, which helps to offset some of its lower energy density. Because of this, the blend’s CHRR pattern develops a little more gradually than pure diesel’s, resulting in a modest difference in the overall heat release profile throughout the course of the combustion cycle.
The introduction of hydrogen at a flow rate of 3 LPM significantly influences the CHRR curve, evident through steeper initial rises during the combustion phase. This behaviour originated from hydrogen’s role in accelerating ignition and promoting more thorough oxidation of the fuel mixture. As a result, the overall energy output from combustion increases, reflecting a higher CHRR compared to the standard diesel-biodiesel blend. The presence of hydrogen enhances the rate at which energy is released, enabling a larger portion of the fuel’s energy to be utilized in a shorter combustion duration. This quicker combustion not only improves efficiency but also helps moderate extreme temperature variations, supporting more stable engine operation. Similar improvements in CHRR were observed at higher hydrogen flow rates of 6 and 9 LPM. However, the elevated heat release at these hydrogen enrichment levels requires precise engine calibration to avoid erratic combustion or mechanically damaging spikes in pressure and temperature, as illustrated in (Fig. 10).
Mean gas temperature
An essential component of a single-cylinder compression ignition (CI) engine is the mean gas temperature inside the combustion chamber, which has a direct effect on emissions, combustion efficiency, and the thermal load on engine parts35. Due to variations in the rate of heat release and combustion properties, different fuel blends have an impact on this mean gas temperature (MGT). MGT is anticipated to be very high in the case of pure diesel fuel (D100), mainly due to diesel’s high energy density and advantageous combustion characteristics. Diesel combustion typically results in higher peak temperatures, particularly during the premixed combustion stage’s rapid energy release. Although high MGT values are advantageous for producing high heat output, they also raise NOx emissions because of the formation of localized hot spots, or high-temperature zones, inside the cylinder. Diesel-only combustion typically produces stable MGTs that can be used as a platform from which to compare other fuel ratios. Adding 20% Dunaliella salina biodiesel to diesel typically brings about a slightly varied MGT since the oxygen content in the biodiesel encourages full combustion with less unburned carbon, but with a slightly reduced peak temperature.
The mean gas temperature (MGT) of a four-stroke, single-cylinder diesel engine is a crucial measure of combustion efficiency and has a big impact on the thermal stress that engine parts endure as well as pollution levels. Different fuel blends have different effects on MGT based on their unique heat release profiles and combustion properties. Because of its high energy content and efficient burning, pure diesel fuel (D100) typically has a higher MGT, as seen in (Fig. 11). Rapid energy release during the premixed phase of diesel combustion results in elevated peak temperatures. High MGT values have a beneficial effect on thermal efficiency, but they also encourage the production of nitrogen oxides (NOx) because they cause hot spots, or high-temperature zones, to form inside the combustion chamber. Diesel combustion usually keeps its MGT fairly constant, which makes it a good starting point for assessing how well different fuel blends operate.
In general, blending 20% algae-based biodiesel with diesel produces a modest variation in mean gas temperature (MGT). This is primarily due to the oxygen-rich nature of biodiesel, which promotes more thorough combustion and reduces unburned carbon emissions, though it slightly lowers the peak temperature during combustion. This reduction in MGT for DuSaBD20 with H2 supplementation at 6 LPM and 9 LPM may also be due to flame quenching effect as a result of fuel particle dissociation especially at elevated engine loads.
Despite higher in-cylinder pressure, the MGT in Du Sa BD20 + 6 LPM H₂ is reduced due to leaner combustion and limited fuel energy. Hydrogen’s fast flame speed leads to quicker combustion but shorter heat transfer duration. Increased water vapor from hydrogen combustion absorbs more heat due to its higher specific heat. Better combustion uniformity spreads heat release, reducing local temperature spikes. Additionally, hydrogen displaces intake air, slightly lowering oxygen availability and overall temperature rise. As noticed, the in-cylinder pressure being higher at 8 degrees after top dead centre; the mean gas temperatures noted in this study for diesel, B20 (80% diesel with 20% Dunaliella salina biodiesel), and hydrogen-enriched B20 blends (3, 6, and 9 LPM) were found to be 61.53, 60.83, 61.18, 59.04 and 61.19 °C. From Literature study it was notable there may be a decrease of 2.9–14.3% in exhaust gas temperatures when hydrogen was added (exact hydrogen fractions unspecified). If the baseline exhaust temperature was ~ 60 °C, this suggests reduced MGT values around/ between 51.4 °C and 58.2 °C, consistent with the lower value observed at 6 LPM H₂ (59.04 °C). Comparably it can be noted that the study values are acceptable36,37.
