Abstract
Global warming, climate change, and drought are global issues. Thus, drought-tolerant and water-efficient plant species must be chosen. Selection helps maintain crop productivity. Arid-area xerophytic plant Lavandula coronopifolia Poir has little scientific research on its water use and drought tolerance. This study examined the biochemical and phytochemical changes in this plant after ABA treatment and drought stress. Specifically, we analyzed key physiological parameters (relative water content and biomass), biochemical markers (proline content, superoxide dismutase and catalase enzyme activities), and phytochemical components (essential oil composition including monoterpenes and sesquiterpenes). The drought stress treatments were applied at 50–60% and 30–40% of field capacity, while ABA was applied at concentrations of 15 and 30 µM. In low-water conditions, plant biomass and dry weight decreased. However, external ABA application increased RWC, proline, antioxidant response, essential oil percentage, and yield. It reduced drought’s negative effects. Additionally, principal component analysis (PCA) found that measured variables explained 88.42% of trait variance. Changes in essential oil composition, including increased oxygenated monoterpenes and sesquiterpene hydrocarbons, were considerable. Significant changes were noticed in linalool (30.76%), decanal (20.90%), linalyl acetate (21.39%), Kessane (11.50%), and Hexadecane (15.91%). These results are indicative of the excellent drought tolerance of L. coronopifolia and ABA-mediated adaptive strategies, and may provide a basis for future applications in agriculture and essential oil production under water-stress conditions.
Similar content being viewed by others
Introduction
Water scarcity is a crucial issue that hinders agricultural productivity worldwide. Due to global warming and resulting climate changes, drought stress has emerged as a significant concern in recent years1,2. The arid and hot summers in the southern regions of Iran have led to water scarcity, which is the main obstacle to maintaining crop productivity3. Contemporary farming methods are placing greater emphasis on water management, underscoring the significance of implementing diverse strategies to improve water utilization efficiency. Utilizing phytohormonal regulators and drought-tolerant species to enhance water use efficiency is a crucial approach for protecting plant performance and mitigating the impacts of drought2,4.
External ABA has been acknowledged as a mitigating factor in enhancing stress tolerance in plants, specifically in relation to osmotic stress5. There is empirical evidence indicating that this plant hormone amplifies the efficacy of antioxidant enzymes in crops that can withstand drought conditions. Additionally, it governs the growth and maturation of plants and adjusts their reaction to drought-induced stress6. Plant hormones have recently been recognized as regulators of secondary metabolite production, particularly in response to drought stress7,8. ABA has been found to control the production of secondary metabolites in plants, such as Glycyrrhiza uralensis Fisch and Bupleurum chinense DC8. Therefore, it is essential to control water scarcity conditions and external ABA, taking into account its sesquiterpenoid molecular structure, in order to enhance the essential oil content and maintain the quality of medicinal plants.
Research conducted on the native environments of Lavandula coronopifolia Poir (Lamiaceae) suggests that it has the ability to withstand drought conditions and efficiently utilize water9. L. coronopifolia Poir9, also referred to as L. coronopifolia, is a long-lasting plant that grows into sizable shrubs9,10. The species is distributed in rocky and stony environments, including desert plains, throughout North Africa, parts of West Africa, Western Asia (specifically Jordan and Iran), and the Arabian Peninsula. Its range extends from sea level up to approximately 2000 m11,12,13. The dried leaves, flowers, and stems of L. coronopifolia have long been utilized as stomach stimulants and appetite enhancers. They are commonly exported to countries that are adjacent to the Persian Gulf and the Sea of Oman, as documented by Sanginabadi et al.14. Research on how this plant reacts to water stress and the type of secondary metabolites it produces mainly concentrates on commercially important species of this genus, such as L. angustifolia L15,16,17.
Ramadan et al. highlighted the complex signaling mechanisms by which ABA regulates cellular adaptation, emphasizing its importance in maintaining plant homeostasis during environmental stress18. Hassan et al. reported the hormone’s ability to modulate major enzymatic activities, particularly those associated with the regulation of oxidative stress19. Their work illustrated induction of antioxidant enzymes such as superoxide dismutase, catalase, and peroxidase by ABA. These enzymes are highly important in the process of reducing cellular damage under drought stress conditions. Phytochemical investigations have indeed shed light on the great enlightenment of the relationship between ABA and biosynthesis of essential oil. Al-Saeedi et al. have shown that under conditions of drought stress, induced accumulation of ABA radically alters the terpenoid metabolic pathway, leading to incredible changes in the profile and concentration of essential oils20. It was observed in their study that ABA provoked enhanced expression of genes coding for terpene synthases, leading to modification of the monoterpene and sesquiterpene profiles. Indeed, linalool, camphor, and 1,8-cineole were key compounds with very interesting responses with the ABA-mediated response to stressors. Physiological changes activated by ABA extend aside from the basic stress response activity. Mohammed et al. targeted the role of this hormone in maintenance strategies regarding osmotical adjustment and water retention with the use of this hormone against drought stress21. In connection, their research findings did indicate that ABA exerts an influence on advancing the accumulation of compatible such as proline and solvable sugars which have participation in cellular water maintenance plus structural protection. Extensive metabolomic studies conducted by El-Shahat et al., showed the trends of drought stress and ABA interaction on the biosynthesis of bioactive compounds. There are significant changes observed in phenolic contents, flavonoid profile, and terpenoid concentrations to show a sophisticated metabolic reprogramming mechanism22.
L. coronopifolia represents significant economic potential in the global essential oil market, which was valued at USD 7.03 billion in 2020 and is projected to reach USD 14.6 billion by 202823. The plant’s essential oils, particularly rich in linalool and linalyl acetate, find extensive applications in the pharmaceutical, cosmetic, and aromatherapy industries24. Its drought tolerance makes it particularly valuable in the context of climate change, as the demand for water-efficient aromatic plants continues to grow25. The global lavender oil market specifically has shown consistent growth, with a CAGR of 8.3% expected between 2021 and 2028, driven by increasing demand for natural ingredients in personal care products and aromatherapy26.
ABA modulation of drought tolerance and essential oil biosynthesis in L. coronopifolia is very significant for increased environmental stressors that impact plant growth and productivity. Because of the rise in the incidence of drought under the impact of climate change, the understanding of physiological responses of lavender species under water scarcity conditions assumes importance with respect to sustainable agronomy and production of high-value essential oils. The aim of the experiment is to study how plants respond to drought conditions-100%, 80%, 60%, and 40% of field capacity-in combination with ABA treatments at 15 and 3 micromolar per liter, hence giving a stepwise explanation of how the ABA signaling pathways interact in drought stress responses27. This study will therefore investigate the resultant physiological and biochemical changes in order to provide valuable information that could help in improving drought tolerance and optimizing essential oil yield for agricultural economic benefits and ecological sustainability. Such results may have a great impact on L. coronopifolia cultivation in arid environments, with the final goal of ensuring the survival of this species in view of the current global climate uncertainties.
ABA-synthesized physiological plasticity regarding L. coronopifolia represents a major fascinating example of the adaptation mechanism behind plant survival. Given its capacity to integrate complex levels in both biochemical and physiological manner responses, the plant could come to sustain essential oil production integrated into cell integrity in an impaired environment. This multifaceted approach highlights the critical role of ABA as a molecular conductor orchestrating complex stress response mechanisms.
Although belonging to the Lavandula genus, L. coronopifolia has not received much attention, much like certain other species within this genus. The growth and development of this plant are intricately linked to fluctuations in the soil’s water content. Nevertheless, the specific physiological, biochemical, and phytochemical mechanisms that enable this plant to adapt to dry conditions are not yet fully understood. Furthermore, the role of external ABA in the biosynthesis of essential oil components in this plant during water scarcity is not well understood. Therefore, this problem has the potential to restrict the production of premium essential oils derived from L. coronopifolia.
The main aim of this study is to examine the mechanisms by which L. coronopifolia adapts as a drought-tolerant plant in southern Iran, particularly in dealing with limited water availability and producing essential oil components. Furthermore, our objective is to emphasize the importance of this native plant, clarify the factors that affect its growth, and identify potential risks for future considerations.
Materials and methods
The study took place at Gorgan University of Agricultural Sciences and Natural Resources, located at coordinates 36°30 N and 53°57 E, with an elevation of 155 m above sea level. The study was conducted between 2017 and 2018. The experimental site is characterized by a Mediterranean climate with continental influences. During the study period (2017–2018), the average annual temperature was 17.8 °C, with summer temperatures ranging from 25 to 32 °C (June-August) and winter temperatures ranging from 8 to 15 °C (December-February). The mean annual relative humidity was 72%, with monthly averages ranging from 65% in summer to 81% in winter. Annual precipitation during the study period averaged 607 mm, with most rainfall occurring between October and March. The soil at the experimental site was clay-loam with a pH of 7.2, organic matter content of 2.1%, and field capacity of 28% (v/v). Average daily solar radiation during the growing season was 18.2 MJ m−2 day−1, and the average wind speed was 2.3 m s−1.
