Abstract
Herpes zoster (HZ) has emerged as a post-viral complication in COVID-19 survivors, but its long-term clinical impact remains uncertain. This retrospective cohort study utilized the TriNetX Global Collaborative Network to evaluate whether HZ reactivation after COVID-19 is associated with increased risks of mortality, cardiovascular, and renal outcomes. Adults diagnosed with HZ within one year of COVID-19 were propensity score–matched 1:1 with controls without HZ. A total of 48,442 matched patients were followed for three years. Compared with controls, HZ patients had significantly higher risks of major adverse cardiovascular events (HR: 1.38, 95% CI: 1.30–1.46), acute kidney injury (HR: 1.67, 95% CI: 1.55–1.80), and renal function decline defined by eGFR < 60 mL/min/1.73 m² (HR: 1.28, 95% CI: 1.20–1.37). Although no significant difference in overall all-cause mortality was observed across the full follow-up period, time-stratified analysis revealed a biphasic pattern: patients with HZ had lower mortality risk during the first 90 days but showed significantly higher mortality from day 91 to three years post-COVID-19 (HR: 1.33, 95% CI: 1.24–1.44). Subgroup analyses consistently identified older age, chronic comorbidities, impaired renal function, and systemic inflammation as risk enhancers across outcomes. These findings suggest that HZ reactivation after COVID-19 signals a vulnerable subgroup predisposed to cardiorenal complications. Targeted follow-up and risk-based interventions are warranted in this population.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been associated with a broad spectrum of long-term complications, collectively referred to as post-acute sequelae of SARS-CoV-2 infection (PASC)1. Beyond its acute respiratory manifestations, COVID-19 has been implicated in multi-organ dysfunction, including increased risks of cardiovascular disease, acute kidney injury (AKI), and chronic kidney disease (CKD)2,3. Early in the pandemic, AKI was reported in approximately 20–40% of hospitalized COVID-19 patients, with many cases progressing to long-term kidney dysfunction4. Recent large cohort studies have shown that even patients who recover from AKI experience accelerated renal function decline and higher risks of major adverse kidney events (MAKE)5.
Interestingly, emerging data suggest that survivors of COVID-19-associated AKI may have relatively lower long-term risks of kidney progression and mortality compared with patients who develop AKI from other illnesses3. Nevertheless, the risk of chronic kidney function decline among COVID-19 survivors who did not initially experience AKI remains a growing concern6. Population-based analyses have demonstrated that even in the absence of overt AKI during the acute phase, COVID-19 survivors are at increased risk of developing estimated glomerular filtration rate (eGFR) decline, advanced CKD, and cardiovascular complications within 6 to 12 months post-infection7,8.
A further complicating factor is the occurrence of secondary viral reactivations, particularly herpes zoster (HZ), after COVID-19 infection9,10. HZ reactivation, driven by immune dysregulation following viral infections, has been increasingly observed among COVID-19 survivors10,11. Previous studies have indicated that HZ is independently associated with heightened risks of cardiovascular events, stroke, and potentially renal dysfunction12,13,14. The underlying mechanisms are thought to involve endothelial damage, inflammatory responses, and thrombotic microangiopathy15,16, which may synergize with COVID-19-induced vascular and immune alterations. However, the impact of post-COVID-19 HZ reactivation on long-term clinical outcomes—including mortality, cardiovascular events, and kidney disease progression—remains poorly understood.
Given the growing global burden of COVID-19 survivors and the increasing recognition of HZ as both a potential post-viral complication and a clinical marker of immunologic vulnerability17,18, it is important to determine whether post-infection or reactivation of HZ further exacerbates adverse clinical outcomes. Clarifying this association has important implications for post-acute care, vaccination strategies, and patient risk stratification. This study aimed to assess whether COVID-19 survivors who develop HZ during this defined post-infection period represent a subgroup at elevated risk for mortality, cardiovascular events, and renal complications, using a large, real-world multicenter dataset and rigorous propensity score matching (PSM). The findings are expected to enhance post-COVID risk profiling and inform targeted prevention and management strategies.
Methods
Study design and data source
This retrospective cohort study utilized data from TriNetX, a federated global health research network providing access to deidentified electronic health records (EHRs) from a diverse group of healthcare organizations. The study specifically employed data from the Global Collaborative Network within TriNetX, comprising 144 healthcare organizations worldwide. TriNetX captures extensive structured patient data, including demographics, diagnoses, procedures, medications, and laboratory values.
The analysis was conducted on April 27, 2025, using the Compare Outcomes module within the TriNetX platform, which complies with the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR). The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was granted by the Institutional Review Board of Taipei Tzu Chi Hospital (Approval Number: 14-IRB043), which waived the requirement for informed consent due to the retrospective nature of the study. All methods were carried out in accordance with relevant guidelines and regulations.
Study population
This study included patients identified from the TriNetX Global Collaborative Network whom had a confirmed diagnosis of COVID-19 in adulthood (aged ≥ 18 years at diagnosis) between January 1, 2020, and January 1, 2022. COVID-19 was defined by either a positive SARS-CoV-2 RNA laboratory result (TNX:9088) or a clinical diagnosis coded as ICD-10-CM U07.1. Patients were subsequently categorized based on the occurrence of HZ within a defined post-COVID time window. Specifically, individuals with an HZ diagnosis recorded from 1 day to 1 year after the initial COVID-19 diagnosis were classified as having COVID-19 with HZ. Patients who had no documented HZ diagnosis during this interval were classified as having COVID-19 without HZ. Diagnoses of HZ were determined using ICD-10-CM codes B02, B02.1, B02.2, B02.3, B02.7, B02.8, and B02.9. To enhance cohort integrity, individuals with any HZ diagnosis prior to COVID-19 or outside the pre-specified window were excluded. After applying these inclusion and exclusion criteria, a total of 24,327 patients with post-COVID HZ and 9,185,214 patients without HZ were identified for initial analysis. The cohort selection process is illustrated in Fig. 1.
