Abstract
Type 2 diabetes mellitus (DM) is associated with impaired host immune responses, increasing the risk for latent tuberculosis (TB) infection (LTBI). This study investigated how DM and associated metabolic dysfunction alter Mycobacterium tuberculosis (Mtb)-specific cytokine and chemokine responses. We analysed a cohort of 164 participants with and without DM and/or LTBI. Mtb-specific cytokine/chemokine responses were measured in QuantiFERON-TB Gold-Plus supernatants using a 17-plex Luminex assay to quantify Th1, Th2, Th17, inflammatory and regulatory responses. DM was associated with decreased Mtb-specific IFN-γ, TNF, IL-12, IP-10 and MIP-1α, with increased IFN-α compared to non-DM, suggesting impairment of Th1 and inflammatory pathways. Additionally, pre-DM was not associated with altered cytokine/chemokine responses, but subtle changes in IL-10, GM-CSF, MIP-1β, and IL-1β may suggest early indicators of immune dysregulation. Poorly controlled DM was associated with increased IL-4 and IFN-α, indicating a shift toward Th2 and inflammatory responses. Compared to borderline high, high total cholesterol levels, indicating dyslipidaemia, were associated with decreased IFN-γ, TNF and IL-2, suggesting impaired Th1 immunity. These findings imply that metabolic disturbances may compromise Mtb-specific immune responses, potentially increasing TB infection susceptibility. Optimising glycaemic and lipid control may be crucial for restoring immune balance and improving TB outcomes in patients with DM-associated metabolic dysfunction.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (DM) is a significant risk factor for the acquisition of latent tuberculosis (TB) infection (LTBI)1 and triples the risk for the development of active pulmonary TB2. Clinically, DM exacerbates TB severity and leads to TB treatment failure and mortality3. Conversely, TB impacts glucose control and may exacerbate DM4,5. Despite the significance of clinical implications for the increased susceptibility to TB infection and progression towards active disease1,2,6, the immunological processes involved remain limited7. Type 2 DM is primarily characterised by insulin resistance and hyperglycaemia, which impact innate and adaptive immune-cell function. This is associated with enhanced susceptibility to TB, leading to the TB-DM dual burden6. Few studies have examined the impact of DM on LTBI. Moreover, existing studies that have assessed the effect of DM on systemic cytokine responses in LTBI participants reported diminished T-helper (Th)-1 and Th17 cytokine responses8,9, with little to no studies on Mycobacterium tuberculosis (Mtb)-specific cytokine and chemokine responses. This hampers our understanding of DM’s increased risk of LTBI in healthy participants. Inflammatory cytokines and chemokines from the innate and adaptive immune system play crucial roles in TB defence10. Systemic Th1, Th17, and interleukin (IL)-1 family of cytokines have been shown to play essential roles in protecting against TB disease8,9, whereas Th2 and anti-inflammatory cytokines have been associated with increased TB risk or enhanced pathology11. Interestingly, participants with both LTBI and DM have been reported to exhibit diminished Mtb-specific Th1 and Th17 cytokine responses9. Because these cytokines and chemokines influence TB susceptibility, we hypothesised that DM could alter the Mtb-specific cytokine and chemokine responses. Using a bead-based multiplex immunoassay (Luminex® 200™), we assessed associations between DM, glycaemic status, DM control, dyslipidaemia, and Mtb-specific cytokine and chemokine concentrations.
Methods
Ethics statement
The TAD study was approved by the School of Biomedical Sciences Research and Ethics Committee (SBS-REC), Makerere University (reference number: SBS-398) and the Uganda National Council for Science and Technology (UNCST) (reference number: HS66ES). Thereafter, Informed consent was obtained from all subjects and/or their legal guardian(s) before enrolling in the Tuberculosis and Diabetes (TAD) study12. Ethical approval for this nested study was obtained from SBS-REC, reference number SBS-2021–41 and UNCST, reference number HS1696ES. The methods that support these results were performed in accordance with relevant guidelines and regulations, and all data were anonymised.
Participants
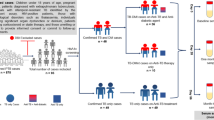
Participants with and without DM and/or LTBI were enrolled in an exploratory nested cohort between October 2017 and March 2018 from Kiruddu National Referral Hospital in Kampala, Uganda. This cohort was part of the TAD study that assessed the frequency of innate lymphoid cells (ILCs) and T and B cells in LTBI and DM participants, as previously described12. Type 2 DM participants were newly diagnosed, and, therefore, were not on DM treatment or TB preventive therapy that may affect immune function. Participants underwent a TB symptom screen to rule out active TB. LTBI was diagnosed using a positive QuantiFERON-TB Gold (QFT)-Plus ELISA assay, following the manufacturer’s recommendations. Type 2 DM was diagnosed based on glycated haemoglobin (HbA1c) levels ≥ 6.5%. All participants were HIV-seronegative. A total of 164 participants were recruited; 64 with DM and 100 without DM. A proportion of participants in each group had LTBI. All samples from these participants were considered for analysis.
