Abstract
Oxidative stress and physical inactivity are regarded as important mechanisms contributing to muscle loss. However, the efficacy of antioxidants and their potential synergy with exercise training remain controversial for older population. The study aimed to evaluate the effects of antioxidants, either combined with or without exercise, on improving muscle strength, mass, and physical performance in older adults. We conducted a comprehensive search of the PubMed, MEDLINE, and Embase databases to identify randomized controlled trials (RCTs) on muscle condition in subjects aged 55 years or older. The search period covered from the inception of each database until June 10, 2024. A total of 39 RCTs involving 1714 participants were included. The meta-analysis presented that antioxidants alone could enhance muscle strength in 1 repetition maximum (RM) in leg press and physical function in the older population. Exercise alone could increase the walking distance of 6 min walk more than antioxidants alone. The combination of antioxidants and exercise further improved 1RM in leg press, usual walking speed and walking distance of 6 min walk compared to antioxidants alone. Additionally, this combination enhanced handgrip strength, 1RM in leg press and walking distance of 6 min walk more than exercise alone. This meta-analysis demonstrated that antioxidants alone had positive effects on muscle condition in older individuals. However, the combination of antioxidants and exercise was more effective than either intervention alone in improving muscle strength and physical function among the older adults.
Similar content being viewed by others
Introduction
The muscle condition generally worsens as individuals age1,2. In severe cases, sarcopenia3can occur, which is a syndrome characterized by progressive, widespread loss of skeletal muscle mass throughout the body, accompanied by decreased muscle strength and/or function4,5. Studies have documented a 40% reduction in total muscle cross-sectional areas from the age of 20 to 606. This poor muscle condition increases the risk of falls, fractures, and postoperative complications in the older population, which can significantly affect their quality of life and survival time4,7,8. The latest research indicated that sarcopenia was also a risk factor of ovarian cancer and a faster usual walking speed was related with a lower risk of ovarian cancer9. Moreover, the impact on health care costs for the older people also contributes to a substantial financial burden on the national healthcare system. With the increasing number and proportion of the older population, the incidence of sarcopenia is rising. Thus, there is an urgent need for further investigation into age-related muscle decline.
It is currently believed that age-related muscle loss is closely related to a variety of factors, such as oxidative stress damage, malnutrition, lack of exercise, chronic inflammation, genetic factors, endocrine disorders and imbalance in neuromuscular regulation. The research has shown that the levels of C-reactive protein (CRP) and IL-1β in patients with sarcopenia were significantly elevated, and the gait speed was negatively correlated with the IL-1β level10. Exosomes derived from Schwann cell-like cells promoted the regeneration of peripheral nerve injuries, alleviated gastrocnemius muscle atrophy and enhanced the recovery of motor function11. The ablation of protein arginine methyltransferase 1 in motor neurons led to elevated cellular stress responses and mitochondrial dysfunction which brought about age-related motor neuron degeneration and muscle loss12. Oxidative stress, as one of the important mechanisms in the process of muscle atrophy, has received extensive attention. As people grow older, the oxidative stress within skeletal muscle cells intensifies, and the production of reactive oxygen species (ROS) significantly increases. The imbalance of the redox system within the body occurs, and leads to oxidative stress damage in muscle tissues. The damage triggers mitochondrial dysfunction through affecting multiple intracellular signaling pathways, leads to a decrease in the number of muscle satellite cells, disrupts the function of the neuromuscular junction and excitation-contraction coupling, and ultimately results in reduced protein synthesis and accelerated protein degradation in muscle tissue4.
Although ample evidence supports the benefits of exercise in improving muscle condition among the older people4,13, some individuals in that age group face challenges in adhering to exercise regimens due to underlying diseases or disabilities. Studies have found that the risk of malnutrition increased with the increase of patients’ age14, and early enteral nutrition can reduce muscle loss in patients with acute exacerbation of chronic obstructive pulmonary disease who require mechanical ventilation15. Consequently, Antioxidants, essential components of nutritional supplements, have become a convenient and economical approach for many older people to prevent or treat age-related muscle loss. Antioxidants can be obtained from various sources, including vitamin C, vitamin E, astaxanthin, as well as polyphenols and flavonoids found in certain fruits, vegetables, and herbs. However, the efficacy of antioxidants and their potential synergy with exercise training remain controversial for older population. A study has demonstrated that long-term intake of antioxidants can alleviate age-related changes in muscle function in mice16. Conversely, another study suggests that creatine, a compound with antioxidant properties, does not clearly improve muscle strength and quality of life among older adults, but the combined use of creatine and exercise training can double the strength obtained in resistance training17. Moreover, many studies evaluating the effects of antioxidants or their combination with exercise on muscle condition has been small-scale and involved different types of antioxidants or a combination of antioxidants in older adults. The purpose of this systematic review and meta-analysis is to evaluate the effects of antioxidants, combined or not with exercise, on improving muscle strength, mass, and physical performance in older adults by synthesizing data from randomized controlled trials (RCTs), and to provide references for the development and clinical practice of nutritional supplement programs.
