Abstract
This study investigated the effect of smoking and regular physical activity (PA) on the composite risk of cardiovascular disease (CVD) in patients with type 2 diabetes mellitus (T2DM) across different steatotic liver disease (SLD) categories. We analyzed data for 1,921,310 patients with T2DM aged 20 years and older. The participants were categorized by SLD status, with hepatic steatosis (fatty liver index ≥ 30). Among current smokers, the adjusted hazard ratios (HR) for composite CVD events significantly increased from the no steatosis to metabolic dysfunction-associated steatotic liver disease (MASLD), metabolic dysfunction and alcohol-related steatotic liver disease (MetALD), and alcohol-related liver disease (ALD) groups, with the highest HR observed in the ALD group (aHR, 2.14; 95% confidence interval [CI], 2.04–2.24). The ALD group without regular PA had the highest risk of composite CVD events (aHR, 1.33; 95% CI, 1.28–1.38). The highest risk of composite CVD events was found among current smokers without regular PA, with the aHR increasing in a stepwise manner from no steatosis to MASLD, MetALD, and ALD groups, with the latter showing the highest risk. Smoking and physical inactivity significantly increased the CVD risk in patients with T2DM, with the highest risk in the ALD group.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the leading cause of death in patients with non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH)1,2,3with NAFLD conferring a cardiovascular (CV) risk greater than the sum of its individual comorbidities4. Recent terminology updates have led to the introduction of the term metabolic dysfunction-associated steatotic liver disease (MASLD), which encompasses NAFLD and highlights its role in driving insulin resistance, hepatic inflammation, and atherogenic dyslipidemia, all of which increase the risk of CVD5,6,7. NAFLD is associated with a 2.2-fold increased risk of incident diabetes mellitus (DM), and its risk is correlated with its severity8. In a previous meta-analysis, patients with NAFLD and type 2 diabetes mellitus (T2DM) were found to have twice the risk of CVD compared to those with T2DM alone9. A recent cohort study found that current smoking significantly increased the risk of T2DM in patients with NAFLD (hazard ratio [HR], 2.05; 95% confidence interval [CI], 1.43–2.94)10while smoking cessation was associated with decreased CVD incidence among T2DM patients11. A recent study indicated that many participants with MASLD did not recognize the role of exercise as an effective treatment option12. Recent evidence suggests that regular physical activity (PA) improves histological outcomes pertaining to the liver in patients with MASLD even in the absence of significant weight loss, reinforcing the importance of exercise in managing metabolic and CV risks13. Furthermore, Balogun et al. demonstrated that the combined effect of smoking and T2DM was associated with an increased prevalence of hepatic fibrosis in MASLD patients14. However, despite established links between modifiable lifestyle factors such as smoking, PA, and CVD, their impact on CVD risk in T2DM patients across different steatotic liver disease (SLD) categories remains underexplored. Given the increasing global prevalence of NAFLD/MASLD among patients with T2DM15this study aimed to address this gap by investigating how smoking and PA affect CVD outcomes, including composite CVD events (myocardial infarction [MI], ischemic stroke, and CV mortality), in these populations.
Results
Baseline characteristics
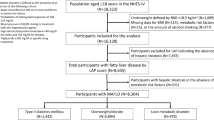
A total of 1,921,310 individuals were categorized into four groups: group 1 (never or former smokers with regular PA, n = 335,222), group 2 (never or former smokers with irregular PA, n = 1,120,489), group 3 (current smokers with regular PA, n = 85,793), and group 4 (current smokers with irregular PA, n = 379,806). Table 1 presents the baseline characteristics of the participants. The median age of the total population was 58 years, with 61.4% being men, and the median follow-up duration was 6.0 years.
The average body mass index (BMI) was approximately 25. In groups 3 and 4, which consisted primarily of current smokers and individuals with new-onset diabetes, most participants were male, with group 4 having a younger average age than the other groups. The waist circumference (WC), fasting glucose, total cholesterol, and triglyceride (TG) levels increased from group 1 to group 4, whereas high density lipoprotein (HDL) cholesterol levels decreased.
