Abstract
Esophageal cancer (EC) is one of the most common malignant tumors in China. EC is characterized by a poor clinical prognosis, with many patients being diagnosed at advanced stages. This study utilized data from the Surveillance, Epidemiology, and End Results (SEER) database. The clinical features, treatment, and prognostic factors of patients with distant metastatic EC were screened and analyzed, and a nomogram was drawn to construct a predictive model. Eligible patients with distant metastatic EC diagnosed from January 2004 to December 2015 were extracted from the SEER database. Propensity score matching (PSM) was used to eliminate group baseline differences.The data were divided into the training cohort (1116 cases) and validation cohort (426 cases) by using R software and random sampling function at the ratio of 7:3. The baseline table was plotted using Chi-square test or Fisher’s exact test. Kaplan–Meier curve, log-rank test, and Cox regression were used for survival analysis. C-index and AUC were used to evaluate the performance of the prognosis model. The calibration curve was used to evaluate the calibration of the model. Using the data of the validation cohort, external validation is used to create a prediction model. After applying the inclusion and exclusion criteria and PSM, a total of 1542 cases diagnosed between 2004 and 2015 were included in the study. We analyzed the Kaplan–Meier survival of patients with metastatic EC before and after PSM, focusing on different treatment methods. The results indicated that radiotherapy, chemotherapy, and surgical treatment provided significant survival benefits to patients with metastatic EC(P < 0.05). Univariate and Multivariate regression analysis showed that T-stage, M-stage, primary site, surgery, chemotherapy, and radiotherapy were independent prognostic factors affecting the prognosis of distant metastatic EC (P < 0.05). Evaluating the predictive ability of the nomogram, the C index of the training cohort was 0.69 (95% CI 0.67–0.71), and the C-index of the validation cohort was 0.659 (95% CI 0.627–0.693)0.6606 patients met the inclusion criteria and were enrolled in the study’s external validation group. In this group, the AUC values of our external validation model for 1-, 2-, and 3-year overall survival (OS) were 0.775 (95% CI 0.762–0.787), 0.790 (95% CI 0.744–0.807). The C-index was 0.726. The AUC values for both the training and validation cohorts for the 1-year OS ranged from 0.50 to 0.70, and the AUC for the rest of the training and validation cohort ranged from 0.70 to 0.90, which suggests that the model is moderately discriminating. The calibration curves of 1 year, 2 years, and 3 years in the two groups are very close to the 45° reference line, suggesting that the models exhibit a good degree of calibration. The C-index, the AUC, and calibration curves suggest that the models have good discriminating and calibration. The results reveal that the T stage, M stage, primary tumor site, surgery, chemotherapy, and radiotherapy play an important role in influencing the treatment effect and prognosis of patients. The nomogram prediction model, which is based on these independent risk factors, shows good discriminative ability and calibration.
Similar content being viewed by others
Introduction
Esophageal cancer ranks seventh globally in cancer incidence and sixth in mortality1. EC was found to be the fourth leading cause of cancer deaths in Chinese men over the age of 45 in the National Cancer Center Registry in 20162. China has a high incidence of esophageal cancer, accounting for more than half of the global EC cases3. The main histological subtype of EC is squamous cell carcinoma2. In 2020, the number of new cases of EC will reach 604,000, and the number of deaths will reach 544,0001. Esophageal cancer is an aggressive type of tumor, which is treated with surgery, chemotherapy, radiotherapy, and various targeted therapies4. The 5-year survival remains low (20%). EC is usually diagnosed in the late stage, mainly due to the lack of early clinical symptoms. With the continuous progress of comprehensive treatment of esophageal cancer, the prognosis of EC has improved using endoscopic treatment, surgical techniques, radiotherapy, chemotherapy, immunotherapy, and targeted therapy. Identifying, exploring, and intervening all potential risk factors may reduce the incidence of esophageal cancer. Risk factors for EC include gastroesophageal reflux disease, obesity, smoking, drinking hot tea and alcohol, eating pickles, low fruit intake, hot food drinks, malnutrition, and low socio-economic status5,6. Studies have shown that Barrett’s esophagus is also an important risk factor for esophageal cancer. The risk factors of esophageal squamous cell carcinoma and adenocarcinoma are still different5. Therefore, identifying risk factors and establishing a prediction model can benefit patients. It can also help clinicians to make more appropriate medical decisions for patients.