Emission characteristics
Unburned hydrocarbon
A single-cylinder diesel engine’s emissions of unburned hydrocarbons (UBHC) are a crucial measure of how well the fuel burns and oxidizes38. Emission reduction potential can be gained by comparing the UBHC outputs of various fuel types, such as pure diesel, a diesel-algae biodiesel blend, and this blend mixed with hydrogen at flow rates of 3, 6, and 9 LPM as shown in (Fig. 12). Diesel naturally tends to produce comparatively larger levels of UBHC since it is a complex hydrocarbon fuel with long molecular chains. The main cause of these emissions is incomplete combustion, which frequently takes place in the combustion chamber’s colder or fuel-rich areas. Even though diesel burns efficiently thanks to its high energy content and quick auto-ignition features, problems such fuel impingement on cylinder walls or build-up in crevice volumes can still result in UBHC.
Pure diesel serves as a useful reference point when evaluating UBHC emissions across different fuel blends. When 20% algae-based biodiesel is mixed with diesel, there is typically a reduction in UBHC emissions. This decrease is largely attributed to the inherent oxygen content in biodiesel, which promotes more effective oxidation and accelerates the combustion process, leading to more complete burning of the fuel. The addition of algae biodiesel results in smoother combustion, with fewer fuel-rich zones where incomplete burning would normally occur, thereby reducing UBHC output. Furthermore, biodiesel tends to shorten the ignition delay, allowing the fuel to ignite and burn more rapidly, which contributes to lower UBHC levels. In terms of emission data, pure diesel (D100) recorded UBHC emissions of approximately 46 ppm, while the 80:20 diesel-biodiesel blend (D80B20) showed a slightly higher value of 48 ppm.
UBHC emissions are further impacted by adding hydrogen to the diesel-biodiesel blend at a flow rate of 3 LPM. Hydrogen’s rapid flame velocity and low ignition energy improve combustion by reducing areas where incomplete burning takes place, which dramatically reduces the formation of UBHC. The amount of unburned hydrocarbons in the cylinder is significantly reduced when hydrogen is present because it causes faster and more even combustion. The value of D80 B20 3LPM, D80 B20 6LPM, and D80 B20 9LPM fuel was 54, 51 and 55 ppm, respectively. Higher UBHC emission was observed for Dunaliella salina fuel blend with H2 9LPM which may be due to poorer dissolution of H2 atoms with Diesel-Biodiesel fuel blend especially at higher engine loads. The quicker atomization of fuel and the shortened ignition delay may also contribute to a rise in UBHC emissions. However, at this level of hydrogen enrichment, UBHC output is nearly eliminated. To maintain these benefits without introducing issues such as engine knock or excessive thermal loading, precise control over injection timing and air–fuel mixture is crucial.
Carbon monoxide
An essential measure of fuel combustion efficiency and the extent of oxidation during combustion is carbon monoxide (CO). CO is usually produced in areas with incomplete combustion, which is frequently brought on by inadequate oxygen availability or inadequate fuel-air mixing. The usage of three distinct fuel types—pure diesel, a combination of diesel and algae biodiesel, and this blend mixed with varied rates of hydrogen flow shows varying levels of CO emissions, which are indicative of variations in oxidative efficiency and combustion dynamics (Fig. 13). Diesel typically produces a moderate quantity of CO under normal combustion settings because of its high carbon content and long-chain hydrocarbon structure.