Interferon-gamma release assay (IGRA; cell stimulation and culture)
The IGRA assay, QFT-plus (Qiagen, Hilden, Germany), was performed following the manufacturer’s recommendations. Briefly, about 1 ml of blood collected by venepuncture was directly drawn into each of the four QFT-plus tubes: Nil; containing only heparin (negative control): TB1; containing Mtb-specific early secreted antigenic target 6 kDa (ESAT-6) and culture filtrate protein 10 kDa (CFP-10) peptides optimised to stimulate CD4+ T cells: TB2; containing Mtb-specific ESAT-6 and CFP-10 peptides optimised to stimulate both CD4+ T and CD8+ T cells: and Mitogen; containing phytohemagglutinin (positive control). The QFT-plus tubes were inverted 10 times after blood collection and were transported within 3-h of blood collection to the Immunology Laboratory, Makerere University, for processing. The samples were again inverted before performing a 24-h incubation at 37°C. The samples were then centrifuged at 2000xg, and the cell culture supernatants were harvested and stored at -80°C until further analysis, including LTBI testing and Luminex assay.
Luminex
Cell culture supernatants from the TB2 stimulations were assayed for the Luminex experiments because the TB2 tube contains Mtb-specific ESAT-6 and CFP-10 peptides optimised to stimulate both CD4+ and CD8+ T-cell responses. Nil supernatants were assayed to provide background-corrected Mtb-specific responses from the TB2 stimulations. The samples were thawed, randomised, and assayed using a 17-plex Luminex kit (R&D Systems, Inc, Minneapolis, MN, USA) to detect the following analytes: tumour necrosis factor (TNF), IL-6, IL-8, IL-10, IL-4, IL-2, IL-12, IL-17A, IL-1β, interferon (IFN)-γ, IFN-α, IFN-γ-inducible protein (IP)-10, vascular endothelial growth factor (VEGF), macrophage inflammatory protein (MIP)-1α, MIP-1β, granulocyte–macrophage colony-stimulating factor (GM-CSF) and c-reactive protein (CRP). The analysis was performed using the Luminex® 200™ system with xPONENT 3.1 software (Merck, Millipore). The concentration values and detection limits were determined and extrapolated from standard curves generated from the Luminex kit’s specific standards using the xPONENT weighted 5PL curve fitting procedure.
Data analysis and visualisation
All data from this study were quality-controlled to remove rows of cytokine concentrations with “NA” values. All raw cytokine concentrations showed skewed distributions; therefore, log2-transformed data were used for analyses. To assess the association between DM and Mtb-specific cytokine and chemokine responses, the HbA1c levels were categorised as no DM (NDM ≤ 6.4%) and DM (≥ 6.5%). To determine the impact of pre-DM, the NDM group was re-categorised as NDM (≤ 5.6%) and pre-DM (≥ 5.7% and ≤ 6.4%). To assess the association between DM control and Mtb-specific cytokine and chemokine responses, the DM group was re-categorised as well-controlled DM (WC-DM; ≥ 6.5% and ≤ 7.9%) and poorly-controlled DM (PC-DM; ≥ 8.0%) based on HbA1c. To assess the association between dyslipidaemia and Mtb-specific cytokine and chemokine responses, total cholesterol levels were categorised as desirable (< 5.2 mmol/L), borderline high (≥ 5.2 and < 6.2 mmol/L) and high (≥ 6.2 mmol/L). Principal component analysis (PCA) was used to visualise cytokine and chemokine expression differences between the categories of comparisons. To identify significant differences in cytokine and chemokine expression levels between individual groups (DM versus NDM, Pre-DM versus NDM, DM versus Pre-DM, Borderline high versus Desirable, High versus borderline high, High versus Desirable), separate linear regression models were fitted for each pairwise combination of groups while adjusting for age and LTBI status. The resulting p-values were corrected for False Discovery Rate (FDR) using the Benjamini–Hochberg procedure. These were log10-transformed and plotted versus the Log2FC values to generate volcano plots. Cytokine and chemokine concentrations among the two-group comparisons with FDR < 0.050 were considered statistically significant. We adjusted for LTBI status in order to isolate DM-modulated Mtb-specific cytokine and chemokine responses. Statistical analysis of participant characteristics was reported after performing the Mann–Whitney U-test, Fisher’s exact test or Chi-squared with Monte Carlo simulation. All data and statistical analyses were performed using R (version 4.4.3; R Foundation for Statistical Computing, Vienna, Austria). The R package, ‘Limma’, was employed for linear regression analysis, utilising an empirical Bayes approach to adjust the standard errors of the estimated log-fold changes13. The package, ‘ggplot2’, was used for visualising the results for all plots14. For completeness, we report p-values (after adjusting for covariates) and adjusted p-values (after adjusting for FDR).