Materials and methods
Search strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov/), MEDLINE (https://www.webofknowledge.com/MEDLINE) and Embase (https://www.embase.com/) databases were searched from their inception time to June 10, 2024. The meta-analysis was registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/; registration number: CRD420251087216).
The search strategy included the following Medical Subject Headings (MeSH) and keywords: Muscle, AND aging OR aged OR geriatric OR elderly, AND antioxidant OR catechin OR quercetin OR resveratrol OR curcumin OR rutin OR flavonoids OR flavanols OR flavones OR anthocyanidins OR flavanones OR flavan-3-ols OR polyphenols OR copper OR Ascorbic Acid OR vitamin C OR Vitamin E OR tocopherol OR tocotrienols OR selenium OR magnesium OR iron OR zinc OR tannins OR quinones OR dexpanthenol OR carotenoids OR retinol OR lycopene OR phytic acid OR ubiquinone OR CoQ10 OR coenzyme Q10 OR xanthophylls OR lutein OR phenolic acid OR luteolin OR flavanone OR naringenin OR anthocyanins OR cyanidin OR Isoflavones OR genistein OR thioctic acid OR lipoic acid OR taurine OR astaxanthin OR oat anthracene amide OR gingerol OR ginger extract OR tea OR zeaxanthin OR pycnogenol OR Allicin OR creatine OR melatonin OR N-acetylcysteine OR carotene OR glutathione OR nitrate OR isothiocyanate OR Vitamin A. An expanded search of the references and related literature was carried out to avoid omission. (See Table S3 for details)
Study selection
The study was conducted in accordance with the PRISMA guidance18.
The inclusion criteria were as follows: ①The study design was a randomized controlled trial. ② Intervention: Participants were treated with antioxidants. And the antioxidants should be clearly defined as certain molecules, regardless of the types of antioxidants. The articles in which participants with or without exercise training were included, but they will would be analyzed in different groups. ③ Subjects were aged 55 years or older. ④ At least one of the following main outcomes were included in the studies: handgrip strength and 1repetition maximum (RM) in leg press, which are related to muscle strength; fat-free mass and body fat percentage, associated with muscle mass; 3 m timed up and go, usual walking speed, and walking distance of the 6-minute walk test, which are indicators of physical function. Secondary outcomes, including body mass index, body weight, systolic blood pressure, diastolic blood pressure and serum malondialdehyde, were not required for inclusion in the article. But if the article had the above secondary outcomes, this outcome would be extracted and analysed. ⑤Articles published in English.
We excluded the articles with the following conditions: ① The researchers didn’t identify the molecules that produce antioxidant effect in the products, or antioxidants mix with other non-antioxidant substances which makes it impossible for us to observe the effect of antioxidants alone. ② Crossover study is excluded. ③ Participants: Participants with serious diseases or irreversible muscular diseases that affect the experimental results were excluded, such as: cancer, poliomyelitis, spinal cord injury, amyotrophic lateral sclerosis, Duchenne muscular dystrophy, etc. Participants younger than 55 years old or athletes were excluded. ④ The study duration less than 1 week is excluded.
Data extraction and quality assessment
Two independent investigators (Y.W. and Z.H.) reviewed the publications and extracted the data. Controversial data would be reviewed by a third investigator (C.L.). We extracted the following data: first author, year of publication, subjects’ characteristic (number, age and physical condition), antioxidants (type, dose and frequency), grouping, combined/non-combined with exercise training, duration of intervention, main outcomes (handgrip strength, 1RM in leg press, fat-free mass, body fat percentage, 3 m timed up and go, usual walking speed and walking distance of the 6-minute walk test) and secondary outcomes. The detailed descriptions of the outcomes were in Supplementary Material 2.
The methodological quality of each study was assessed by two independent researchers (Y.W. and Z.H.). We assessed the risk of bias in the included randomized trials using the Cochrane Revised “Risk of bias” tool for randomized trials (RoB 2.0). Each trial was judged as having a “low risk of bias”, “some concerns”, or “high risk of bias” overall.
Statistical analysis
The outcomes were expressed as mean difference (MD) or standard mean difference (SMD) with 95% confidence intervals (CIs). We quantified heterogeneity using the I2 statistic. Then, based on the I2 value, the random-effects model (I2 ≥ 50%, indicating significant heterogeneity) or the fixed-effect model (I2 < 50%, indicating low heterogeneity) would be chosen. Whenever significant heterogeneity was present, we searched for potential sources of heterogeneity. For example, if one study showed results that were completely out of range of the others, we searched for likely reasons explaining the difference and performed a sensitivity analysis excluding that study, when deemed appropriate. We didn’t distinguish antioxidant types or exercise modalities when we included the RCTs. So, we conducted a limited subgroup analysis of creatine, resveratrol and antioxidant Vitamin that frequently appear in the RCTs on the main outcome fat-free mass and body fat. The potential for bias was assessed by inspecting a funnel plot. The presence of publication bias was also evaluated by using the Begg and Egger tests. Sensitivity analyses were performed to assess the impact of high-risk studies to the pooled analyses. The results were considered statistically significant at two-sided p-values of less than 0.05. All statistical analyses were performed using RevMan 5.4.1 and Stata 15.0.