Diesel combustion is very pressure and temperature-sensitive for the maximum oxidation; but low-temperature or fuel-rich regions of the combustion chamber can still be responsible for CO production. Diesel-alone combustion is used as a base reference for CO emission in comparison to other fuel blends. Blending 20% algae biodiesel into diesel fuel usually decreases CO emissions. Biodiesel’s inherent oxygen content encourages fuller combustion since the extra oxygen limits the chances of CO formation due to more complete carbon oxidation to CO₂. Incorporating hydrogen into the diesel-biodiesel blend lowers the ignition energy and raises flame speed, which improves overall combustion efficiency and influences CO emission levels. However, the accelerated combustion process can sometimes result in slightly elevated CO emissions, as there may be insufficient time for complete oxidation. This is reflected in CO emission values of 0.25%, 0.225%, and 0.313% at hydrogen flow rates of 3, 6, and 9 LPM, respectively. Higher CO emission could be noticed for Dunaliella salina biodiesel blend with addition and increase in H2 supplemtation. This may be due to quicker fuel vaporisation in the premixed combustion period leading to relatively lower atomization rate along with increase in cetane number. Availability of lesser oxygen atom may also be a reason for elevated CO emission. Moreover, the improved combustion characteristics and enhanced fuel-air mixing provided by biodiesel reduce the presence of fuel-rich regions, thereby lowering CO emissions compared to pure diesel. Pure diesel (D100) recorded a CO emission level of 0.11%, while the blend containing 20% algal biodiesel showed a slightly higher value of 0.132%. Comparable findings were reported in the studies conducted by Jayaraman et al.23 and Kesharvani et al.10.
Carbon dioxide
CO₂ emissions in a single-cylinder engine indicate the extent of complete fuel combustion and oxidation efficiency. Unlike CO, CO₂ is produced when carbon in the fuel is fully oxidized. This study compares CO₂ levels from pure diesel, a 20% algae biodiesel blend, and blends enriched with hydrogen. Diesel, with its high carbon content, naturally produces more CO₂ during combustion. The biodiesel blend shows a slight increase in CO₂ (9.87%) over neat diesel (8.98%) due to improved oxidation. Biodiesel’s oxygen content enhances combustion, influencing CO₂ output slightly. Hydrogen addition (3 LPM) lowers CO₂ emissions per energy unit, as hydrogen contains no carbon. Thus, hydrogen-enriched blends offer cleaner energy with reduced carbon emissions.
This blend is seen to achieve full combustion due to the extreme reactivity of hydrogen that it leads to favourable oxidation and might decrease CO₂ emission even further as it generates energy without the increase of carbon content, and specifically, the value of the emission was stated to be 8.83%, whereas for 6 LPM and 9 LPM, it was 8.54 and 8.88% respectively as shown in (Fig. 14). Unavailability of more free oxygen atom at elevated engine load especially also during the stepped-up hydrogen supplementation for the conversion of CO to CO2 may be a reason for higher decrement in CO2 emission with higher H2. Faster vaporization of fuel atoms and reduced progressive combustion duration in the later stages of combustion may also be a reason for reduced CO2 emission. Similar outcomes were reported by Venkatesan et al.32 and Zhong et al.13 in their studies. Therefore, incorporating hydrogen into diesel-biodiesel blends helps lower CO₂ emissions while maintaining combustion efficiency; however, careful consideration is needed to monitor potential increases in NOx emissions and thermal effects at varying hydrogen concentrations.
Oxides of nitrogen
NOx emissions from diesel engines are a major environmental concern due to their contribution to air pollution and severe health risks. These gases form mainly during high-temperature combustion, where nitrogen and oxygen in the air react inside the cylinder. This study compares NOx emissions across five fuel types: pure diesel, a diesel–algae biodiesel blend, and the same blend with varying hydrogen levels. Diesel combustion typically results in high NOx levels due to rapid heat release and elevated temperatures. Optimized diesel engines often exceed the critical temperature threshold for thermal NOx formation, especially during the premixed combustion phase. This makes diesel the reference point for NOx emissions. Introducing 20% Dunaliella salina biodiesel to diesel helps reduce NOx output by a noticeable margin. The oxygen content in biodiesel promotes cleaner combustion with less soot. Despite improved oxidation, it does not significantly raise combustion temperatures. Thus, biodiesel blending offers a slight NOx reduction without compromising combustion efficiency.
The inherent oxygen content in algae-based biodiesel promotes more complete combustion, typically at slightly lower temperatures, which can lead to a mild reduction in NOx formation. However, the effect of biodiesel on NOx emissions is complex and highly dependent on specific combustion conditions. In some cases, the added oxygen can locally increase temperatures, potentially boosting NOx production. Under full load, pure diesel produced NOx emissions of 1744 ppm, while the diesel–algal biodiesel blend showed a slightly higher value of 1762 ppm. When hydrogen was introduced into the blend at flow rates of 3, 6, and 9 LPM, NOx levels increased marginally, though the rise was minimal and within acceptable limits (Fig. 15). Hydrogen’s high flame speed leads to quicker and more complete combustion, which can elevate peak temperatures within the cylinder. This temperature rise promotes thermal NOx formation, a common trait in hydrogen-enriched combustion. As a result, NOx emissions were recorded at 1789 ppm, 1801 ppm, and 1699 ppm for hydrogen flow rates of 3, 6, and 9 LPM, respectively. Hydrogen supplementation up to 6 LPM increased the engine NOx, but increasing H2 concentration beyond 6 LPM reduced NOx formation notably. This could be due to poorer atomization of fuel parcels especially at higher engine loads and reduction in time period during the premixed combustion phase. Presence of surplus O2 and oxidation of available fuel particles completely at higher H2 concentration with enhanced flammability characteristics may have brought down the incylinder peak temperature leading to produce reduced NOx emission. Similar outcomes were reported by Saravanan et al.39, Venu et al.17 and Rajkumar et al.19 in their investigations.