Studies have shown that concurrent chemoradiotherapy (CCRT) is the preferred treatment for unresectable locally advanced esophageal cancer7. Radiotherapy is one of the main cancer treatments8. At present, 5-fluorouracil or capecitabine combined with oxaliplatin or cisplatin is commonly used to treat locally advanced or metastatic esophageal squamous cell cancer9. One study found that definitive radiotherapy improved overall survival (OS) in newly diagnosed metastatic esophageal cancer10. Short-term radiotherapy is used to relieve dysphagia, malnutrition, and chronic bleeding in esophageal cancer11. Comprehensive treatment is crucial for managing esophageal cancer. Endoscopic therapy and surgical intervention are options for early-stage cancer. Chemotherapy, targeted drug therapy, and immunotherapy are used for the treatment of unresectable locally advanced or advanced patients. Currently, there are limited options for targeted therapy in esophageal cancer, mostly targeting human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), and inositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR)12. It is reported that adoptive T cell transfer technology is also used for distant metastatic esophageal cancer13. Of course, it also needs a lot of clinical trial data to verify. Clinical trials such as KEYNOTE-590, CheckMate648, KEYNOTE-811, and ATTRACTION-3 demonstrate that immunotherapy can improve the prognosis of advanced EC patients.
Preference score matching (PSM) is a statistical method to reduce data bias and the influence of confounding variables in observation and research. PSM is often used to balance baseline characteristics and eliminate the bias between the treatment population and the control population. The SEER database is a large tumor database established by the National Cancer Institute. It is a public database commonly used clinically for surveillance and research in the field of cancer. Nomograms are widely used for cancer prognosis.
Nomograms are widely utilized in clinical care. They play a crucial role in predicting patient survival and informing clinical decision-making by physicians. For instance, one study used nomogram to evaluate the survival probability of patients with metastatic lung cancer who received radiotherapy for bone metastases14. This suggests that the use of a nomogram can assist radiation oncologists in tailoring treatment to a patient’s life expectancy, including the initiation of end-of-life discussions and hospice referrals when appropriate. The same principle applies to initiating hospice care for patients with distant metastatic esophageal cancer. Nomograms can provide valuable clinical treatment support with a degree of credibility.
In recent years, there have been few prognostic models for patients with metastatic esophageal cancer. Metastatic EC is not clear in this field at present. The prognosis model has been constructed, which has a good prediction effect on patients with metastatic EC and also has certain clinical references.
Methods
Patients and selection criteria
Patients with distant metastatic EC from January 2004 to December 2015 were selected from the SEER database. Data used in this research are publicly available to qualified researchers on application to the SEER database(https://seer.cancer.gov/). Training cohort: Incidence-SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018). External validation. Incidence-SEER Research Data, 17 Registries, Nov 2023 Sub (2000–2021).
Patients with metastatic EC were identified based on the following inclusion criteria: From January 2004 to December 2015, patients with esophageal cancer classified as M1 stage according to the AJCC 6th edition were included. Patients must have complete survival time data, and the effectiveness of follow-up must be guaranteed; EC was diagnosed by pathology. The exclusion criteria: Patients with missing or insufficient information such as staging, survival rate, follow-up time, or cause of death; The treatment is not clear; The patient has not only one primary malignant tumor; The patients diagnosed only by autopsy results or death certificates. SEER database data is publicly available, so it does not need ethical approval.
Ethical approval
was exempt from this retrospective study since SEER data are de-identified and publicly available for research use.
Study variables
The following patient information was collected: age, race, sex, primary site, histological type, degree of differentiation, TNM stage, radiotherapy, chemotherapy, surgery, survival time, survival status, and so on. The end point of our research is OS. OS was defined as the time from the start of diagnosis to the time of death from any cause or the time of the last follow-up cutoff.
Construction of the nomogram
After screening the data from the SEER database to meet the inclusion and exclusion criteria, baseline differences were eliminated by the PSM, and then the matched data were randomly divided into a training cohort and a validation cohort in a 7:3 ratio using the R software and plotted a baseline table for each variable. Kaplan–Meier method was used to draw the survival curves of the radiotherapy group and chemotherapy group. The log-rank test was used to compare the differences between OS. Univariate Cox regression model was used to analyze the prognostic factors of metastatic EC (P < 0.05), The variables with p < 0.05 were analyzed by multivariate Cox regression. The risk ratio (HR) and the corresponding 95% confidence interval (95%CI) are calculated. The index of independent risk factors for metastatic EC was obtained (P<0.05). Based on these independent prognostic factors, we use R software to construct a nomogram, which can predict the probability of OS in patients with metastatic EC for 1 year, 2 years, and 3 years.
Discrimination and calibration of the nomogram
C-index and calibration curves are often used to evaluate the performance and accuracy of nomograms. The discrimination degree of the prediction model is the degree of evaluating the ability of the model to predict events. The degree of discrimination can reflect the prediction ability of constructing this prediction model. The nomogram was validated using C-index, AUC, and calibration curve. In the ROC curve, the abscissa is specificity, and the ordinate is sensitivity. C index and AUC of 0.5 indicate that the model has no predictive ability. The range of 0.50–0.70 indicates that the discrimination of the prediction model is low. A range of 0.70–0.90 indicates that the prediction discrimination is moderate. More than 0.90 indicates that the prediction model has high discrimination.