Risk of CVD incidence according to smoking status
Table 2 presents the association between CVD outcomes (composite CVD events, MI, ischemic stroke, and CV mortality) and smoking status, stratified by SLD subtype. Overall, current smoking significantly increased the CVD outcomes across all SLD subtypes. For current smokers, the HRs for composite CVD event, MI, stroke, and CV mortality significantly increased from the no steatosis group to the MASLD, metabolic dysfunction and alcohol-related steatotic liver disease (MetALD), and alcohol-related liver disease (ALD) groups. With the never smoker and no steatosis group as the reference, the current smokers in the ALD group demonstrated the highest HRs: composite CVD event (aHR, 2.14; 95% CI, 2.04–2.24), MI (aHR, 2.14; 95% CI, 1.99–2.30), stroke (aHR, 1.99; 95% CI, 1.86–2.13), and CV mortality (aHR, 2.91; 95% CI, 2.57–3.30). Former smokers generally showed increased aHRs for CVD risk compared to never smokers without steatosis. However, when referenced to never smokers within each SLD subtype, the aHRs for former smokers were reduced or neutral, suggesting the potential benefit of smoking cessation. Additionally, when never smoker status was used as the reference within each SLD group, the effect of smoking on CVD risk was more pronounced in individuals with ALD and no steatosis. Significant interactions were observed between smoking and SLD subtypes for composite CVD events, MI, and stroke (P for interaction < 0.001, 0.028, and < 0.001, respectively), indicating that the effect of smoking on these outcomes differed across SLD groups. In contrast, no significant interaction was observed for CV mortality.
Risk of CVD incidence according to regular PA and intensity
Table 3 presents the associations between regular PA and CVD outcomes stratified by the SLD subtype. As shown in Table 3, compared to the no steatosis group without regular PA (reference group), the ALD group without regular PA had the highest risk of composite CVD event (aHR, 1.33; 95% CI, 1.28–1.38), MI (aHR, 1.32; 95% CI, 1.24–1.39), stroke (aHR, 1.29; 95% CI, 1.23–1.36), and CV mortality (aHR, 1.55; 95% CI, 1.41–1.70). Additionally, when irregular PA was used as the reference within each SLD group, overall regular PA was associated with a reduced risk of composite CVD events, MI, ischemic stroke, and CV mortality across all SLD groups. This association was consistent across MI, ischemic stroke, and CV mortality (P for interaction = 0.161, 0.103, and 0.387, respectively). However, we observed an interaction between the PA and SLD subtypes for composite CVD event (P for interaction = 0.005). Furthermore, we stratified patients into three categories according to energy expenditure: <500, 500–1499, ≥1500 metabolic equivalent of task (MET)-minutes/week (Supplementary Table 1). The association between CVD outcomes and intensity of regular PA, measured in MET-minutes per week, is shown in Supplementary Table 1. Across all SLD groups, higher PA levels were significantly associated with reduced risks of composite CVD events, MI, ischemic stroke, and CV mortality. When <500 MET-min/week was used as the reference within each SLD group, increased PA intensity (500–1499 and ≥1500 MET-min/week) consistently reduced the CVD risk in a dose-dependent manner. Notably, the highest PA group (≥1500 MET-min/week) showed the highest risk reduction for composite CVD events evidenced by aHRs of 0.84 (95% CI, 0.81–0.87), 0.84 (95% CI, 0.76–0.94), and 0.79 (95% CI, 0.70–0.88) in the MASLD, MetALD, and ALD groups, respectively, with the most prominent reduction observed in the ALD group. Significant interactions were observed between PA levels and SLD subtypes with regard to the risk of composite CVD events and CV mortality (P for interaction < 0.001 and 0.048, respectively), suggesting that the protective effect of PA intensity varied across the SLD groups.