Results
Description of participant characteristics
The study included 164 participants, of whom 64 (39.0%) had DM, and 100 (61.0%) were non-diabetic. The median age was 40 years (IQR: 32–48), with DM participants being significantly older than non-DM participants (46 versus 38 years, p < 0.0001). The majority were female (72.6%), with no significant difference in sex distribution between groups (p = 1.000). A history of TB exposure differed significantly between groups (p < 0.001), with one DM participant reporting TB exposure. The LTBI prevalence was high (69.5%) and markedly greater among DM compared to non-DM participants (79.7% versus 63.0%, p = 0.025). DM participants had significantly higher waist-to-hip ratios (p = 0.001), blood pressure (p < 0.001), fasting blood glucose (13.4 versus 5.0 mmol/L, p < 0.0001) and HbA1c (9.6% versus 4.0%, p < 0.0001). Lipid profiles, including total cholesterol, LDL and triglycerides, were significantly elevated in the DM group (p < 0.001) compared to the no-DM group. Haematological parameters were largely non-significant, except for a higher neutrophil count in DM participants (p = 0.033), as shown in Table 1. These findings suggest that DM participants exhibit a greater burden of metabolic risk factors and LTBI, highlighting potential interactions between metabolic dysfunction and TB infection susceptibility.
DM is associated with altered Mtb-specific Th1 and inflammatory cytokine and chemokine responses
We determined the influence of DM on Mtb-specific cytokine and chemokine responses by measuring the panel of 17 cytokines. All data were grouped into two categories based on the HbA1c levels regardless of LTBI status: (i) no DM (NDM ≤ 6.4%) and (ii) DM (≥ 6.5%). First, the PCA of cytokine and chemokine concentrations showed extreme overlap between DM and NDM (Fig. 1A), indicating similar overall marker expression between the two groups. However, the volcano plot derived from the linear regression model indicated that, compared to NDM, DM was associated with decreased Mtb-specific IFN-γ (adjusted regression coefficient estimate β: -0.64 [95% CI: 0.36–0.78], p = 0.002, padjusted = 0.013), TNF (-0.86 [0.26–0.70], p = 0.001, padjusted = 0.013), IL-12 (-0.26 [0.63–0.94], p = 0.012, padjusted = 0.035), IP-10 (-0.42 [0.49–0.89], p = 0.008, padjusted = 0.032) and MIP-1α (-0.58 [0.36–0.88], p = 0.012, padjusted = 0.035) responses (Fig. 1B, Supplementary Fig. S1). Conversely, DM was associated with increased Mtb-specific IFN-α (0.43 [1.17–2.02], p = 0.002, padjusted = 0.013) responses (Fig. 1B, Supplementary Fig. S1). All other cytokines and chemokines were not significantly associated with alteration in DM after adjusting for FDR, as shown in Supplementary Table S1.
Association between DM and Mtb-specific Th1 and inflammatory cytokine and chemokine responses. (A) PCA of supernatant cytokines and chemokines using the first two principal components. The ellipses were centred based on the median of PC1 and PC2 for NDM (blue, n = 100) and DM (red, n = 64). (B) Volcano plot showing differentially expressed cytokines and chemokines for DM versus NDM. The analysis was performed using a linear regression model with age and LTBI status as covariates. Each dot represents a cytokine or chemokine, with the x-axis showing the Log2FC and the y-axis showing the -Log10[adj.P.Val]. The upregulated cytokines or chemokines are shown in red, downregulated in blue, and the non-significant in grey. The dashed horizontal line represents the threshold for statistical significance (FDR-adjusted [adj.P.Val] < 0.050). Abbreviations: DM–Diabetes mellitus; NDM–No diabetes mellitus.
Pre-DM is not associated with altered Mtb-specific Th1, Th2, Th17, inflammatory and regulatory cytokine and chemokine responses
Next, we assessed changes in Mtb-specific cytokine and chemokine responses from NDM through pre-DM and DM to determine immune impairment before participants developed DM. We regrouped all the data into three categories based on the HbA1c levels regardless of LTBI status: (i) NDM (≤ 5.6%), (ii) pre-DM (≥ 5.7% and ≤ 6.4%) and (iii) DM (≥ 6.5%). The PCA of cytokine and chemokine concentrations showed extreme overlap between DM, pre-DM and NDM (Fig. 2A), indicating similar marker expression between the three groups. Upon linear regression analysis, no differences were observed for pre-DM versus NDM [IL-10 (-0.69 [0.28–0.88], p = 0.017, padjusted = 0.181), GM-CSF (-0.68 [0.27–0.93], p = 0.028, padjusted = 0.181), MIP-1β (-0.99 [0.14–0.97], p = 0.044, padjusted = 0.181), and IL-1β (-0.88 [0.17–0.99], p = 0.048, padjusted = 0.181)] (Fig. 2B, Supplementary Fig. S2), and DM versus pre-DM (Fig. 2C, Supplementary Fig. S2) for all cytokines and chemokines after adjusting for FDR. However, within this comparison, DM remained associated with decreased Mtb-specific TNF (-0.80 [95% CI: 0.27–0.74], p = 0.002, padjusted = 0.017), IL-12 (-0.26 [0.63–0.94], p = 0.011, padjusted = 0.047) and CRP (-0.44 [0.47–0.88], p = 0.006, padjusted = 0.036) responses compared to NDM (Fig. 2D, Supplementary Fig. S2). In addition, DM was associated with increased Mtb-specific IFN-α (0.50 [1.26–2.17], p < 0.001, padjusted = 0.006) responses compared to NDM (Fig. 2D, Supplementary Fig. S2). The results of these comparisons are reported in Supplementary Table S2.