Index date and Follow-Up period
The index date was defined as the first occurrence of COVID-19 diagnosis or positive laboratory result. Each patient’s observation window began 1 day after the index date and continued for up to 3 years (1,095 days) or until death or loss to follow-up, whichever occurred first. Outcomes that occurred before the index date or within the first day were excluded from risk analysis.
Outcome measures
The primary outcomes assessed in this study were all-cause mortality, major adverse cardiovascular events (MACE), sepsis, AKI, and different stages of renal dysfunction defined by GFR thresholds. All-cause mortality was identified either through a recorded deceased status in the demographic records or by a diagnosis code for ill-defined and unknown cause of mortality (ICD-10-CM: R99). MACE was defined as a composite endpoint comprising acute myocardial infarction (I21), cardiac arrest (I46), other cardiac arrhythmias (I49), heart failure (I50), nontraumatic intracerebral hemorrhage (I61), cerebral infarction (I63), and ischemic heart diseases (I20–I25), or death (R99). AKI was identified using the diagnosis code for acute kidney failure (ICD-10-CM: N17). Renal function decline was defined as eGFR < 60 mL/min/1.73 m², based on the most recent value during the observation window, calculated using the CKD-EPI 2021 formula (UMLS: LNC:98979-8). For all outcome analyses, patients who experienced the specific outcome prior to the index date were excluded to ensure temporal clarity between exposure and outcome occurrence.
Propensity score matching
Due to platform constraints of the TriNetX database, full-scale PSM with extensive clinical or laboratory variables was not feasible in very large cohorts. In the main analysis of the entire eligible COVID-19 cohort (initial source population > 9 million), we conducted 1:1 PSM limited to seven demographic variables: age, female sex, male sex, White, Black or African American, and Asian race. These variables represent the maximum allowable number of covariates permitted by TriNetX for matching in large-scale cohorts exceeding one million individuals.
Because detailed covariate balance diagnostics and export functions are not available for these demographically matched cohorts, we extracted a representative age range (40–50 years) to illustrate the matching process. This illustrative sample allowed us to include a broader set of clinical and laboratory covariates and is presented in Table 1. PSM in this cohort was performed using a greedy nearest-neighbor algorithm. Covariate balance was assessed using standardized mean differences (SMDs), with an SMD < 0.1 considered acceptable. While most variables achieved adequate balance, residual differences remained in iron levels (SMD = 0.29) and CRP (SMD = 0.16), potentially reflecting persistent inflammation and metabolic burden in the HZ group.
To ensure the robustness and generalizability of our findings, all primary outcome analyses—including all-cause mortality, MACE, AKI, and renal function decline (eGFR < 60 mL/min/1.73 m²)—were conducted in the demographically matched overall cohort. The 40–50 year sample was used to determine if balancing of comorbidities or laboratory parameters altered the outcomes of the study. Outcome patterns remained consistent across both cohorts (Table 2), supporting the validity of our main results.
Statistical analyses
Following PSM, two primary analytic approaches were employed. First, absolute risks, risk differences, risk ratios, and odds ratios (ORs) were calculated for each outcome using the TriNetX Risk Analysis module. Patients with prior occurrence of the outcome before the index event were excluded from risk calculations. Second, Kaplan-Meier survival analyses were performed to estimate cumulative incidence and survival probabilities. Survival curves between cohorts were compared using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated through Cox proportional hazards models to assess time-to-event differences. Statistical significance was defined as a two-sided p-value < 0.05. All analyses were conducted within the TriNetX platform environment.
Sensitivity analysis
To test the robustness of our findings regarding the impact of HZ in patients with COVID-19, we conducted three sets of sensitivity analyses (see Supplementary data; Supplementary Tables S3–11). First, to minimize confounding from pre-existing kidney impairment, we restricted the cohort to patients without a diagnosis of chronic kidney disease (CKD) within one year before the index COVID-19 event (Supplementary Table S3). Second, to focus on incident kidney outcomes and reduce the possibility of reverse causality, we excluded patients who had experienced acute kidney injury (AKI) in the year preceding their COVID-19 diagnosis (Supplementary Table S4). Third, to isolate the effect of HZ from concurrent or overlapping viral illnesses, we excluded patients diagnosed with influenza within one year before or after their COVID-19 diagnosis (Supplementary Table S5).
In addition, to account for residual imbalance in baseline characteristics after initial PSM, we performed further sensitivity analyses with expanded covariate adjustment. Specifically, we conducted 1:1 PSM using an extended set of demographic, comorbidity, medication, and laboratory variables. Supplementary Table S6 presents analyses adjusted for age, sex, race, and major comorbidities including hypertension, cerebrovascular disease, and diabetes mellitus. Supplementary Table S7 further incorporated key laboratory parameters such as C-reactive protein, hemoglobin, and iron levels into the matching algorithm.