Calibration degree, also called fitting degree, which can be evaluated by calibration curve to evaluate the degree of accurate risk estimation by the model. The higher the overlap of the model prediction curve with the diagonal standard line, the better the calibration of the model.
Results
Case inclusion and exclusion process
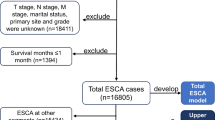
The inclusion and exclusion criteria of the study cases are shown in Fig. 1. The study’s external validation group is shown in Supplementary Table 1. We selected patient cases from the SEER database between January 2004 and December 2015, and these patients were diagnosed with EC in a total of 10,792 cases. Patients with EC with M1 distant metastases were subsequently included. After that, we excluded patients with missing race (3 cases); patients with missing marital status (77 cases); patient cases with missing grading (388 cases); patient cases with missing tumor size (2165 cases); patients with incomplete information on TNM staging (1318 cases); and patients diagnosed only by autopsy results or death certificates (89 cases), which left 2068 patients. These patients were treated with radiotherapy in 1087 cases, and there were 981 patients who did not receive radiotherapy.
Baseline characteristics
Table 1 shows the clinical baseline characteristics of all patients with metastatic esophageal cancer. From the analysis of the table, it can be seen that the number of patients in the radiotherapy group was 1087, the number of patients in the non-radiotherapy group was 981, and the number of patients receiving radiotherapy in metastatic EC accounted for 52.6% of the total number of patients. Before the use of PSM, it was possible to observe some differences in the distribution of some variables between the two groups, which included marital status, chemotherapy treatment, tumor location, surgical treatment, and degree of differentiation. The proportion of unmarried patients who received radiotherapy increased relative to those who did not receive radiotherapy; patients who received radiotherapy were more likely to receive chemotherapy and surgery; patients with upper and mid-stage EC were more likely to receive radiotherapy; and receipt of radiotherapy was increased in patients with lower levels of differentiation; of these, there were no statistically significant differences in the variables of gender, age, ethnicity, and histology on whether or not they received radiotherapy. By matching using the PSM method, we categorized patients into a group that did not receive radiotherapy and a group that received radiotherapy, each including 771 patients. By analyzing the baseline characteristics of the two groups, we can observe that there is no statistically significant difference in the data of patients who received radiotherapy or not after matching by the PSM method, and the data after balancing for the same variable satisfy P > 0.05, which indicates that PSM has well balanced the baseline characteristics between the group that received radiotherapy and the group that did not receive radiotherapy. With this method, we eliminated the interference of confounding factors on the study results during the research process and improved the reliability of the study results.
Survival analyses
To further demonstrate the therapeutic effects of radiotherapy, chemotherapy, and surgery in patients with distant metastatic esophageal cancer, we grouped the patients according to whether they received radiotherapy, chemotherapy or not, or surgery or not, and then we performed Kaplan–Meier survival analysis of metastatic EC patients before and after PSM, and the specific results are detailed in Fig. 2. Figure 2A showed that the survival graph of metastatic EC patients before PSM, and there was a significant improvement in the chemotherapy group OS compared with no chemotherapy ([HR] 0.324, 95% CI 0.294 –0.358, p < 0.0001). Figure 2B shows the survival graph after PSM in patients with metastatic esophageal cancer, and there was a significant improvement in OS in the chemotherapy group compared with no chemotherapy ([HR] 0.369, 95% CI 0.330–0.413, p < 0.0001). Figure 2C shows the survival graph of metastatic EC patients before PSM, and there was a significant improvement in OS in the radiotherapy group compared with the no-radiotherapy group ([HR] 0.681, 95% CI 0.624–0.744, p < 0.0001). Figure 2D shows the survival graph of metastatic EC patients before PSM, and there was a significant improvement in OS in the radiotherapy group compared with no radiotherapy ([HR] 0.879, 95% CI 0.795–0.973, p = 0.0084). Figure 2E shows the survival graph of metastatic EC patients before PSM, and there was a significant improvement in OS in the surgical group compared with the non-surgical group ([HR] 0.455, 95% CI 0.383–0.540, p < 0.0001). Figure 2F shows the survival graph after PSM in patients with metastatic esophageal cancer, and there was a significant improvement in OS in the surgical group compared with the non-surgical group ([HR] 0.482, 95% CI 0.379–0.612, p < 0.0001). According to the results of our study, patients who had been treated with radiotherapy, chemotherapy, and surgery demonstrated longer survival than those who did not receive these treatment modalities, regardless of whether or not a propensity score matching process was performed.