Results
Literature search
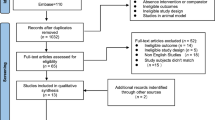
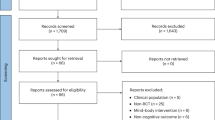
A total of 2,904 articles were retrieved from the databases. Then, through screening titles and abstracts, 167 relevant articles were obtained by excluding duplicate publications and articles that did not conform to the research subject and design. After a thorough reading of the full texts, 39 articles19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 were finally included, having eliminated those with incomplete data and inconsistent subject age, et cetera. The detailed selection process is shown in Fig. 1.
Basic characteristics and quality assessment
The characteristics of the included trials are presented in Table 1. Seven RCTs23,25,35,37,41,43,48 conducted experiments using four groups: a control group, an antioxidant group, an exercise training group, and a combined antioxidant and exercise training group. Twenty studies19,20,24,26,27,28,29,30,31,32,33,36,38,42,49,51,53,54,55,56 used two groups: an exercise training group and a combined antioxidant and exercise training group. Twelve articles21,22,34,39,40,44,45,46,47,50,52,57 included only a control group and an antioxidant group.
We used the Cochrane Collaboration’s tool to assess the bias of the 39 articles (supplement Figure S1 and S2). Among the items assessed, the blinding of participants and personnel, incomplete outcome data, and other biases had a higher percentage of high-risk bias.
Antioxidant versus control
Data on the efficacy of the antioxidant group compared to the control group were available from 19 RCTs21,22,23,25,34,35,37,39,40,41,43,44,45,46,47,48,49,52,57. The antioxidant group was associated with a significant improvement in 1RM in leg press (MD = 1.90 kg; 95% CI, [1.39 kg to 2.41 kg]; P < 0.00001; Fig. 2a) ,3 m timed up & go (MD = 0.22s; 95% CI, [0.08s to 0.36s]; P = 0.003; Fig. 2b) and walking distance of 6 min walk (MD = 22.63 m; 95% CI, [6.82 m to 38.43 m]; P = 0.005; Fig. 2c) compared to the control group. However, the antioxidants showed no significant effect on secondary outcomes or muscle mass (Table 2 and supplement Figure S3-6).
Antioxidant versus exercise training
Data regarding the efficacy between the antioxidant group and the exercise group were sourced from 7 RCTs23,25,35,37,41,43,48. In comparison to exercise training, the antioxidants demonstrated a significant reduction in walking distance of 6 min walk (MD = -122.50 m; 95% CI, [-201.74 m to -43.26 m]; P = 0.002; Fig. 3). However, no significant differences were observed in secondary outcomes, muscle strength or muscle mass (Table 2 and supplement Figure S7-10).
Antioxidant versus antioxidant + exercise training
Data on the efficacy of antioxidants alone versus the combination of antioxidants and exercise training were available from 7 RCTs23,25,35,37,41,43,48. Compared with antioxidants alone, the combination of antioxidants and exercise training was associated with a significant improvement in 1RM in leg press (MD = 15.28 kg; 95% CI, [11.50 kg to 19.06 kg]; P < 0.00001; Fig. 4a), usual walking speed (MD = 0.13 m/s; 95% CI, [0.02 m/s to 0.24 m/s]; P = 0.03;Figure 4b) and walking distance of 6 min walk (MD = 122.10 m; 95% CI, [48.42 m to 195.78 m]; P = 0.001; Fig. 4c). Interestingly, body mass index (MD = 0.15 kg; 95% CI, [0.04 kg to 0.25 kg]; P = 0.007; Fig. 4d) significantly increased in antioxidant + exercise training group compared with antioxidant group. However, no significant changes were observed in other outcomes (Table 2 and supplement Figure S11-14).
Exercise training versus antioxidant + exercise training
Data on the efficacy of exercise training alone versus the combination of antioxidants and exercise training were available from 27 RCTs19,20,23,24,25,26,27,28,29,30,31,32,33,35,36,37,38,41,42,43,48,49,51,53,54,55,56. Compared with exercise training alone, the antioxidant + exercise training was associated with a significant improvement in handgrip strength (MD = 1.02 kg; 95%CI, [0.37 kg to 1.68 kg]; P = 0.002; Fig. 5a), 1RM in leg press (MD = 5.21 kg; 95%CI, [0.14 kg to 10.28 kg]; P = 0.04; Fig. 5b) and walking distance of 6 min walk (MD = 25.55 m; 95%CI, [15.37 m to 35.74 m]; P < 0.00001; Fig. 5c). Non-significant impacts were shown in secondary outcomes and muscle mass (Table 2 and supplement Figure S15-17).