Additional sensitivity analyses were conducted to evaluate specific sources of potential bias. To assess whether the observed early survival benefit among HZ patients might be confounded by preexisting renal vulnerability, we reanalyzed post-COVID mortality (days 1–90) after excluding individuals with CKD or AKI prior to COVID-19 (Supplementary Table S8). To examine the role of COVID-19 vaccination status, we performed subgroup analyses stratified by vaccination status (Supplementary Table S9). To address concerns about time-varying confounding due to epidemic phase or circulating variants, we examined temporal alignment by comparing the age at index and current age between matched groups (Supplementary Table S10). Finally, to assess whether post-COVID HZ was associated with increased susceptibility to reinfection, we compared SARS-CoV-2 reinfection risk and frequency between matched cohorts (Supplementary Table S11).
Results
All-cause mortality
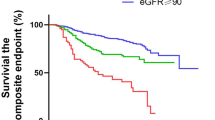
In the overall matched cohort, patients diagnosed with HZ within one year following COVID-19 exhibited a higher all-cause mortality rate of 8.0%, compared to 6.1% in those without HZ. However, Kaplan–Meier survival analysis showed no significant difference in overall survival curves between the two groups over the full three-year follow-up period (log-rank p = 0.260), and the corresponding HR was 1.04 (95% CI: 0.97–1.11) (Fig. 2A).
Kaplan–Meier survival curves comparing COVID-19 patients with and without herpes zoster (HZ) infection for (A) all-cause mortality, (B) MACE, (C) AKI, and (D) decline in eGFR to less than 60 mL/min/1.73 m² over a three-year follow-up period. The blue line represents patients with HZ development, and the red line represents patients without HZ. The log-rank test was used to compare survival distributions between groups, with p-values provided in each panel. Abbreviation: AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; HZ, herpes zoster; MACE, major adverse cardiovascular events.
Notably, the proportional hazards assumption was violated (global test p < 0.001), suggesting that the effect of post-COVID HZ on mortality varied over time. Time-stratified survival analyses were therefore performed (Supplementary Table S1). During the first 90 days after COVID-19 diagnosis, patients with HZ had a significantly lower mortality risk (HR = 0.30, 95% CI: 0.25–0.36, p < 0.001), potentially reflecting increased medical attention during acute HZ episodes (Supplementary Table S2). From day 91 onward, this early survival advantage reversed. Between day 91 and three years, the mortality risk became significantly higher in the HZ group (HR = 1.33, 95% CI: 1.24–1.44; log-rank p < 0.001), and the proportional hazards assumption was no longer violated (p = 0.897) (Supplementary Figure S1). Further stratified analyses showed that the excess risk emerged most prominently between days 181–270 (HR = 1.45, 95% CI: 1.13–1.87) and 271–360 (HR = 1.55, 95% CI: 1.24–1.94), indicating a delayed but persistent adverse impact of HZ on long-term survival.
In subgroup analyses (Fig. 3A), several factors were associated with increased all-cause mortality among COVID-19 patients who developed HZ, including older age (HR: 3.83; 95% CI: 3.42–4.29), diabetes mellitus (HR: 1.39; 95% CI: 1.25–1.54), hypertension (HR: 1.34; 95% CI: 1.15–1.54), smoking (HR: 1.45; 95% CI: 1.25–1.69), chronic obstruction pulmonary disease (COPD) (HR: 1.85; 95% CI: 1.63–2.11), reduced eGFR < 60 mL/min/1.73 m² (HR: 2.08; 95% CI: 1.85–2.34), CRP ≥ 10 mg/L (HR: 3.10; 95% CI: 2.68–3.58), and vitamin D deficiency < 30 ng/mL (HR: 1.77; 95% CI: 1.43–2.20). In contrast, female sex (HR: 0.69; 95% CI: 0.62–0.77) and higher body mass index (BMI) (HR: 0.65 for BMI ≥ 30; 95% CI: 0.57–0.74) were associated with lower mortality risk.
Subgroup analysis of adverse clinical outcomes in COVID-19 patients with herpes zoster (HZ). Hazard ratios (HRs) with 95% confidence intervals (CIs) are shown for key risk factors associated with (A) all-cause mortality, (B) MACE, (C) AKI, and (D) renal function decline (eGFR < 60 mL/min/1.73 m²). Abbreviations: AKI, acute kidney injury; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; MACE, major adverse cardiovascular events.
Major adverse cardiovascular events
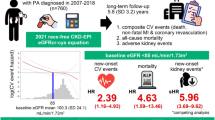
Patients diagnosed with HZ within one year after COVID-19 exhibited a higher risk of MACE compared to those without HZ. The MACE incidence was 18.1% in the post-COVID HZ group versus 10.6% in the non-HZ group. Kaplan–Meier analysis demonstrated a significantly lower MACE-free survival probability in the HZ group (80.27%) relative to the non-HZ group (84.95%), with a log-rank p < 0.001. The corresponding HR was 1.38 (95% CI: 1.30–1.46), indicating a sustained increase in cardiovascular risk throughout the follow-up period (Fig. 2B).
Subgroup analysis among COVID-19 patients with HZ (Fig. 3B) revealed that several risk factors were significantly associated with elevated MACE risk. These included older age (HR: 2.94; 95% CI: 2.72–3.18), diabetes mellitus (HR: 1.67; 95% CI: 1.53–1.83), hypertension (HR: 2.18; 95% CI: 1.96–2.42), smoking (HR: 1.58; 95% CI: 1.51–1.98), COPD (HR: 2.03; 95% CI: 1.81–2.27), reduced eGFR < 60 mL/min/1.73 m² (HR: 1.70; 95% CI: 1.52–1.89), and elevated CRP ≥ 10 mg/L (HR: 1.41; 95% CI: 1.26–1.59). Conversely, female sex was associated with a lower MACE risk (HR: 0.77; 95% CI: 0.71–0.84). BMI ≥ 30 (HR: 0.95; 95% CI: 0.87–1.05) and vitamin D deficiency < 30 ng/mL (HR: 1.01; 95% CI: 0.86–1.18) were not significantly associated with MACE.