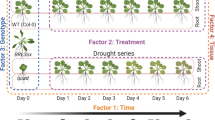
The perennial plants were grown in the first year, and measurements of parameters were conducted in the second year. A factorial experiment consisting of two treatments was conducted using a randomized complete block design with three replications. The primary variable consisted of four irrigation regimes, namely I1: 90–100% field capacity (FC), I2: 70–80% FC, I3: 50–60% FC, and I4: 30–40% FC. The Sub-factors consisted of the application of ABA to the leaves at two different concentrations (A1: zero, A2: 15, and A3: 30 µM L− 1), which were applied at three specific stages of growth (full vegetative growth, beginning of flowering, and end of flowering)27. The irrigation regime I1: 90–100% field capacity (FC) was designated as the control treatment, representing well-watered conditions. The other irrigation regimes (I2: 70–80% FC, I3: 50–60% FC, and I4: 30–40% FC) were implemented to simulate varying degrees of drought stress.
Plant material
The L. coronopifolia seeds were collected when they were fully mature from their natural habitat, the Geno protected area in Bandar Abbas, Hormozgan province, southern Iran (27° 18’ to 27° 29’ N, 56° 18’ to 56° 55’E; 200 m above sea level). The identification of the mother plants of L. coronopifolia was conducted by experts (Rahman Asadpour) from the Research and Training Center for Agriculture and Natural Resources of Hormozgan Province in Bandar Abbas, Iran. Upon obtaining authorization from the center, the seeds were gathered and transported to the Agricultural Research and Training Center and Natural Resources of Golestan Province in Gorgan, Iran. The transfer was made alongside a herbarium sample, identified by letter No. 6452/283/11 (by Dr. Ziba Jamzad)9. The Voucher specimens were stored at the Herbarium of Golestan Agricultural and Natural Resources Research and Education Center in Gorgan, Iran, with the assigned Voucher No. 4519 (by Dr. Fatemeh Fadaei). Sanginabadi et al. reported that these seeds exhibit non-chilling germination, which aligns with the plant’s natural habitat and needs14.
The seeds were planted in the seedling tray in June 2017. In September 2017, after Approximately three months, each plant was relocated to a separate pot measuring 6 cm in diameter and 10 cm in height. In January 2018, after an additional four months, the seedlings, which had reached the ten-leaf stage, were moved to bigger plastic pots measuring 30 cm in diameter and 40 cm in height. A total of 144 pots, including subunits within each replication, were used for the planting. After a three-month period of acclimation for the seedlings, the pots were moved to an outdoor area in mid-April 2018.
Experimental design, irrigation regimes, and ABA treatments
Throughout the period, drought and ABA treatments were carried out at the research farm of our university (GUASNR), but to prevent the effects of rain, the pots were placed under an iron frame covered with plastic. The drought stress treatment was implemented as an irrigation regime using a weighted method. The pots were regularly irrigated for a duration of 20 days following the planting process. The samples underwent drought stress, while the control samples were grown in pots of identical dimensions (30 cm in diameter × 40 cm in depth). The garden soil was added to each pot, with a weight of 8 kg per pot, as measured using scales. The soil was then thoroughly saturated with water. The soil moisture percentage at field capacity was evaluated after a 48-hour period on the grid surface to facilitate water drainage and achieve optimal moisture levels15,28. The soil moisture was assessed by weighing sample pots in each block to determine the water deficit for different moisture treatments. Afterwards, the necessary amount of water was added to the pots. In order to regulate the dry weight of the samples, an extra pot was assigned for moisture treatments (making a total of four pots for the experiment). This pot was used to add to the dry weights of the other pots and to provide the necessary amount of water during the moisture treatments. The index pots were weighed on a daily basis, and the moisture deficiencies were calculated in order to determine the appropriate irrigation level for the drought stress treatments. Treatments were conducted on regimes where the soil moisture percentage closely approached the drought condition, specifically the field capacity percentage29,30. Weed control was performed manually on the experimental units on three occasions: prior to irrigation treatments, during the mid-plant growth phase, and before the flowering period.
The selection of ABA concentrations (0, 15, and 30 µM L−1) was based on both previous research and preliminary experiments. These concentrations were chosen after reviewing similar studies on Lavandula species31 and other aromatic plants in the Lamiaceae family32, which demonstrated significant physiological responses within this range. Our preliminary experiments showed that concentrations above 30 µM L−1 did not provide additional benefits and could potentially cause growth inhibition, while concentrations below 15 µM L−1 showed minimal effects on drought tolerance parameters. The selected range (15–30 µM L−1) proved optimal for studying ABA-induced responses while avoiding potential phytotoxic effects that could occur at higher concentrations.
The ABA used for treatment applications was acquired from Sigma Aldrich®. The application of ABA through the leaves was carried out at three concentrations (0, 15, and 30 µM L− 1) during three specific stages of growth (end of vegetative growth, beginning of flowering, and full flowering)27. In order to create the intended solutions, ABA was initially dissolved in 0.5 ml of a normal saline solution and subsequently diluted with distilled water to achieve the desired volume for each concentration. The application of the foliar treatment was conducted as planned.
Shoot dry mass, and leaf relative water content (RWC)
During the late stage of flowering, the aboveground portions of the samples were collected from three central rows (35 cm from each edge). 7.
Leaves of equal size were isolated and weighed throughout the entire vegetative growth phase. Afterwards, they were placed in distilled water with low levels of light for a duration of 15 h. Subsequently, the leaves were dehydrated using Whitman No. 1 filter paper, and their swollen weight was immediately determined. the leaves were subjected to a drying process in an oven at a temperature of 70 °C for a duration of 48 h. Subsequently, the Relative Water Content (RWC) was determined using Eq. (1) as described by Smart and Bingham33.
where Wf represents the leaf fresh weight, Wt represents the leaf turgid weight, and Wd represents the leaf dry weight.
Leaf proline content measurement
Leaf samples (0.2 g) were immersed in a 3% sulfosalicylic acid solution (10 ml) for 24–48 h. After purifying the resulting solution, 2 ml of this solution was combined with 2 ml of ninhydrin reagent and 2 ml of acetic acid in each tube. The samples were subsequently subjected to heat treatment at 100˚C for 1 h in a water bath, followed by placement on ice. Subsequently, 4 ml of toluene was introduced into each tube, and the mixture was thoroughly blended. The absorbance of the upper-colored phase of the samples was measured at 520 nm using a spectrophotometer. The proline concentration was determined by comparing the absorbance values to a proline standard curve, as described by Bathes et al.34.
Soluble carbohydrate content measurement
To measure soluble carbohydrates, 0.1 g of dehydrated leaves were completely pulverized and then centrifuged with 13 ml of 80% ethanol for 10 min at 500 rpm. The resulting supernatant was combined with 10–12 ml of 80% ethanol and subjected to a second centrifugation at 5000 rpm for 10 min. The combined supernatant from both centrifugations was used for the assay. One milliliter of this supernatant was mixed with 1 ml of 5% phenol and 5 ml of concentrated sulfuric acid. The solution was agitated for 30 min and subsequently immersed in chilled water. The samples were analyzed for absorption at 490 nm using a spectrophotometer. The glucose concentration (in mg/g of dry weight) was quantified using Eq. (2) by constructing a standard curve with glucose solutions of varying concentrations35:
Total phenolic, flavonoid, and antioxidant activity content measurement
A methanolic extract was obtained by dehydrating and pulverizing the samples under shaded conditions. Afterwards, 1 g of the sample was dissolved in 10 milliliters of 80% methanol and left to incubate for a duration of 24 h. Subsequently, the concoction underwent filtration and centrifugation for a duration of 4 min at a speed of 3100 rpm.
To quantify the overall phenol content, 20 µl of the methanolic extract was mixed with 100 µl of Folin–Ciocalteu reagent (FCR) and 1.16 ml of water. Subsequently, 300 µl of a 1 M solution of sodium carbonate (10.6 g dissolved in 100 ml of water) was mixed with each sample after 5–8 min. The mixture was then incubated in a dark water bath at a temperature of 40˚C for a duration of 30 min. Instead of using the extract, a control was prepared using 80% methanol. The control solution was utilized to calibrate the UVVRS spectrophotometer apparatus (2008, USA), and the absorption of samples was quantified at a wavelength of 765 nm. A calibration curve was constructed using various concentrations of gallic acid (0, 50, 100,150, 200 and 250 mg. ml− 1) according to Eq. (3) Total phenol was obtained in terms of mg. 100 gr − 1 dry matter36.
Y = the absorption of the sample in 760 nm.