Heterogeneity and sensitivity analyses
We found that there’s no significant difference in the respective effects of the common antioxidant types (creatine, resveratrol and antioxidant Vitamin). The reason why we failed to subgroup analyze the other main outcomes and exercise modalities is that the related studies were insufficient. We looked forward to more publications about other main outcomes and different exercise modalities, and we will incorporate more mechanism-related outcomes to analyze the mechanisms of different antioxidant types and exercise modalities to muscle condition in future (See Supplementary file 2 for details).
What’s more, we conducted a sensitivity analysis on the main outcomes with enough number of articles and divided them into high-risk and non-high-risk groups for subgroup analysis. The main reason why subgroup analysis was not conducted for other outcomes is that the number of articles is too small or there was no high-risk article applied in this outcome. The results could be seen in Supplementary file 2 and for all the outcomes of our analysis, none of the high-risk groups had a significantly different effect from the non-high-risk subgroups.
Publication bias
Funnel plot was used to assess publication bias for a certain outcome and five or more studies for this outcome were required to detect funnel plot asymmetry58. Funnel plot inspection and Begg’s/Egger’s tests revealed no significant publication bias, except for usual walking speed in comparisons between exercise training versus antioxidant + exercise training group. (supplement Figure S18-19, Table 2)
Discussion
This meta-analysis of 39 RCTs assessed the effects of antioxidants, with or without exercise training, on muscle strength, mass, and physical function in older adults. We discovered that antioxidants in the older population can enhance their muscle strength, as measured by 1RM in leg press, and physical function, as indicated by the 3 m timed up & go and the walking distance of 6 min walk test. Exercise training can increase the walking distance of 6 min walk more than antioxidants alone. Furthermore, the combination of antioxidants and exercise training could further improve 1RM in leg press, usual walking speed, and walking distance of 6 min walk compared to antioxidants alone. Additionally, it could further enhance handgrip strength, 1RM in leg press, and the walking distance of 6 min walk compared to exercise training alone.
In this study, we found that antioxidants provided a positive effect on muscle condition in the older population and, at least, did no harm to muscle mass. Some of recent studies supported our view. Talebi et al. showed that coenzyme Q10 effectively reduced exercise-induced muscle damage and biomarkers of oxidative stress in Asian adults59. Two studies on athletes showed that exogenous administration of melatonin can alleviate oxidative stress, inflammation, and muscle damage, and might improve exercise performance60,61. Another umbrella review of systematic reviews and meta-analyses suggested that some antioxidants, such as curcumin, polyphenols, and anthocyanins, can alleviate muscle soreness62. Antioxidant intake has been believed to inhibit oxidative stress and play an effective role in managing sarcopenia.
Mitochondrial dysfunction is a hallmark feature of both cellular senescence and organ aging63. Mitochondrial antiviral signaling protein is a factor widely present in mitochondria that can regulate the aging of human stem cells. Its absence in the elderly population can impair mitochondrial dynamics, manifesting as an increase in mitochondrial fragmentation, accumulation of mitochondrial DNA copies, a decrease in mitochondrial membrane potential, and an increase in ROS levels63. The mitochondria with age-related functional decline produced excessive ROS, which aggravated the oxidative stress induced by ROS. This, in turn, damaged the mitochondria of muscles and motor neurons12,63. Therefore, antioxidants may prevent the vicious cycle of mitochondrial damage by reducing the level of oxidative stress. Antioxidants may also reduce the occurrence of NLRP3-mediated skeletal muscle inflammation and pyroptosis by regulating molecules with redox-sensitive domains, such as caspase-1 and gasdermins B and D64.
However, serum malondialdehyde (MDA), an indicator of lipid peroxidation to reflect ROS levels, didn’t become lower in antioxidants group compared with the control group in our study (Supplement Figure S3.E). This may be due to the limited number of included studies, or because changes in MDA were not reflected in serum. Zubaidah et al.65 found that Gynura procumbens leaf and root (contain phenolics and flavonoids) with antioxidant effect significantly reduced lipid peroxidation in liver and kidneys, but did not in the serum. It is expected that more randomized controlled experiments will include indicators related to mitochondria and inflammatory factors, so that meta-analysis can have more results related to mechanisms.
Nevertheless, some previous studies have mentioned the dual role of ROS66,67. They mentioned that though excessive ROS cause oxidative damage and promote functional decline in skeletal muscle, a moderate amount of ROS can also act as a signaling molecule to stimulate the body’s defense response67,68, which results in the controversial effect of antioxidants66. We didn’t find that antioxidants had negative effects on the muscle condition of the elderly. Perhaps this is because, as age progresses, ROS are continuously produced in large amounts, and simultaneously, the endogenous antioxidant system becomes increasingly inefficient in responding to oxidative stress. Thus, in the older population, exogenous supplement of antioxidants demonstrates favorable effects when there is an imbalance in oxidant/antioxidant homeostasis.