Survival curve of metastatic esophageal cancer before and after PSM. A Pre-PSM chemotherapy overall survival. B Post-PSM chemotherapy overall survival. C Pre-PSM radiotherapy overall survival. D Post-PSM radiotherapy overall survival. E Pre-PSM surgery overall survival. F Post-PSM surgery overall survival.
Comparison of clinical case data between training and validation cohort
After eliminating baseline differences by the PSM method described above, a total of 1542 eligible patients were included, and we randomly divided the 1542 patients into a training cohort and a validation cohort according to the ratio of 7:3, in which 1116 patients were in the training cohort, and 426 patients were in the validation cohort. Among them, the case data of patients in the overall training cohort and validation cohort are shown in Table 2.
Of the 1542 patients with distant metastatic esophageal cancer, gender included 1288 male patients (83.5%) and 254 female patients (16.5%). Among races, there were 1274 cases of Caucasians, accounting for up to 82.6%, and 268 cases of non-Caucasians, accounting for 17.4%. Age < 65 years had 877 patients (56.9%), and age ≥ 65 years had 665 patients (43.1%). In marriage, there were 861 patients (55.8%) who were married and 681 patients (44.2%) who were other (single, divorced, separated, widowed). Of chemotherapy, there were 1064 patients (69.0%) who received chemotherapy and 478 patients (31.0%) who did not receive chemotherapy. The number of patients who received radiotherapy and those who did not was 771 cases, accounting for 50%, and the histologic types were adenocarcinoma in 1032 cases, accounting for 66.9%, and non-adenocarcinoma in 510 cases, accounting for 33.1%. The tumor location was located in the upper EC in 61 cases, accounting for 4.0%, in the middle EC in 279 cases, accounting for 18.1%, in the lower EC in 1119 patients, accounting for 72.6%, and in the other patients in 83 cases, accounting for 5.4%. In surgical treatment, there were 1459 patients without surgery, accounting for 94.6%, and 83 patients with surgery, accounting for 5.4%. There were a total of 990 patients in grade III + IV tumor differentiation, accounting for 64.2%, and a total of 552 patients in grade I + II, accounting for 35.8%.There were 414 patients in the T1 stage in T staging, accounting for 26.8%, 84 patients in the T2 stage, accounting for 5.4%, 390 patients in the T3 stage, accounting for 25.3%, 399 patients in the T4 stage, accounting for 25.9%, 255 patients in Tx stage, accounting for 16.5%, and 417 patients in lymph node stage N0, accounting for 27.0%, 998 patients in N1, accounting for 64.7%, and 127 patients in Nx, accounting for 8.2%. Among distant metastases, there were 74 patients with M1a (4.8%) and 1468 patients with M1b (95.2%). A total of 1508 (97.8%) of the 1542 patients died, and 34 (2.2%) survived.
The ROC curves for the 1-year, 2-year, and 3-year OS for in the study’s external validation group are shown in Supplementary Fig. 1. In the external validation group, we used the Kaplan–Meier survival curves to compare the effects of several combination treatment regimens (Supplementary Figs. 2A, B, 3A, B, 4A, B). Radiotherapy combined with chemotherapy showed a median OS of 8 months (95% CI 7.16–8.84 months, p < 0.001), which was significantly superior to the 2 months observed in the radiotherapy-alone group (Supplementary Fig. 2A). The median OS reached 16 months (95% CI 7.70–24.30, p < 0.001) in the radiotherapy combined with the surgery group, which was prolonged by 10 months compared with the radiotherapy alone group (Supplementary Fig. 2B). median OS was 14 months (95% CI 5.53–22.47, p < 0.001) in the chemotherapy combined with surgery group, which was an improvement of 6 months compared with the chemotherapy alone group (Supplementary Fig. 3A). Median OS was 8 months in both groups for chemotherapy combined with radiotherapy and radiotherapy alone (p = 0.250). This result may be that radiotherapy is used for palliative radiotherapy in advanced metastatic esophageal cancer to alleviate painful symptoms (Supplementary Fig. 3B). Surgery combined with radiotherapy showed the median OS was 16 months (95% CI 7.70–24.30, p = 0.011), which was significantly better than the surgery alone group. This result suggests that postoperative radiotherapy may reduce the risk of recurrence (Supplementary Fig. 4A). Surgery combined with chemotherapy showed the Median OS was 14 months (95% CI 5.53–22.47, p = 0.032), but the sample size in the surgery alone group was too small (n ≈ 21) with a wide confidence interval (5–40 months) (Supplementary Fig. 4B).