Risk of CVD incidence according to regular PA and smoking status
We categorized the participants into four subgroups based on smoking status and regular PA to explore the joint impact on CVD outcomes (Table 4). The risk of composite CVD events, MI, stroke, and CV mortality increased in a stepwise manner from group 1 (never or former smokers with regular PA) to group 4 (current smokers without regular PA) and from no steatosis to MASLD, MetALD, and ALD, with the latter (ALD, group 4) showing the highest risk: composite CVD event (aHR, 2.67; 95% CI, 2.53–2.82), MI (aHR, 2.53; 95% CI, 2.34–2.75), stroke (aHR, 2.47; 95% CI, 2.29–2.66), and CV mortality (aHR, 4.36; 95% CI, 3.79–5.01). Furthermore, with group 1 as reference, the aHRs for CVD risk were consistently higher in group 3 (current smokers with regular PA) than in group 2 (never or former smokers without regular PA) across all SLD groups. On assessing the interactions among smoking, regular PA, and the risk of composite CVD events and stroke, significant differences were observed across the SLD groups (P for interaction = 0.002 and < 0.001, respectively).
Discussion
This population-based retrospective study investigated the associations among smoking, regular PA, and composite CVD outcomes in patients with T2DM across different SLD categories. Both current smoking and lack of regular PA were independently and significantly associated with an increased CVD risk across all SLD groups. Notably, current smokers without regular PA, particularly those with ALD, exhibited the highest risk.
In a previous study, the combination of T2DM and tobacco use was found to be associated with a higher prevalence of fibrosis (adjusted odds ratio [OR], 3.04; 95% CI, 1.62–5.76) in patients with MASLD than T2DM alone (aOR, 2.28; 95% CI, 1.37–3.85)14. In one Korean study, smoking was linked to a high risk of NAFLD (OR, 1.38; 95% CI, 1.08–1.76), with the risk decreasing as the smoking cessation duration increased and increasing as the pack years increased, suggesting that smoking cessation can help manage NAFLD16. Furthermore, a meta-analysis demonstrated a significant association between smoking and NAFLD, with passive smoking increasing the risk approximately 1.38 times, highlighting a public health concern that warrants attention17. Smoking is associated with an increased risk of CVD in individuals with NAFLD18. Consistent with these findings, our study suggests that current smoking significantly increases the risks of composite CVD events, MI, stroke, and CV mortality across all SLD subtypes, with the highest risk observed among current smokers in the ALD group. In addition, although former smokers showed a higher CVD risk than never smokers without steatosis, this risk was attenuated or neutral when referenced within each SLD subtype, suggesting the potential benefits of smoking cessation. Therefore, smoking cessation should be emphasized as part of CV risk management, especially for individuals with varying degrees of liver disease and T2DM. According to previous studies, regular PA has been associated with reduced CV mortality and has been shown to improve the fatty liver index (FLI) in patients with MASLD in a randomized controlled trial19,20. Similarly, in our study, regular PA was associated with a significant reduction in CVD risk across all SLD groups, with increasing benefits observed as the PA intensity increased. Notably, achieving ≥1500 MET-min/week was linked to the lowest risk of composite CVD events and CV mortality, even in high-risk groups such as MASLD and ALD.
In our study, both current smoking and physical inactivity independently increased the risks of composite CVD events, MI, ischemic stroke, and CV mortality across all SLD groups, with the highest risk observed in the ALD group. A recent study suggested that alcohol contributes to an increased risk of significant fibrosis through a dose-dependent supra-additive interaction with cardiometabolic risk factors21. Previous studies have shown that patients with advanced liver fibrosis have a high risk of developing CVD22,23. In a recent Korean cohort study, individuals with ALD exhibited a significantly increased risk of CVD compared to those without SLD (HR, 1.95; 95% CI, 1.01–3.77)24and among those with MASLD, the risk of CVD was markedly higher in participants with DM than in those with prediabetes (HR, 1.69; 95% CI, 1.32–2.15). A recent study reported that individuals with both MASLD and T2DM had significantly high CVD mortality25. Therefore, in individuals with high-risk fibrosis, stringent management of metabolic conditions and adherence to alcohol abstinence should be prioritized to mitigate the elevated CVD risk. Previous studies have shown that exercise reduces liver fat and enzyme levels and improves insulin sensitivity26,27,28whereas smoking, through nicotine, contributes to insulin resistance both directly and indirectly by promoting abdominal obesity and impairing cell-mediated immunity29,30. Recent research has emphasized that the mechanisms underlying MASLD involve lipotoxicity; therefore, the interaction between exercise and smoking could play a significant role in managing the condition31,32,33,34.