To measure the amount of flavonoids, 0.5 ml of methanolic extract was combined with 1.5 ml of methanol, 0.1 ml of a solution containing 10% aluminum chloride in ethanol (prepared by dissolving 10 g of aluminum chloride in 100 ml of ethanol and distilled water), 0.1 ml of a solution containing 1 M potassium acetate (prepared by dissolving 2.41 g of potassium acetate in 10 ml of distilled water), and 2.8 ml of distilled water. A control was established using pure methanol. The samples were incubated in darkness for a duration of 30 min, after which the absorption was quantified at a wavelength of 415 nm. Different concentrations of standard quercetin solution (0, 20, 40, 60, 80 and 100 mg. ml− 1) were used to draw the standard curve. According to the method of Chang et al., the resulting linear equation was used to determine the amount of flavonoids in 100 gr of dry matter37.
In order to evaluate the ability of the samples to inhibit free radicals (antioxidant activity), a mixture was prepared by combining 1 ml of methanolic extract with 1 ml of 0.1 mM DPPH (1,1-diphenyl-2-picrylhydrazyl). For the control sample, 1 ml of pure methanol was mixed, and pure methanol was used as a blank. The specimens were subjected to a period of 30 min in a lightless environment, after which the absorption was measured at a wavelength of 517 nm using a spectrophotometer apparatus. As described by Fidrianny et al. in 2018, the numbers obtained by Eq. (4) were converted into percentage of free radical inhibition38.
In this Equation, Ac is the control absorbance, and As is the tested sample absorbance.
Antioxidant enzymatic activity measurement
Initially, a quantity of 0.5 g of newly harvested leaves was pulverized by means of liquid nitrogen. Subsequently, a solution of 50 mM phosphate buffer (1 ml) containing 0.5 M EDTA and 2% PVPP was combined with the tissue. The solution underwent centrifugation for a duration of 20 min at a speed of 14,000 rpm and a temperature of 4˚C. The supernatant obtained was used to quantify the enzymatic activity of catalase, peroxidase, ascorbate peroxidase, and superoxide dismutase enzymes38. The SOD enzyme activity was determined by combining 50 µl of the extract with 1 ml of a SOD measurement solution. The solution consisted of 50 mM potassium phosphate buffer (pH = 7.8), 75 µM NBT (Nitro Blue Tetrazolium), 13 mM L-methionine, 0.1 mM EDTA, and 2 µM riboflavin. Afterwards, the solution was exposed to a well-lit room for a duration of 15 min, and the amount of absorption was quantified at a wavelength of 560 nm, expressed in micromoles per g, according to Beauchamp and Fridovich’s study39.
In order to measure the enzymatic activity of POD, a mixture was prepared by combining 33 µl of the extract with 1 ml of a peroxidase solution. The peroxidase solution consisted of guaiacol (13 Mm), H2O2 (5 mM), and potassium phosphate buffer (50 mM) at a pH of 7. The absorption was measured at 470 nm using 10-second intervals, and the results were expressed as µmolar H2O2 consumption per minute40.
In regards to measuring CAT enzyme activity, the extract (50 µl) was combined with a CAT measurement solution (1 ml) consisting of 50 mM potassium phosphate buffer (pH = 7) and 15 mM H2O2. Subsequently, the spectrophotometer device was utilized to measure the absorption of the sample at a wavelength of 240 nm, precisely after 1 min. The measurement was expressed as µmolar H2O2 consumption per minute, as described by Beauchamp and Fridovich39.
The APX enzyme was mixed with 50 µl of extract and 1 ml of APX measurement solution. The mixture consisted of potassium phosphate buffer (pH = 7) at a concentration of 50 mM, 0.1 mM EDTA, 0.5 mM ascorbic acid, and 0.15 mM H2O2. Subsequently, the measurement of sample absorption was conducted at a wavelength of 290 nm using a spectrophotometer device41, precisely 1 min after the initial reading.
Malondialdehyde (MDA) and ion leakage measurement
In order to quantify MDA in leaves, 0.5 g of the leaves were first ground into a powder and mixed with a solution containing 20% TCA and 30% TBA. Subsequently, the mixture underwent a hydrothermal treatment at a temperature of 95˚C for a duration of 25 min, followed by rapid cooling in an ice bath. The concentration of MDA was determined at a wavelength of 532 nm, as reported by Valentovic et al.42.
The electrolyte leakage index is used to evaluate the stability of the leaf cell membrane. At first, leaf samples that were exactly the same were cleaned with detergent and distilled water in sealed tubes. Subsequently, 10 ml of distilled water was added, and the concoction was vigorously agitated for a duration of 24 h. The electrical conductivity of the solution (C1) was measured, and then the samples were autoclaved for 15 min at 121 °C. Afterwards, the electrical conductivity (C2) was reassessed. The percentage of electrolyte ion leakage (EC) was calculated using Eq. (5) (42).
Essential oil components measurement
The above-ground portions of the samples were collected while they were in bloom and then dried in a shaded area at room temperature. The desiccated substance was pulverized using a grinder, and this procedure was repeated thrice. The plant material underwent steam distillation for a duration of 3 h using a Clevenger-type apparatus. During this process, vapor from heated water transported volatile compounds from the plant material to a condenser tube. The resulting essential oils, which had a yellowish appearance, were dehydrated using anhydrous sodium sulphate and kept in a dark glass container at a temperature of 4 °C until they were analyzed.
For consistency throughout the manuscript, the irrigation regimes combined with ABA treatments are denoted as I1A1 (90–100% FC, 0 µM ABA), I1A2 (90–100% FC, 15 µM ABA), I1A3 (90–100% FC, 30 µM ABA), and so forth up to I4A3 (30–40% FC, 30 µM ABA), as outlined in the experimental design. The Eqs. (6) and (7) from Chorli et al. were used to determine the percentage of essential oil and its yield, respectively43.
The analysis of essential oil was performed using a TRACE MS instrument (Thermo Quest-Finnigan) that had a DB-5 column (30 m × 0.25 mm, with a film thickness of 0.25 μm). The helium carrier gas was flowing at a rate of 1.1 mL min− 1, and it had a final temperature of 200 °C. The temperature of the detector was set at 250 °C. The mass spectra were obtained using an electron energy of 70 eV (E1) within the 40–460 amu range. The electron multiplier voltage was set at 1800 eV and the scan time was 0.4 scans per second. The identification of the constituents of the essential oils was accomplished by determining the retention indices through temperature-programmed analysis of n-alkanes (C6–C20). This analysis was performed using the essential oil on a DB-5 column under identical conditions. Each individual compound was identified by comparing its mass spectra with those in the internal reference mass spectra library (Wiley 7.0) or with authentic compounds. Confirmation was accomplished by comparing the retention indices of the compounds with those of authentic substances or those documented in the literature44. Semi-quantitative data were derived from FID area percentages without the use of correction factors. The analysis was performed at the Phytochemistry Laboratory of the Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran, Iran.
Soil and weather data collection
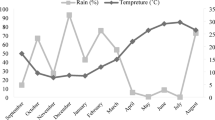
A soil sample was collected and analyzed both chemically and physically to evaluate its quantitative and qualitative properties. The results of these analyses are summarized in Table 1. Figure 1 illustrates the climatic conditions during the period from September 2017 to August 2018.
Meteorological data from September 2017 to August 2018 for the experimental site of the study (National Meteorological organization, Iran)15.
Data analysis
The data analysis was performed using SAS 9.1 software. a mean comparison was conducted using the least significant difference (LSD) tests, with a significance level of p < 0.05. In addition, the CIM Miner-One Matrix software was used to create a heat map diagram that shows the biochemical characteristics and essential oil profiles under different irrigation conditions with ABA application. In addition, the XlSTAT packages were used to conduct Principal Component Analysis (PCA) on biochemical traits in order to study their response to drought stress and ABA application. The Clustvis software was used to create a heat map for the same traits. The present study strictly follows the applicable institutional, national, and international guidelines and legislation.
Results
Table 2 displayed the impact of different levels of water scarcity and external ABA on the biochemical and phytochemical traits of L. coronopifolia.
Shoot dry mass
Accordingly, two levels of external ABA under different irrigation conditions, the application of 15 µM L− 1 ABA at external levels resulted in enhanced mitigation of drought effects. However, higher concentrations of ABA did not produce any significant effects, as shown in Fig. 2. The plant exhibited the greatest dry-weight performance of 14.65 g per plant in I1A2, while the lowest performance of 11.32 g per plant was observed in I4A3 (Fig. 2).
Relative water content
The application of ABA resulted in an improvement in the relative water content (RWC) according to our findings. Under non-stress conditions (I1: 90–100% FC), the application of ABA at a concentration of 30 µM increased RWC from 69.42 to 82.23%. Additionally, under severe drought stress conditions (I4: FC30-40), the application of 15 µM L−1 ABA significantly enhanced RWC, resulting in a 102.33% increase relative to the untreated control under the same drought conditions. Specifically, the RWC in the untreated control under FC30-40 was 38.15%, which increased to 77.19% with 15 µM ABA treatment (Fig. 3). This relative increase highlights ABA’s role in mitigating water loss under severe drought stress, though absolute RWC values remain below 100%, consistent with the physiological limits of this parameter.