Acute kidney injury
Patients diagnosed with HZ within one year after COVID-19 had a significantly higher risk of AKI compared to those without HZ. The incidence of AKI was 9.5% in the post-COVID HZ group versus 4.6% in the non-HZ group. Kaplan–Meier analysis showed a lower AKI-free survival probability in the HZ group (89.71%) compared to the non-HZ group (93.55%), with a significant difference confirmed by the log-rank test (p < 0.001). The HR for AKI associated with HZ was 1.67 (95% CI: 1.55–1.80), suggesting a sustained increased renal risk following the occurrence of HZ during the post-acute COVID-19 phase (Fig. 2C).
Subgroup analysis (Fig. 3C) demonstrated that the risk of AKI was markedly elevated among patients aged ≥ 65 years (HR: 3.04; 95% CI: 2.75–3.37), those with diabetes mellitus (HR: 2.10; 95% CI: 1.90–2.33), hypertension (HR: 3.02; 95% CI: 2.59–3.53), smoking history (HR: 1.67; 95% CI: 1.43–1.96), and COPD (HR: 1.96; 95% CI: 1.72–2.24). Furthermore, pre-existing eGFR < 60 mL/min/1.73 m² (HR: 4.24; 95% CI: 3.76–4.81) and elevated CRP ≥ 10 mg/L (HR: 2.35; 95% CI: 2.06–2.67) were strongly associated with increased AKI risk. Vitamin D deficiency < 30 ng/mL was also significantly associated (HR: 1.37; 95% CI: 1.14–1.64). In contrast, female sex was protective (HR: 0.63; 95% CI: 0.57–0.70), and BMI ≥ 30 was not significantly associated with AKI (HR: 1.07; 95% CI: 0.95–1.20).
Renal function decline by eGFR < 60 mL/min/1.73 m²
Among patients with a diagnosis of HZ within one year following COVID-19, the incidence of renal function decline (defined as eGFR < 60 mL/min/1.73 m²) was 12.2%, compared to 7.6% in the matched COVID-19 cohort without HZ. Kaplan–Meier survival analysis demonstrated a lower renal function preservation rate in the HZ group, with 86.56% of patients maintaining eGFR ≥ 60 mL/min/1.73 m² at three years, compared to 89.23% in the non-HZ group. The log-rank test indicated a significant difference in renal outcomes between groups (p < 0.001), with a corresponding HR of 1.28 (95% CI: 1.20–1.37) (Fig. 2D).
Subgroup analysis (Fig. 3D) showed that renal function decline was more pronounced in patients aged ≥ 65 years (HR: 2.86; 95% CI: 2.62–3.13), those with diabetes mellitus (HR: 1.47; 95% CI: 1.33–1.63), hypertension (HR: 1.80; 95% CI: 1.60–2.04), smoking history (HR: 1.18; 95% CI: 1.03–1.36), COPD (HR: 1.25; 95% CI: 1.10–1.43), and elevated CRP ≥ 10 mg/L (HR: 1.41; 95% CI: 1.24–1.60). Female sex (HR: 0.91; 95% CI: 0.82–1.00) showed a marginal protective effect. Notably, BMI ≥ 30 (HR: 1.05; 95% CI: 0.94–1.17) and vitamin D deficiency (HR: 1.03; 95% CI: 0.87–1.22) were not significantly associated with eGFR decline in this subgroup.
Sensitivity analysis
In the matched cohorts, excluding patients with CKD within one year prior to COVID-19 diagnosis, HZ was significantly associated with increased risks of MACE, AKI, and decreased renal function, but not with all-cause mortality (Supplementary Table S3). When patients with AKI within one year before COVID-19 were excluded, HZ remained significantly associated with increased risks of MACE, AKI, and reduced kidney function, while the association with all-cause mortality remained nonsignificant (Supplementary table S4). In a third sensitivity analysis excluding patients with influenza one year before or after COVID-19, the associations between HZ and both cardiovascular and renal outcomes persisted, whereas the association with all-cause mortality was not statistically significant (Supplementary Table S5).
Furthermore, in analyses using expanded covariate adjustment, HZ remained independently associated with elevated risks of MACE, AKI, and eGFR decline. In the model adjusted for age, sex, race, and major comorbidities (Supplementary Table S6), the HRs for MACE, AKI, and renal impairment ranged from 1.25 to 1.44, with no significant association for all-cause mortality (HR 0.98, p = 0.520). These findings remained consistent when key laboratory parameters (CRP, hemoglobin, and iron) were included in the matching algorithm (Supplementary Table S7), showing robust associations with cardiorenal outcomes and a nonsignificant association with mortality (HR 0.968, p = 0.310).
In an additional sensitivity analysis focused on the early post-COVID period (days 1–90), we assessed whether the observed short-term survival advantage among HZ patients was confounded by baseline renal vulnerability. After excluding individuals with CKD or AKI in the year prior to COVID-19, the early mortality benefit associated with post-COVID HZ remained statistically significant (HR = 0.36, 95% CI: 0.29–0.45, p < 0.001) compared to matched non-HZ controls (Supplementary Table S8).