A key strength of our nationwide study in an Asian population is its focus on the effects of smoking and regular PA on CVD outcomes in patients with T2DM across different SLD subtypes, which, to our knowledge, has not been previously explored. However, this study had several limitations. First, owing to the lack of biopsy or imaging data in routine health screenings, the FLI was used as a simple, cost-effective tool used for noninvasive diagnosis in clinical settings35,36. Nonetheless, its limited sensitivity and specificity compared to imaging modalities including ultrasonography and magnetic resonance imaging (MRI) may have led to misclassification of hepatic steatosis, and the findings should be interpreted carefully. Second, self-reported questionnaires may introduce recall bias, and the study design may not fully capture long-term behavioral changes, potentially missing shifts in SLD subtypes over time. Third, the unavailability of glycated hemoglobin (HbA1c) data from the National Health Insurance Service (NHIS) database may have led to an underestimation of T2DM incidence, and the lack of platelet count data limited the calculation of liver fibrosis biomarker scores such as Fibrosis-4 index and aspartate aminotransferase (AST)-to-platelet ratio index. Finally, because the study included only Korean participants, our findings should be applied to the general population with caution, taking genetic and environmental differences into consideration37.
In conclusion, current smoking and physical inactivity significantly increased the risks of composite CVD events, MI, ischemic stroke, and CV mortality in all SLD subtypes among T2DM patients, with the highest risk noted in the ALD group. This result highlights the need for targeted lifestyle changes, particularly smoking cessation, regular exercise, and alcohol abstinence, to reduce the CVD risk in this vulnerable population, where no established therapies for MASLD currently exist.
Methods
Study population and design
We obtained data from the comprehensive Korean NHIS database, which includes the entire Korean population from January 2015 to December 2022. In the NHIS, the National Health Insurance covers approximately 97% of the population, whereas Medical Aid supports the remaining 3% with the lowest income, providing comprehensive health coverage. This government-operated database comprises a claims dataset with patient demographics, health check-up records, diagnoses coded according to the International Classification of Diseases, 10th revision (ICD-10), and prescription details. The health examination dataset included responses to health-related behavior questionnaires, comprehensive medical histories, anthropometric data, and laboratory results. Mortality records from the Korean National Statistical Office including death dates and causes were coded according to the revised 10th International Statistical Classification of Diseases. This study was approved by the Institutional Review Board of Samsung Medical Center (approval no. SMC 2024-03-104), Seoul, Republic of Korea. The requirement for informed consent was waived because all data provided to the researchers were de-identified.
The study included patients with T2DM aged 20 years and older who underwent general health examinations between 2015 and 2016 (N = 2,616,828). The exclusion criteria were age under 20 years (n = 323), missing data (n = 88,559), prior diagnosis of liver cancer before the index date (n = 10,438), history of liver transplantation (n = 946), previous diagnosis of concomitant liver disease (n = 174,667), MI (n = 132,121), stroke (n = 264,506), and individuals who died or were diagnosed with MI or stroke within 1 year of screening (n = 23,958). The final cohort consisted of 1,921,310 individuals (Supplementary Fig. 1).
