Abstract
Understanding the physicochemical and structural properties of the dietary fiber present in date fruits is useful for its onward processing for food and non-food applications. This research fractionated dietary fiber from four date fruit cultivars into soluble and insoluble fractions and studied their structural features and physicochemical and thermal properties. Total dietary fibers content ranged 3.2–7.4 g/100 g with > 90% being insoluble. Insoluble fiber was crystalline and mainly composed of lignin, cellulose, and hemicellulose while the soluble fiber was amorphous and mainly contained pectin (> 50%). The lignin content was higher in Barakawi and Neghal (3.6 & 3.7 g/100 g) than in Shishi and Barhi (1.4 & 2.1 g/100 g). Guaiacyl lignin was present in the sclereid and parenchyma cells and syringyl lignin was present in the xylem vessels. The pectin in the date fruit soluble fiber is highly branched possibly by oligosaccharides such as type II arabinogalactan. Several furan and pyran-type residues with different linking patterns were observed in the soluble fiber fraction (α-L-arabinofuranose, α-L-rhamnopyranose, β-D-galactopyranose, α-L-glucopyranose). The insoluble fiber fraction was thermally more stable than the soluble fraction. Overall, this research provides a knowledge-base for onward valorization of date fruit soluble and insoluble fibers for various food applications.
Similar content being viewed by others
Introduction
Date palm (Phoenix dactylifera L., family: Arecaceae family) is one of the oldest fruit crops in the world, with a history of more than 6000 years1. The total production of date fruits in 2022 was 9.8 million tonnes, with Egypt, Iran, Saudi Arabia, United Arab Emirates, and Iraq ranking as the top producers2. Mature date fruits contain soluble sugars (60–75%), dietary fiber (2–15%), moisture (10–30%), protein (1.6–4.7%) and fat (0.2–0.7%)3,4. It provides health benefits including antioxidant, anticancer, antiviral, antimutagenic, antihyperlipidemic, immunomodulatory, and cholesterol-lowering effects5. Date fruits are consumed fresh, dried or processed into products like date syrup (or dibs), and date paste6. Date fruit pomace, a by-product of the date syrup processing, is rich in dietary fiber (42–49%) and can be used as a supplement in a wide range of foods including cereal7 meat8 snack bars9 and dairy products10.
Dietary fiber is defined as the portion of food that is resistant to digestion by human enzymes in the stomach and small intestine and it includes polysaccharides and lignin11. Depending on its physical and chemical composition, dietary fiber acts as a polymer matrix in the large intestine and affects various phenomena such as bacterial fermentation, water holding, and adsorptive and cation-exchange processes12. It contributes no calories to our diet but the metabolites of bacterial metabolism in the colon provide several health benefits13. The consumption of diet containing high levels of dietary fiber prevents constipation and inhibits obesity and diabetes and decreases the risk of coronary heart disease14. The recommended daily intake of dietary fiber varies across age and gender; in male: 19–50 years (38 g/day) and > 50 years (30 g/day), and in female: 19–50 years (25 g/day) and > 50 years (21 g/day)15.
Dietary fibers are broadly classified into soluble fractions including pectin, β-ᴅ glucan, fructan, and certain hemicelluloses and insoluble fractions including cellulose, hemicellulose, and lignin16,17. Total dietary fibers (TDF) in date fruit ranges 4.8–13.6 g/100 g dry matter18,19,20,21,22,23,24,25. The main fraction was the insoluble fiber (5.9–9.1 g/100 g dry matter) while the soluble fiber was a minor fraction (0.4–1.2 g/100 g dry matter)25. Lignin is the major reason for the differences in total dietary fiber amongst the date fruit cultivars23. Lignin exists independently of silica in the secondary cell walls of parenchyma and sclereid cells; silica exists independently of lignin in the spheroid phytoliths surrounding the sclereid cells, and there is a co-existence between lignin and silica in the spiral coils of the tracheid phytoliths26. Furthermore, lignin, arabinoxylan, galactomannan, and pectin significantly correlate with the hardness of date fruits27. Linear, β-glycosidically linked ᴅ-glucan containing both (1→3)- and (1→4)-linkages was previously isolated from date fruits28.
The importance of dietary fibers, including those derived from date fruits, is well accepted and is well known to the scientist and public. However, significant knowledge gaps exist in the in-depth understanding of their detailed physicochemical characteristics that are critical to the understanding of their nutritional value and processing and conversion to value added-products. In this study, we fractionated date fruit fibers into soluble and insoluble fractions and conducted further structural, physicochemical, and thermal analyses of both fiber types.
Materials and methods
Chemicals and reagents
Acetone, arabinose, ethanol, fucose, galactose, glucose, mannose, 1-methyl imidazole, myo-inositol, rhamnose, sodium hydroxide, sulfuric acid, xylose, citrus peel pectin, and cellulose were purchased from Sigma Chemical Company (St Louis, MO, USA), and are all analytical grades. Oat beta-glucan and wheat arabinoxylan were purchased from Megazyme, Neogen Europe Limited, Scotland, United Kingdom. Gelatin was purchased from Hassani Food Industries, Dubai, United Arab Emirates.
Sample Preparation and composition of the date fruit
Three date fruit cultivars (Barhi, Neghal, and Shishi) were collected from the Al-Foah Date Factory (Al ain, United Arab Emirates), and one cultivar from Sudan (Barakawi). These 4 date cultivars were selected to include soft, semi-hard, and hard date cultivars. The date fruits were manually de-seeded with knife, minced (electric meat grinder, 175026, ShipServ, London, SE1 7TY, United Kingdom), and desugared with 80% ethanol (6 times, 1:10 w/v). The desugared fruit was first dried at ambient condition (20–25 °C, 30–60% relative humidity, and 24 h) and then in a hot air oven (Binder GmbH, Im Mittleren Ösch 5, 78532 Tuttlingen, Germany) at 50 °C for 18 h. The dried fruit was ground (high speed electric dry food grinder, HC-700, Shuangfeng County, Loudi, Hunan Province, China) to 106–250 μm and stored in a desiccator. The protein content of the fiber was analyzed by Kjeldahl equipment with 6.25 as a nitrogen conversion factor. Desugared fruit powder (0.5 g) was ashed (550 °C, 5 h) in a muffle furnace (Carbolite, CWF 1100, Chamber furnace, Derbyshire, UK), allowed to cool, and then weighed.
Microscopy and staining of the lignin in date fruit
Elemental mapping was carried out on the silica phytoliths of the date fruit (Neghal variety) using a scanning electron microscopy-Energy X-ray dispersive spectroscopy (Jeol Analytical Scanning Electron Microscope, JEOL JSM-6010PLUS/LA, Tokyo, Japan) accelerating at 20 kV23. Lignin was localized in the date fruit using Mäule and Weisner staining29,30. Briefly, Mäule staining was conducted as follows: the slide was immersed in 1% (w/v) potassium permanganate solution for 5 min at room temperature, then washed with 3% HCl till the tissue color changed from black or dark brown to light brown, after which the tissue was treated with 0.1 M ammonia solution to produce a yellow-brown color for guaiacyl lignin units (softwood) and red-purple color for syringyl lignin units (hardwood). The stained section was dried and mounted with DPX (dibutyl phthalate polystyrene xylene) and covered lid and used for onward microscopic studies. Weisner staining was carried out by dipping the slide into 1% phloroglucinol for 5 min and 95% ethanol for 5 min. Then, a few drops of concentrated HCl were added to the tissue surface. The slide was then dried and mounted with DPX and a cover lid for onward microscopic studies. The Weisner stained section produced a red-purple color, characterizing guaiacyl units. Microscopic images were taken at 50x and 80x magnification (scale bars of 200 μm and 50 μm).