After statistical tests, it was found that there was no statistically significant difference between the training and validation cohort on the baseline balance (p > 0.05), and the differences in the various study indicators were not statistically significant, see Table 2. After plotting the OS survival curves for the training and validation cohort, it was found that there was also no statistically significant difference between the two groups (p = 0.77), and the detailed data are shown in Fig. 3.
Independent prognostic factors in the training cohort
The results of univariate and multivariate Cox regression analysis are shown in Table 3. Univariate Cox regression analysis was performed on all variables to explore the effects of different factors on the prognosis of metastatic esophageal cancer, and the results showed that “race, age, marriage, chemotherapy, radiotherapy, histological grading, tumor site, surgery, T stage, N stage, and M stage” were the prognostic factors affecting the OS of metastatic EC (P < 0.05). In the multifactorial Cox risk proportional regression model, chemotherapy, radiotherapy, tumor site, surgery, T stage, and M stage were independent prognostic factors affecting OS in metastatic EC (Table 3).
From the results of multifactorial Cox regression, we can learn that receiving chemotherapy was an independent prognostic factor for improving OS of metastatic EC compared with not receiving chemotherapy (HR = 0.038, 95% CI 0.266–0.357, p < 0.001); receiving radiotherapy was also an independent prognostic factor for improving overall survival of metastatic EC compared with not receiving radiotherapy (HR = 0.835, 95% CI 0.740–0.942, p = 0.003); receiving surgery was also an independent prognostic factor for improving OS in distant metastatic EC compared to not receiving surgery (HR = 0.446, 95% CI 0.333–0.596, p < 0.001); and in the upper esophagus (HR = 0.611,95% CI 0.407–0.918, p = 0.018) and lower esophagus (HR = 0.788, 95% CI 0.612–1.015, p = 0.065) had a poorer prognosis than patients with other tumor sites; patients with stage T4 (HR = 1.227, 95% CI 1.038–1.451, p = 0.017) had a poorer prognosis than patients with T1; patients with stage Nx (HR = 1.226,9 5% CI 0.958–1.568, p = 0.105) patients had a worse prognosis than N0 patients; M1b stage (HR = 1.799, 95% CI 1.325–2.444, p < 0.001) patients had a worse prognosis than M1a stage patients (Table 3).
Prognostic nomogram for OS
The statistically significant study variables obtained from the multifactorial Cox regression analysis above, such as the six independent risk factors of radiotherapy, T-stage, M-stage, surgery, chemotherapy, and tumor site, were plotted on a column-line graph (Fig. 4). The OS at 1 year, 2 years, and 3 years can be easily calculated, and the score value at the very top is the score obtained from the upwardly-directed vertical line made by each of the study variables below, and an individual risk score can be calculated by summing the scores obtained by all of them. The corresponding points were then found on the survival scale. The scores obtained can be summed to give a total score, an individual risk score is calculated, and the corresponding points are then found on the survival scale.
Calibration and validation of the nomogram
The accuracy and discriminative power of the column line drawings of distant metastatic EC could be evaluated based on C-index and AUC. In the prediction model, the C-index of OS in the training cohort was 0.690 (95% CI 0.672–0.709), and that of OS in the validation cohort was 0.659 (95% CI 0.627–0.693), and the AUCs of OS for metastatic EC in the training cohort for 1 year, 2 years, and 3 years were 69%, 73%, and 77.3%, respectively. The AUCs of 1, 2, and 3 years for OS of metastatic EC in the validation cohort were 69.4%, 73.4%, and 78.1%. The AUCs of 1, 2, and 3 years for OS of metastatic EC in the external validation cohort were 77.5%, 79.0%, and 80.4 (Supplementary Fig. 1). In the constructed model, the AUC values of the area under the curve for 1-year OS in the training cohort and validation cohort were between 0.50 and 0.70, and the AUCs of the rest of the training and validation cohorts were between 0.70 and 0.90, which indicated that the model had a medium degree of differentiation, and, it could be observed that the AUC of the area under the curve might get bigger and bigger with the prolongation of the time and that the predictive model was getting more and more reliable, and the prediction model was getting differentiation is getting stronger. The calibration curve showed a good agreement between the predictions of the risk model and the observed results, indicating that the model had good sensitivity and specificity (Supplementary Fig. 1). The ROC curves for the 1-year, 2-year, and 3-year OS for the training and validation cohort are shown in Fig. 5. The calibration of the prediction model is assessed by the calibration curve, and the calibration curve is similar to the 45° diagonal line, which indicates that the calibration of the prediction model is good, and the calibration curves of the OS for 1, 2, and 3 years for the training and validation cohort are shown in Fig. 6.