Measurements and definitions
Height, body weight, and WC were recorded during health examinations. BMI was calculated by dividing the body weight (kg) by the square of height (m2). Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2. T2DM was defined as fasting blood glucose level ≥ 126 mg/dL; the use of specific drugs (oral hypoglycemic agents (OHAs), glucagon-like peptide-1 receptor agonist (GLP-1 RA), and insulin; or at least one claim using codes E11-14. OHAs include metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, meglitinides, alpha-glucosidase inhibitors, and sodium-glucose co-transporter (SGLT)-2 inhibitors. Insulin use was defined as one or more insulin prescriptions per year or three or more prescriptions per year in an outpatient setting. New-onset DM was defined as fasting blood glucose level of 126 mg/dL or higher at the time of health screening, with no prior DM medication prescriptions before the health check-up, and with HbA1c data not provided in the screening records. Hypertension (HTN) was defined by systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, use of antihypertensive medication, or at least one claim with codes I10-13 and I15. Dyslipidemia was defined as at least one claim per year with code E78, at least one claim per year for the prescription of a lipid-lowering agent, or total cholesterol level of ≥ 240 mg/dL. Alcohol abuse/misuse or ALD was defined by ICD-10 codes E24.4, F10, G31.2, G62.1, G72.1, I42.6, K29.2, K70, K86.0, Q35.4, R78.0, T51.0, T51.8, T51.9, X65, Y15, Y57.3, Y90, Y91, Z50.2, and Z71.4. Concomitant liver diseases were also defined using ICD-10 codes, including viral hepatitis (B15–B19), drug-induced (toxic) liver disease (K71), hepatic veno-occlusive disease (I82), liver abscess (K75.0, A06.4), hemochromatosis (E83.1), Wilson’s disease (E83.0), alpha-1 antitrypsin deficiency (E88.0), autoimmune hepatitis (K75.4), primary biliary cholangitis (K74.3, K74.4), other cholangitis (K83), and glycogen storage disease (E74). Liver cancer was defined using codes C22 and V193, and liver transplantation was defined using code Z94.4.
A composite CVD event was defined as hospitalization for MI, diagnosed using ICD-10 codes I21 or I22; hospitalization for ischemic stroke, diagnosed using I63 or I64, accompanied by claims for brain computed tomography (CT) or MRI; or CV mortality, diagnosed using ICD-10 codes I00–99.
The Charlson Comorbidity Index (CCI) comprises 12 conditions. Low income was defined as the lowest quartile of income distribution within the study population. PA was evaluated using a questionnaire and defined according to the following criteria: (i) light-intensity PA (i.e., slow walking) for > 30 min, (ii) moderate-intensity PA (i.e., brisk-pace walking or bicycling leisurely) for > 30 min, and (iii) vigorous-intensity PA (i.e., running or climbing) for > 20 min during a recent week. In this study, participants were classified as engaging in regular PA if they performed vigorous-intensity PA for > 20 min or moderate-intensity PA for > 30 min at least once a week. Conversely, participants were classified as having ‘no regular PA’ if they did not participate in any moderate- or vigorous-intensity PA, irrespective of the duration of light-intensity PA. Energy expenditure (MET-minutes/week) was calculated as: 7.0 × (average daily minutes of vigorous-intensity PA × number of days) + 4.0 × (average daily minutes of moderate-intensity PA × number of days) + 2.7 × (average daily minutes of light-intensity PA × number of days). Since the exact duration of activity in minutes was not available from the questionnaire, we assumed 20 min for vigorous-intensity PA and 30 min for moderate- and light-intensity PA, based on the response categories in the survey. Based on these calculations, patients were stratified into <500, 500 to <1500, and ≥1500 MET-min/week groups. Smoking status was categorized as never smoked, former smoker, and current smoker. Alcohol consumption was assessed using questionnaires in which participants reported their average frequency (days per week) and amount consumed (in standard glasses). Standard glass was defined as a glass containing 8 g of pure alcohol, and the reported consumption was converted into daily pure alcohol intake (g).
Definition of hepatic steatosis and MASLD
Hepatic steatosis was assessed using the FLI, calculated using the following equation:
(e0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (gamma-glutamyl transferase (GGT)) + 0.053 × WC − 15.745)/(1 + e0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × WC − 15.745).
Participants with hepatic steatosis (FLI ≥ 30)22 were diagnosed with MASLD if they also presented with at least one of the following cardiometabolic risk factors: (1) BMI ≥ 23 kg/m2 or WC ≥ 90 cm in men and ≥ 80 cm in women; (2) fasting blood glucose levels ≥ 100 mg/dL or T2DM or specific drug treatment; (3) blood pressure (BP) ≥ 130/85 mmHg or specific drug treatment; (4) TG ≥ 150 mg/dL or specific drug treatment; and (5) HDL cholesterol < 40 mg/dL for men and < 50 mg/dL for women or specific drug treatment.