Soluble and insoluble fiber fractionation
The soluble and insoluble dietary fiber were fractionated (Fig. 1) according to the AOAC method31. Briefly, desugared fruit (10 g) was mixed with distilled water (140 ml) and heated at 95 °C for 15 min, then at 60 °C for 60 min. The mixture was vacuum filtered to collect the soluble fraction, and the extraction was repeated. The filtrates were pulled and evaporated to a total volume of 40 ml, and ethanol (95%, 80 ml, 60 °C) was then added to the filtrate and left to stand overnight to precipitate the soluble fraction. The soluble fraction was then recovered and freeze-dried (Bioevopeak, LYO60B-1P, Shandong, China). The insoluble fraction was washed twice with ethanol (78% v/v and 95% v/v) and acetone and dried in a hot air oven overnight at 100 °C (Binder GmbH, Im Mittleren Ösch 5, 78532 Tuttlingen, Germany). Furthermore, to determine the soluble dietary fiber (SDF) and insoluble dietary fiber (IDF), the soluble and insoluble fractions were analyzed for protein and ash and removed from each fraction (Eqs. 1 and 2)32.
Chemical properties of soluble and insoluble fractions
The monosaccharide composition was determined according to Uppsala method33. Briefly, the soluble and insoluble fractions were subjected to acid hydrolysis to release the monosaccharides, then reduced to alditols and acetylated to convert the neutral sugars into volatile alditol acetate, which was then analyzed in the gas chromatography (YL Instrument, 6500GC System, Hogye-dong, Anyang, Korea). The gas chromatographic system was equipped with a capillary column (15 m×0.25 mm id, 0.15 μm film thickness, DB 225), a split mode injector (ratio, 1:50), and a flame ionization detector. The temperature program was set at 160 °C (6 min initial) and increased to 220 °C at a rate of 4 °C/min and kept at 220 °C for 4 min. Uronic acids in the hydrolyzed filtrate were analyzed by UV–visible spectrophotometer (Shimadzu, Columbia, Maryland, USA). Klasson lignin was determined according to the National Renewable Energy Laboratory (NREL) method34. Briefly, the insoluble samples (0.2 g) were hydrolyzed in sulfuric acid (12 M, 3 mL), allowed to stand in a water bath (30 °C, 1 h) with intermittent shaking, diluted with water (74 mL), autoclaved (1 h at 120 °C), vacuum filtered, and the acid insoluble residue was dried overnight (105 °C) and ashed (550 °C, 5 h) in a muffle furnace (Carbolite, CWF 1100, Chamber furnace, Derbyshire, UK), and then the klasson lignin was calculated.
Fourier transform infrared spectroscopy (FTIR) of soluble and insoluble fractions, commercial β-glucan, arabinoxylan, pectin, cellulose, and protein were carried out on a Spectrum Two FT-IR spectrometer (DTGS Detector, Perkin Elmer, New York, USA)35. The absorbance was captured between 4000 cm− 1 to 400 cm− 1 for each sample. Magic Angle Spinning (MAS) solid-state nuclear magnetic resonance (NMR) of soluble and insoluble fractions was carried out on a Bruker Avance-HD 600 MHz spectrometer operating at a static field of 14.1 T using a 4.0 mm MAS probe36. Powdered dry samples were packed into 4.0 mm zirconia rotors and spanned at a MAS frequency of 14 kHz. Cross-Polarization Magic Angle Spinning (CP/MAS) NMR experiments were performed using a standard linearly ramped cross-polarization pulse sequence13. C chemical shifts were externally referenced to the adamantane CH2 signal at 38.48 ppm on the TMS scale. NMR data were processed using TopSpin software.
Soluble fiber from Barhi date fruits was analyzed by nuclear magnetic resonance spectroscopy in D2O at 60 °C1. H NMR spectrum of the sample was analyzed using zgpr to repress the residual water peak with 16 scans, 2 dummy scans, and d1 set to 5 s13. C NMR spectrum of the sample was analyzed with standard proton decoupled NMR for 8192 scans. To determine structural information on the carbohydrate rings, proton correlation spectroscopy1H COSY) and proton total correlation spectroscopy1H TOCSY) NMR experiments were used to verify ring structures. The COSY pulse program was cosygppppf F1 and has 128 points and 4 scans. The TOCSY pulse program was mlevphpp F1 and has 256 points with a total of 64 scans. Heteronuclear single quantum correlation (HSQC) NMR was used to enhance resolution and find the basic rudimentary assignments. The pulse program used for the HSQC is hsqcedetgpsisp2.3 in the Bruker spectrum library ns 64. Heteronuclear multiple bond correlation (HMBC) NMR was used to determine long-range couplings. The pulse program used was hmbcetgpl3nd from the Bruker library using 64 scans and a dwell time of 50 us. Diffusion Ordered Spectroscopy (DOSY) was used to determine the approximate molecular weights of the polysaccharide mixture and potentially characterize the nature of the molecule in solution37. DOSY spectrum was obtained using Bruker pulse program dstebpgp3s, 32 scans and a linear ramp for 128 points, D20 was set to 100ms (big Delta) and p30 was set to 1000 µs (little Delta). The plot was processed on MestReNova using the Bayesian approximations.
Physical and thermal analysis of soluble and insoluble fiber
Thermal properties of soluble and insoluble fractions, commercial Beta-glucan, arabinoxylan, pectin, cellulose, and protein were analyzed by differential scanning calorimeter (DSC Q100, TA instruments, Delaware, USA)38. The heating and nitrogen flow rates were 5 °C/min and 50 mL/min, respectively. During each run the sample (5 mg) was first equilibrated to 20 °C and then heated to 400 °C, after which the thermogram was analyzed. Alos, thermo gravimetric analyzer (TGA Q500, TA Instruments, Delaware, USA) was used to analyze the thermal properties of the soluble and insoluble fractions and commercial cellulose. The heating and nitrogen flow rates were 20 °C/min and 50 mL/min, respectively. For each run, sample (10 mg) were heated from 20 to 700 °C38. Thermogravimetric (TG) and derivative thermogravimetry (DTG) curves were then plotted. X-ray diffractometer (X’Pert PRO MRD XL XRD System from PANalytical, EA Almelo, Netherlands) was used to analyze the soluble and insoluble fractions and cellulose. Cu K-α radiation at a wavelength of 1.54 Å was used for this experiment and the minimum step size was 2θ39. The morphology of the soluble and insoluble fractions was analyzed by scanning electron microscopy (JEOL Ltd., Tokyo, Japan). The samples were mounted on the specimen stub using an adhesive carbon tape and coated with gold (80 s at 40 mA). The images were captured at different magnifications (500× and 1000 × )40.