In a further sensitivity analysis stratified by COVID-19 vaccination status, we evaluated whether vaccination modified the associations between post-COVID HZ and adverse outcomes. Among vaccinated patients, HZ was significantly associated with increased risks of all-cause mortality (HR = 1.25, 95% CI: 1.06–1.47, p < 0.05), MACE (HR = 1.36, 95% CI: 1.21–1.52, p < 0.05), AKI (HR = 1.38, 95% CI: 1.20–1.60, p = 0.07), and eGFR < 60 mL/min/1.73 m² (HR = 1.29, 95% CI: 1.14–1.46, p = 0.08), although the associations for AKI and renal impairment did not reach conventional statistical significance. In contrast, among unvaccinated individuals, HZ was significantly associated with increased risks of MACE (HR = 1.29, 95% CI: 1.22–1.37, p < 0.001), AKI (HR = 1.65, 95% CI: 1.53–1.77, p < 0.001), and renal function decline (HR = 1.20, 95% CI: 1.13–1.28, p < 0.05), while the association with all-cause mortality was not statistically significant (HR = 1.05, 95% CI: 0.98–1.12, p < 0.001) (Supplementary Table S9).
Risk estimates in the overall and age-restricted cohorts
Because the overall cohort included more than one million patients, conducting PSM across the full dataset was computationally infeasible. To provide a representative example of matched baseline characteristics, we selected the 40–50-year-old subgroup for presentation in Table 1. To support the generalizability of our findings, Table 2 presents outcome estimates from both the overall cohort and this age-restricted subgroup. In both groups, HZ was consistently associated with significantly increased risks of MACE, AKI, and renal function decline, while the association with all-cause mortality was not statistically significant. These concordant results support the validity of our matching approach and the robustness of the observed associations.
To further address concerns regarding the temporal alignment of COVID-19 diagnosis dates between groups, we evaluated the distributions of age at index (i.e., date of COVID-19 diagnosis) and current age before and after matching. As shown in Supplementary Table S10, the age at index was perfectly matched between HZ and non-HZ patients after PSM (mean 58.3 ± 15.6 years in both groups), resulting in nearly identical current age distributions (62.8 ± 15.6 years).
COVID-19 reinfection risk and frequency
To explore whether post-COVID HZ was associated with increased vulnerability to SARS-CoV-2 reinfection, we compared reinfection risk and frequency between matched cohorts. As shown in Supplementary Table S11, patients with HZ experienced a significantly higher risk of COVID-19 reinfection than those without HZ (71.0% vs. 53.2%). Moreover, the mean number of reinfection episodes was higher in the HZ group (2.90 ± 5.13) compared to the non-HZ group (1.66 ± 3.65), with a statistically significant difference (p < 0.001).
Discussion
In this large-scale, propensity score–matched cohort study, we found that patients diagnosed with HZ within one year after COVID-19 infection experienced significantly increased risks of MACE, AKI, and decline in renal function, as defined by an eGFR falling below 60 mL/min/1.73 m². Although the crude all-cause mortality rate was higher in the HZ group, this association was not statistically significant in the adjusted time-to-event analysis across the entire three-year follow-up period. These findings suggest that the occurrence of HZ in the post-COVID period may serve as a clinical indicator of heightened susceptibility to adverse cardiovascular and renal outcomes, emphasizing the importance of long-term risk monitoring in this population.
Subgroup analyses among COVID-19 patients with HZ revealed that older age, diabetes mellitus, hypertension, smoking, COPD, reduced baseline renal function, and elevated CRP were consistently associated with higher risks across all outcomes—including all-cause mortality, MACE, AKI, and renal function decline. Pre-existing renal impairment and systemic inflammation emerged as strong, reproducible predictors of poor prognosis. Female sex was protective against mortality, MACE, and AKI, but not renal decline, while higher BMI was only inversely associated with mortality. Notably, vitamin D deficiency was linked to increased risks of mortality and AKI, but not MACE or renal decline, suggesting differential organ vulnerability. Furthermore, to address the potential for time-varying confounding arising from differences in epidemic phase, healthcare practices, or circulating viral variants, we conducted a supplementary analysis to evaluate temporal alignment between matched groups. As detailed in Supplementary Table S10, the age at index was perfectly balanced between the HZ and non-HZ groups after matching, and their current age distributions were likewise identical. This temporal alignment supports the robustness of our matching approach in minimizing bias from time-sensitive external factors. Altogether, our results emphasize the multifactorial drivers of adverse post-COVID outcomes and support the need for targeted surveillance in high-risk individuals.
The association between HZ and all-cause mortality warrants particular attention. Although patients with HZ exhibited a higher crude risk of all-cause mortality following COVID-19, this association did not reach statistical significance in adjusted time-to-event models across the full three-year period. Kaplan–Meier survival analysis showed comparable cumulative survival probabilities between the HZ and non-HZ groups, and the proportional hazards assumption was violated, indicating non-uniform effects over time. Interestingly, patients with HZ demonstrated a lower mortality risk during the initial 90 days post-COVID-19, potentially reflecting increased medical attention at the time of HZ diagnosis, as supported by higher healthcare utilization rates. However, this early survival advantage diminished over time and was reversed during the second and third years. Stratified analysis starting from day 91 revealed a significantly increased long-term mortality risk among patients with HZ.
These findings suggest a biphasic risk trajectory: short-term protection potentially driven by enhanced acute-phase care, followed by a delayed increase in mortality. This later effect may be attributable to immunosenescence, HZ-related systemic inflammation, and endothelial dysfunction, all of which are known contributors to adverse long-term outcomes after viral infections19,20,21. Prior studies have also suggested that HZ may serve as a clinical marker of underlying immune dysregulation, especially among older adults or those with cardiometabolic comorbidities14,17. Consistent with this, a recent systematic review reported that herpesvirus reactivation in COVID-19 was associated with worse outcomes, including prolonged ICU stays and increased mortality, reinforcing the potential long-term impact of viral reactivation22.