Discussion
EC remains an aggressive disease with a low survival rate15, with a low 5-year survival rate and distant metastases in 20–30% of patients with EC at initial diagnosis16. It is necessary to construct a prognostic model for metastatic esophageal cancer. Through univariate and multivariate Cox regression analysis, we found that radiotherapy, chemotherapy, surgery, tumor site, T-stage, and M-stage were independent risk factors affecting OS.
Some studies have indicated that metastatic EC treated with surgery or radiotherapy can provide survival benefits for patients. Zhao et al. showed that the median survival of patients with M1a stage metastatic squamous EC undergoing R0 esophagectomy after simultaneous radiotherapy could reach 36.9 months17. Elk et al. found that after the use of a multimodal treatment approach, including surgery in patients with extraintestinal metastases or liver metastases from esophageal cancer, recurrence occurred in about 64% of R0 resection patients, and 50% of the operated patients were still alive after a median follow-up of 22 months18. This suggests that palliative resection or radiotherapy may be beneficial for survival. There are also some case reports suggesting that a combination of treatments, such as surgery or radiotherapy treatment, improves the overall survival of patients with metastatic esophageal cancer19,20,21,22,23. The above studies are consistent with the findings of the present study.
Surgery is an option for earlier staging of esophageal cancer, and simultaneous radiochemotherapy is considered to be the preferred treatment option for locally advanced patients who are inoperable or unresectable. The results of a prospective phase II study showed that among patients with inoperable squamous esophageal cancer, those who received simultaneous radiotherapy with a chemotherapy regimen of cisplatin and doxorubicin had a more prolonged OS24. Seyedin et al. found that patients with non-visceral and non-osteosynovial metastatic EC who underwent radical radiotherapy in combination with surgery had a higher median OS than those who were treated with radiotherapy alone25. The results of the study conducted by David et al. showed that chemotherapy combined with radical radiotherapy improved the OS of patients even more10. The results of the study in our SEER database also showed that radiotherapy improved the OS of patients with metastatic esophageal cancer.
This study also has some limitations; the phase III study KEYNOTE-590 evaluated the efficacy of pembrolizumab in combination with chemotherapy as a first-line treatment for patients with advanced esophageal cancer26. ORIENT-15, also a phase III trial, compared to placebo in the first-line treatment of patients with advanced or metastatic esophageal squamous cell carcinoma, sindelizumab in combination with cisplatin in combination with paclitaxel showed significant benefits in terms of OS and progression-free survival27. In addition, similar benefits of sindilizumab in combination with cisplatin plus 5-fluorouracil showed significant potential27. The ESCORT-1st randomized clinical trial, a phase III trial designed to investigate the efficacy of chemotherapy combined with karelizumab treatment compared to placebo-combined chemotherapy in patients with advanced or metastatic esophageal squamous cell carcinoma, demonstrated that the chemotherapy-combined karelizumab treatment group significantly improved patients’ OS and progression-free survival28. A multicenter phase III trial, the JUPITER-06 study, demonstrated that teraplizumab in combination with paclitaxel plus cisplatin chemotherapy significantly improved overall and progression-free survival in patients with primary advanced esophageal squamous carcinoma29. Cetuximab, targeting epidermal growth factor receptor (EGFR), and bevacizumab, targeting vascular endothelial growth factor (VEGF), have been shown to significantly improve the prognosis of patients with esophageal cancer30,31. Pabolizumab (PD-L1 inhibitor) has been approved for the treatment of patients with PD-L1-positive or advanced esophageal squamous cell carcinoma (ESCC), showing significant efficacy32.
In contrast, the strategy of palliative chemotherapy combined with immunotherapy should be considered for those patients with distant metastatic disease7. For example, pembrolizumab combined with chemotherapy is used as first-line treatment for patients with advanced esophageal cancer. Immunotherapy is performed by activating one’s T-lymphocytes so that they recognize and kill tumor cells33. There are nationally renowned large clinical trials of immunotherapy for esophageal cancer, such as the study in KEYNOTE-028, which showed that pembrolizumab PD-L1-positive patients with advanced EC demonstrated manageable toxicity and continued to exhibit antitumor activity34. The study in CheckMate648 showed that in patients with advanced esophageal squamous cell carcinoma, either Nivolumab in combination with chemotherapy or in Nivolumab combination with ibritumomab as first-line treatment regimens both demonstrated significant benefits and were able to significantly prolong OS35. ATTRACTION-3, a phase III clinical trial, demonstrated that Nivolumab was associated with a significantly higher OS in patients with advanced esophageal squamous cell carcinoma who had received prior therapy compared to chemotherapy and a favorable safety profile36.
There are also some limitations in this study, First, the SEER database does not contain information such as immunotherapy, targeted therapy, clinical manifestations, basic diseases, and surgical types. Second, although we consider chemotherapy in the nomogram, the specific scheme is unknown, different treatment schemes may lead to different results. In addition, we used SEER data mainly in America for validation and analysis but did not use data from patients in other countries. Therefore, the reliability of the findings would be higher if data from domestic and foreign multicenter studies were integrated for external validation.