Statistical analysis
OriginPro 9.0 (OriginPro 9.0, Northampton, Massachusetts, USA) was used for plotting the graphs. Standard deviation was computed for the gravimetric weight of soluble and insoluble fibers and for monosaccharides composition using OriginPro software.
Results and discussion
Date fruits total dietary fiber
The total dietary fiber content (TDF, g/100 g dry weight) in the four date cultivars analyzed in this study: (Barhi (3.2), Shishi (4.5), Neghal (7.2), and Barakawi (7.4)) were within the values reported previously (4.8–13.6)18,19,20,21,22,23,24,25. The variation in TDF was attributed to cultivar, genetics, growing condition, stage of maturation, and the point of harvest41. There are two pools of date fruits: a Middle East pool dominated by soft varieties and a North African pool containing semi-dry and dry cultivars41. The Barhi and Shishi cultivars can be classified as soft cultivars, while Neghal and Barakawi can be classified as semi-dry and dry cultivars, respectively. Date fruits can be considered a good source of TDF compared to other fruits, e.g., banana (1.8 g/100 g dry weight), jackfruit (3.5 g/100 g dry weight), pear (4.3 g/100 g dry weight), guava (7.2 g/100 g dry weight), and sapota (10.9 g/100 g dry weight)42.
Dates insoluble fiber
Neghal and Barakawi showed a higher proportion of insoluble carbohydrates (2.8 and 2.9 g/100 g) compared to Barhi and Shishi (1.5 and 2.0 g/100 g) (Fig. 2). Thus, date fruit fiber was mostly insoluble (85–90%) in agreement with previous findings18,25,43. The variation in the insoluble fiber could be related to genetics, growing conditions, and stage of maturation21,24,41. Lignin and insoluble carbohydrate polymers are the major components of the date dietary fiber (Fig. 2). The total dietary fiber and lignin content were previously found to be correlated23 and to significantly contribute to fruit hardness27. The harder Neghal and Barakawi cultivars had higher lignin contents (3.6 and 3.7 g/100 g) than the softer Barhi and Shishi cultivars (1.4 and 2.1 g/100 g).
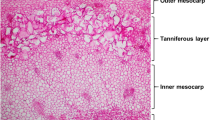
Scanning electron microscopy shows four major cell layers in date fruits: the exocarp, outer mesocarp, tanniferous layer, and inner mesocarp, including vascular bundles23. Light microscopy examination of the date fruits after staining with Mäule stain showed yellow to brown guaiacyl (G) and red to purple syringyl (S) lignin units and staining with Wiesner stain showed red to purple G-lignin units (Fig. 3). The G-units were deposited in the sclerenchyma and parenchyma cell walls and were especially concentrated in the sclereid (or stone) cells of the pericarp, while the S-units were deposited in the xylem vessels. It was reported that G-units exist in the primary cell walls of sclerenchyma and S-units are deposited in the secondary walls of the xylem vessels44,45. It was also revealed that G-units are deposited at the early stage of plant development, while S-units are deposited at the later stage, and there is the formation of a lignified sclerenchyma ring46. The presence of lignin in the epicarp and mesocarp of most fruits gives rigidity to the plant cell walls, especially the xylem vessels and the epicarp23,47,48. Most of the lignin in date fruits are linked with silica, forming association complexes that also contribute to the hardness of the fruits23. The majority is G-lignin in the sclerids or stone cells of the exocarp (stained red in Fig. 3), while the remaining is S-lignin associated with silica in phytoliths. Silica represented about 15% of the spheroid echinate phytoliths of the exocarp and the tracheary phytoliths in the xylem vessels confirming the coexistence silicification lignification in but their presence was negligible in the parenchyma cells and sclerids (results not shown).
Light microscopic images of stained sections of mature date fruit (50x) showing the distribution of guaiacyl (G) and syringyl (S) monomers: (A) Mäule staining of G-monomers in the sclereid cells yellow to brown and of S-monomers in the xylem vessels red to purple color and (B) Wiesner staining G-monomers red-purple color.
Fourier transform infrared spectroscopy (FTIR) fingerprinting of the insoluble fiber (Fig. 4) revealed signals at 1037 cm− 1 (C–O stretching vibration peak of C–O–C bond for cellulose or hemicellulose), 1245 cm− 1 (O–H and/or C–O groups vibration of lignin/hemicellulose), 1443 cm− 1 (bending or scissoring vibrations of CH2 or CH3 groups or C–O stretching of lignin, cellulose, and hemicellulose), 1515 cm− 1 (aromatic skeletal vibrations in lignin), and 1645 cm− 1 (C= O stretching vibration), which confirms that insoluble fiber contains lignin, cellulose, and hemicellulose. Solid-state nuclear magnetic resonance13C NMR) revealed that the insoluble fibers contain aliphatic signals (10 to 110 ppm) at varying intensities, and there was a signal between 62 and 65 ppm, which is assigned to C-6 resonance of crystalline and amorphous regions of a glucose backbone49. There was also an anomeric carbon signal (C1) at 104–106 ppm (Fig. 5), indicating that insoluble fiber contains cellulose50. In addition to aliphatic signals in the NMR spectrum of the insoluble fiber, there were aromatic (110 to 160 ppm) and carbonyl carbons (160 to 180 ppm). The aromatic signals are related to the presence of lignin and/or other phenolic compound50,51. The higher intensity of aromatic signals in Neghal insoluble fiber compared to Barhi insoluble fiber is explained by the higher lignin content51. The carbonyl groups can come from lignin, cellulose/hemicellulose, insoluble pectin, etc52. The insoluble fiber was also characterized by CH3 carbon of methyl ester groups (~ 54 ppm), CH2 groups from wax (~ 33 ppm), and CH3 groups from acetyl ester groups (~ 21 ppm).
The monosaccharides components of the insoluble fiber (Fig. 6 and supplementary Table S1 online) were glucose (40.6%), xylose (27.2%), galactose (9.2%), uronic acid (8.7%), arabinose (8.2%), mannose (3.0%), rhamnose (2%), and fucose (1%). This confirms that insoluble fiber contains insoluble pectin, which may be associated with proteins53. The glucose in the insoluble fiber comes majorly from cellulose polymer50,51. Arabinose and xylose in insoluble fiber are from arabinoxylan54 and mannose is from glucomannan55. Barakawi and Neghal had higher uronic acid and galactose and lower xylose and glucose than Barhi and Shishi.
Dates soluble fiber
The FTIR signals in the date soluble fiber appeared at 1017 cm− 1, 1239 cm− 1, 1423 cm− 1, 1609 cm− 1, and 1750 cm− 1 corresponding to C–H plane deformation, C–C, C–O, and C=O stretching, asymmetric C–H deformation, ring conjugate C=C stretching, and C=O stretching of unconjugated carboxyl/ester groups (Fig. 4), respectively. Similar signals were observed in soluble fiber from mushrooms56 tomato peels57 apples, kale, and celery51.