With regard to cardiovascular outcomes, our findings revealed a strong association between HZ development and increased MACE risk among COVID-19 survivors. Patients with HZ had a 37.8% higher risk of MACE compared to those without HZ. This observation aligns with prior population-based studies. A nationwide Taiwanese cohort of 19,483 HZ patients reported a 16% increased risk of arrhythmia and an 11% increased risk of coronary artery disease23. Similarly, a retrospective cohort of 41,930 individuals showed a 19% increase in composite major adverse cardiac and cerebrovascular events following HZ diagnosis24. Notably, short-term risks are especially pronounced: a large U.S. Medicare analysis identified a 2.4-fold increase in stroke and a 1.7-fold increase in myocardial infarction within the first week after HZ onset25, with elevated risk persisting—though gradually attenuating—over the subsequent six months26.
Several mechanisms may explain this heightened cardiovascular risk. Varicella-zoster virus (VZV) reactivation promotes systemic inflammation, endothelial injury, and a hypercoagulable state, which together can destabilize atherosclerotic plaques and increase thrombogenic potential27,28,29. VZV can also directly invade vascular endothelium, causing immune-mediated vasculitis and reductions in anticoagulant proteins such as protein S30. Moreover, plasma exosomes from patients with HZ have been shown to exhibit prothrombotic activity by stimulating platelet aggregation and inducing IL-6 and IL-8 release from cerebrovascular cells, sustaining vascular inflammation for up to three months post-infection29. Given that COVID-19 itself induces endothelial damage and systemic inflammation, the occurrence of HZ in this setting may act as a synergistic amplifier of cardiovascular injury. Clinically, these findings support the need for proactive cardiovascular monitoring in post-COVID-19 patients, especially among older adults and those with metabolic or vascular risk factors.
Regarding renal outcomes, our findings demonstrated that HZ was significantly associated with increased renal complications in COVID-19 survivors. Specifically, patients with HZ exhibited a 67.4% higher risk of AKI and a 27.8% greater likelihood of eGFR decline below 60 mL/min/1.73 m². These results highlight the dual impact of HZ on both acute kidney injury and progressive renal dysfunction.
SARS-CoV-2 has been shown to directly affect renal tubular and endothelial cells, promote microvascular thrombosis, and sustain systemic inflammation, all of which contribute to renal damage31,32,33. In parallel, VZV may potentiate these mechanisms via vascular inflammation and immune-mediated vasculopathy16,34. Furthermore, treatment of HZ often involves nephrotoxic agents such as intravenous acyclovir, which has been associated with AKI. Prior studies reported AKI rates of 5.0% in 3,273 hospitalized HZ patients and up to 17.8% in a smaller inpatient cohort of 287 individuals35,36. The risk is further compounded in patients concurrently receiving NSAIDs or vancomycin. One pharmacovigilance study noted a 19.4% incidence of AKI in valacyclovir plus NSAID users, compared to 8.6% in those on valacyclovir alone37.
Even in the absence of overt AKI, 5–15% of COVID-19 survivors may develop incident eGFR < 60 mL/min/1.73 m² within 6 to 12 months38, and accelerated renal function decline has been observed relative to uninfected controls39. These outcomes likely result from persistent fibrotic, ischemic, and immune-mediated injury, including tubular-interstitial fibrosis, glomerulosclerosis, and microvascular rarefaction40,41,42. When coupled with HZ-associated endothelial dysfunction, the risk of renal deterioration may be further amplified.
Persistent kidney impairment after COVID-19 has been associated with elevated risks of proteinuria, ESRD, cardiovascular events, and mortality5,6,43. Our findings suggest that COVID-19 survivors who subsequently develop HZ represent a particularly vulnerable population who may benefit from enhanced renal surveillance and earlier nephrology involvement. Special attention should be given to older adults and individuals with diabetes, and caution is warranted when prescribing nephrotoxic agents in this context.
This study has several limitations that warrant consideration. First, although PSM was used to minimize confounding, residual imbalances remained in certain laboratory parameters, particularly serum iron and CRP levels, which may reflect unmeasured differences in inflammatory or nutritional status. Second, the observational nature of this retrospective cohort study precludes any causal inference between HZ occurrence and subsequent cardiorenal outcomes. Third, diagnosis codes were used to define both exposure (HZ) and outcomes (e.g., MACE, AKI, eGFR decline), which may be subject to misclassification or underreporting. Fourth, the study relied on deidentified electronic health records across multiple healthcare systems, which may have variability in coding practices, follow-up duration, and laboratory availability. Fifth, granular clinical information such as antiviral treatment for HZ, adherence to medications, vaccination status (e.g., HZ or COVID-19 vaccines), and COVID-19 severity (e.g., hospitalization, ICU stay) were not available in the TriNetX platform and thus could not be adjusted for. Sixth, although we performed time-stratified analyses, the observed early protective effect (days 1–90 post-COVID) may be influenced by residual or unmeasured confounding, such as differences in healthcare accessibility or baseline disease severity, which are not fully captured in the available dataset. Seventh, the age-restricted matching (40–50 years) performed to address TriNetX platform extraction limits may limit generalizability to broader age groups, despite consistent results in sensitivity analyses. Eighth, smoking status was not available for inclusion in the propensity score model due to missingness within the TriNetX dataset, and BMI was not incorporated as a matching variable. Although stratified subgroup analyses showed consistent associations across smoking and BMI categories, the lack of direct adjustment for these important confounders in the matching process may have introduced residual confounding. Ninth, information on SARS-CoV-2 reinfection timing was not available in the TriNetX platform due to the aggregated and deidentified nature of the data. As such, reinfected individuals could not be excluded or censored during follow-up, and our analyses followed an intention-to-treat approach based on the index COVID-19 diagnosis. While this reflects real-world clinical practice, it also limits the ability to account for reinfection-related variability in long-term outcomes. Finally, the findings reflect associations during a defined follow-up period and may not capture delayed complications beyond three years.