This study demonstrated that T-stage, M-stage, primary tumor site, surgery, chemotherapy, and radiotherapy are independent prognostic risk factors affecting the survival of patients with distant metastatic esophageal cancer. A study showed that the use of local therapy and local radiotherapy in oligometastatic EC patients proved to be beneficial for survival37. Again using the nomogram approach, this supports our findings. the nomogram can guide individualized management survival and provide valuable evidence for clinical decision-making38. Some studies have shown that age, gender, diagnostic grading, number of metastatic organs at the time of diagnosis, type of pathology, local treatment and chemotherapy are independent predictors of CSS in patients39. Our study did not find an effect of age, sex, and diagnostic grading on OS in distant metastatic esophageal cancer, which may be due to the heterogeneity of their selection of patients with initially diagnosed metastatic esophageal cancer. The majority of esophageal cancer diagnoses occur in the elderly. Additionally, We used propensity score matching (PSM) to effectively balance baseline differences caused by confounders such as age and gender. We nomogram can be utilized to design clinical trials for patients with metastatic esophageal cancer and to identify individuals eligible for trials of new therapies.
The strength of our study lies in the selection of a large sample of metastatic EC patients and the adequate statistical analysis of a column line graph of distant metastatic EC patients, which has a better predictive effect on the prognosis of the patients and also has some clinical reference value. Moreover, with the continuous progress of immunotherapy and targeted therapies, the prediction of survival of cancer patients with different treatment combinations may be more challenging. Therefore, more multicenter studies and prospective data collection with other potential variables are needed to improve this column line graph.
Conclusions
We developed and validated a column-line diagram based on the SEER database, which can assist clinicians in predicting patient prognosis and also provide a convenient and reliable individualized survival prediction tool for patients with distant metastatic esophageal cancer. Our study demonstrated that T-stage, M-stage, primary tumor site, surgery, chemotherapy, and radiotherapy were independent prognostic risk factors affecting the survival of patients with distant metastatic esophageal cancer. Meanwhile, radiotherapy, chemotherapy, as well as surgical treatment can prolong the survival time of patients with distant metastatic esophageal cancer, which can bring survival benefits to patients with distant metastatic esophageal cancer.
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249 (2021).
Zhu, H. et al. Esophageal cancer in china: Practice and research in the new era. Int. J. Cancer. 152 (9), 1741–1751 (2023).
Chen, W. et al. Cancer statistics in china, 2015. CA Cancer J. Clin. 66 (2), 115–132 (2016).
Liu, R. et al. Annexin A2 combined with TTK accelerates esophageal cancer progression via the akt/mtor signaling pathway. Cell. Death Dis. 15 (4), 291 (2024).
Yang, J. et al. Understanding esophageal cancer: The challenges and opportunities for the next decade. Front Oncol. 10, 1727 (2020).
Blot, W. J. Invited commentary: More evidence of increased risks of cancer among alcohol drinkers. Am. J. Epidemiol. 150 (11), 1138 (1999).
van Rossum, P. S. N., Mohammad, N. H., Vleggaar, F. P. & van Hillegersberg, R. Treatment for unresectable or metastatic oesophageal cancer: Current evidence and trends. Nat. Rev. Gastroenterol. Hepatol. 15 (4), 235–249 (2018).
Tang, J. et al. CDK2-activated TRIM32 phosphorylation and nuclear translocation promotes radioresistance in triple-negative breast cancer. J. Adv. Res. 61, 239 –2 51 (2024)
Bleiberg, H. et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur. J. Cancer. 33 (8), 1216–1220 (1997).
Guttmann, D. M. et al. Improved overall survival with aggressive primary tumor radiotherapy for patients with metastatic esophageal cancer. J. Thorac. Oncol. 12 (7), 1131–1142 (2017).
Rusthoven, C. G. et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J. Clin. Oncol. 34 (24), 2835–2842 (2016).
Dutton, S. J. et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 15 (8), 894–904 (2014).
Lee, S. & Cohen, D. J. Pharmacotherapy for metastatic esophageal cancer: Where do we need to improve?. Expert Opin. Pharmacother. 20 (3), 357–366 (2019).
Yap, W-K. et al. Development and validation of a nomogram for assessing survival in patients with metastatic lung cancer referred for radiotherapy for bone metastases. JAMA Netw. Open. 1 (6), e183242 (2018).
Mao, W-M., Zheng, W-H. & Ling, Z-Q. Epidemiologic risk factors for esophageal cancer development. Asian Pac. J. Cancer Prev. 12 (10), 2461–2466 (2011).