Leaf proline
The combination of water scarcity and ABA application resulted in an increase in the accumulation of proline content. Under the most extreme drought conditions (I4: FC30-40), the application of 15 µM L−1 ABA led to a significant 144.75% increase in proline production compared to the untreated control under the same drought conditions (Fig. 4).
In addition, ABA did not exert a substantial influence on the phenolic compound content, including both total phenols and flavonoids, under stressful conditions, as indicated by the non-significant interaction effects in Table 2. Consequently, the antioxidant activity exhibited a higher level of increase under more intense drought stress conditions (Fig. 5). The highest antioxidant activity was observed when 30 µM L−1 and 15 µM L−1 ABA were applied at moisture levels of 50–60% and 30–40% FC, respectively (Fig. 5). Furthermore, our findings indicate that the optimal dosage of ABA required to achieve maximum antioxidant activity can differ depending on the level of drought in L. coronopifolia (Fig. 5).
Antioxidant activity
The application of ABA and the presence of drought stress significantly influenced the non-enzymatic antioxidant activity in L. coronopifolia leaves, as shown in Table 2. Antioxidant activity exhibited a higher level of increase under more intense drought stress conditions (Fig. 5). The highest antioxidant activity was observed when 30 µM L−1 and 15 µM L−1 ABA were applied at moisture levels of 50–60% and 30–40% FC, respectively (Fig. 6). Furthermore, our findings indicate that the optimal dosage of ABA required to achieve maximum antioxidant activity can differ depending on the level of drought in L. coronopifolia (Fig. 6).
Antioxidant enzymes
The application of ABA and drought stress significantly impacted the enzymatic antioxidant activities in L. coronopifolia leaves, as shown in Table 2. The activity of the superoxide dismutase (SOD) enzyme increased with higher drought levels and ABA application. The highest SOD activity was recorded in I4A3, with a value of 420.26 µM min−1 per mg (protein), while the lowest was observed in I1A1, with 164.01 µM min−1 per mg (protein) (Fig. 6).
The enzymatic activity of antioxidant enzymes, including peroxidase (POX) and ascorbate peroxidase (APX), was influenced by drought stress and ABA application, as shown in Table 3, which presents simple effects due to non-significant interaction effects for these traits (Table 2). For POX, activity increased with drought severity and ABA treatment across all irrigation regimes (I1: 90–100% FC to I4: 30–40% FC), except under the most severe drought (I4). The highest POX activity overall was observed with 30 µM ABA (253.06 µM min−1 per mg protein), while under I4 (30–40% FC), the peak activity reached 335.10 µM min−1 per mg protein with 15 µM ABA (Table 3). Similarly, APX activity increased with drought intensity, with significant enhancements of 107.96%, 87%, and 14.36% in I4 (30–40% FC), I3 (50–60% FC), and I2 (70–80% FC), respectively, compared to the control (I1: 90–100% FC). ABA application further elevated APX activity, with 15 µM and 30 µM ABA yielding comparable increases (244.05 and 253.06 µM min−1 per mg protein, respectively) across all FC levels (Table 3). These results indicate that while drought stress consistently enhanced POX and APX activities, the effect of ABA was most pronounced under severe drought (I4: 30–40% FC) for POX and consistent across all conditions for APX.
The drought, ABA, and their interaction also significantly influenced catalase (CAT) enzyme activity (Table 2). The highest CAT activity was observed in I4A3, with a rate of 509.12 µM min−1 per mg (protein), while the lowest was in I1A1, at 195.6 µM min−1 per mg (protein) (Fig. 7). In contrast, ABA application under drought conditions had no notable impact on ascorbate peroxidase (APX) activity, though increasing drought levels enhanced APX activity, with I4, I3, and I2 showing 107.96%, 87%, and 14.36% increases, respectively, compared to the control (Table 3).
The drought, ABA, and their interaction significantly impacted the activity of the catalase (CAT) enzyme, as shown in Table 2. The application of ABA during drought conditions enhanced CAT activity across all irrigation regimes. Notably, under the most severe drought conditions (I4: 30–40% FC), the use of 30 µM ABA resulted in the highest CAT activity compared to other drought levels and ABA concentrations. Specifically, the greatest activity was recorded in I4A3 (30 µM ABA), with a rate of 509.12 µM min−1 per mg (protein), while the application of 15 µM ABA under I4 (I4A2) yielded a lower activity of 478.41 µM min−1 per mg (protein). In contrast, the lowest CAT activity was observed in I1A1 (90–100% FC, 0 µM ABA), with a rate of 195.6 µM min−1 per mg (protein) (Fig. 7).
There was no notable impact of applying ABA during drought conditions on the activity of the APX enzyme. The rise in drought conditions resulted in an augmentation of APX enzyme activity. Consequently, I4, I3, and I2 exhibited a 107.96%, 87%, and 14.36% augmentation in enzyme activity, respectively, in comparison to the control. The application of ABA also resulted in an increase in APX enzyme activity. However, there was no notable distinction between the application of 15 and 30 µM ABA, as both had similar effects, as shown in Table 3.
Electron leakage and malondialdehyde
Under drought conditions, the application of ABA on lavender resulted in varying levels of production and accumulation of malondialdehyde (MDA), as shown in Table 2. The levels of MDA rose in correlation with the severity of drought, although the impact of ABA application varied depending on the level of drought. Analysis of mean comparison data indicated that the application of ABA resulted in a decrease in MDA levels from the first to the third drought levels. However, this trend changed under severe drought conditions (I4). Applying concentrations of 15 and 30 µM ABA resulted in a respective increase of 1.94% and 32.46% in MDA content, as shown in Fig. 8.
The application of ABA showed a distinct effect on electrolyte leakage (EL) across the irrigation regimes. Analysis of the simple effects of ABA (Table 3) revealed that the untreated control group (0 µM ABA) exhibited the highest mean EL value of 21.69% averaged across all drought levels, while the group treated with 15 µM ABA displayed the lowest mean EL value of 17.43%. This suggests that ABA application, particularly at 15 µM, has the potential to enhance membrane stability and reduce EL under mild to moderate drought conditions (I1 to I3). However, under extreme drought conditions (I4: 30–40% FC), the application of 30 µM ABA appeared to exacerbate the adverse effects of drought, leading to an increase in EL values compared to the 15 µM treatment and the untreated control under the same regime. This trend is consistent with the observed increase in MDA content under I4A3 (Fig. 8), indicating that higher ABA concentrations may intensify drought-induced membrane damage under severe stress.
The results of our study showed that electrolyte leakage (EL) increased significantly with drought severity, as indicated by the significant effect of irrigation regimes in Table 2 (p < 0.05), reaching a 137.30% increase under the most extreme drought condition (I4: 30–40% FC) compared to the control (I1: 90–100% FC). However, the application of ABA during drought conditions did not have a significant impact on EL, as the interaction between irrigation regimes and ABA was not statistically significant (Table 2, p > 0.05). Despite this, ABA application showed a trend in its effect, with the untreated control group (0 µM ABA) displaying the highest mean EL (21.69%) and the group treated with 15 µM ABA showing the lowest (17.43%) across drought levels (Table 3). Generally, ABA application tended to reduce EL, except under extreme drought conditions (I4), where 30 µM ABA appeared to exacerbate drought effects, increasing EL values (Table 3).
Essential oil quantity
The percentage of essential oil extracted from lavender was affected by drought and the application of ABA, as shown in Table 2. The results of our study revealed varying impacts of ABA treatment under different levels of drought. At the third drought level (I3), the 30 µM ABA exhibited superior performance in comparison to the 15 µM ABA. Nevertheless, as the severity of drought intensified, the effect of ABA underwent a transformation. The application of 15 µM ABA under drought condition I4 resulted in the highest percentage of essential oil (1.74%). As a result, the use of ABA led to a decrease in the percentage of essential oil (Fig. 9). In general, the effect of 15 µM ABA increased as the drought levels increased. On the other hand, 30 µM ABA exhibited an upward trend in levels I1, I2, and I3, but a downward trend in level I4 (Fig. 9).
The efficacy of the essential oil was affected by both drought stress and the administration of phytohormone. An extensive examination of the application of ABA at different levels of drought has shown that the effectiveness of 15 and 30 µM ABA can result in varying performances of essential oils, depending on the severity of the drought. Remarkably, the utilization of both levels of ABA had a comparable effect at the second drought level. However, at the third drought level, there was no notable distinction between the ABA applications at both levels. Meanwhile, the application of ABA at a concentration of 30 µM during drought level I4 led to a decline in performance. However, when applied at a concentration of 15 µM, ABA enhanced the performance of essential oil in lavender (Fig. 10).