The monosaccharides of date soluble fiber included uronic acid (57.6%), galactose (13.7%), arabinose (10.0%)/glucose (10.0%), rhamnose (4.1%), xylose (3.6%), and mannose (1.0%) (Fig. 6). Rhamnose, arabinose, galactose, uronic acids, glucose, xylose have been found in the date fruit soluble fiber58. Uronic acid was the highest, suggesting that pectin was the major component of date fruit-soluble fiber19,59. Pectin is made up of galacturonic acid with galacturonans backbone and substituted with various neutral sugars (such as arabinose, galactose, and xylose)60. Furthermore, the greater proportion of uronic acid (57.6%) compared to rhamnose sugar (4.1%) suggests that the pectin in the date fruit soluble fiber was highly branched61. The greater proportion of the arabinose and galactose residues (23.7%) compared to rhamnose sugar (4.1%) suggests that these sugars are attached as side chains to the rhamnose residues, thereby confirming that date fruit pectin is majorly rhamnogalacturonan I60. The galactose and arabinose chains of the rhamnosyl residues may also exist as large oligosaccharides like galactan, arabinan, or arabinogalactan61. The glucose in the water-soluble fiber extract might be from β-D-glucan28. The slightly higher amount of galactose (13.7%) compared to arabinose (10%) might indicate a possible arabinogalactan59.
Solid-state NMR (Fig. 5) showed signals appearing from aliphatic (10–110 ppm) and carbonyl carbons (160–180 ppm), which might be due to the presence of pectin and soluble hemicellulose. The absence of aromatic signals (110–160 ppm) indicates the absence of lignin or other phenolic compounds like melanin36,41. Signal appearing at ~ 171 ppm could be assigned to the carboxyl group, ~ 101 ppm correlate to C-1 carbon, intense signals between 60 and 90 ppm are assigned to carbon atoms of the pyranoid ring (C-2,3,5) and signal at ~ 80 ppm could be assigned to C-4 carbon of (1 → 4)-linked-β-ᴅ-galactopyranosyl units. Similar aliphatic signals were also identified in soluble fiber from prunes and barley62. The downfield shift of C-4 signal with respect to other methyl β-ᴅ-galactopyranoside shows that β-ᴅ-Galp units carry a 4-O-substituted carbon63. Signal at ~ 54 ppm is assigned to the CH3 carbon of the methyl ester groups (COOCH3), ~ 33 ppm is assigned to the CH2 groups (wax) and signal at ~ 21 ppm is assigned to the CH3 groups from the acetyl ester groups (OCOCH3). In comparison to other signals, methyl and acetyl signals were shorter, confirming that date fruit contain lower levels of methyl and acetyl groups61.
In the1H NMR (Fig. 7a and see supplementary Fig. S1 online), anomeric hydrogen resonated between 6.0 and 4.3, carbohydrate rings resonated between 4.5 and 3.3, methylated region of the acetylated carbohydrates resonated between 1.9 and 2.2, and rhamnose H6 signals resonated between 1.17–1.3164,65,66,67. Five arabinose, three rhamnose, two galactose, one glucan and two uronic acid residues were tentatively identified in the date fruit soluble fiber (Table 1). α-L-arabinofuranose residues (α-L-Araf) were identified in the most downfield of the 13C and 1H region with some variations depending on substitutions (Fig. 7b–d; Table 1, and see supplementary Fig. S2-S4 online). α-L-rhamnopyranose (α-L-Rhap) anomeric correlations (H1-C1) were somewhat upfield in both1H and 13C. Somewhat upfield in1H and downfield in 13C is the β-D-galactopyranose (β-D-Galp) and α-L-glucopyranose (α-L-glup) anomeric signal anomeric resonances. Uronic acid was relatively upfield in the13C and 1H region. The multiple arabinose resonances are indicative of a complex polysaccharide sample with multiple structural motifs. Acetylation and methylation signals were abundant, as well as a highly aliphatic environment in the terminal of α-L-rhamnopyranose65,67,68,69,70,71.
1D and 2D Nuclear magnetic resonance of date fruit soluble fiber: (A)1H NMR, (B)1H–13C Heteronuclear single quantum correlation (HSQC) spectrum, (C) Correlation spectroscopy1H COSY) spectrum, (D) total correlation spectroscopy1H TOCSY) spectrum, (D) Heteronuclear multiple bond correlation (HMBC) spectrum, (E) Diffusion Ordered Spectroscopy (DOSY) (See supplementary Figure S1-S6 online for more clearer view).
Some possible connections amongst the sugar residues were revealed from the HMBC spectra (Fig. 7e and see supplementary Fig. S5 online). For instance, α-L-Araf-1 linked to position 2 on the α-L-Rhap-1 (5.77/73.0), acetylation of α-L-Araf-1 with carbonyl (uronic acid) (5.77/148.5), arabinose linked to the O-4 position of a branched galactose (5.45/86.8), a branch of α-L-Araf-3 linked to O-2 of the side chain of →2,4)-α-L-Rhap-(1→ (5.25/79.5), anomeric arabinose linked to the O-3 position of an adjacent arabinose (5.14/85.28), O-4 linkage of rhamnose to the C4 of an arabinose (5.28/86.6), rhamnose anomeric proton that has a C3 arabinose linkage (5.25/83.4), rhamnose anomeric proton linked to another saccharide, possibly galactose (5.10/82.1), and a 1,3-β-D-galactan linked to a 1,6-β-D-galactan (80.4), which is typical of a Type II arabinogalactan. Furthermore, there was a possible correlation of methyl acetate signals to the carbonyl region of acetylated sugars (2.01–2.29/175.9)67,72,73,74.
DOSY easily resolves a small molecule in the mix with signals at δ 1.17, 1.16, 1.18, 1.94, 3.34, and 8.72 ppm with an estimated molecular weight of ~ 72 Da based-on projections from the MNOVA software (Fig. 7f and see supplementary Fig. S6 online). The polysaccharides have a broad diffusion range of −9.3 to −9.8 log(m2/s) which is equivalent to the expected molecular weight of 1000–36,000 Da. The regions indicated higher degrees of freedom (diffusion), agreeing with our expectations of flexibility and rigidity with respect to the molecule. The anomeric signal for arabinose at δ5.78 has the highest mobility in the system, corresponding to the proposed branching of arabinose linkages. The Rhamnose C6 protons have a slower diffusion rate being in the backbone of the Rhamnogalacturonan motif. The ring regions also have a slower diffusion and were more confined in the macromolecule. The solution is a mixture of polysaccharides, with minimal aggregation at elevated temperatures.
Overall, the NMR results are consistent with the possible presence of Arabinogalactan, particularly Type II. The multiple arabinose anomeric peaks are indicative of multiple states of arabinose sugars. This is consistent with the nature of arabinose as it forms branches from the Arabinogalactan. The presence of rhamnose resonances is also consistent with the presence of Rhamnogalacturonans (RGs) along with the methylations and acetylation’s identified in the NMR spectra, including carbonyls correlated to the H4 Rhamnose as shown by the HMBC.
Properties of dates insoluble and soluble fibers
Thermal properties of soluble and insoluble fiber
The date fruit insoluble fiber showed both glass transition (Tg: 43–50 °C) and melting (Tm: 313–325 °C) (Fig. 8), indicating that it is a semi-crystalline material. The DSC curve of cellulose, which is one of the main components of the insoluble fiber, showed a similar curve. However, the melting peak of cellulose had greater intensity compared to the insoluble fiber, which might be because the insoluble fiber contains other macromolecules like lignin, hemicellulose (arabinoxylan), and protein, which have more amorphous region75. Furthermore, the glass transition temperature reveals that there would be a change in phase in the material after 50 °C, particularly cellulose or protein. The melting temperature (313–325 °C) reveals the point in which the material starts degrading, and this was contributed by cellulose and lignin76. This agrees with the TGA curve.