We classified the participants into four groups: (1) no steatosis (FLI < 30); (2) MASLD, defined as FLI ≥ 30 with alcohol consumption < 30 g/d for men and < 20 g/d for women and at least one cardiometabolic risk factor; (3) MetALD, characterized by alcohol consumption (≥ 30 g/d but < 60 g/d for men and ≥ 20 g/d but < 50 g/d for women) with FLI ≥ 30 and at least one cardiometabolic risk factor; and (4) ALD, defined as FLI ≥ 30 and at least one cardiometabolic risk factor with alcohol consumption (≥ 60 g/d for men and ≥ 50 g/d for women) or any ICD code related to ALD.
Outcomes
The primary outcome was the occurrence of a composite CVD event—MI, ischemic stroke, and CV mortality. The participants were monitored from the initial health examination until the incidence of one of these primary outcomes. The baseline period was defined as the period between 2015 and 2016, and the date of the last examination was used as the reference point. The study cohort was followed from baseline until death or December 31, 2022, whichever was earlier.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as counts and percentages (%). One-way ANOVA was used to compare continuous variables, and the chi-squared test was used for categorical variables. The incidence rates were calculated by dividing the number of incident cases by the total follow-up duration (person-years). We evaluated the risks of incident composite CVD events (MI, ischemic stroke, and CV mortality) by incorporating a 1-year lag period in our analysis.
We used Cox proportional hazards models to assess the HRs and 95% CIs for incidence. The final model was adjusted for age, sex, income, regular PA, CCI score, fasting glucose levels, DM duration, insulin use, OHA use, CKD, BMI, alcohol consumption, HTN, and dyslipidemia. Covariates were selected based on clinical relevance and evidence from previous studies in T2DM populations38,39. Statistical significance was defined as P < 0.05, and all analyses were conducted using the SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Data availability
The data used in this study are available from the Korean NHIS, but restrictions apply to the availability of these data, which were provided for research purposes during a limited access period. Data are therefore not publicly available. However, data may be available from the authors upon reasonable request and with permission from the NHIS. Please contact the corresponding author for inquiries.
Abbreviations
- ALD:
-
Alcohol-related liver disease
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CCI:
-
Charlson Comorbidity Index
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CT:
-
Computed tomography
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- DM:
-
Diabetes mellitus
- DPP-4:
-
Dipeptidyl peptidase-4
- eGFR:
-
Estimated glomerular filtration rate
- FLI:
-
Fatty liver index
- GGT:
-
Gamma-glutamyl transferase
- GLP-1 RA:
-
Glucagon-like peptide-1 receptor agonist
- HbA1c:
-
Glycated hemoglobin
- HDL:
-
High density lipoprotein
- HR:
-
Hazard ratio
- HTN:
-
Hypertension
- ICD-10:
-
International classification of diseases 10th revision
- LDL:
-
Low density lipoprotein
- MASLD:
-
Metabolic dysfunction-associated steatotic liver disease
- MET:
-
Metabolic equivalent of task
- MetALD:
-
Metabolic dysfunction and alcohol-related steatotic liver disease
- MI:
-
Myocardial infarction
- MRI:
-
Magnetic resonance imaging
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NHIS:
-
National health insurance service
- OHA:
-
Oral hypoglycemic agent
- OR:
-
Odds ratio
- PA:
-
Physical activity
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SGLT:
-
Sodium glucose co-transporter
- SLD:
-
Steatotic liver disease
- TG:
-
Triglyceride
- TZD:
-
Thiazolidinedione
- T2DM:
-
Type 2 diabetes mellitus
- WC:
-
Waist circumference
References
Lonardo, A., Ballestri, S., Targher, G. & Loria, P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 9, 629–650. https://doi.org/10.1586/17474124.2015.965143 (2015).
Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 6, 903–913. https://doi.org/10.1016/s2468-1253(21)00308-3 (2021).
Oh, R. et al. Metabolic dysfunction-associated steatotic liver disease and all-cause and cause-specific mortality. Diabetes Metab. J. https://doi.org/10.4093/dmj.2024.0042 (2024).
Allen, A. M. et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology 67, 1726–1736. https://doi.org/10.1002/hep.29546 (2018).