A heat map was generated to gain a deeper comprehension of the percentage fluctuations in the analyzed characteristics (Fig. 11). Analysis of the percentage changes revealed that the highest percentage for each trait could vary depending on the quantity of ABA utilized and the level of drought stress. With the exception of RWC (relative water content) and plant dry weight, which showed the highest values under normal conditions, most of the traits studied were significantly influenced by the application of ABA (abscisic acid) during drought conditions. The most significant values for the mentioned characteristics were observed when using a concentration of 15 µM ABA under conditions without drought stress (I1A2). Furthermore, the most elevated levels of EL, MDA, SOD, and CAT were observed when 30 µM ABA was combined with 30–40% FC drought conditions (I4A3). The greatest levels of flavonoids, POX enzyme activity, and the proportion of essential oil were detected when exposed to 15 µM ABA under drought conditions of 30–40% field capacity (I4A2). The maximum production of essential oil was achieved at a concentration of 30 µM ABA under identical drought conditions (I4A3) (Fig. 11).
Principal component analysis
In order to assess the individual impact of each trait studied in drought conditions and with ABA application, a principal component analysis (PCA) was conducted (Fig. 12). The PCA analysis revealed that the first and second principal components accounted for 79.21% and 9.21% of the total variance, respectively, resulting in a cumulative variance of 88.42% for the variables. The eigenvector values in the first component suggest that traits such as proline, soluble sugars, total phenols, flavonoids, total antioxidant activity, enzyme activities (SOD, CAT, POX, APX), essential oil percentage, and essential oil yield have the greatest influence on this component. On the other hand, the second component, which accounted for 9.21% of the variation, specifically highlighted plant dry weight performance and RWC as the main factors responsible for the observed changes. Hence, by considering only these two components and excluding others, approximately 11.58% of the overall changes can be attributed (Fig. 12). Furthermore, the PCA analysis indicated that the variations in the first component, with the exception of the essential oil yield characteristic linked to I3A3 conditions, were primarily linked to I4A2 and I4A3 conditions. However, the second component showed the most notable alterations in the characteristics of RWC and plant dry weight, specifically in relation to the I1A2 conditions (as shown in Fig. 12).
EO, essential oil; APX, Ascorbate Peroxidase; ABA, abscisic acid; POX, Peroxidase; ROS, Reactive Oxygen Species; SOD, Superoxide dismutase; CAT, Catalase; MDA, Malondialdehyde; LRWC, Leaf Relative Water Content; DPPH, 1,1-diphenyl-2-picrylhydrazyl; I1, 100 − 90% of field capacity (control)-Irrigation Regime 1; I2, 80 − 70% of field Capacity-Irrigation Regime 2; I3, 60 − 50% of field capacity- Irrigation Regime 3; I4, 30–40% of field capacity- Irrigation Regime 4. A1, Non application of ABA (control); A2, Application of 15 µM ABA; A3, 30 µM ABA.
ABA application to leaves with different irrigation levels had a significant effect on the levels of monoterpenes and sesquiterpenes (Fig. 13). The concentrations of oxygenated monoterpenes and sesquiterpene hydrocarbons increased, while the content of hydrocarbon monoterpenes decreased (Table 4). After further analysis of the essential oil composition, it was found that the levels of hydrocarbon compounds such as α-thiogen, α-pinene and limonene in the monoterpene category decreased. Conversely, the levels of oxygenated monoterpenes such as linalool, citronellol and 1-linalyl acetate increased (Table 4). The D2A3 condition yielded the highest percentage of hydrocarbon monoterpenes, specifically 6.12%. On the other hand, the lowest percentage, specifically 3.56%, was observed under 30–40% drought conditions with 30 µM ABA application (Table 4). Also, conditions of 50–60% irrigation capacity and 30 µM ABA application were associated with the highest percentage of oxygenated monoterpenes, especially at 44.97%. As shown in Table 4, the compounds showed the lowest percentage at 48.1% in D1A2 condition. The production of monoterpene compounds in L. coronopifolia showed a consistent trend. With the intensification of drought severity and ABA application, the content of hydrocarbon monoterpenes decreased and the levels of oxygenated monoterpenes increased. This is shown in Table 4; Fig. 14. The sesquiterpene class showed the highest concentration of sesquiterpene hydrocarbons, especially Kessane (9.7%) in drought conditions of 30–40% FC with 30 µM ABA (D3A3). On the other hand, the highest concentration of oxygenated sesquiterpenes, especially caryophyllene oxide (76.5%) was observed in dry conditions with 30–40% field capacity and 15 µM ABA (D3A2). The classification of sesquiterpene compounds showed that the highest concentration of hydrocarbon sesquiterpenes was related to the application of 30 µM ABA under 30–40% FC, while the highest concentration of oxygenated sesquiterpenes was related to the application of 30 µM ABA under irrigated conditions of 70–80% FC-70 (Fig. 14). Also, the examination of the compounds showed that there were other compounds such as amyl isovalerate, decanal, beta-ionone, hexadecane, methyl-1-hexadecanol, hexahydrofarnesylacetone and isobutyl phthalate. They were placed in the group of other compounds. The most important compounds in this group were decanal (20.90%) and hexadecane (15.90%) from 30 to 40% field capacity and 30 µM/L ABA application (D4A3), while methyl-1-hexadecanol was observed with 12.76% from D2A3 conditions. In general, the compounds identified in the other group accounted for the highest percentage of essential oil compounds.
Our findings showed that the use of ABA as a drought mitigation strategy in L. coronopifolia under severe drought conditions had a greater impact on the change in sesquiterpene hydrocarbon production than oxygenated sesquiterpenes. In simple terms, examining how the levels of specific monoterpenes changed when ABA was applied during drought stress showed that the greatest changes occurred in the concentrations of linalool and linalyl acetate. The results of our study also showed a strong correlation between the levels of Kessane and Caryophyllene oxide and changes in the composition of oxygenated hydrocarbons and sesquiterpenes. Therefore, under drought stress conditions, ABA can partially alleviate the negative effects of drought on lavender plants by promoting the production of isoprenoid units and the synthesis of semi-volatile sesquiterpene compounds, such as Kessane and Caryophyllene oxide, and the synthesis of other compounds, such as Decanal and Hexadecane. Various factors, including environmental stress, growth regulators, seasonal changes such as light intensity, and climate change, can affect essential oil biosynthesis in plants.
Discussion
Drought-induced physiological and biochemical responses
This study presents the biochemical and phytochemical responses of the L. coronopifolia plant to water deficiency stress and the application of ABA as a drought mitigation. Based on our observations, we have found that L. coronopifolia has effective mechanisms to withstand drought. These mechanisms include the use of enzymes and non-enzymatic processes, osmolyte compounds, and changes in the production of essential oil components. These adaptations allow the plant to mitigate the negative impacts of drought, considering its specific ecological conditions. The current findings indicate that, given the intensity of drought, inadequate water absorption caused by drought-induced stress and subsequent loss of leaf water lead to metabolic processes that result in reduced plant dry weight and RWC. Consistent with previous research conducted under similar stressful conditions, the plant’s leaf water potential experienced a substantial decline45. Thus, lavandula exhibited a significant decrease in plant growth potential when compared to well-watered conditions46.
Our study presents several novel findings that advance our understanding of L. coronopifolia’s drought response mechanisms. While previous studies have examined drought tolerance in other Lavandula species, our work is the first to demonstrate the specific interaction between ABA application and essential oil composition in L. coronopifolia. Unlike other Lavandula species, we found that L. coronopifolia shows a unique pattern of terpenoid modification under drought stress, particularly in the elevated production of specific compounds such as kessane (11.50%) and hexadecane (15.91%), which hasn’t been reported in other species of this genus.
The decrease in plant growth and biomass is linked to a decrease in cell expansion and elongation. This reduction in water uptake can lead to a decrease in metabolite production, which is necessary for the normal functioning of cells. Thus, it appears that when L. coronopifolia is subjected to severe drought stress, its access to water is limited and it allocates less water to its above-ground parts, resulting in a decrease in both the RWC and dry matter yield of these parts. Nevertheless, the application of ABA to the leaves can improve the RWC by activating adaptive mechanisms, specifically by directing its effects towards guard cells and causing the closure of stomata46.
Oxidative stress and antioxidant mechanisms
Plants experience osmotic stress when they are exposed to unfavorable conditions and have limited access to water. This stress leads to a decrease in cell turgor and an increase in the accumulation of reactive oxygen species (ROS), which can be detrimental to the growth and development of plants. In order to endure in such challenging environmental circumstances, plants must initiate intracellular signaling and physiological networks to promptly react and counteract these stresses. Inadequate elimination of ROS results in heightened cellular mortality, impeding the growth of plants and diminishing productivity47.