In this meta-analysis, we found that exercise training showed more improvement in physical function compared to antioxidants. Exercise can reduce age-related oxidative stress and inflammation, promote mitochondrial biosynthesis pathways in skeletal muscle, and contribute to better neuromuscular performance10,68,69,70,71. A systematic review suggests that resistance training is the ‘gold standard treatment’ for early sarcopenia71. Insulin-like growth factor 1 activates intracellular signaling pathways, sequentially activating phosphatidylinositol 3-kinase and protein kinase B, thereby activating the downstream mammalian target of rapamycin (mTOR), and subsequently promoting protein synthesis. However, this mechanism is weakened in aged skeletal muscle. The Muscle-tendon progenitor cells in the myotendinous junction are pluripotent and have a certain potential to differentiate into muscle cells72. The mTOR is an important molecule that maintains the functions of muscle-tendon progenitor cells. Exercise can improve mTOR colocalization with the cell periphery which may result in the improvement of muscle function72,73.
Exercise and antioxidants may have a synergistic effect and there are multiple mechanisms for this synergy. One review found that exercise increases markers of mitochondrial fusion and fission, converting defective mitochondria into efficient ones, thereby increasing cellular oxidative stress levels69. Exercise increased mitochondrial biogenesis by activating the genes affected by peroxisome proliferator activated receptor gamma coactivator 1-β (PGC1-β), regulated the ATP-AMP ratio and cellular stress, and formed a new mitochondrial network69. Morawin et al. found an inverse correlation between gait speed and IL-1β11, suggesting that excessively high levels of inflammation may be associated with poor muscle condition, whereas exercise can reduce the levels of inflammatory indicators such as tumor necrosis factor α, IL-6, and CRP74. The inflammatory factor IL-6 can also increase the production of mitochondrial ROS dependent on the activation of the JAK/STAT pathway, but this effect can be weakened by the mitochondrial-targeted antioxidant MitoQ75. ROS can promote host defense mechanisms such as the activation of signaling pathways, including Keap1-Nrf2-antioxidant response element signaling pathway. Nrf2 is activated, inducing antioxidant genes transcription68. A recent study conducted on aged mice has shown that long-term exercise intervention regulated drp-1-dependent mitochondrial fission, increased the mRNA expression of Nrf2 in skeletal muscle, and improved mitochondrial quality76. This might be another manifestation of how exercise enhances the antioxidant effect.
Our study has confirmed that combining antioxidants and exercise training can offer additional advantages for muscle strength and physical function compared to exercise training or the antioxidants alone. A comprehensive systematic review also found that the combination of resveratrol and exercise improves exercise adaptation and muscle function in healthy older adults77.Another 2018 review reported that exogenous natural antioxidants such as curcumin, quercetin, and resveratrol, together with physical activity, could protect the body from age-related disorders78. Their findings aligned with our conclusion that the combination of antioxidants and exercise training yields better effects on muscle condition than either exercise training or antioxidants alone.
Our analysis differed in some ways from other meta-analyses. Our search was the most comprehensive to date, aiming to include as many relevant antioxidant studies as possible, and we only included RCTs to ensure high-quality and reliable evidence. Our results provided new insights into the role of antioxidants and demonstrated the synergistic relationship between antioxidants and exercise in addressing skeletal muscle aging. However, our research has some limitations that should be addressed in future studies. Firstly, some of the included RCTs had small sample sizes, and more relevant RCTs are needed to strengthen the convincingness of our results. Secondly, although our meta-analysis detected statistically significant effects for several outcomes, the clinical relevance of small effect sizes (e.g., walking speed MD = 0.13 m/s) remains uncertain. Future trials should prioritize clinically meaningful endpoints over purely statistical significance. Thirdly, there is a paucity of RCTs that systematically evaluate oxidative stress biomarkers (e.g., SOD, glutathione peroxidase), inflammatory factors (e.g., IL-6), and skeletal muscle-specific signaling pathways (e.g., mTOR) as outcomes, resulting in a lack of direct evidence investigating the mechanisms by which antioxidants with/without exercise improve muscle condition in older populations. Therefore, future studies should consider the detection of the above common biomarkers. Finally, we included articles from different countries with varying lifestyles and dietary habits, so the results should be interpreted with caution. More high-quality, well-designed randomized controlled trials are needed to gain insight into the effects of antioxidants, with or without exercise, on the prevention and treatment of senile muscular atrophy.
Conclusions
This meta-analysis demonstrated that antioxidants alone or exercise alone had some positive effects on muscle condition in older individuals. The combination of antioxidants and exercise was more effective than either supplements or exercise alone in improving muscle strength and physical function among the older population.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- CRP:
-
C-reactive Protein
- ROS :
-
Reactive oxygen species
- RCTs:
-
Randomized controlled trials
- MeSH:
-
Medical subject headings
- MD:
-
Mean difference
- SMD:
-
Standard mean difference
- CIs:
-
Confidence intervals
- RM:
-
Repetition maximum
- MDA :
-
Malondialdehyde
- mTOR :
-
Mammalian target of rapamycin
- PGC1-β:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-βAs
References
Fukumoto, Y. et al. Age-related ultrasound changes in muscle quantity and quality in women. Ultrasound Med. Biol. 41, 3013–3017 (2015).