Targher, G., Day, C. P. & Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl. J. Med. 363, 1341–1350. https://doi.org/10.1056/NEJMra0912063 (2010).
Targher, G., Byrne, C. D. & Tilg, H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 69, 1691–1705. https://doi.org/10.1136/gutjnl-2020-320622 (2020).
Driessen, S. et al. Metabolic dysfunction-associated steatotic liver disease and the heart. Hepatology https://doi.org/10.1097/hep.0000000000000735 (2023).
Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 70, 962–969. https://doi.org/10.1136/gutjnl-2020-322572 (2021).
Zhou, Y. Y. et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 30, 631–636. https://doi.org/10.1097/meg.0000000000001075 (2018).
Liu, C., Wu, Y., Duan, W. & Xu, W. Cigarette smoking increases the risk of type 2 diabetes mellitus in patients with non-alcoholic fatty liver disease: A population-based cohort study. Exp. Clin. Endocrinol. Diabetes. 130, 793–800. https://doi.org/10.1055/a-1813-7435 (2022).
Jeong, S. M. et al. Smoking behavior change and risk of cardiovascular disease incidence and mortality in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 22, 193. https://doi.org/10.1186/s12933-023-01930-4 (2023).
Deshpande, K., Olynyk, J., Ayonrinde, O. & Nosaka, K. Barriers to exercise in patients with metabolic dysfunction-associated steatotic liver disease: A patient survey. J. Clin. Med. Res. 16, 94–105. https://doi.org/10.14740/jocmr5113 (2024).
Cuthbertson, D. J. et al. Exercise improves surrogate measures of liver histological response in metabolic dysfunction-associated steatotic liver disease. Liver Int. 44, 2368–2381. https://doi.org/10.1111/liv.15947 (2024).
Balogun, O. et al. Effect of combined tobacco use and type 2 diabetes mellitus on prevalent fibrosis in patients with MASLD. Hepatol. Commun. 7 https://doi.org/10.1097/hc9.0000000000000300 (2023).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among patients with type 2 diabetes. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2024.03.006 (2024).
Jang, Y. S., Joo, H. J., Park, Y. S., Park, E. C. & Jang, S. I. Association between smoking cessation and non-alcoholic fatty liver disease using NAFLD liver fat score. Front. Public. Health. 11, 1015919. https://doi.org/10.3389/fpubh.2023.1015919 (2023).
Akhavan Rezayat, A. et al. Association between smoking and non-alcoholic fatty liver disease: A systematic review and meta-analysis. SAGE Open. Med. 6, 2050312117745223. https://doi.org/10.1177/2050312117745223 (2018).
Åberg, F. & Färkkilä, M. Drinking and obesity: alcoholic liver disease/nonalcoholic fatty liver disease interactions. Semin Liver Dis. 40, 154–162. https://doi.org/10.1055/s-0040-1701443 (2020).
Kim, D., Wijarnpreecha, K., Dennis, B. B., Cholankeril, G. & Ahmed, A. Types of physical activity in nonalcoholic fatty liver disease and All-Cause and cardiovascular mortality. J. Clin. Med. 12 https://doi.org/10.3390/jcm12051923 (2023).
Couret, A. et al. Effect of different modalities of exercise on fatty liver index in patients with metabolic syndrome: The RESOLVE randomized trial. Clin. Res. Hepatol. Gastroenterol. 48, 102461. https://doi.org/10.1016/j.clinre.2024.102461 (2024).
Marti-Aguado, D. et al. Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 81, 930–940. https://doi.org/10.1016/j.jhep.2024.06.036 (2024).
Lee, H. H. et al. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut 73, 533–540. https://doi.org/10.1136/gutjnl-2023-331003 (2024).
Vilar-Gomez, E. et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 155, 443–457e417. https://doi.org/10.1053/j.gastro.2018.04.034 (2018).
Choe, H. J., Moon, J. H., Kim, W., Koo, B. K. & Cho, N. H. Steatotic liver disease predicts cardiovascular disease and advanced liver fibrosis: A community-dwelling cohort study with 20-year follow-up. Metabolism 153, 155800. https://doi.org/10.1016/j.metabol.2024.155800 (2024).