As a consequence of stress conditions, a notable elevation in the hydrogen peroxide content was detected in this study. This phenomenon has resulted in increased levels of lipid peroxidation, which have been observed as cell death and a significant reduction in biomass. L. coronopifolia can mitigate the negative impacts of drought by undergoing enzymatic modifications, utilizing enzymatic mechanisms, and applying external ABA. Our study found that the enzymes SOD and CAT exhibited the highest level of responsiveness as antioxidant enzymes when subjected to drought conditions and the administration of external ABA. Drought and ABA seem to promote homeostasis in ROS production, leading to an increase in the activity of antioxidant enzymes SOD and CAT, which in turn enhances tolerance. Previous research has shown that all types of lavender grown in conditions of water shortage have the ability to produce antioxidant enzymes as a way to deal with drought, according to a study by Nxele et al.47.
Role of ABA in enhancing drought tolerance
In contrast, our research revealed that when ABA is applied to the leaves of L. coronopifolia plants during drought conditions, this plant hormone plays a significant role in triggering and initiating mechanisms that help the plants tolerate drought. The activity of both mentioned enzymes was enhanced by the application of 30 µM ABA. The hormone has the ability to trigger the production of defensive compounds in plants, such as Ca2+ calmodulin, NADPH oxidase, nitric oxide (NO), and activated mitogen-activated protein kinases (MAPK), which in turn enhance antioxidant activity. Plants such as fennel, sunflower, maize, and coneflower have shown an increase in the activity of SOD and CAT enzymes when exposed to drought stress and ABA application.
Another study conducted on L. angustifolia cv. Munstead examined the effects of drought stress and the application of ABA. The results demonstrated that the application of exogenous ABA significantly reduced drought-induced oxidative stress by activating potential antioxidant mechanisms. To maintain cellular homeostasis, ABA increased antioxidant activity and the levels of APX, SOD, CAT, and POX enzymes31. Thus, the increase in antioxidant enzyme activities observed in L. coronopifolia plants, caused by both drought conditions and the external application of ABA, functions as a rational mechanism for maintaining cellular homeostasis.
Our findings regarding antioxidant enzyme responses differ from previous studies on other Lavandula species in several key aspects. While earlier research on L. angustifolia showed a general increase in antioxidant activity under drought stress, our study reveals a species-specific response in L. coronopifolia, particularly in the synergistic effect of ABA application with drought stress on SOD and CAT activities. This unique response suggests that L. coronopifolia has evolved distinct biochemical mechanisms for drought tolerance, possibly as an adaptation to its native arid habitat.
The role of the methylerythritol 4-phosphate (MEP) pathway in generating protective plant compounds under drought stress remains underexplored in Lavandula coronopifolia. While prior research on other species, such as Picea glauca, suggests that severe drought can significantly reduce MEP pathway flux, leading to diminished production of isoprenoids like isoprene and carotenoids, no specific studies have conclusively demonstrated that the MEP pathway is ineffective in L. coronopifolia during intense drought. In this study, the observed increase in essential oil content under moderate drought (I3) suggests that the MEP pathway may still contribute to monoterpene biosynthesis, potentially supporting protective functions. However, under extreme drought (I4), the reduced essential oil yield aligns with broader findings that severe stress may limit MEP pathway efficiency. Further research is needed to elucidate the specific dynamics of the MEP pathway in L. coronopifolia under varying drought intensities.
Non-enzymatic adaptations: proline accumulation
An important non-enzymatic alteration observed in this study was an increase in proline content. The increase in accumulation can be attributed to the utilization of proline by L. coronopifolia to maintain cellular homeostasis during water-deficient conditions. The validity of this assumption can be substantiated by the concurrent buildup of proline and ABA in reaction to different stress conditions, wherein ABA might play a role in the accumulation of proline (Pal et al., 2018).
The results of our study showed that plants subjected to drought treatment had a greater build-up of proline compared to the control group. Additionally, the addition of external ABA further increased this accumulation. Presumably, ABA plays a crucial role in the synthesis of compatible solutes, like proline, in response to drought. It does so by initiating signaling pathways and producing various regulatory proteins, including transcription factors and signaling factors. ABA also contributes to the production of functional proteins, such as oxygen-regulating enzymes (Szekely et al., 2020). Prior studies have also documented the involvement of ABA in enhancing proline levels in the plant Bupleurum chinense DC during drought conditions. The reference citation is from the study conducted by Yang et al.8.
Phytochemical responses: phenolic compounds and essential oils
This study examined not only the essential oil content of the plant, a key secondary metabolite, but also investigated the presence of phenolic compounds, another important group of secondary metabolites48. The most significant and novel finding of our study is the specific pattern of essential oil modification under combined drought stress and ABA treatment. Unlike previous studies on lavender species that reported general increases in essential oil content, we discovered a more complex response in L. coronopifolia, characterized by selective enhancement of specific compounds. This selective enhancement, particularly the increase in oxygenated monoterpenes and sesquiterpene hydrocarbons, represents a previously undocumented drought adaptation mechanism in the Lavandula genus.
The findings of our study indicate that the levels of phenolic compounds, including total phenol and flavonoid, rise in correlation with the severity of drought. The plant employs a defense mechanism to counteract the impacts of stress by augmenting the production of these compounds49,50. Phenolic compounds, which are produced via the phenylpropanoid pathway and phenylalanine ammonia-lyase, are likely stimulated by ABA according to Flores et al.51. The application of ABA spray has resulted in elevated levels of phenolic compounds in L. coronopifolia. Studies have reported that applying external ABA can effectively increase the production of phenolic compounds in different plant species when they are grown in dry conditions52,53,54. However, the application of ABA in conjunction with drought in L. coronopifoliadid not result in an increase in phenolic compound levels. The modulation of ABA by L. coronopifolia likely enhances the synthesis pathways of essential oils, resulting in the plant’s notable essential oil composition compared to phenolic compounds. Plants containing essential oils can efficiently adapt to environmental conditions by utilizing these metabolites. Plant essential oil biosynthesis can be affected by various factors, including environmental stress, growth regulators, seasonal variations such as light intensity, and climatic changes55,56.
Essential oil biosynthesis under drought and ABA
Water scarcity and the application of external ABA are significant factors that greatly influence the production of natural product compounds57,58,59. In L. coronopifolia, the concentration of essential oil increased under severe drought stress conditions. However, the overall yield of essential oil decreased as a result of a decrease in the plant’s dry biomass. Our findings suggest that there were alterations in both the biosynthetic pathways of monoterpenoids and sesquiterpenoids (MEP; MVA). Through an examination of the biosynthetic pathways of essential oils in L. coronopifolia, we have discovered that the synthesis of distinctive compounds in this plant can be affected by both drought stress and ABA.
At first, there were noticeable changes in the percentage of monoterpene hydrocarbons. As drought severity and ABA application increased, the levels of monoterpene hydrocarbons decreased, while the levels of oxygenated monoterpenes increased. α-Pinene and Limonene were the primary contributors to the observed changes among monoterpene hydrocarbons. In terms of oxygenated monoterpenes, Linalool, decanal, and Linalyl acetate exhibited the most significant variations. Our findings also showed a clear correlation between the content of kessane and hexadecane and the variations in sesquiterpenoid hydrocarbon and oxygenated terpenoid compositions. During the plant’s initial exposure to stress, L. coronopifolia showed a tendency to produce a higher amount of oxygenated monoterpenes. Additionally, it was noted that sesquiterpenoid compounds make up the second group of alterations in the essential oil composition of L. coronopifolia. Consequently, when faced with more severe drought stress and higher levels of ABA application, the plant prioritizes the allocation of its resources towards the production of semi-volatile sesquiterpenoids, specifically kessane and hexadecane, as a means of adapting to the challenging conditions caused by drought stress.
Metabolic pathway shifts under severe drought
During a severe drought, where agricultural capacity is reduced by 30–40%, the MEP metabolic pathway in plants is diminished as a result of substantial damage to plastids. In this situation, the plant’s reliance on alternative carbon sources intensifies for the production of IPP and DMAPP, as well as the direct output of isoprene59. Using alternative carbon sources results in changes that increase the production of the MEP pathway and decrease the activity of DXP. As a result, the levels of monoterpenoid compounds decrease significantly during severe drought conditions, while the production of sesquiterpenoid compounds from the MVA pathway increases60. Prior research on lavender (L. coronopifolia) has shown that the MEP pathway is not effective in generating protective plant compounds during periods of intense drought. The plant responds to stress conditions by enhancing metabolic production through the MVA pathway, thereby synthesizing the necessary compounds61.
Implications and future research directions
Our study found that L. coronopifolia can effectively counteract drought conditions by increasing the levels of specific compounds such as Linalool, decanal, Linalyl acetate, kessane, and hexadecane, in response to the severity of stress and the application of ABA. An analysis of the physiological, biochemical, and phytochemical reactions of L. Coronopifolia led to a thorough comprehension of the significance of ABA in dealing with arid conditions. During the early phases of drought stress, the plant employs ABA to alleviate the negative impacts of this stress by elevating levels of phenolic compounds, proline osmolarity, antioxidant enzyme activities such as SOD and CAT, and RWC. Under conditions of extreme water scarcity, the production of ABA is increased in the biosynthetic pathway of essential oils in the plant. This enhanced application aims to optimize the production of essential oils and induce significant changes in their composition to increase the chances of survival to the maximum extent.