Smoke
Smoke emissions primarily result from incomplete combustion, where fuel burns but fails to fully oxidize into particulate-free gases. This study presents smoke emission data using various fuels: pure diesel, a diesel–algae biodiesel blend, and the same blend supplemented with different hydrogen flow rates, highlighting how fuel composition and hydrogen addition influence smoke levels (Fig. 16). Diesel, with its complex hydrocarbon structure and high aromatic content, has a strong tendency to generate significant smoke, especially in fuel-rich zones. Under high engine loads, inadequate mixing of air and fuel during diesel combustion often leads to localized rich regions, which are a major source of increased smoke output. Diesel fuel’s high carbon content and extended combustion duration contribute significantly to its baseline smoke emission levels. When algae-based biodiesel is blended with diesel, a noticeable reduction in smoke output is observed. This is largely due to the biodiesel’s oxygen content, which enhances combustion efficiency and promotes more complete oxidation of hydrocarbons, thereby lowering soot production. Compared to pure diesel, the diesel–biodiesel blend produces fewer particulates during combustion. This improvement is further supported by biodiesel’s lower aromatic content and the oxygenated nature of the fuel. Additionally, the higher cetane number and smoother combustion phase of the blend—especially under heavy engine load—also aid in minimizing smoke. Experimental results showed that smoke emissions from neat diesel reached 61.3%, while the diesel–algal biodiesel mixture recorded a reduced value of 48.9%. Introducing hydrogen into the blend further cuts down smoke levels. Hydrogen, being carbon-free and highly diffusive, burns cleanly and does not contribute to soot formation, leading to a cleaner combustion process overall. Introducing hydrogen into the diesel–biodiesel blend improves air-fuel mixture uniformity and promotes cleaner, more complete combustion, significantly lowering smoke emissions. Compared to the base diesel–biodiesel blend, smoke levels dropped to 53.6, 45.7, and 59.2% at hydrogen flow rates of 3, 6, and 9 LPM, respectively. The most notable reduction occurred at 6 and 9 LPM, attributed to hydrogen’s clean-burning nature and its ability to create a more homogeneous combustion environment. These blends demonstrate strong potential for smoke suppression in diesel engines, although higher hydrogen levels may require additional NOx control strategies.
Performance analysis
Brake specific fuel consumption
Brake specific fuel consumption is the measure of useful work produced by a unit quantity of fuel consumed or admitted. It can be determined using the Eq. (5)
Where \(\:{{m}^{o}}_{fuel}\) is the mass flow rate of the fuel admitted and T is the engine torque. Figure 17 depicts the variation in BSFC of Dunaliella salina biodiesel blend with hydrogen induction at 3, 6 and 9 LPM and compared against straight diesel. It can be noticed that initially at low load, the BSFC of all test fuel was higher, especially with straight diesel and DuSaBD20 + 3 LPM H2 which showcased inferior combustion performance. With the escalation in engine load, the BSFC gradually reduced upto 3.52 kg/kWhr across all fuel blends. Reduced calorific value and higher fuel density of DuSaBD20 may have increase the BSFC considerably. At part load condition, straight diesel and DuSaBD20 fuel showed a BSFC of 0.284 kg/kWhr and 0.279 kg/kWhr respectively. Hydrogen induction with DuSaBD20 at 3 LPM, 6 LPM and 9 LPM produced a BSFC of 0.321 kg/kWhr, 0.3102 kg/kWhr and 0.3057 kg/kWhr respectively. Higher engine load further decreased the BSFC especially with H2 supplementation at 6 LPM and 9 LPM upto 0.252 kg/kWhr and 0.240 kg/kWhr respectively. This 2.25% reduction in BSFC may be due to higher oxygen concentration of DuSaBD20 with relatively lower kinematic viscosity. Supplementing H2 with Dunaliella salina biodiesel blend may have elevated the calorific value of the admitted fuel, thereby reducing the BSFC notably especially at high load condition. Similar results were reported by John et al.31 and Adu-Mensah et al.40 in their studies.