Quint, L. E. et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer 76 (7), 1120–1125 (1995).
Chao, Y-K. et al. Distant nodal metastases from intrathoracic esophageal squamous cell carcinoma: Characteristics of long-term survivors after chemoradiotherapy. J. Surg. Oncol. 102 (2), 158–162 (2010).
Van Daele, E. et al. Long-term survival after multimodality therapy including surgery for metastatic esophageal cancer. Acta Chir. Belg. 118 (4), 227–232 (2018).
Tokairin, Y., Kumagai, Y. & Yamazaki, S. A case of postoperative liver metastasis of esophageal cancer remains in progression free after successfully resected. Gan Kagaku Ryoho. 36 (12), 2462–2464 (2009).
Ikebe, M., Kitamura, M., Saitoh, G. & Hasegawa, H. Multimodality therapy containing hepatic arterial infusion chemotherapy for liver metastasis of esophageal cancer-a case report. Gan Kagaku Ryoho. 39 (10), 1555–1557 (2012).
Ida, S. et al. A case of recurrent esophageal cancer with lymph node and lung metastases, successfully treated with systemic chemotherapy and radiofrequency-ablation. Gan Kagaku Ryoho. 39 (1), 107–110 (2012).
Iitaka, D. et al. Case involving long-term survival after esophageal cancer with liver and lung metastases treated by multidisciplinary therapy: Report of a case. Surg. Today. 43 (5), 556–561 (2013).
St-Amour, P. et al. Correction to: The ‘’real R0’’: A resection margin smaller than 0.1 cm is associated with a poor prognosis after oncologic esophagectomy. Ann. Surg. Oncol. 28 (Suppl 3), 882 (2021).
Tang, H-R. et al. A phase II study of concurrent chemoradiotherapy with paclitaxel and cisplatin for inoperable esophageal squamous cell carcinoma. Am. J. Clin. Oncol. 39 (4), 350–354 (2016).
Seyedin, S. N., Parekh, K. R., Ginader, T. & Caster, J. M. The role of definitive radiation and surgery in metastatic esophageal cancer: An NCDB investigation. Ann. Thorac. Surg. 112 (2), 459–466 (2021).
Kato, K. et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 15 (10), 1057–1066 (2019).
Lu, Z. et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ. 377, e068714 (2022).
Luo, H. et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA. 326 (10), 916–925 (2021).
Wang, Z-X. et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell., 40(3), 277 (2022).
Ruhstaller, T. et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: A randomized, open-label, phase III trial (SAKK 75/08). Ann. Oncol. 29 (6), 1386–1393 (2018).
Cunningham, D. et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK medical research Council ST03): Primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 18 (3), 357–370 (2017).
Shah, M. A. et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol. 5 (4), 546–550 (2019).
Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Invest. 125 (9), 3335–3337 (2015).
Doi, T. et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J. Clin. Oncol. 36 (1), 61–67 (2018).
Doki, Y. et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl. J. Med. 386 (5), 449–462 (2022).
Kato, K. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (11), 1506–1517 (2019).
Li, B. et al. Development and validation of a nomogram prognostic model for esophageal cancer patients with oligometastases. Sci. Rep. 10 (1), 11259 (2020).
Jung, H. A. et al. Nomogram to predict treatment outcome of fluoropyrimidine/platinum-based chemotherapy in metastatic esophageal squamous cell carcinoma. Cancer Res. Treat. 45 (4), 285–294 (2013).
Tang, X. et al. A novel nomogram and risk classification system predicting the cancer-specific survival of patients with initially diagnosed metastatic esophageal cancer: A SEER-Based study. Ann. Surg. Oncol. 26 (2), 321–328 (2019).
Acknowledgements
The authors thank all patients, especially for the ability to have open access to the SEER database.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82473238 to Haibo Zhang; 82203377 to Yanwei Lu), Zhejiang natural science foundation of China (Grant num-ber: LQ22H160036, to Yanwei Lu; LY24H160022, to Haibo Zhang), Zhejiang Health Science and Technology Project (2025KY031 to Haibo Zhang).
Author information
Authors and Affiliations
Contributions
Shuang Li and Yanwei Lu wrote the main manuscript text, and Ruiqi Liu, Luanluan Huang and Ding Nan prepared Figs. 1, 2 and 3. Xiaoyan Chen and Wenjie Xia prepared Figs. 4 and 5. Xiaodong Liang and Haibo Zhang prepared Fig. 6. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Lu, Y., Liu, R. et al. Clinical features, treatment and prognosis analysis of distant metastatic esophageal cancer. Sci Rep 15, 30977 (2025). https://doi.org/10.1038/s41598-025-16890-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16890-w