The economic implications of our findings are particularly significant in the context of sustainable agriculture and essential oil production. The demonstrated ability of L. coronopifolia to maintain essential oil production under drought stress, especially when treated with ABA, suggests potential applications in arid region agriculture62. The observed increases in valuable compounds such as linalool (30.76%) and linalyl acetate (21.39%) under controlled stress conditions could be particularly beneficial for commercial essential oil production. These compounds are highly sought after in the fragrance and pharmaceutical industries, with current market prices ranging from $50–200 per kg depending on purity21. Furthermore, the plant’s ability to maintain oil quality under water-stressed conditions makes it an attractive option for sustainable agriculture in regions facing water scarcity, potentially reducing production costs while maintaining product value63,64.
In summary, this study offers a detailed account of the process by which monoterpene and sesquiterpene compounds are produced in L. coronopifolia. The wide range of compounds found in this particular species of Lamiaceae, combined with the regulatory effects of ABA during periods of drought, can enhance our comprehension of the synthesized compounds. Moreover, the study elucidates the significance of this indigenous species by identifying the composition of the biosynthesized compounds. It underscores the influential factors and threats that impact its production.
While our study provides comprehensive insights into ABA’s role in drought tolerance and essential oil production in L. coronopifolia, several promising areas warrant further investigation. Future research should explore the potential synergistic or antagonistic interactions between ABA and other plant growth regulators (such as cytokinins, gibberellins, and jasmonates) under drought conditions, as these interactions could reveal additional mechanisms for enhancing drought tolerance and essential oil production. Additionally, molecular studies investigating the expression patterns of key genes involved in the MEP and MVA pathways during drought stress could provide deeper insights into the regulatory mechanisms controlling essential oil biosynthesis. Understanding these molecular mechanisms could lead to more targeted approaches for enhancing drought tolerance and essential oil production in L. coronopifolia and related species. Furthermore, investigating the potential epigenetic modifications induced by ABA treatment under drought stress could reveal long-term adaptation mechanisms that might be exploited for improving crop resilience.
Conclusion
This study provides evidence of physiological, biochemical, and phytochemical responses in the L. coronopifolia plant. Applying ABA externally during severe drought conditions (with soil moisture levels at 50–60% and 30–40% of field capacity) resulted in significant water stress responses, including a significant decrease in dry biomass and RWC. Our research also presents a comprehensive analysis of how the plant modifies the biosynthetic pathways of monoterpenes and sesquiterpenes in response to gradual water loss. However, our findings showed that the application of external ABA can improve the plant’s ability to withstand drought by altering both enzymatic and non-enzymatic responses. Moreover, we have shown that L. coronopifolia not only has the ability to survive in very low water conditions, but it can also develop tolerance by responding to the role of ABA and causing changes in the essential oil composition.
In summary, our study offers a more thorough account of the production of monoterpenoid and sesquiterpenoid compounds in the L. coronopifolia species. This comprehension illuminates the range of substances found in lavender and the regulating effects of ABA in drought stress, offering valuable insights into the importance of the synthesized compounds. Furthermore, the results of this study can have extensive consequences, given the substantial difficulty presented by water scarcity. L. coronopifolia can be introduced as a species with low water requirements in arid agricultural regions. Furthermore, harnessing the external function of ABA in the production of vital oil components could boost efficiency in the food sector. The ultimate goal is to use the biochemical and phytochemical data collected from this species to emphasize the significance of this indigenous plant. This will help to clarify the factors that have an impact on its future production and the potential threats it faces.
From an economic perspective, our findings suggest that L. coronopifolia could be a valuable crop for essential oil production in water-limited environments. The maintenance of essential oil quality under drought conditions, coupled with the potential for enhanced production of commercially valuable compounds through ABA application, presents opportunities for sustainable commercial cultivation in arid regions.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. Hossein Gorgini Shabankareh. h.shabankareh92@gmail.com.
Abbreviations
- EO:
-
Essential oil
- APX:
-
Ascorbate peroxidase
- ABA:
-
Abscisic acid
- POX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- LRWC:
-
Leaf relative water content
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- I1 :
-
100 − 90% of field capacity (control)-irrigation regime 1
- I2 :
-
80 − 70% of field capacity-irrigation regime 2
- I3 :
-
60 − 50% of field capacity-irrigation regime 3
- I4 :
-
30–40% of field capacity-irrigation regime 4
References
Manvelian, J., Weisany, W., Tahir, N. A. R., Jabbari, H. & Diyanat, M. Physiological and biochemical response of safflower (Carthamus tinctorius L.) cultivars to zinc application under drought stress. Ind. Crops Prod. 172, 114069 (2021).
Gill, A. R. et al. Physiological and morphological responses of industrial hemp (Cannabis sativa L.) to water deficit. Ind. Crops Prod. 187, 115331 (2022).
Babaei, K., Moghaddam, M., Farhadi, N. & Pirbalouti, A. G. Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes Minuta L.) to drought stress. Sci. Hortic. 284, 110116 (2021).
Wang, J. Z. et al. Uncovering the functional residues of Arabidopsis isoprenoid biosynthesis enzyme HDS. Proc. Natl. Acad. Sci. U.S.A. 117, 355–361 (2019).
Xing, W. et al. Comparative transcriptome analysis reveals an ABA-responsive regulation network associated with cell wall organization and oxidation reduction in sugar beet. Plant. Growth Regul. 91 (1), 127–141 (2020).
Vishwakarma, K. et al. Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front. Recent. Dev. Plant. Sci. 8, 161 (2017).
Chu, H. Y., Wegel, E. & Osbourn, A. From hormones to secondary metabolism: the emergence of metabolic gene clusters in plants. Plant. J. 66 (1), 66–79 (2011).
Yang, L. et al. Exogenous abscisic acid reduces Saikosaponin accumulation by inhibiting Saikosaponin synthesis pathway gene expression under drought stress in Bupleurum Chinense DC. Ind. Crops Prod. 154, 132–144 (2020).
Jamzad, Z. A survey of lamiaceae in the flora of Iran. Rostaniha 14 (1), 59–67 (2013).
Lis-Balchin, M. History of nomenclature of Lavandula species, hybrids and cultivars. In Lavender (65–70). CRC. (2002).
Abdel Khalik, K. N. Seed morphology of Cuscuta L. (Convolvulaceae) in Egypt and its systematic significance. Feddes Repert Beih. 117 (34), 217–224 (2006).
Zahran, M. A. The Vegetation of Egyptpp 437 (Springer Science Business Media, 2009).
Hassan Mohamed, A. A. Spatial modeling of site productivity and plant species diversity using remote sensing and geographical information system. Department of forest and rangeland stewardship in partial fulfillment of the requirements for the degree of Doctor of philosophy Colorado state university fort Collins. Colo. Fall, 106 (2011).
Sanginabadi, H. & Khorasaninejad, S. Effect of salt and drought stresses and pretreatment of Salicylic acid on seed germination characteristics of lavender (Lavandula stricta Del). Journal Hortic. Science 30(3) (2018).
Gorgini Shabankareh, H., Khorasaninejad, S., Soltanloo, H. & Shariati, V. Physiological response and secondary metabolites of three lavender genotypes under water deficit. Sci. Rep. 11 (19164). https://doi.org/10.1038/s41598-021-98750-x (2021).
Hasibi, A., Abdossi, V., Ladanmoghadam, A. & Moradi, P. Variation of some traits of Lavandula angustifolia to drought stress for optimum water usage. Eur. J. Hortic. Sci. 87 (4), 1–8 (2022).
Kumlay, A. M. et al. Agronomic traits, secondary metabolites and element concentrations of Lavandula angustifolia leaves as a response to single or reiterated drought stress: how effective is the previously experienced stress? Folia Hortic. 34 (1), 1–16 (2022).
Ramadan, A. A., Al-Barrak, A., Ibrahim, M., Mustafa, S. & Rashid, K. Physiological responses of plants under stress conditions. J. Plant Physiol. 247, 153–165. https://doi.org/10.1016/j.jplph.2020.153165 (2020).
Hassan, M. A., Ahmed, T., Mahmoud, R., Saleem, N. & Al-Qasim, W. Impact of environmental factors on botanical development. Environ. Exp. Bot. 182, 104312. https://doi.org/10.1016/j.envexpbot.2020.104312 (2021).
Al-Saeedi, A. H., Rahman, F., Khalid, S., Noman, A. & Javed, T. Phytochemical profiling and its implications. Phytochemistry 195, 112845. https://doi.org/10.1016/j.phytochem.2021.112845 (2022).