Ballak, S. B., Degens, H., de Haan, A. & Jaspers, R. T. Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res. Rev. 14, 43–55 (2014).
Falcon, L. J. & Harris-Love, M. O. Sarcopenia and the new ICD-10-CM code: screening, staging, and diagnosis considerations. Fed. Pract. 34, 24–32 (2017).
Cruz-Jentoft, A. J., Sayer, A. A. & Sarcopenia Lancet 393, 2636–2646 (2019).
Sayer, A. A. & Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. 51, afac220 (2022).
Doherty, T. J. Invited review: aging and sarcopenia. J. Appl. Physiol. (1985). 95, 1717–1727 (2003).
Yuan, S. & Larsson, S. C. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism 144, 155533 (2023).
Tournadre, A. et al. Joint Bone Spine 86, 309–314 (2019).
Wu, M. et al. Sarcopenia-related traits, body mass index and ovarian cancer risk: investigation of causal relationships through multivariable Mendelian randomization analyses. BIOI 5, 1–8 (2024).
Morawin, B., Tylutka, A., Bielewicz, F. & Zembron-Lacny, A. Diagnostics of inflammaging in relation to sarcopenia. Front. Public. Health. 11, 1162385. https://doi.org/10.3389/fpubh.2023.1162385 (2023).
Hu, T. et al. Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats. Burns Trauma. 11, tkad013 (2023).
So, H. K. et al. Protein arginine methyltransferase 1 ablation in motor neurons causes mitochondrial dysfunction leading to Age-related motor neuron degeneration with muscle loss. Research 6, 0158 (2023).
Borde, R., Hortobágyi, T. & Granacher, U. Dose-response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med. 45, 1693–1720 (2015).
Zhou, H. J., Zuo, D. J., Zhang, D., He, X. H. & Guo, S. B. Nutritional status and prognostic factors for mortality in patients admitted to emergency department observation units: a national multi-center study in China. World J. Emerg. Med. 14, 17–24 (2023).
Li, Y., Xie, Y. P., Li, X. M. & Lu, T. Effects of early standardized enteral nutrition on preventing acute muscle loss in the acute exacerbation of chronic obstructive pulmonary disease patients with mechanical ventilation. World J. Emerg. Med. 14, 193–197 (2023).
Tsukamoto-Sen, S. et al. Effect of antioxidant supplementation on skeletal muscle and metabolic profile in aging mice. Food Funct. 12, 825–833 (2021).
Amiri, E. & Sheikholeslami-Vatani, D. The role of resistance training and creatine supplementation on oxidative stress, antioxidant defense, muscle strength, and quality of life in older adults. Front. Public. Health. 11, 1062832 (2023).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71 (2021).
Aguiar, A. F. et al. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 113, 987–996 (2013).
Alway, S. E. et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J. Gerontol. Biol. Sci. Med. Sci. 72, 1595–1606 (2017).
Amstrup, A. K., Sikjaer, T., Mosekilde, L. & Rejnmark, L. The effect of melatonin treatment on postural stability, muscle strength, and quality of life and sleep in postmenopausal women: a randomized controlled trial. Nutr. J. 14, 102 (2015).
De Benedetto, F. et al. Supplementation with Qter® and creatine improves functional performance in COPD patients on long term oxygen therapy. Respir. Med. 142, 86–93 (2018).
Bermon, S., Venembre, P., Sachet, C., Valour, S. & Dolisi, C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol. Scand. 164, 147–155 (1998).
Bjørnsen, T. et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports. 26, 755–763 (2016).
Bobeuf, F., Labonte, M., Dionne, I. J. & Khalil, A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J. Nutr. Health Aging. 15, 883–889 (2011).
Brose, A., Parise, G. & Tarnopolsky, M. A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. Biol. Sci. Med. Sci. 58, 11–19 (2003).
Candow, D. G. et al. Low-dose creatine combined with protein during resistance training in older men. Med. Sci. Sports Exerc. 40, 1645–1652 (2008).
Chrusch, M. J., Chilibeck, P. D., Chad, K. E., Davison, K. S. & Burke, D. G. Creatine supplementation combined with resistance training in older men. Med. Sci. Sports Exerc. 33, 2111–2117 (2001).
Collins, J., Longhurst, G., Roschel, H. & Gualano, B. Resistance training and co-supplementation with creatine and protein in older subjects with frailty. J. Frailty Aging. 5, 126–134. https://doi.org/10.14283/jfa.2016.85 (2016).
Cooke, M. B. et al. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur. J. Appl. Physiol. 114, 1321–1332 (2014).