Association between pre-DM and Mtb-specific cytokine and chemokine responses. (A) PCA of supernatant cytokines and chemokines using the first two principal components. The ellipses were centred based on the median of PC1 and PC2 for NDM (green, n = 92), pre-DM (blue, n = 8) and DM (red, n = 64). (B-D) Volcano plots showing differentially expressed cytokines and chemokines for NDM versus pre-DM, DM versus pre-DM and DM versus NDM. The analysis was performed using a linear regression model with age and LTBI status as covariates. Each dot represents a cytokine or chemokine, with the x-axis showing the Log2FC and the y-axis showing the -Log10[adj.P.Val]. The upregulated cytokines or chemokines are shown in red, downregulated in blue, and the non-significant in grey. The dashed horizontal line represents the threshold for statistical significance (FDR-adjusted [adj.P.Val] < 0.050). Abbreviations: DM–Diabetes mellitus; PreDM–Prediabetes mellitus; NDM–No diabetes mellitus.
Poorly controlled DM is associated with upregulated Mtb-specific Th2 and inflammatory cytokine responses
Next, we assessed the association between DM control and Mtb-specific cytokine and chemokine responses. We grouped all the DM participant data into two categories based on the HbA1c levels regardless of LTBI status: (i) WC-DM (≥ 6.5% and ≤ 7.9%) and (ii) PC-DM (≥ 8.0%). The PCA of the cytokine and chemokine concentrations showed extreme overlap between PC-DM and WC-DM (Fig. 3A), indicating similar marker expression between the two groups. However, the volcano plot from the linear regression model showed that compared to WC-DM, PC-DM was associated with increased Mtb-specific IL-4 (0.51 [1.30–2.14], p < 0.001, padjusted = 0.002) and IFN-α (0.74 [1.26–2.17], p < 0.002, padjusted = 0.018) responses (Fig. 3B, Supplementary Fig. S3). All other cytokines and chemokines were not significantly associated with alteration in PC-DM after adjusting for FDR, as shown in Supplementary Table S3.
Association between DM control and Mtb-specific Th2 and inflammatory cytokine responses. (A) PCA of supernatant cytokines and chemokines using the first two principal components. The ellipses were centred based on the median of PC1 and PC2 for WC-DM (blue, n = 15) and PC-DM (red, n = 49). (B) Volcano plots showing differentially expressed cytokines and chemokines for PC-DM versus WC-DM. The analysis was performed using a linear regression model with age and LTBI status as covariates. Each dot represents a cytokine or chemokine, with the x-axis showing the Log2FC and the y-axis showing the -Log10[adj.P.Val]. The upregulated cytokines or chemokines are shown in red, and the non-significant in grey. The dashed horizontal line represents the threshold for statistical significance (FDR-adjusted [adj.P.Val] < 0.050). Abbreviations: WC-DM–Well-controlled diabetes mellitus; PC-DM–Poorly-controlled diabetes mellitus.
Dyslipidaemia is associated with decreased Mtb-specific Th1 cytokine responses
Finally, we assessed changes in Mtb-specific cytokine and chemokine responses from normal through borderline high and high total cholesterol levels to determine their association with dyslipidaemia. All the data were regrouped into three categories based on total cholesterol levels regardless of LTBI status: (i) desirable (< 5.2 mmol/L), (ii) borderline high (≥ 5.2 and < 6.2 mmol/L) and (iii) high (≥ 6.2 mmol/L). The PCA of the cytokine and chemokine concentrations showed extreme overlap between desirable, borderline high and high total cholesterol (Fig. 4A), indicating similar marker expression between the three groups. Following linear regression analysis, no differences were observed for borderline high versus desirable (Fig. 4B, Supplementary Fig. S4) and high versus desirable (Fig. 4D, Supplementary Fig. S4) for all cytokines and chemokines after adjusting for FDR. Interestingly, compared to borderline high, high total cholesterol levels were associated with decreased Mtb-specific IL-2 (-0.82 [0.27–0.73], p = 0.002, padjusted = 0.020), TNF (-1.16 [0.14–0.68], p = 0.003, padjusted = 0.020) and IFN-γ (-0.91 [0.22–0.74], p = 0.003, padjusted = 0.020) responses (Fig. 4C, Supplementary Fig. S4). The results of these comparisons are reported in Supplementary Table S4.
Association between dyslipidaemia and Mtb-specific Th1 cytokine responses. (A) PCA of supernatant cytokines and chemokines using the first two principal components. The ellipses were centred based on the median of PC1 and PC2 for desirable (green, n = 103), borderline high (blue, n = 34) and high (red, n = 27) total cholesterol levels. (B-D) Volcano plots show differentially expressed cytokines and chemokines for borderline high versus desirable, high versus borderline high, and high versus desirable. The analysis was performed using a linear regression model with age and LTBI status as covariates. Each dot represents a cytokine or chemokine, with the x-axis showing the Log2FC and the y-axis showing the -Log10[adj.P.Val]. The downregulated cytokines or chemokines are shown in blue, and the non-significant in grey. The dashed horizontal line represents the threshold for statistical significance (FDR-adjusted [adj.P.Val] < 0.050).