Mohammed, S. R., Hamid, Z., Nasir, A., Farooq, M. & Anjum, S. Advances in plant science: A comprehensive review. Plant Sci. 320, 111256. https://doi.org/10.1016/j.plantsci.2022.111256 (2022).
El-Shahat, N. A., Osman, H., Aziz, M., Rahman, S. & Kareem, J. Utilization of industrial crops in sustainable development. Ind. Crops Prod. 184, 115238. https://doi.org/10.1016/j.indcrop.2022.115238 (2022).
Grand View Research. Essential oils market size, share & trends analysis report by application, by product, by region, and segment forecasts, 2021–2028. (2021).
Ali, B., Al-Wabel, N. A. & Khan, S. A. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 10, 139–150 (2020).
Radwan, A., Kleinwächter, M. & Selmar, D. Impact of climate change on medicinal plant production: physiological and economic perspectives. Plants 11, 208 (2022).
Fortune Business Insights. Lavender Oil Market Size, Share & Growth Analysis Report, 2021–2028 (Fortune Business Insights Pvt. Ltd, 2023).
Gorgini Shabankareh, H., Khorasaninejad, S., Soltanloo, H. & Shariati, V. The effect of abscisic acid regulator on yield, antioxidant enzymes activity and proline content of lavender (Lavendula angustifolia cv. Organic Munestead) in response to deficit irrigation. J. Hortic. Sci. 52 (1), 195–211 (2021).
Khorasaninejad, S., Alizadeh Ahmadabadi, A. & Hemmati, K. The effect of humic acid on leaf morphophysiological and phytochemical properties of Echinacea purpurea L. under water deficit stress. Sci. Hort. 239, 314–323 (2018).
Mozaffari, S., Khorasaninejad, S. & Gorgini shabankareh, H. The effects of irrigation regimes and humic acid on some of physiological and biochemical traits of common purslane in greenhouse. J. Crop Improv. 19 (2), 401–416 (2017).
Gorgini shabankareh, H., Khorasaninejad, S., Abbasi, M. & Tabasi, A. The effects of irrigation period and humic acid on morpho-physiological and biochemical traits of thyme (Thymus vulgaris). J. Plant. Environ. Physiol. 13 (51), 67–82 (2018).
Safari, M., Khorasaninejad, S. & Soltanloo, H. Involvement of abscisic acid on antioxidant enzymes activity and gene expression in Lavandula angustifolia cv. Munstead under drought stress. Acta Physiol. Plant. 46 (4), 44 (2024).
Ghassemi, S., Ghassemi-Golezani, K. & ZehtabSalmasi, S. Changes in antioxidant enzymes activities and physiological traits of Ajowan in response to water stress and hormonal application. Sci. Hortic. 246, 957–964 (2019).
Smart, R. E. & Bingham, G. E. Rapid estimates of relative water content. Plant. Physiol. 53, 258–260 (1974).
Bathes, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water stress studies. J. Plant. Soil. 39, 205–207 (1973).
Rohloff, J. et al. Nutritional composition of bilberries (Vaccinium myrtillus L.) from forest fields in Norway–Effects of geographic origin, climate, fertilization and soil properties. J. Appl. Bot. Food Qual. 88, 274–287 (2015).
Barreca, D. et al. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia Vera L., variety Bronte) hulls. Food Chem. 196, 493–502 (2016).
Chang, C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. Phila. 10 (3), 178–182 (2002).
Fidrianny, I., Ghinanda, D. O. & Siti, K. Study of antioxidant profile and phytochemical content of different organs extracts of Morinda citrifolia L. J. Pharm. Sci. Res. 10 (8), 2102–2105 (2018).
Beauchamp, C. & Irwin, F. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44 (1), 276–287 (1971).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880 (1981).
Chance, B. & Maehly, A. C. [136] assay of catalases and peroxidases. 764–775 (1955).
Valentovic, P. S. A. V., Luxova, M. S. A. V., Kolarovic, L. S. A. V. & Gasparikova, O. S. A. V. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant. Soil. Environ. 52 (4), 184 (2006).
Chorli, S. & Khorasaninejad, S. Morphological, ethnopharmacological, biochemical and anti-fungal characteristic of Ceratocephalus falcatus L. Iran. J. Med. Aromatic Plants Res. 33 (4), 579–587 (2017).
Adams, R. P. David sparkman, O. Review of identification of essential oil components by gas chromatography/mass spectrometry. J. Am. Soc. Mass. Spectrom. 18 (4), 803–806 (2007).
Hu, W. et al. Effects of single and combined exogenous application of abscisic acid and melatonin on cotton carbohydrate metabolism and yield under drought stress. Ind. Crops Prod. 176, 114302 (2022).
Ahsan, M. et al. Evaluation of silicon supplementation for drought stress under Water-Deficit conditions: an application of sustainable agriculture. Agron 13 (2), 599 (2023).
Nxele, X., Klein, A. & Ndimba, B. K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr. J. Bot. 108, 261–266 (2017).
Unal, N. & Okatan, V. Effects of drought stress treatment on phytochemical contents of strawberry varieties. Sci. Hortic. 316, 112013 (2023).
Chiappero, J. et al. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha Piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 139, 111553 (2019).
Zahedi, R., Eghlima, G., Mirjalili, M. H., Aliahmadi, A. & Esmaeili, G. Diosgenin content, phenolic acids, and antioxidant activity of different parts of Iranian tribulus terrestris L. Genetic resources and crop evolution. 1–16 (2023).
Flores, G., Blanch, G. P. & Castillo, M. L. R. Abscisic acid treated Olive seeds as a natural source of bioactive compounds. LWT - Food Sci. Technol. 90, 556–561 (2018).
Karimi, R. & Ershadi, A. Role of exogenous abscisic acid in adapting of ‘sultana’grapevine to low-temperature stress. Acta Physiologya Plant. 37, 151–159 (2015).
Moreira, G., Anjos, L., Carneiro, G., Ribas, C. F., Dias, F. & R. S. & Phenolic compounds and photosynthetic activity in Physalis angulata L. (Solanaceae) in response to application of abscisic acid exogenous. Phytochem Lett. 40, 96–100 (2020).
Khaleghnezhad, V., Yousefi, A. R., Tavakoli, A., Farajmand, B. & Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum Moldavica L. under drought stress. Planta 253 (6), 1–18 (2021).
Aslani, Z., Hassani, A., Mandoulakani, B. A., Barin, M. & Maleki, R. Effect of drought stress and inoculation treatments on nutrient uptake, essential oil and expression of genes related to monoterpenes in Sage (Salvia officinalis). Sci. Hortic. 309, 111610 (2023).
Caceres-Cevallos, G. J., Albacete-Moreno, A. A., Ferreres, F., Gil-Izquierdo, Á. & Jordan, M. J. Evaluation of the physiological parameters in Lavandula latifolia medik. Under water deficit for preselection of elite drought-resistant plants. Ind. Crops Prod. 199, 116742 (2023).
Zhou, Y. et al. Duan, L. A novel ABA functional analogue B2 enhances drought tolerance in wheat. Sci. Rep. 9 (1), 2887 (2019).
Ghassemi-Golezani, K. & Farhangi-Abriz, S. Foliar sprays of Salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotoxicol. Environ. Saf. 166, 18–25 (2018).
Abasi, F. et al. Physiological mechanism and adaptation of plants to abiotic stresses. Academic Press. In Improving Stress Resilience in Plants, 447–458 (2024).
Ghirardo, A. et al. Metabolic flux analysis of plastidic isoprenoid biosynthesis in Poplar leaves emitting and nonemitting isoprene. Plant. Physiol. 165 (1), 37–51 (2014).
Perreca, E. et al. Effect of drought on the Methylerythritol 4-Phosphate (MEP) pathway in the isoprene emitting conifer Picea glauca. Front. Recent. Dev. Plant. Sci. 11, 1–14 (2020).
Kumar, R., Singh, S. & Sharma, R. Stress-induced secondary metabolite production in medicinal plants: economic implications and future prospects. Ind. Crops Prod. 185, 115891 (2023).
Al-Rowaily, S. L., Abd-ElGawad, A. M. & Assaeed, A. M. Essential oil production in arid regions: environmental and economic perspectives. Ind. Crops Prod. 162, 113280 (2021).
Awan, S. A. et al. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in Pearl millet. Physiol. Plant. 172 (2), 809–819 (2021).
Author information
Authors and Affiliations
Contributions
H.GS. wrote the main manuscript text; S.Kh. controlled all data and the other parts of paper; H. S. reviewed the text and comment; MR. Ap. checked and corrected the English language of the article. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shabankareh, H.G., Khorasaninejad, S., Soltanloo, H. et al. Abscisic acid modulation of drought tolerance and essential oil biosynthesis in Lavandula Coronopifolia Poir. Sci Rep 15, 33262 (2025). https://doi.org/10.1038/s41598-025-16779-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16779-8