Conclusion
HZ reactivation after COVID-19 may signal a state of heightened immunologic or vascular vulnerability that predisposes individuals to long-term cardiovascular and renal complications. The consistent associations with MACE, AKI, and renal function decline suggest that HZ serves as more than a benign dermatologic event—it may be an early clinical marker of systemic risk. Given the delayed increase in mortality observed after the acute phase, clinicians should remain vigilant beyond the initial months of recovery. Incorporating HZ occurrence into post-COVID risk profiling may help identify patients who would benefit from closer monitoring, earlier intervention, and targeted preventive strategies.
Data availability
Due to licensing and privacy restrictions, the data used in this study from the TriNetX Global Health Research Network are not publicly available. TriNetX provides access to de-identified, aggregate-level data obtained from a global network of healthcare organizations. Researchers may request access through the TriNetX website (https://trinetx.com) or by contacting Privacy@TriNetX.com. Data are available on reasonable request from the corresponding author.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- AKI:
-
Acute kidney injury
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- HR:
-
Hazard ratio
- HZ:
-
Herpes zoster
- LDL:
-
Low-density lipoprotein
- MACE:
-
Major adverse cardiovascular events
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PASC:
-
Post-acute sequelae of SARS-CoV-2 infection
- PSM:
-
Propensity score matching
- PTH:
-
Parathyroid hormone
- RNA:
-
Ribonucleic acid
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SD:
-
Standard deviation
- SMD:
-
Standardized mean difference
- VZV:
-
Varicella-Zoster virus
References
Jiao, T. et al. Research progress of post-acute sequelae after SARS-CoV-2 infection. Cell. Death Dis. 15 (4), 257 (2024).
Sabaghian, T. et al. COVID-19 and acute kidney injury: A systematic review. Front. Med. (Lausanne). 9, 705908 (2022).
Aklilu, A. M. et al. COVID-19-Associated acute kidney injury and longitudinal kidney outcomes. JAMA Intern. Med. 184 (4), 414–423 (2024).
Fu, E. L. et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin. Kidney J. 13 (4), 550–563 (2020).
Bowe, B. et al. Kidney outcomes in long COVID. J. Am. Soc. Nephrol. 32 (11), 2851–2862 (2021).
Schiffl, H. & Lang, S. M. Long-term interplay between COVID-19 and chronic kidney disease. Int. Urol. Nephrol. 55 (8), 1977–1984 (2023).
Xie, Y. et al. Burdens of AKI and CKD among COVID-19 survivors: PO0019. J. Am. Soc. Nephrol. 32 (10S), 61 (2021).
Copur, S. et al. Post-acute COVID-19 syndrome and kidney diseases: what do we know? J. Nephrol. 35 (3), 795–805 (2022).
Wang, F. et al. A systematic review and meta-analysis of herpes Zoster occurrence/recurrence after COVID-19 infection and vaccination. J. Med. Virol. 96 (5), e29629 (2024).
Narasimhan, M. et al. Association between COVID-19 infection and herpes zoster: A case series. J. Family Med. Prim. Care. 12 (10), 2516–2519 (2023).
Fathy, R. A. et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an international dermatology registry. J. Eur. Acad. Dermatol. Venereol. 36 (1), e6–e9 (2022).
Erskine, N. et al. A systematic review and meta-analysis on herpes Zoster and the risk of cardiac and cerebrovascular events. PLoS One. 12 (7), e0181565 (2017).
Curhan, S. G. et al. Herpes Zoster and Long-Term risk of cardiovascular disease. J. Am. Heart Assoc. 11 (23), e027451 (2022).
Yamaoka-Tojo, M. & Tojo, T. Herpes Zoster and cardiovascular disease: exploring associations and preventive measures through vaccination. Vaccines (Basel) 12(3), 252 (2024).
Wu, P. H., Chuang, Y. S. & Lin, Y. T. Does herpes Zoster increase the risk of stroke and myocardial infarction?? A comprehensive review. J. Clin. Med. 8(4), 547 (2019).
Yawn, B. P. et al. Risk of, and risk factors for, vasculopathy associated with acute herpes Zoster. J. Stroke Cerebrovasc. Dis. 32 (2), 106891 (2023).
Soyuncu, S. et al. Herpes Zoster as a useful clinical marker of underlying cell-mediated immune disorders. Ann. Acad. Med. Singap. 38 (2), 136–138 (2009).
Sandor, E. V. et al. Herpes Zoster ophthalmicus in patients at risk for the acquired immune deficiency syndrome (AIDS). Am. J. Ophthalmol. 101 (2), 153–155 (1986).
Lin, T. Y. et al. Herpes Zoster infection increases the risk of peripheral arterial disease: A nationwide cohort study. Med. (Baltim). 95 (35), e4480 (2016).