The date fruit soluble fiber showed both glass transition (Tg: 35–45 °C) and crystallization (Tc: 240–255 °C) (Fig. 8), indicating that it is an amorphous material and can form crystals when cooling after melt. The amorphous nature of soluble fiber is due to the change of pectin from crystalline to amorphous structure and water evaporation75. The DSC curve of soluble fiber was like the DSC curve of pectin, however there was a slight deviation in the Tg and Tc, probably because of other compositions like protein, glucan, etc. Barakawi soluble fiber has lower Tg than other varieties (Barhi, Neghal, and Shishi), and this may be because of lesser amount of pectin.
The insoluble fiber showed 3-step thermal decomposition at 20–100 °C, 200–350 °C, 350–400 °C (Fig. 9), corresponding to moisture evaporation, strong thermal lysis reactions such as dehydroxylation, deoxygenation, or decarboxylation of hemicellulose/pectin, and thermal decomposition of cellulose/lignin, respectively77. The thermal degradation of cellulose occurred between 300 °C and 400 °C (Fig. 9). Additionally, after 400 °C, Neghal insoluble fiber showed lower degradation compared to Barhi insoluble fiber, which might be because of the higher lignin content, the nature and molecular weight of the lignin. The soluble fiber showed 2-step thermal decomposition at 20–100 °C and 200–300 °C (Fig. 9). The first peak corresponds to moisture evaporation and thermal salvation of pectin, and the second peak corresponds to vaporization of volatile components and the oxidative decomposition of polymers like pectin and hemicellulose78. Neghal soluble fiber had greater weight loss compared to Barhi soluble fiber, which might be because of the nature and amount of the pectin. The thermal transition and stability results revealed by DSC and TGA are vital for retaining the date fruit soluble and insoluble fibers functionality during food processing or high temperature processes.
X-ray diffraction analysis
The insoluble and soluble fibers showed two characteristic peaks at 11° and 21.5° (Fig. 10). The insoluble fiber showed a sharp peak at 21.5°, indicating a crystalline region, which is a typical cellulose I structure77,78,79. The crystalline peak (21.5°) in the insoluble fiber was also observed in the XRD pattern of the standard cellulose. Additionally, 2 other peaks (11° and 35°) were observed in the standard cellulose, which was almost negligible in the XRD pattern of the insoluble fiber, and this might be because of the complexation of cellulose with lignin and hemicellulose polymers and the less exposure of the cellulose crystalline structure80,81. The soluble fiber showed a relatively amorphous peak at 11° and a short crystalline peak at 21.5°. A similar pattern was observed by Zou et al.59 for soluble fibers. The amorphous region (11°) may be due to pectic polysaccharides, while the short crystalline peak might be due to glucan75,82. This agrees with the DSC curve, which shows that soluble fiber can form crystals during cooling. The crystalline and amorphous characteristics of dietary fiber, revealed by XRD, will influence the date fruit soluble and insoluble fibers digestibility, solubility, and modification to functional food ingredients or biomaterials.
Morphological features of date fruit fibers
The dates insoluble fibers showed an intact-compact fiber bundle (Fig. 11). There were some similarities between the Barhi, Shishi, and Neghal insoluble fibers, which are vascular bundles with intact xylem vessel, however, their packing was different. Moreover, the microstructure of Barakawi insoluble fiber was different from other cultivars, it showed a dense packing of irregular size particles. The presence of vascular bundles in the parenchyma cells with lignified xylem vessel was found in date fruits23,27. Similar irregular size particles observed in Barakawi insoluble fiber was reported in the water-insoluble fiber from Rubus chingii Hu. fruit, but with less compaction77.
Barhi and Barakawi date fruit soluble fibers showed compact irregular-size particles; however, the soluble fiber from Barhi was smoother compared to Barakawi date fruits (Fig. 11). Similar smooth irregular-size particles in the Barhi soluble fiber were observed in the commercial pectin, but the particles in commercial pectin were less dense, and this might be because the extracted soluble fiber contains other non-pectin components. Shishi and Neghal soluble fibers showed a web-like network/structure, which is different from Barhi and Barakawi soluble fibers, this might be because of high methyl-esterification observed by the solid-state NMR. Similar irregular-size smooth particles were noticed in the soluble fiber from flaxseed and orange, and commercial pectin59 Rubus chingii Hu. fruit77 and proso millet bran. The water extracted soluble fiber from peach pomace showed a similar sponge-like or web-like network found in this study, and it was related to the degree of esterification60. The morphology, porosity, and structural changes revealed by scanning electron microscopy will affect the date fruit soluble and insoluble fiber digestibility, hydration, and processing behavior.
Comparison of date fruits fibers to other fruits in the Arecaceae family
Compared to other fruits in the Arecaceae family, the TDF in date fruit (3.2–7.4 g/100 g dry weight) was close to peach palm fruit (5.5–10.8 g/100 g dry weight)84 but less than coconut fiber (9.0–17.6 g/100 g dry weight)85,86. Like date fruit, peach palm, coconut, Açaí and Buriti fruits all showed that insoluble fiber was the major component (> 80%)84,85,87,88. Lignin and insoluble carbohydrates were the major component of date fruit and Açaí fruit fibers85 but for coconut insoluble carbohydrates was greater than lignin85,86. The chemical composition of insoluble and soluble carbohydrates in date, Açaí and coconut fruits were different. Date insoluble carbohydrates were majorly from glucose, xylose, galactose, uronic acid and arabinose while soluble carbohydrates were majorly from uronic acid, galactose, arabinose and glucose, Açaí fruit insoluble carbohydrates were majorly uronic acid, xylose, and arabinose, while soluble carbohydrates were majorly uronic acid, glucose, galactose and arabinose87 coconut insoluble carbohydrates were mainly mannose, galactose, galactosamine, arabinose, and ribose, while soluble carbohydrates were mainly mannose, galactose, arabinose, and glucuronic acid89 and peach palm fruit soluble carbohydrates were mainly uronic acid, arabinose, xylose, rhamnose, and glucose90. Therefore, Açaí fruit insoluble fiber will have greater digestibility and prebiotic potential than date insoluble fiber and coconut fiber, and can be used as a gelling agent, emulsifiers, stabilizers, and fat replacers in food industries. Moreover, date insoluble fiber will serve as a better texturizer in baked foods and meat analogs and bulking agent in low calorie foods. Aside the pectin and glucan in the Açaí fruit soluble fiber87 there is also a possible occurrence of arabinogalactan, like in date fruit soluble fiber. Date soluble fiber microstructure differs from coconut soluble fiber, the coconut soluble fiber had a compact texture with a wrinkled surface, cracks and holes whereas, the date fruit soluble fiber showed a dense and compact irregular size particles89,91. The implication is that date fruit soluble fiber will have good bulking ability, which will be well suitable for bakery foods, cereal bars, or fiber fortification, whereas the coconut soluble fiber will have high water holding and oil binding capacity, greater gelling ability, better texture, which will be better suited for fat replacers, stabilizers and emulsifier, and dairy products. The crystallinity of the date soluble fiber was less than that of coconut soluble fiber. Furthermore, the major thermal decomposition in soluble fiber occurred between 200 and 400 °C for both date and coconut fruits89,91. Monosaccharides and NMR studies revealed that date soluble polysaccharides have highly branched rhamnogalacturonan I and possible arabinogalactan II, whereas peach palm soluble polysaccharides have a linear highly methyl esterified homogalacturonan with small amounts of xylogalacturonan and rhamnogalacturonan I90 and Açaí fruit soluble polysaccharides has high methoxyl homogalacturonan, arabinogalactan II and small portion of glucomannan92. Therefore, the date soluble fiber will offer excellent prebiotic and immunomodulatory properties (e.g. nutraceuticals), and good emulsification ability just like Açaí fruit soluble fiber, but more than peach palm soluble fiber. Moreover, peach palm and Açaí fruit soluble fibers will offer better gelling (e.g. jams) and encapsulation ability (e.g. for controlled drug release).