Córdova-Martínez, A. et al. l-Arginine and beetroot extract supplementation in the prevention of sarcopenia. Pharmaceuticals (Basel). 15, 290 (2022).
Eijnde, B. O. et al. Effects of creatine supplementation and exercise training on fitness in men 55–75 year old. J. Appl. Physiol. (1985). 95, 818–828 (2003).
Gliemann, L. et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 591, 5047–5059 (2013).
Gotshalk, L. A. et al. Creatine supplementation improves muscular performance in older men. Med. Sci. Sports Exerc. 34, 537–543. https://doi.org/10.1097/00005768-200203000-00023 (2002).
Gualano, B. et al. Creatine supplementation and resistance training in vulnerable older women: a randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 53, 7–15 (2014).
Harper, S. A. et al. Resveratrol and exercise combined to treat functional limitations in late life: A pilot randomized controlled trial. Exp. Gerontol. 143, 111111 (2021).
Kim, H. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. Geriatr. Gerontol. Int. 13, 458–465 (2013).
Kinoshita, T., Maruyama, K., Yamamoto, N. & Saito, I. The effects of dietary licorice flavonoid oil supplementation on body balance control in healthy middle-aged and older Japanese women undergoing a physical exercise intervention: a randomized, double-blind, placebo-controlled trial. Aging Clin. Exp. Res. 33, 3099–3108 (2021).
Kuhlman, A. B. et al. Coenzyme Q10 does not improve peripheral insulin sensitivity in statin-treated men and women: the LIFESTAT study. Appl. Physiol. Nutr. Metab. 44, 485–492 (2019).
Kumar, P. et al. Supplementing glycine and N-acetylcysteine (GlyNAC) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: A randomized clinical trial. J. Gerontol. Biol. Sci. Med. Sci. 78, 75–89 (2023).
Labonté, M. et al. Effects of antioxidant supplements combined with resistance exercise on gains in fat-free mass in healthy elderly subjects: a pilot study. J. Am. Geriatr. Soc. 56, 1766–1768 (2008).
Liu, S. Z. et al. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachexia Sarcopenia Muscle. 9, 826–833 (2018).
Mafi, F., Biglari, S., Ghardashi Afousi, A. & Gaeini, A. A. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-Week resistance training and epicatechin supplementation in sarcopenic older adults. J. Aging Phys. Act. 27, 384–391 (2019).
Mankowski, R. T. et al. Effects of curcumin C3 complex® on physical function in moderately functioning older adults with low-grade inflammation—a pilot trial. J. Frailty Aging. 12, 143–149 (2023).
McDermott, M. M. et al. Effect of resveratrol on walking performance in older people with peripheral artery disease: the RESTORE randomized clinical trial. JAMA Cardiol. 2, 902–907 (2017).
McDermott, M. M. et al. Cocoa to improve walking performance in older people with peripheral artery disease: the COCOA-PAD pilot randomized clinical trial. Circ. Res. 126, 589–599 (2020).
Munguia, L. et al. High Flavonoid Cocoa Supplement Ameliorates Plasma Oxidative Stress and Inflammation Levels While Improving Mobility and Quality of Life in Older Subjects: A Double-Blind Randomized Clinical Trial. The journals of gerontology. Series A, Biological sciences and medical sciences. 74, 1620–1627 (2019).
Nalbant, O. et al. Vitamin E and aerobic exercise: effects on physical performance in older adults. Aging Clin. Exp. Res. 21, 111–121 (2009).
Pinto, C. L., Botelho, P. B., Carneiro, J. A. & Mota, J. F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cachexia Sarcopenia Muscle. 7, 413–421 (2016).
Rawson, E. S., Wehnert, M. L. & Clarkson, P. M. Effects of 30 days of creatine ingestion in older men. Eur. J. Appl. Physiol. Occup. Physiol. 80, 139–144 (1999).
Roschel, H. et al. Supplement-based nutritional strategies to tackle frailty: A multifactorial, double-blind, randomized placebo-controlled trial. Clin. Nutr. 40, 4849–4858 (2021).
Søndergård, S. D. et al. The effects of 3 weeks of oral glutathione supplementation on whole body insulin sensitivity in obese males with and without type 2 diabetes: a randomized trial. Appl. Physiol. Nutr. Metab. 46, 1133–1142 (2021).
Tokuda, Y. & Mori, H. Effect of ingestion of essential amino acids and tea catechins after resistance exercise on the muscle mass, physical performance, and quality of life of healthy older people: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 30, 213–233 (2021).
Tokuda, Y. & Mori, H. Essential amino acid and tea catechin supplementation after resistance exercise improves skeletal muscle mass in older adults with sarcopenia: an open-label, pilot, randomized controlled trial. J. Am. Nutr. Assoc. 42, 255–262 (2023).
Veronese, N. et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: a randomized controlled trial. Am. J. Clin. Nutr. 100, 974–981 (2014).
Woessner, M. et al. Beet the best?? Circ. Res. 123, 654–659 (2018).