Brake thermal efficiency
Brake thermal efficiency is the measure of engine effectiveness in converting the admitted fuel into power produced by the engine’s crank shaft. Figure 18 shows the variation of BTE when the CI engine is fuelled with Straight diesel, Dunaliella salina biodiesel blend with H2 supplementation at 3 LPM, 6 LPM and 9 LPM. Generally, BTE followed an increasing path with escalation in engine load. Straight diesel represented BTE of 0.35, 31.765 and 35.87% at low, part and full load operation whereas DuSaBD20 fuel showcased 0.47, 29.88 and 33.221% at similar loading condition. This may be due to reduced calorific value of DuSaBD20 and poor atomization due to higher relative density. Addition of H2 with DuSaBD20 fuel blend significantly increased the BTE upto 37.78% for DuSaBD20 + 6 LPM H2 at full load condition which may be due to enhanced calorific value of H2 and better mixing of air and fuel parcels especially at higher engine loads. The homogeneity of the air-fuel mixture was achieved by inducting the H2 through the throttle body thereby thorough mixing of air-fuel mixture takes place due to diffusivity. Further increase in H2 concentration upto 9 LPM marginally diminished the BTE by 2.152% upto 36.98% at similar loads.
Operational cost
With the establishment period of 12 months, the operational cost was assessed considering the operation time duration and test fuel blends consumption at low, part and higher loading condition using Eq. (6).
Where ρf is the cost of fuel, tp is the operational time period and BSFC is Brake Specific Fuel Consumption. The maximum operation cost of 10,900 Rs/kWhr and 11,250 Rs/kWhr was noticed for straight diesel and DuSaBD20 test fuels. The blend of DUSaBD with straight diesel at 20% increased the Cp by 1.983%. Further supplementing Hydrogen (3 LPM, 6 LPM and 9 LPM) escalated the operational cost by 2.98% with a 47 kg Hydrogen cylinder costing Rs. 7450/-. Marginal rise in the operational cost was compromised by reduced BSFC, improved combustion efficiency and enhanced engine performance greatly thereby the life of the engine was significantly increased in the present investigation.
Conclusion
This research highlighted the viability of Dunaliella salina microalgae as an eco-friendly and efficient source for biodiesel production, achieving a high conversion rate of 94.8% using an optimized one-step transesterification method. The formation of fatty acid methyl esters (FAME) was validated through advanced characterization techniques such as FTIR and NMR spectroscopy. Additionally, performance and emission assessments carried out on a single-cylinder diesel engine using pure diesel, algae-based biodiesel, and hydrogen-enriched fuel blends revealed key findings regarding combustion behavior and emission profiles, offering valuable data for cleaner alternative fuel strategies. The results rendered the following outcomes.
-
The harvested Dunaliella salina biomass was subjected to ultrasonic bio-oil extraction process at 50 Hz in 15 batches producing 645 ml of Dunaliella salina bio-oil.
-
Single stage transesterification process with NaOH and methanol in an optimized condition (i.e.) 1:8 molar ratio, 0.6% by weight of catalyst concentration, 50 °C reaction temperature and 120 min reaction duration yielded 612 ml of Dunaliella salina biodiesel with an transesterification efficiency of 94.8%.
-
The physio-chemical analysis on Dunaliella salina bio-oil and its biodiesel revealed its suitability in CI engine and were within ASTM standards.
-
The characterization studies include GCMS, FTIR and NMR spectroscopic analysis revealed the presence of 14, 17 – Octadecadienoic acid methyl ester (commonly called Linoleic acid) in prominent proportions.
-
The standardized Dunaliella salina biodiesel fuelled in Kirloskar 240PE single cylinder test engine exposed promising combustion, performance and emission characteristics.
-
The In-cylinder pressure and rate of pressure rise was improved by 2.14% and 1.98% respectively. H2 induction through throttle body further escalated the combustion characteristics appreciably.
-
The Net heat release of DuSaDB20 blend was found as 20.89 J/CAD. Hydrogen supplementation by 3, 6 and 9 LPM escalated the NHR rate by 2.53–3.12% significantly. Cumulative heat release also showed an appreciable curve with H2 induction.