Discussion
Diabetes mellitus can impair the immune system, causing a shift in immune cell phenotypes and cytokine and chemokine expression differences8,9,15. Pre-diabetes is indicated by elevated blood glucose and glycated haemoglobin levels and has similar impacts on the immune system16,17. Though the mechanisms are unclear, immune response impairment, including cytokines and chemokines in diabetic or pre-diabetic participants, may increase the risk of TB infection acquisition or progression to active TB7. We analysed several cytokines (proinflammatory, Th1, Th2, Th17 and regulatory) involved in the immune responses to LTBI and DM. Our data showed that (i) DM is associated with altered Mtb-specific Th1 and inflammatory responses, (ii) Pre-DM is not associated with alteration of Mtb-specific responses, (iii) PC-DM is associated with upregulated Mtb-specific Th2 and inflammatory responses, and (iv) Dyslipidaemia is associated with decreased Mtb-specific Th1 responses. These data supplement our current understanding of Mtb-specific cytokine and chemokine immune responses in DM, glycaemic control, DM control and dyslipidaemia during LTBI.
Cytokines and chemokines are pivotal in the host’s immune defence against TB infection in several mouse models and human infection and disease10. First, when we assessed the impact of DM on Mtb-specific cytokine responses based on whether participants had DM or not, we reported that DM is associated with decreased Mtb-specific production for IFN-γ, TNF, IL-12, IP-10 and MIP-1α, as well as increased Mtb-specific production for IFN-α, highlighting the immunomodulatory effects of DM on these responses. Although we adjusted for the effect of LTBI status in our analysis, these results agree with another study that reported decreased antigen-specific Th1 responses during DM-LTBI9. T-helper cytokines, including IFN-γ, TNF, IL-2 and IL-12, are essential for controlling Mtb infection18,19,20,21,22,23,24. Specifically, TNF maintains the integrity of granulomatous structures, coordinates the chemokine response within the lung20,21 and induces the apoptosis of Mtb-infected macrophages25. IFN-γ gene knock-out mice exhibit poor macrophage activation and exacerbated granulocytic inflammation18. IL-12 is essential for the induction of IFN-γ and protective granulomas through differentiation and activation of antigen-specific lymphocytes23. Additionally, chemokines, including CXCL10/IP-10, function in the recruitment of lymphocytes to areas of Mtb infection26, whereas MIP-1α and MIP-1β induce the proliferation and activation of T cells27. MIP-1α promotes Th1 differentiation during Mtb infection28. Interestingly, MIP-1α and MIP-1β have been reported to restrict intracellular Mtb growth within alveolar macrophages29. Conversely, IFN-α heightened levels are associated with increased Mtb burden, with the cytokine reported to perturb CD4+ Th1 IFN-γ cytokine production30 and impair bacterial control of Mtb-infected macrophages31. It is probable that upregulated IFN-α levels impact Th1 and inflammatory responses in DM. The non-significant levels of IL-2 could indicate that while certain aspects of the immune response are impaired in DM, a level of Th1 functionality is retained. However, this paradox warrants careful interpretation. It is plausible that while the overall production of IL-2 is maintained, the cellular state or the signalling cascade downstream of its receptor might be altered, leading to a functionally compromised response32. Alternatively, the decrease in IFN-γ, TNF, IL-12, MIP-1α and IP-10 could impair the overall immune response, even in the presence of robust IL-2 levels. This dichotomy emphasises the need for further studies that examine the functional capacity of these cytokines in the context of DM rather than merely their concentrations.
When we evaluated cytokine and chemokine responses across the spectrum of glycaemic control, from no DM to pre-DM and DM, we did not observe differences between these states, especially for pre-DM versus NDM and DM versus pre-DM after FDR correction. This could be attributable to the very small pre-DM sample size (n = 8), which may have limited the power of our study to detect a significant difference33. However, it still revealed that pre-DM could be associated with a subtle decrease in IL-10, GM-CSF, MIP-1β and IL-1β compared to NDM. These cytokines, particularly IL-10, prevents immunopathology through immune regulation34, GM-CSF maintains macrophage protective differentiation and metabolic state against Mtb35, MIP-1β suppresses macrophage intracellular Mtb growth29, and IL-1β maintains macrophage anti-mycobacterial immunity during early TB infection36,37. The role of IL-10 is rather paradoxical, as studies have reported that IL-10 induces Mtb susceptibility38,39. Nevertheless, our data suggests that although early metabolic disturbances might not be sufficient to induce marked immune dysfunction (requiring sustained hyperglycaemia), they could represent early indicators of immune dysregulation17,40. Such findings reinforce the importance of early intervention and glycaemic control as strategies to mitigate the risk of TB reactivation.