Conclusions
In this study, we fractionated dietary fiber from four date fruit cultivars (Barhi, Shishi, Neghal, and Barakawi) into soluble and insoluble components and explored their structural and physicochemical characteristics. In all cultivars, the insoluble fiber was dominated by lignin. Guaiacyl lignin units are present in the sclerenchyma, while syringyl lignin units are present in the xylem vessels. Solid-state nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy, thermogravimetric analysis, and gas chromatography analysis revealed that insoluble fiber contains mainly lignin, cellulose, and hemicellulose, whereas the soluble fiber contains majorly pectic polysaccharides. The soluble fiber contains highly branched pectin and the side chains of rhamnose residues could contain galactose and arabinose sugars or large oligosaccharides like galactan, arabinan, or arabinogalactan. Proton NMR and 2D-NMR revealed several sugar residues and potential linkages, and a possible occurrence of Type II arabinogalactan in the side chains. Differential scanning calorimeter and X-ray diffraction revealed that the soluble fiber is amorphous, and insoluble fiber is semi-crystalline. Date fruit fibers as other fruits in the Arecaceae family agrees that dietary fiber is majorly insoluble (> 80%), the insoluble fiber will serve as a better texturizer and bulking agents in food products (meat analogs, baked foods, etc.) while the soluble fiber will offer excellent prebiotic and immunomodulatory properties compared to the other fruits in the Arecaceae family due to rhamnogalacturonan and arabinogalactan II polysaccharides. This study provides a knowledge base on the properties of the soluble and insoluble fibers from date fruits for its onward valorization to value-added food products. The soluble fiber-rich pectin can be used for various food products: jam and jellies, gummy candies, dairy products, sauces and dressings, etc., and insoluble fiber or isolated compounds can be valorized to products like bakery products, antimicrobial films, antioxidant agents, emulsifiers, etc.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Mohamed, R. M., Fageer, A. S. & Eltayeb, M. M. Mohamed ahmed, I. A. Chemical composition, antioxidant capacity, and mineral extractability of Sudanese date palm (Phoenix dactylifera L.) fruits. Food Sci. Nutr. 2, 478–489 (2014).
FAOSTAT. from Crops and livestock products. Food and Agriculture Organization of the United Nations (FAO). Retrieved May 09 (2024). https://www.fao.org/faostat/en/#data/QCL/visualize (2023).
Ghnimi, S., Umer, S., Karim, A. & Kamal-Eldin, A. Date fruit (Phoenix dactylifera L.): an underutilized food seeking industrial valorization. NFS J. 6, 1–10 (2017).
Alam, M. Z., Alhebsi, M. S., Ghnimi, S. & Kamal-Eldin, A. Inability of total antioxidant activity assays to accurately assess the phenolic compounds of date palm fruit (Phoenix dactylifera L). NFS J. 22, 32–40 (2021).
Rambabu, K. et al. Nutritional quality and physico-chemical characteristics of selected date fruit varieties of the united Arab Emirates. Processes 8, 256 (2020).
Kamal-Eldin, A., Hashim, B., Mohamed, O. & I. & Processing and utilization of palm date fruits for edible applications. Recent. Pat. Food Nutr. Agric. 4, 78–86 (2012).
Almoumen, A. et al. Harnessing date fruit pomace: extraction of high fibre dietary ingredient and its impact on high fibre wheat flour dough. NFS J. 35, 100178 (2024).
Besbes, S. et al. Date fiber concentrate: chemical compositions, functional properties and effect on quality characteristics of beef burgers. JFDA 18, 3 (2010).
Aljaloud, S., Colleran, H. L. & Ibrahim, S. A. Nutritional value of date fruits and potential use in nutritional bars for athletes. FNS 11, 463–480 (2020).
Almusallam, I. A. et al. Effect of date palm (Phoenix dactylifera L.) spikelets extract on the physicochemical and microbial properties of set-type yogurt during cold storage. LWT 148, 111762 (2021).
Anderson, J. W. et al. Health benefits of dietary fiber. Nutr. Rev. 67, 188–205 (2009).
Karim, A., Raji, Z., Habibi, Y. & Khalloufi, S. A review on the hydration properties of dietary fibers derived from food waste and their interactions with other ingredients: opportunities and challenges for their application in the food industry. Crit. Rev. Food Sci. Nutr. 64, 11722–11756 (2024).
Makki, K., Deehan, E. C., Walter, J. & Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell. Host Microbe. 23, 705–715 (2018).
Ötles, S. & Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 13, 191–202 (2014).
Turner, N. D. & Lupton, J. R. Dietary fiber. Adv. Nutr. 2, 151–152 (2011).
Chen, T. et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. AFST 260, 114335 (2020).
Howarth, N. C., Saltzman, E. & Roberts, S. B. Dietary fiber and weight regulation. Nutr. Rev. 59, 129–139 (2001).
Al-Farsi, M., Alasalvar, C., Morris, A., Baron, M. & Shahidi, F. Compositional and sensory characteristics of three native sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 53, 7586–7591 (2005).
Elleuch, M. et al. Date flesh: chemical composition and characteristics of the dietary fibre. Food Chem. 111, 676–682 (2008).
Habib, H. M. & Ibrahim, W. H. Nutritional quality of 18 date fruit varieties. Int. J. Food Sci. Nutr. 62, 544–551 (2011).
Mrabet, A. et al. Dietary fiber from Tunisian common date cultivars (Phoenix dactylifera L.): chemical composition, functional properties, and antioxidant capacity. J. Agric. Food Chem. 60, 3658–3664 (2012).
Parvin, S. et al. Nutritional analysis of date fruits (Phoenix dactylifera L.) in perspective of Bangladesh. Am. J. Life Sci. 3, 274–278 (2015).
George, N., Antony, A., Ramachandran, T., Hamed, F. & Kamal-Eldin, A. Microscopic investigations of silicification and lignification suggest their coexistence in tracheary phytoliths in date fruits (Phoenix dactylifera L). Front. Plant. Sci. 11, 977 (2020a).
Ali, H. I. et al. Total dietary fiber analysis in dates and other dry fruits without starch and protein hydrolyzing enzymes. J. Food Compos. Anal. 108, 104415 (2022).