-
UBHC and CO emission showed significant reduction at low and part load with DuSaBD20 against Straight diesel whereas with increase in engine load, the UBHC emission was higher. H2 supplementation notably increased UBHC emission at part and full load for 9 LPM whereas 3 LPM and 6 LPM showed reduced UBHC emission. CO2 emission also showed similar trend across all fuel blends at low, part and full load operation.
-
NOx emission generally showed increasing trend with escalation in engine load. DuSaBD20 showed higher NOx emission than Straight diesel. H2 induction further increased NOx emission with the increase in its concentration notably. Higher NOx emission of 1795 ppm was noticed for DuSaBD20 H2 6 LPM at full load condition.
-
Smoke emission was lower for DuSaBD20 fuel at all loads than Straight diesel. H2 6 LPM with the DuSaBD20 recorded lowest smoke opacity of 39.75% at full load condition.
-
H2 induction notably decreased the BSFC by 2.25% whereas the BTE was significantly increase at 6 LPM.
In summary, fuel blends combining Dunaliella salina biodiesel with hydrogen present a strong potential for enhancing combustion performance while significantly lowering emissions such as carbon monoxide and smoke. These characteristics make them a compelling option for more environmentally sustainable diesel engine applications. Although a minor rise in NOx emissions is observed with hydrogen enrichment, this issue can be effectively managed by fine-tuning hydrogen injection rates and incorporating suitable exhaust after-treatment systems.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Compression ignition engine
- NMR:
-
Nuclear magnetic resonance
- GCMS:
-
Gas chromatography mass spectrometry
- FTIR:
-
Fourier transform infra-red spectrometry
- NOx :
-
Oxides of nitrogen
- CO:
-
Carbon monoxide
- UBHC:
-
Unburned hydrocarbon
- CO2 :
-
Carbon dioxide
- HB:
-
Hydrogenated biodiesel
- FFA:
-
Free fatty acid
- KOH:
-
Potassium hydroxide
- H2SO4 :
-
Sulphuric acid
- NaOH:
-
Sodium hydroxide
- ASTM:
-
American society for testing and materials
- TDC:
-
Top dead centre
- BDC:
-
Bottom dead centre
- FAME:
-
Fatty acid methyl ester
- BBO:
-
Broadband gradient probe
- LPM:
-
Litres per minute
- HRR:
-
Heat release rate
- ROPR:
-
Rate of pressure rise
- CHRR:
-
Cumulative heat release rate
- MGT:
-
Mean gas temperature
- BSFC:
-
Brake specific fuel consumption
References
Qiu, Z., Chen, R., Gan, X. & Wu, C. Torsional damper design for diesel engine: theory and application. Phys. Scr. 99 (12), 125214. https://doi.org/10.1088/1402-4896/ad8af8 (2024).
Venkatesan, H., Sivamani, S., Premkumar, T. M. & Tharun, P. Reduction of exhaust emissions using a nanometallic enriched Lemongrass biodiesel blend. Energy Sour. Part A Recover. Utilization Environ. Eff. 39 (21), 2065–2071 (2017).
Guo, H. et al. Transient lubrication of floating Bush coupled with dynamics and kinematics of cam-roller in fuel supply mechanism of diesel engine. Phys. Fluids. 36 (12), 123103. https://doi.org/10.1063/5.0232226 (2024).
Christopher Selvam et al., (2025). Improving diesel engine performance with hydrogen-fumigated soapnut biodiesel. Results in Engineering, 27, 106287. https://doi.org/10.1016/j.rineng.2025.106287
Naureen, R., Tariq, M., Yusoff, I., Chowdhury, A. J. & Ashraf, M. A. Synthesis, spectroscopic and chromatographic studies of sunflower oil biodiesel using optimized base catalyzed methanolysis. Saudi J. Biol. Sci. 22 (3), 332–339 (2015).
Sun, X. et al. Heat transfer augmentation, endothermic pyrolysis and surface coking of hydrocarbon fuel in manifold microchannels at a supercritical pressure. Int. Commun. Heat Mass Transfer. 161, 108564. https://doi.org/10.1016/j.icheatmasstransfer.2024.108564 (2025).
Sui, M., Zhu, Z., Li, F., Wang, H. & Tang, C. Effect of oxidation on the combustion flame characteristics of Jatropha biodiesel. Fuel Process. Technol. 252, 107972 (2023).