Next, we determined whether glycaemic control may modulate the immune responses during DM, further adding to the complex interplay of metabolic dysfunction and immune responses. Poorly controlled DM is associated with defects in IL-12 production by antigen-presenting cells during bacterial infections, resulting in lower IFN-γ production by CD4+ T cells41. Our study reports that PC-DM is associated with upregulated IL-4 and IFN-α responses compared to the WC-DM participants. We did not observe differences in the Th1 responses for PC-DM versus WC-DM. This contrasts with another study that reported increased purified protein derivative (PPD)-specific IFN-γ, IL-2, TNF and IL-1β cytokine responses in the PC-DM participants42. The contrasting responses could be attributable to differences in study design, including participants (Hispanics versus Africans; TB versus LTBI; long-term versus newly diagnosed DM) and stimulants used (PPD versus ESAT-6 and CFP-10). IL-4 subverts Mtb containment in Mtb-infected macrophages43, and together with IFN-α, they perturb CD4+ Th1 IFN-γ30,43 and TNF cytokine production43. Whereas IL-8 was non-significant after FDR adjustment, its upregulation might suggest an attempt by the host to compensate for impaired macrophage function by recruiting additional neutrophils44. However, although neutrophils play an important role in early pathogen defence, their excessive recruitment can contribute to tissue damage and may interfere with the effective clearance of Mtb7. Taken together, the data indicate that not only does DM affect the extent of Mtb-specific cytokine responses, but the extent of glycaemic control creates an immune milieu characterised by an inflammatory response that may not be sufficiently regulated, potentially compromising the host’s ability to effectively control Mtb, consequently increasing the risk for the acquisition of TB infection.
Lastly, our study highlights the role of dyslipidaemia as a modulator of immune responses to Mtb. In particular, when compared to borderline high, high total cholesterol levels were associated with decreased expression of key Th1 cytokines: IFN-γ, TNF and IL-2. These observations imply that dyslipidaemia may adversely affect the Mtb-specific Th1 response. It is well established that dyslipidaemia can alter the structure and function of lipid rafts, which are microdomains critical for T-cell receptor signalling and subsequent cytokine production45,46. Cytokine signalling specificity depends on lipid raft compartmentalisation of receptor subunits, which concentrate kinases and adaptors to enhance signalling efficiency. Disrupting lipid rafts impairs this process, impairing cytokine responses47,48. Given the crucial role of Th1 cytokines in orchestrating protective immunity against Mtb18,19,20,21,22,23,24, their downregulation by dyslipidaemia could partially explain the enhanced susceptibility to TB infection observed in dyslipidaemic patients. Moreover, IL-2 drives the expansion of immune-activated effector and memory T-cell responses49 and restores Mtb-induced T-cell dysfunction22. It could imply that there is impairment of T-cell differentiation and proliferation, as dyslipidaemia has been associated with impaired CD8+ T-cell differentiation and effector function50. Interestingly, diet-induced dyslipidaemia induces T-cell apoptosis, impairing their ability to control Mtb infection in vivo. These patients might experience additional immune dysfunction on top of that conferred by hyperglycaemia, rendering them even more susceptible to TB infection. It is worth noting that the positive correlation between lower cholesterol levels and cytokine secretion supports the notion that a healthy lipid profile may induce optimal Th1 cytokine function51, with extremely low levels attenuating cytokine signalling and production52. Given that DM is usually accompanied by dyslipidaemia53,54,55, the combined effect of these metabolic disturbances (including poor glycaemic control) could synergistically exacerbate the risk for the acquisition of TB infection.
However, it is important to interpret these findings within the context of our study’s limitations. Firstly, the relatively limited number of supernatant samples (especially in DM, its control and dyslipidaemia) and the study’s observational nature warrant a cautious interpretation of the results. In addition, our Luminex panel did not include all inflammatory markers, and we could have missed cytokines and chemokines that may have implications for the Mtb-related DM pathology.
Overall, the findings presented in this study contribute to our understanding of Mtb-specific immune dysregulation associated with DM and highlight the importance of metabolic health in the management of TB. While our findings support the hypothesis that DM impairs the immune response against Mtb, they also raise important questions regarding the underlying mechanisms and the potential for therapeutic interventions aimed at restoring immune balance.
Data availability
Data from this article is available from the corresponding author upon reasonable request.
References
Liu, Q. et al. The association between diabetes mellitus and the risk of latent tuberculosis infection: a systematic review and meta-analysis. Front. Med. 9, 899821 (2022).
Al-Rifai, R. H., Pearson, F., Critchley, J. A. & Abu-Raddad, L. J. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS ONE 12(11), e0187967 (2017).
Huangfu, P., Ugarte-Gil, C., Golub, J., Pearson, F. & Critchley, J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int. J. Tuberculosis Lung Dis. 23(7), 783–796 (2019).
Chen, H. et al. The impact of pulmonary tuberculosis on immunological and metabolic features of diabetic patients. Front. Immunol. 13, 973991 (2022).
Rissaadah, S., Nursiswati, N. & Pahria, T. Glycemic profile and clinical treatment in patients with diabetes mellitus-tuberculosis: an update scoping review. J. Multidiscip. Healthc. 18, 747–758 (2025).
Hensel, R. L. et al. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int. J. Tuberc. Lung Dis. 20(1), 71–78 (2016).