Zamani, P. et al. Isosorbide dinitrate, with or without hydralazine, does not reduce wave reflections, left ventricular hypertrophy, or myocardial fibrosis in patients with heart failure with preserved ejection fraction. J. Am. Heart Assoc. 6, e004262 (2017).
Sutton, A. J., Duval, S. J., Tweedie, R. L., Abrams, K. R. & Jones, D. R. Empirical assessment of effect of publication bias on meta-analyses. Bmj 320, 1574–1577 (2000).
Talebi, S. et al. The effects of coenzyme Q10 supplementation on biomarkers of exercise-induced muscle damage, physical performance, and oxidative stress: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN. 60, 122–134 (2024).
Almendros-Ruiz, A. et al. The effects of melatonin supplementation on professional football player performance: A systematic review. Nutrients 15, 4467 (2023).
Celorrio San Miguel, A. M. et al. Impact of melatonin supplementation on sports performance and circulating biomarkers in highly trained athletes: A systematic review of randomized controlled trials. Nutrients 16 (1), 1011 (2024).
Talebi, S. et al. Nutritional interventions for exercise-induced muscle damage: an umbrella review of systematic reviews and meta-analyses of randomized trials. Nutr. Rev. 82, 639–653. https://doi.org/10.1093/nutrit/nuad078 (2024).
Wang, C. et al. MAVS antagonizes human stem cell senescence as a mitochondrial stabilizer. Research 6, 0192 (2023).
Rusetskaya, N. Y., Loginova, N. Y., Pokrovskaya, E. P., Chesovskikh, Y. S. & Titova, L. E. Redox regulation of the NLRP3-mediated inflammation and pyroptosis. Biomeditsinskaia Khimiia. 69, 333–352 (2023).
Zubaidah, U. et al. Gynura procumbens adventitious root ameliorates oxidative stress and has cytotoxic activity against cancer. Bio 16, 1–9 (2024).
Fougere, B., van Kan, G. A., Vellas, B. & Cesari, M. Redox systems, antioxidants and sarcopenia. Curr. Protein Pept. Sci. 19, 643–648 (2018).
Damiano, S. et al. Dual role of reactive oxygen species in muscle function: can antioxidant dietary supplements counteract Age-Related sarcopenia?? Int. J. Mol. Sci. 20, 3815 (2019).
El Assar, M., Álvarez-Bustos, A., Sosa, P., Angulo, J. & Rodríguez-Mañas, L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int. J. Mol. Sci. 23, 8713 (2022).
Alizadeh Pahlavani, H., Laher, I., Knechtle, B. & Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. Physiol. 13, 1040381 (2022).
Miller, R. M., Bemben, D. A. & Bemben, M. G. Skeletal muscle adaptations following 80 weeks of resistance exercise in older adults. J. Geriatr. Phys. Ther. 45, 117–124 (2022).
Talar, K. et al. Benefits of resistance training in early and late stages of frailty and sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Clin. Med. 10, 1630 (2021).
Yan, R. et al. Discovery of Muscle-Tendon Progenitor Subpopulation in Human Myotendinous Junction at Single-Cell Resolution. Research 9760390 (2022). (2022).
Abou Sawan, S. et al. Trained integrated postexercise myofibrillar protein synthesis rates correlate with hypertrophy in young males and females. Med. Sci. Sports. Exerc. 54, 953–964 (2022).
Visser, M. et al. A healthy lifestyle in old age and prospective change in four domains of functioning. J. Aging Health. 31, 1297–1314 (2019).
Abid, H., Ryan, Z. C., Delmotte, P., Sieck, G. C. & Lanza, I. R. Extramyocellular interleukin-6 influences skeletal muscle mitochondrial physiology through canonical JAK/STAT signaling pathways. FASEB J. 34, 14458–14472 (2020).
Yan, X. et al. Nrf2 contributes to the benefits of exercise interventions on age-related skeletal muscle disorder via regulating Drp1 stability and mitochondrial fission. Free Radic. Biol. Med. 178, 59–75 (2021).
Yadegar, S. et al. Effects and safety of resveratrol supplementation in older adults: A comprehensive systematic review. Phytother Res. 38, 2448–2461 (2024).
Simioni, C. et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9, 17181–17198 (2018).
Funding
This research was funded by Natural Science Foundation of Hunan Province of China (No. 2021JJ30932), National College Students’ Innovation and Entrepreneurship Training Program of China (No. 202210533090X).
Author information
Authors and Affiliations
Contributions
Y. W., T. H. and Y. Y. designed the study. Y. W., Z. H. and C. L. contributed to the acquisition, analysis and interpretation of data. Y. W. and Y. L. wrote the manuscript. Y. Y. and T. H. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., He, Z., Long, C. et al. Systematic review and meta-analysis of antioxidants with or without exercise training improving muscle condition in older adults. Sci Rep 15, 34356 (2025). https://doi.org/10.1038/s41598-025-16917-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16917-2