Chen, Y. et al. Utilization of renewable biodiesel blends with different proportions for the improvements of performance and emission characteristics of a diesel engine. Heliyon 1 (9), 9 (2023).
Qiu, Z. et al. An iterative approach to solve the viscous damper temperature and the torsional vibration amplitude of a diesel engine. J. Vib. Eng. Technol. 13 (6), 437. https://doi.org/10.1007/s42417-025-01983-7 (2025).
Kesharvani, S. et al. Enhancing diesel engine performance and emission reduction through hydrogen enrichment in algal biodiesel blends. Environ. Sci. Pollut Res. https://doi.org/10.1007/s11356-024-34531-z (2024).
Avadhoot et al. Maximizing efficiency and environmental benefits of an algae biodiesel-hydrogen dual fuel engine through operational parameter optimization using response surface methodology. Int. J. Hydrog. Energy. 52, 1395–1407. https://doi.org/10.1016/j.ijhydene.2023.10.134 (2024).
Vellaiyan, S., Kandasamy, M., & Devarajan, Y. (2023). Optimization of Bauhinia parviflora biodiesel production for higher yield and its compatibility assessment with water and Di-tert-butyl peroxide emulsion. Waste Management, 162, 63–71. https://doi.org/10.1016/j.wasman.2023.03.012
Zhong, W. et al. Experimental study on combustion and emission characteristics of fatty acid Methyl esters and hydrogenated catalytic biodiesel/diesel blends under world harmonized steady state cycle. Fuel 343, 127887 (2023).
Tsanaktsidis, C. et al. Variation of the physicochemical properties diesel-biodiesel blends–range 0–100%. Pet. Sci. Technol. 36 (11), 772–780 (2018).
Hu, Q. Industrial production of microalgal cell mass and secondary products-major industrial species. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology (Vol. 264). United States: Blackwell Publishing Ltd. (2004).
Hariram, V. et al. Enhanced combustion and emission characteristics of diesel-algae biodiesel-hydrogen blends in a single-cylinder diesel engine. Results Eng. 26 https://doi.org/10.1016/j.rineng.2025.104676 (2025).
Venu, H. et al. Performance and emission prediction using ANN (artificial neural network) on H2-assisted garcinia gummi-gutta biofuel doped with nano additives. Sci. Rep. 15, 5911. https://doi.org/10.1038/s41598-025-90165-2 (2025).
Rao, T. S. S. B. et al. Sustainable synthesis and advanced optimization of Prosopis Juliflora biomass catalyst for efficient biodiesel production and environmental impact assessment. Sci. Rep. 15, 4472. https://doi.org/10.1038/s41598-025-88355-z (2025).
Zhang, W., Shi, L., Xia, W., Du, Y. & Yao, L. Research on the anharmonic effect of main reactions of important intermediate species in NH3/DME mixed combustion. Chem. Phys. Lett. 874–875. https://doi.org/10.1016/j.cplett.2025.142170 (2025).
Pattanaik, S. et al. Combined mixture process design approach for flexible fuel maps development of ternary blends operated gasoline engine. Process Saf. Environ. Prot. 180, 1104–1117 (2023).
Balasubramanian, D. et al. Engine behavior analysis on a conventional diesel engine combustion mode powered by low viscous Cedarwood oil/waste cooking oil biodiesel/diesel fuel mixture–An experimental study. Process Saf. Environ. Prot. 184, 560–578 (2024).
Hariram, V. & Vasanthaseelan, S. Optimization of base catalysed transesterification and characterization of brassica Napus (Canola seed) for the production of biodiesel. Int. J. ChemTech Res. 8 (9), 418–423 (2015).
Jayaraman, J., Alagu, K., Appavu, P., Joy, N. & Mariadhas, A. Impact of methyl, ethyl, and Butyl ester blends of freshwater algae oil on the combustion, performance, and emissions of a CI engine. Energy Fuels. 34 (8), 9763–9770 (2020).
Yano, J., Aoki, T., Nakamura, K., Yamada, K. & Sakai, S. I. Life cycle assessment of hydrogenated biodiesel production from waste cooking oil using the catalytic cracking and hydrogenation method. Waste Manag. 38, 409 – 423. (2015).
Souza, B. S., Pinho, D. M., Leopoldino, E. C., Suarez, P. A. & Nome, F. Selective partial biodiesel hydrogenation using highly active supported palladium nanoparticles in imidazolium-based ionic liquid. Appl. Catal. A. 433, 109–114 (2012).