Ssekamatte, P., Sande, O. J., van Crevel, R. & Biraro, I. A. Immunologic, metabolic and genetic impact of diabetes on tuberculosis susceptibility. Front. Immunol. 14, 1122255 (2023).
Kumar, N. P. et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann. Am. Thorac. Soc. 10(5), 441–449 (2013).
Kumar, N. P. et al. Diminished systemic and antigen-specific type 1, type 17, and other proinflammatory cytokines in diabetic and prediabetic individuals with latent Mycobacterium tuberculosis infection. J. Infect. Dis. 210(10), 1670–1678 (2014).
Domingo-Gonzalez, R., Prince, O., Cooper, A. & Khader, S. A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectrum. 4(5), 23 (2016).
Ashenafi, S. et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin. Immunol. 151(2), 84–99 (2014).
Ssekamatte, P. et al. Type 2 diabetes mellitus and latent tuberculosis infection moderately influence innate lymphoid cell immune responses in Uganda. Front. Immunol. 12, 3551 (2021).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43(7), e7-e (2015).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis 2nd edn. (Springer, 2016).
Hu, R., Xia, C.-Q., Butfiloski, E. & Clare-Salzler, M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: Implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clin. Immunol. 195, 139–148 (2018).
Grossmann, V. et al. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care 38(7), 1356–1364 (2015).
Eckold, C. et al. Impact of intermediate hyperglycemia and diabetes on immune dysfunction in tuberculosis. Clin. Infect. Dis. 72(1), 69–78 (2020).
Maciag, K., Plumlee, C. R., Cohen, S. B., Gern, B. H. & Urdahl, K. B. Reappraising the role of T cell-derived IFN-γ in restriction of mycobacterium tuberculosis in the murine lung. J. Immunol. 213(3), 339–346 (2024).
Green, A. M., DiFazio, R. & Flynn, J. L. IFN-γ from CD4 T Cells Is essential for host survival and enhances CD8 T cell function during mycobacterium tuberculosis infection. J. Immunol. 190(1), 270–277 (2013).
Allie, N. et al. Prominent role for T cell-derived tumour necrosis factor for sustained control of mycobacterium tuberculosis infection. Sci. Rep. 3(1), 1809 (2013).
Clay, H., Volkman, H. E. & Ramakrishnan, L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29(2), 283–294 (2008).
Liu, X. et al. IL-2 restores T-cell dysfunction induced by persistent mycobacterium tuberculosis antigen stimulation. Front. Immunol. 10, 2350 (2019).
Cooper, A. M., Magram, J., Ferrante, J. & Orme, I. M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 186(1), 39–45 (1997).
Chen, Y.-Y. et al. Recombinant bacille Calmette-Guerin coexpressing Ag85b, CFP10, and interleukin-12 elicits effective protection against Mycobacterium tuberculosis. J. Microbiol. Immunol. Infect. 50(1), 90–96 (2017).
Roca, F. J., Whitworth, L. J., Redmond, S., Jones, A. A. & Ramakrishnan, L. TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell 178(6), 1344–61.e11 (2019).
Blauenfeldt, T. et al. Interplay of DDP4 and IP-10 as a potential mechanism for cell recruitment to tuberculosis lesions. Front. Immunol. 9, 1456 (2018).
Taub, D. D., Turcovski-Corrales, S. M., Key, M. L., Longo, D. L. & Murphy, W. J. Chemokines and T lymphocyte activation: Beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 156(6), 2095–2103 (1996).
Karpus, W. J. et al. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 158(9), 4129–4136 (1997).
Saukkonen, J. J. et al. β-chemokines are induced by mycobacterium tuberculosis and inhibit its growth. Infect. Immun. 70(4), 1684–1693 (2002).
McNab, F. W. et al. Type I IFN Induces IL-10 production in an IL-27–independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in mycobacterium tuberculosis–infected macrophages. J. Immunol. 193(7), 3600–3612 (2014).
Kotov, D. I. et al. Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis. Cell 186(25), 5536–53.e22 (2023).
Permanyer, M. et al. Efficient IL-2R signaling differentially affects the stability, function, and composition of the regulatory T-cell pool. Cell. Mol. Immunol. 18(2), 398–414 (2021).
Ristić-Djurović, J. L., Ćirković, S., Mladenović, P., Romčević, N. & Trbovich, A. M. Analysis of methods commonly used in biomedicine for treatment versus control comparison of very small samples. Comput. Methods Programs Biomed. 157, 153–162 (2018).
Redford, P. S., Murray, P. J. & O’Garra, A. The role of IL-10 in immune regulation during M tuberculosis infection. Mucosal Immunol. 4(3), 261–270 (2011).
Mishra, A. et al. Human macrophages exhibit GM-CSF dependent restriction of mycobacterium tuberculosis infection via regulating their self-survival. Diff. Metab. Front. Immunol. 13, 859116 (2022).
Fremond, C. M. et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to mycobacterium tuberculosis infection. J. Immunol. 179(2), 1178–1189 (2007).