Gambichler, T. et al. Prognostic clinical characteristics and systemic immune inflammatory biomarkers in 520 inpatients with herpes zoster: a retrospective analysis based on tertiary care hospitals. Eur. J. Dermatol. 34 (5), 502–508 (2024).
Staikov, I. et al. Herpes Zoster as a systemic disease. Clin. Dermatol. 32 (3), 424–429 (2014).
Talukder, S. et al. Clinical features and outcomes of COVID-19 patients with concomitant herpesvirus co-infection or reactivation: A systematic review. New. Microbes New. Infect. 58, 101233 (2024).
Wu, P. Y. et al. Increased risk of cardiovascular events in patients with herpes zoster: a population-based study. J. Med. Virol. 86 (5), 772–777 (2014).
Horev, A. et al. Herpes Zoster and long-term vascular risk: a retrospective cohort study. Sci. Rep. 13 (1), 2364 (2023).
Minassian, C. et al. Acute cardiovascular events after herpes zoster: A Self-Controlled case series analysis in vaccinated and unvaccinated older residents of the united States. PLoS Med. 12 (12), e1001919 (2015).
Wise, J. Shingles is linked to increased risk of cardiovascular events. Bmj 351, h6757 (2015).
Salvotti, F. et al. Atypical Varicella-Zoster virus reactivation: A case report. Eur. J. Case Rep. Intern. Med. 10 (9), 003945 (2023).
Cersosimo, A. et al. Varicella Zoster virus and cardiovascular diseases. Monaldi Arch. Chest Dis., 93(2). (2022).
Bubak, A. N. et al. Zoster-Associated prothrombotic plasma exosomes and increased stroke risk. J. Infect. Dis. 227 (8), 993–1001 (2023).
Khan, R. et al. Cerebral venous thrombosis and acute pulmonary embolism following varicella infection. Eur. J. Case Rep. Intern. Med. 6 (10), 001171 (2019).
da Mata, G. F. et al. Inflammation and kidney involvement in human viral diseases caused by SARS-CoV-2, HIV, HCV and HBV. J. Venom. Anim. Toxins Incl. Trop. Dis. 27, e20200154 (2021).
Kasal, D. A., De Lorenzo, A. & Tibiriçá, E. COVID-19 and microvascular disease: pathophysiology of SARS-CoV-2 infection with focus on the Renin-Angiotensin system. Heart Lung Circ. 29 (11), 1596–1602 (2020).
Chen, X. et al. COVID-19 associated thromboinflammation of renal capillary: potential mechanisms and treatment. Am. J. Transl Res. 12 (12), 7640–7656 (2020).
Bandeira, F. et al. Herpes Zoster ophthalmicus and varicella Zoster virus vasculopathy. Arq. Bras. Oftalmol. 79 (2), 126–129 (2016).
Lee, E. J. et al. The incidence, risk factors, and clinical outcomes of acute kidney injury (staged using the RIFLE classification) associated with intravenous acyclovir administration. Ren. Fail. 40 (1), 687–692 (2018).
Xu, R. et al. Associations between different antivirals and Hospital-Acquired acute kidney injury in adults with herpes Zoster. Clin. J. Am. Soc. Nephrol. 19 (6), 694–703 (2024).
Yue, Z. et al. Association between concomitant use of acyclovir or valacyclovir with NSAIDs and an increased risk of acute kidney injury: data mining of FDA adverse event reporting system. Biol. Pharm. Bull. 41 (2), 158–162 (2018).
Atiquzzaman, M. et al. Long-term effect of COVID-19 infection on kidney function among COVID-19 patients followed in post-COVID-19 recovery clinics in British columbia, Canada. Nephrol. Dial Transpl. 38 (12), 2816–2825 (2023).
Nugent, J. et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw. Open. 4 (3), e211095 (2021).
Ahmadian, E. et al. Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev. Med. Virol. 31 (3), e2176 (2021).
Smith, K. D. & Akilesh, S. Pathogenesis of coronavirus disease 2019-associated kidney injury. Curr. Opin. Nephrol. Hypertens. 30 (3), 324–331 (2021).
Silva, A. et al. COVID-19 and acute kidney Injury - Direct and indirect pathophysiological mechanisms underlying lesion development. Acad. Bras. Cienc. 94 (suppl 3), e20211501 (2022).
Matsumoto, K. & Prowle, J. R. COVID-19-associated AKI. Curr. Opin. Crit. Care. 28 (6), 630–637 (2022).
Acknowledgements
The authors would also like to thank the Core Laboratory at the Department of Research, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, for their technical support and the use of their facilities.
Funding
This study was supported by grants from the Fu Jen Catholic University Hospital (PL-202508008-V) and Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-113-RT-3(1/3)).
Author information
Authors and Affiliations
Contributions
Chien-Lin Lu, Kuo-Cheng Lu and Ya-Chieh Chang: conceptualization; Chien-Lin Lu: literature search; Chien-Lin Lu and Kuo-Cheng Lu: data collection; Kuo-Cheng Lu and Joshua Wang: data analysis; Joshua Wang and Kuo-Cheng Lu: statistical analysis; Chien-Lin Lu: manuscript preparation; Chien-Lin Lu, Joshua Wang and Kuo-Cheng Lu: manuscript editing; Chien-Lin Lu, Joshua Wang and Kuo-Cheng Lu: manuscript review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, CL., Wang, J., Chang, YC. et al. Cardiorenal outcomes after herpes zoster reactivation in COVID-19 survivors from a global TriNetX study. Sci Rep 15, 30036 (2025). https://doi.org/10.1038/s41598-025-16398-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16398-3