Stojanovska, L. et al. Soluble and insoluble dietary fibre in date fruit varieties: an evaluation of methods and their implications for human health. Foods 12, 1231 (2023).
George, N., Andersson, A. A., Andersson, R. & Kamal-Eldin, A. Lignin is the main determinant of total dietary fiber differences between date fruit (Phoenix dactylifera L.) varieties. NFS J. 21, 16–21 (2020b).
Kamal-Eldin, A. et al. Dietary fiber components, microstructure, and texture of date fruits (Phoenix dactylifera, L). Sci. Rep. 10, 21767 (2020).
Ishurd, O., Sun, C., Xiao, P., Ashour, A. & Pan, Y. A neutral β-d-glucan from dates of the date palm, Phoenix dactylifera L. Carbohydr. Res. 337, 1325–1328 (2002).
Chateigner-Boutin, A. L. et al. Ferulate and lignin cross-links increase in cell walls of wheat grain outer layers during late development. Plant. Sci. 276, 199–207 (2018).
Liu, B. et al. Lignin distribution on cell wall Micro-Morphological regions of fibre in developmental phyllostachys pubescens culms. Polymers 14, 312 (2022).
AOAC. AOAC Official Method 991.43. Total, Soluble, and Insoluble Dietary Fiber in Foods. Chapter 32 (page 7) in Cereal Foods, Gaithersburg, MD, USA (1995).
Mohapatra, D., Patel, A. S., Kar, A., Deshpande, S. S. & Tripathi, M. K. Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chem. 271, 129–135 (2019).
Theander, O., Åman, P., Westerlund, E., Andersson, R. & Pettersson, D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J. AOAC Int. 78, 1030–1044 (1995).
Ren, M. et al. Green, one-pot biomass hierarchical utilization strategy for lignin-containing cellulose nanofibrils and fractionated lignin Preparation. Ind. Crop Prod. 203, 117193 (2023).
Lv, X. et al. Structure characterization and pyrolysis behavior of organosolv lignin isolated from corncob residue. J. Anal. Appl. Pyrol. 136, 115–124 (2018).
Alam, M. Z. et al. Date fruit melanin is primarily based on (–)-epicatechin Proanthocyanidin oligomers. Sci. Rep. 14, 4863 (2024).
Groves, P. Diffusion ordered spectroscopy (DOSY) as applied to polymers. Polym. Chem. 8, 6700–6708 (2017).
Han, T. et al. Characterization of lignin at pre-pyrolysis temperature to investigate its melting problem. Fuel 235, 1061–1069 (2019).
De, S., Mishra, S., Poonguzhali, E., Rajesh, M. & Tamilarasan, K. Fractionation and characterization of lignin from waste rice straw: biomass surface chemical composition analysis. Int. J. Biol. Macromol. 145, 795–803 (2020).
Haris, S. et al. Characterization analysis of date fruit pomace: an underutilized waste bioresource rich in dietary fiber and phenolic antioxidants. Waste Manage. 163, 34–42 (2023).
Alam, M. Z. et al. Contributing factors to quality of date (Phoenix dactylifera L.) fruit. Sci. Hortic. 321, 112256 (2023).
Ramulu, P. & Rao, P. U. Total, insoluble and soluble dietary fiber contents of Indian fruits. J. Food Compos. Anal. 16, 677–685 (2003).
Borchani, C. et al. Effect of date flesh fiber concentrate addition on dough performance and bread quality. J. Texture Stud. 42, 300–308 (2011).
Vallet, C., Chabbert, B., Czaninski, Y. & Monties, B. Histochemistry of lignin deposition during sclerenchyma differentiation in alfalfa stems. Ann Bot 625–632 (1996).
Hawkins, S. & Boudet, A. Defence lignin’and hydroxycinnamyl alcohol dehydrogenase activities in wounded Eucalyptus Gunnii. Pathol. 33, 91–104 (2003).
De Micco, V. & Aronne, G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech. Histochem. 82, 209–216 (2007).
Vanholme, R., Morreel, K., Ralph, J. & Boerjan, W. Lignin engineering. Curr. Opin. Plant. Biol. 11, 278–285 (2008).
Zhang, L., Kamitakahara, H., Murayama, H., Ohsako, T. & Itai, A. Analysis of fruit lignin content, composition, and linkage types in Pear cultivars and related species. J. Agric. Food Chem. 68, 2493–2505 (2020).
Luo, X. et al. Modification of insoluble dietary fibers from bamboo shoot shell: structural characterization and functional properties. Int. J. Biol. Macromol. 120, 1461–1467 (2018).
Cantu-Jungles, T. M., Iacomini, M. & Cordeiro, L. M. Investigation of structural features of prunes (Prunus domestica) insoluble dietary fibers. J. Food Sci. Nutr. 3, 001–006 (2017).
Zhang, H., Zhang, S., Xie, M., Lu, F. & Yue, F. UV resistance properties of lignin influenced by its Oxygen-Containing groups linked to aromatic rings. Biomacromolecules 26, 428–436 (2024).
Cheng, P. et al. Emission characteristics and formation pathways of carbonyl compounds from the combustion of biomass and their cellulose, hemicellulose, and lignin at different temperatures and oxygen concentrations. Atmos. Environ. 291, 119387 (2022).
Mudarisova, R. K., Kukovinets, O. S., Kolesov, S. V. & Novoselov, I. V. Role of functional groups in the complexation of structural analogs of aromatic amino acids with pectin. Russ J. Phys. Chem. A. 98, 805–811 (2024).
Karra, S. et al. Effect of extraction methods on the physicochemical, structural, functional, and antioxidant properties of the dietary fiber concentrates from male date palm flowers. J. Food Biochem. 44, e13202 (2020).
Mudgil, D. The interaction between insoluble and soluble fiber. In Dietary Fiber Prev. Cardiovasc. Disease, 35–59 (2017).
Xue, Z. et al. Structure, thermal and rheological properties of different soluble dietary fiber fractions from mushroom lentinula Edodes (Berk.) pegler residues. Food Hydrocoll. 95, 10–18 (2019).
Niu, Y., Li, N., Xia, Q., Hou, Y. & Xu, G. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT 88, 56–63 (2018).
Djaoud, K. et al. UV-C treatment impact on the availability of Water-Soluble carbohydrates, polyphenols, and antioxidant capacity of an Algerian underutilized date fruit (Phoenix dactylifera L). Foods 13, 893 (2024).
Zou, X. et al. Properties of plant-derived soluble dietary fibers for fiber-enriched foods: A comparative evaluation. Int. J. Biol. Macromol. 223, 1196–1207 (2022).
Xu, H., Jiao, Q., Yuan, F. & Gao, Y. In vitro binding capacities and physicochemical properties of soluble fiber prepared by microfluidization pretreatment and cellulase hydrolysis of Peach pomace. LWT 63, 677–684 (2015).
Gribaa, A. et al. Effect of water deficit on the cell wall of the date palm (Phoenix dactylifera ‘deglet nour’, A recales) fruit during development. Plant. Cell. Environ. 36 (5), 1056–1070 (2013).
Wang, Y. X. et al. Fractionation, physicochemical and structural characterization of polysaccharides from barley water-soluble fiber. Food Hydrocoll. 113, 106539 (2021).