Mayer-Barber, K. D. et al. Cutting edge: Caspase-1 Independent IL-1β Production Is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184(7), 3326–3330 (2010).
Moreira-Teixeira, L. et al. T cell-derived IL-10 impairs host resistance to mycobacterium tuberculosis infection. J. Immunol. 199(2), 613–623 (2017).
Park, H.-S. et al. Mycobacterium tuberculosis Rv2145c promotes intracellular survival by STAT3 and IL-10 receptor signaling. Front. Immunol. 12, 666293 (2021).
Sinha, R. et al. Pre-diabetes increases tuberculosis disease severity, while high body fat without impaired glucose tolerance is protective. Front. Cell. Infect. Microbiol. 11, 691823 (2021).
Tan, K. S. et al. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J. Clin. Invest. 122(6), 2289–2300 (2012).
Restrepo, B. I. et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin. Infect. Dis. 47(5), 634–641 (2008).
Pooran, A. et al. IL-4 subverts mycobacterial containment in Mycobacterium tuberculosis-infected human macrophages. Eur. Respir. J. 54(2), 1802242 (2019).
Krupa, A. et al. Binding of CXCL8/IL-8 to Mycobacterium tuberculosis modulates the innate immune response. Mediators inflamm. 1, 124762 (2015).
Amersfoort, J. et al. Diet-induced dyslipidemia induces metabolic and migratory adaptations in regulatory T cells. Cardiovasc. Res. 117(5), 1309–1324 (2020).
Varshney, P., Yadav, V. & Saini, N. Lipid rafts in immune signalling: current progress and future perspective. Immunology 149(1), 13–24 (2016).
Legler, D. F., Micheau, O., Doucey, M.-A., Tschopp, J. & Bron, C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα-Mediated NF-κB activation. Immunity 18(5), 655–664 (2003).
Sen, S., Roy, K., Mukherjee, S., Mukhopadhyay, R. & Roy, S. Restoration of IFNγR subunit assembly, IFNγ signaling and parasite clearance in Leishmania donovani Infected Macrophages: role of membrane cholesterol. PLoS Pathog. 7(9), e1002229 (2011).
Ross, S. H. & Cantrell, D. A. Signaling and function of Interleukin-2 in T lymphocytes. Ann. Rev. Immunol. 36, 411–433 (2018).
Bensussen, A., Santana, M. A. & Rodríguez-Jorge, O. Metabolic alterations impair differentiation and effector functions of CD8+ T cells. Front Immunol. 13, 945980 (2022).
Mahammad, S., Dinic, J., Adler, J. & Parmryd, I. 2010 Limited cholesterol depletion causes aggregation of plasma membrane lipid rafts inducing T cell activation Biochimica et Biophysica Acta (BBA). Mol. Cell Biol. Lipids. 6, 625–634 (1801).
Zhu, Y. et al. lowering Glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J. Biol. Chem. 286(17), 14787–14794 (2011).
Lokpo, S. Y. et al. The pattern of dyslipidaemia and factors associated with elevated levels of non-HDL-cholesterol among patients with type 2 diabetes mellitus in the Ho municipality: A cross sectional study. Heliyon. 8, 8 (2022).
Abdissa, D. & Hirpa, D. Dyslipidemia and its associated factors among adult diabetes outpatients in West Shewa zone public hospitals, Ethiopia. BMC Cardiovasc. Disord. 22(1), 39 (2022).
Brake, J. et al. Inflammation and dyslipidaemia in combined diabetes and tuberculosis; a cohort study. iScience. 28, 112760 (2025).
Acknowledgements
We are grateful to the study participants under the Kiruddu National Referral Hospital, PROTID clinical trial (grant number: RIA2018CO-2514), an EDCTP2 programme supported by the European Union and the Government of Uganda through the Uganda Independence Scholarship Trust Fund (UISTF) for the support.
Funding
This project was mainly supported by postdoctoral fellowships from Makerere University/UVRI Centre of Excellence in Infection and Immunity Research and Training (MUII Plus; grant number 084344) and the MRC/UVRI and LSHTM Uganda Research Unit training grant, both held by Prof. Irene Andia Biraro. Further funding and support was through the Makerere University Research and Innovations Fund (MakRIF) and the MRC/UVRI and LSHTM Uganda Research Unit training fellowship, which is jointly funded by the UK Medical Research Council (MRC) part of UK Research and Innovation (UKRI) and the UK Foreign, Commonwealth and Development Office (FCDO) under the MRC/FCDO Concordat agreement and also part of the EDCTP2 programme supported by the European Union.
Author information
Authors and Affiliations
Contributions
PS, OJS, RvC, SC, and IAB conceptualised the study. PS, DS and RN performed the experiments. PS analysed the data and drafted the manuscript. All authors reviewed the manuscript for intellectual content input and approved its submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ssekamatte, P., Sitenda, D., Nabatanzi, R. et al. Metabolic dysfunction impairs Mycobacterium tuberculosis-specific cytokine and chemokine responses in latent tuberculosis and type 2 diabetes mellitus. Sci Rep 15, 30474 (2025). https://doi.org/10.1038/s41598-025-16385-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16385-8