Cernat, A. et al. Aspects of an experimental study of hydrogen use at automotive diesel engine. Heliyon 9 (3), e13889. https://doi.org/10.1016/j.heliyon.2023.e13889 (2023).
Hosmath, R. S. et al. Performance, emission and combustion characteristics of dual fuel (DF) engine fuelled with hydrogen induction and injection of Honne and Honge Methyl esters. Energy Power Eng. 07 (09), 384–395. https://doi.org/10.4236/epe.2015.79036 (2015).
Yuvarajan, D., & Venkata Ramanan, M. (2016). Experimental analysis on neat mustard oil methyl ester subjected to ultrasonication and microwave irradiation in four stroke single cylinder Diesel engine. Journal of Mechanical Science and Technology, 30(1), 437–446. https://doi.org/10.1007/s12206-015-1248-x
Hu, C. et al. Catalytic hydrogenation of C [double bond, length as m-dash] C and C [double bond, length as m-dash] O in unsaturated fatty acid Methyl esters. Catal. Sci. Technol. 4 (8), 2427–2444 (2014).
Kongprawes, G., Wongsawaeng, D., Ngaosuwan, K., Kiatkittipong, W. & Assabumrungrat, S. Low-temperature and atmospheric pressure plasma for palm biodiesel hydrogenation. Sci. Rep. 11 (1), 14224 (2021).
John, G., Hariram, V. & Seralathan, S. Emission reduction using improved fuel properties of algal oil biodiesel and its blends. Energy Sour. Part A Recover. Utilization Environ. Eff. 40 (1), 45–53 (2018).
Venkatesan, H., Sivamani, S., Sampath, S., Gopi, V. & Kumar, D. A comprehensive review on the effect of nano metallic additives on fuel properties, engine performance and emission characteristics. International J. Renew. Energy Research (IJRER). 7 (2), 825–843 (2017).
Tang, H., Salley, S. O. & Ng, K. S. Fuel properties and precipitate formation at low temperature in soy-, cottonseed-, and poultry fat-based biodiesel blends. Fuel 87 (13–14), 3006–3017 (2008).
Choubey, G., Gaud, P., Mahmood Fatah, A. & Devarajan, Y. Numerical investigation on geometric sensitivity and flame stabilisation mechanism in H2 fueled two-strut based scramjet combustor. Fuel 312, 122847. https://doi.org/10.1016/j.fuel.2021.122847 (2022).
Choubey, G., Solanki, M., Patel, O., Devarajan, Y. & Huang, W. Effect of different strut design on the mixing performance of H2 fueled two-strut based scramjet combustor. Fuel 351, 128972 (2023).
Reitmayr, C. & Hofmann, P. Strategies to increase hydrogen energy share of a Dual-Fuel Hydrogen–Kerosene engine for sustainable general aviation. Hydrogen 6 (1), 17. https://doi.org/10.3390/hydrogen6010017 (2025).
Karagöz, Y., Sandalcı, T., Yüksek, L., Dalkılıç, A. S. & Wongwises, S. Effect of hydrogen–diesel dual-fuel usage on performance, emissions and diesel combustion in diesel engines. Adv. Mech. Eng. https://doi.org/10.1177/1687814016664458 (2016).
Madhu, S., Lionus Leo, G. M., Prathap, P., Devarajan, Y. & Jayabal, R. Effective utilization of waste pork fat as a potential alternate fuel in CRDI research diesel engine – Waste reduction and consumption technique. Process Saf. Environ. Prot. 172, 815–824. https://doi.org/10.1016/j.psep.2023.02.057 (2023).
Saravanan, B., Musthafa, B. & Asokan, M. A. Assessment of CI engine vibration at various injection timing and injection pressure with Ceiba Pentandra biodiesel. Int. J. Green Energy. 21 (4), 719–731 (2024).
Adu-Mensah, D., Mei, D., Zuo, L., Zhang, Q. & Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel properties. Fuel 251, 660–668 (2019).
Author information
Authors and Affiliations
Contributions
All authors have equally contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hariram, V., Sathishbabu, R., John, J.G. et al. Maximizing the combustion phenomenon with reduced emission of a compression ignition engine fulled with Dunaliella salina biodiesel and hydrogen blends. Sci Rep 15, 30372 (2025). https://doi.org/10.1038/s41598-025-16285-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16285-x