Cantu-Jungles, T. M. et al. Polysaccharides from prunes: gastroprotective activity and structural Elucidation of bioactive pectins. Food Chem. 146, 492–499 (2014).
Ketha, K. & Gudipati, M. Purification, structural characterization of an Arabinogalactan from green gram (Vigna radiata) and its role in macrophage activation. J. Funct. Foods. 50, 127–136 (2018).
Hamed, M. et al. Isolation and identification of an Arabinogalactan extracted from pistachio external hull: assessment of immunostimulatory activity. Food Chem. 373, 131416 (2022).
Wu, D. T. et al. Microwave-assisted deep eutectic solvent extraction, structural characteristics, and biological functions of polysaccharides from sweet tea (Lithocarpus litseifolius) leaves. Antioxidants 11, 1578 (2022).
Tan, L. et al. Most of the rhamnogalacturonan-I from cultured Arabidopsis cell walls is covalently linked to arabinogalactan-protein. Carbohydr. Polym. 301, 120340 (2023).
Sun, Y., Cui, S. W., Tang, J. & Gu, X. Structural features of pectic polysaccharide from Angelica sinensis (Oliv.) diels. Carbohydr. Polym. 80, 544–550 (2010).
Kaczmarek, A., Maciejewska, A., Kasperkiewicz, K., Noszczyńska, M. & Łukasiewicz, J. Structure of the O-specific polysaccharide of Asaia Bogorensis ATCC BAA-21 lipopolysaccharide. Carbohydr. Res. 545, 109266 (2024).
Laplanche, V. et al. The human gut symbiont Ruminococcus gnavus displays strain-specific exopolysaccharides modulating the host immune response. Carbohydr. Polym. 347, 122754 (2025).
Hao, J. et al. Structural characterization and hypolipidemic activity of a hetero-galactan purified from Sanghuangporus vaninii based on modulation of TLR4/NF-κB pathway. Carbohydr. Polym. 347, 122702 (2025).
Zheng, Y. & Mort, A. Isolation and structural characterization of a novel oligosaccharide from the rhamnogalacturonan of Gossypium hirsutum L. Carbohydr. Res. 343, 1041–1049 (2008).
Tan, L., Qiu, F., Lamport, D. T. & Kieliszewski, M. J. Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in Transgenic Nicotiana tabacum. J. Biol. Chem. 279, 13156–13165 (2004).
Liu, J. et al. Structure characterisation of polysaccharides in vegetable Okra and evaluation of hypoglycemic activity. Food Chem. 242, 211–216 (2018).
Tan, X. et al. Characterization and function analysis of soluble dietary fiber obtained from radish pomace by different extraction methods. Molecules 29, 500 (2024).
Yang, X. et al. Characterization of insoluble dietary fiber from three food sources and their potential hypoglycemic and hypolipidemic effects. Food Funct. 12, 6576–6587 (2021).
Wang, S. et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from rubus Chingii hu. fruits. J. Funct. Foods. 93, 105081 (2022).
Tang, X. et al. Physicochemical, structure properties and in vitro hypoglycemic activity of soluble dietary fiber from adlay (Coix lachryma-jobi L. Var. ma-yuen Stapf) Bran treated by steam explosion. Front. Nutr. 10, 1124012 (2023).
Liu, M., Zhou, S., Li, Y., Tian, J. & Zhang, C. Structure, physicochemical properties and effects on nutrients digestion of modified soluble dietary fiber extracted from sweet potato residue. Food Res. Int. 150, 110761 (2021).
Alotaibi, M. D. et al. Characterization of natural fiber obtained from different parts of date palm tree (Phoenix dactylifera L). Int. J. Biol. Macromol. 135, 69–76 (2019).
Sang, J. et al. Evaluation of the structural, physicochemical and functional properties of dietary fiber extracted from Newhall navel orange by-products. Foods 10, 2772 (2021).
Lutz, R., Aserin, A., Wicker, L. & Garti, N. Structure and physical properties of pectins with block-wise distribution of carboxylic acid groups. Food Hydrocoll. 23, 786–794 (2009).
Li, Y. et al. Effects of fermentation with lactic bacteria on the structural characteristics and physicochemical and functional properties of soluble dietary fiber from prosomillet Bran. LWT 154, 112609 (2022).
Pires, M. B., Amante, E. R., Lopes, A. S., Rodrigues, A. M. D. C. & Silva, L. H. M. D. Peach palm flour (Bactris Gasipae KUNTH): potential application in the food industry. Food Sci. Technol. 39, 613–619 (2019).
Lund, E. D., Smoot, J. M. & Hall, N. T. Dietary fiber content of eleven tropical fruits and vegetables. J. Agric. Food Chem. 31, 1013–1016 (1983).
Gunathilake, K. D. P. P., Thilakahewa, C. & Kumara, A. A. N. Nutritional composition of Dikiri coconut. Cord 25, 7–7 (2009).
Maria do Socorro, M. R. et al. Açaí (Euterpe oleraceae)‘BRS Pará’: A tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res. Int. 44, 2100–2106 (2011).
Resende, L. M., Franca, A. S. & Oliveira, L. S. Buriti (Mauritia flexuosa L. f.) fruit by-products flours: evaluation as source of dietary fibers and natural antioxidants. Food Chem. 270, 53–60 (2019).
Du, X., Bai, X., Gao, W. & Jiang, Z. Properties of soluble dietary fibre from defatted coconut flour obtained through subcritical water extraction. IJFST 54, 1390–1404 (2019).
Cantu-Jungles, T. M., Cipriani, T. R., Iacomini, M., Hamaker, B. R. & Cordeiro, L. M. A pectic polysaccharide from Peach palm fruits (Bactris gasipaes) and its fermentation profile by the human gut microbiota in vitro. Bioact Carbohydr. Diet. Fibre. 9, 1–6 (2017).
Du, X. et al. Effects of different extraction methods on structure and properties of soluble dietary fiber from defatted coconut flour. LWT 143, 111031 (2021).
Cantu-Jungles, T. M., Iacomini, M., Cipriani, T. R. & Cordeiro, L. M. Extraction and characterization of pectins from primary cell walls of edible Açaí (Euterpe oleraceae) berries, fruits of a monocotyledon palm. Carbohydr. Polym. 158, 37–43 (2017).
Acknowledgements
This study was funded by United Arab Emirates University (12R158). Solid state and solution NMR analysis were carried out at the Core Technologies Platform (CTP) in New York University Abu Dhabi, United Arab Emirates. Published with the approval of the Director of the Louisiana Agricultural Experiment Station as manuscript # 2025-232-39888.
Author information
Authors and Affiliations
Contributions
C.E.O, Investigation, Formal analysis, Data curation, Writing—original draft. N.S., Investigation, Formal analysis, Data curation. H.M., Methodology, Investigation. M.J.O., Investigation, Writing—original draft. S.V., Investigation, Writing—original draft. A.H.A., M.A., & D.B., Supervision, Writing–review & editing. A.K.E, Conceptualization, Resources, Supervision, Project administration, Funding acquisition, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Okonkwo, C.E., Samad, N., Mohamed, H. et al. Characterization of dietary fiber and soluble carbohydrates in date fruits (Phoenix dactylifera L.). Sci Rep 15, 31616 (2025). https://doi.org/10.1038/s41598-025-16812-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16812-w