Riley, D. R. et al. The synergistic impact of type 2 diabetes and MASLD on cardiovascular, liver, diabetes-related and cancer outcomes. Liver Int. 44, 2538–2550. https://doi.org/10.1111/liv.16016 (2024).
Smart, N. A., King, N., McFarlane, J. R., Graham, P. L. & Dieberg, G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: A systematic review and meta-analysis. Br. J. Sports Med. 52, 834–843. https://doi.org/10.1136/bjsports-2016-096197 (2018).
Zou, T. T. et al. Lifestyle interventions for patients with nonalcoholic fatty liver disease: A network meta-analysis. Eur. J. Gastroenterol. Hepatol. 30, 747–755. https://doi.org/10.1097/meg.0000000000001135 (2018).
Sabag, A. et al. The effect of high-intensity interval training vs moderate-intensity continuous training on liver fat: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 107, 862–881. https://doi.org/10.1210/clinem/dgab795 (2022).
Artese, A., Stamford, B. A. & Moffatt, R. J. Cigarette smoking: an accessory to the development of insulin resistance. Am. J. Lifestyle Med. 13, 602–605. https://doi.org/10.1177/1559827617726516 (2019).
Moszczyński, P. et al. Immunological findings in cigarette smokers. Toxicol. Lett. 118, 121–127. https://doi.org/10.1016/s0378-4274(00)00270-8 (2001).
Hejazi, K. & Hackett, D. Effect of exercise on liver function and insulin resistance markers in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 12. https://doi.org/10.3390/jcm12083011 (2023).
Li, Y. et al. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 23, 117. https://doi.org/10.1186/s12944-024-02108-x (2024).
Gastaldelli, A., Folli, F. & Maffei, S. Impact of tobacco smoking on lipid metabolism, body weight and cardiometabolic risk. Curr. Pharm. Des. 16, 2526–2530. https://doi.org/10.2174/138161210792062858 (2010).
Lee, Y. et al. The effects of PPAR agonists on atherosclerosis and nonalcoholic fatty liver disease in ApoE-/-FXR-/- mice. Endocrinol. Metab. (Seoul) 36, 1243–1253. https://doi.org/10.3803/EnM.2021.1100 (2021).
Cho, E. J. et al. Fatty liver index for predicting nonalcoholic fatty liver disease in an asymptomatic Korean population. Diagnostics (Basel) 11. https://doi.org/10.3390/diagnostics11122233 (2021).
Lee, H., Lee, Y. H., Kim, S. U. & Kim, H. C. Metabolic Dysfunction-Associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin. Gastroenterol. Hepatol. 19, 2138–2147e2110. https://doi.org/10.1016/j.cgh.2020.12.022 (2021).
Liu, X. R., Yin, S. C., Chen, Y. T. & Lee, M. H. Metabolic dysfunction-associated steatotic liver disease and its associated health risks. J. Chin. Med. Assoc. 88, 343–351. https://doi.org/10.1097/jcma.0000000000001230 (2025).
Moon, J. H., Jeong, S., Jang, H., Koo, B. K. & Kim, W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: A nationwide cohort study. EClinicalMedicine 65, 102292. https://doi.org/10.1016/j.eclinm.2023.102292 (2023).
Cho, S. H. et al. Impact of steatotic liver disease categories on atrial fibrillation in type 2 diabetes: A nationwide study. Sci. Rep. 15, 11430. https://doi.org/10.1038/s41598-025-94783-8 (2025).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
G.K., K.H., and J.H.K. conceived and designed the study. S.H.C. and K.L. collected and analyzed the data. S.H.C. interpreted the results and wrote the manuscript. G.K., K.H., and J.H.K. edited the manuscript and contributed to discussion. All authors contributed to the discussion and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All procedures involving human participants were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement for informed consent was waived because the data were publicly available and de-identified.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cho, S.H., Kim, G., Lee, Kn. et al. Impact of smoking and physical activity on cardiovascular outcomes in type 2 diabetes across steatotic liver disease categories. Sci Rep 15, 31029 (2025). https://doi.org/10.1038/s41598-025-16750-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16750-7