Abstract
This research explored the fabrication and characterization of bacterial cellulose (BC), with a distinct emphasis on leveraging indigenous Vietnamese biomass sources. A diverse sample library consisting of 150 BC samples was prepared, with six samples selected for objective evaluation, based on the standard test methods. These samples were subjected to characterization techniques including Scanning electron microscopy (SEM), Energy dispersive X-ray (EDX), Fourier Transformation Infrared (FTIR) and Differential scanning calorimetry (DSC) to explore potential applications of BC in fashion and textiles. Moreover, the growth medium (or SCOBY- Symbiotic Culture of Bacteria and Yeast), from which the BC was cultivated, was analyzed to identify the constituent bacterial and yeast strains. The EDX analysis showed the major elements of BC were Carbon (C) followed by Oxygen (O), which accounted for 99% of the elemental composition of BC. The cellulosic structure was confirmed by the FTIR results, which indicated the characteristics bonds of BC. The DSC results showed that thermal stability can be achieved for the fashion and textiles produced from BC. Bacterial analysis showed the presence of Acetobacter Indonesiensis, a gram-negative bacterium, in all the BC samples. The outcomes of this study established a deeper comprehension of the morphological, thermal, biological, and chemical attributes of BC, as well as the microbial dynamics within the SCOBY mother. This exploration not only augments the existing knowledge on BC’s potential in material design but also paves the way for further research on the influence of local ingredients on biomaterial production, thereby contributing to the burgeoning field of sustainable material innovation within a localized context.
Similar content being viewed by others
Introduction
Biobased materials are gaining significant importance in the field of material science, offering the promise of sustainable, biodegradable, and functional materials1. They originate from biological resources and have the potential to serve as alternatives to their petroleum-based equivalents in the field of material science. Some biobased products are already being commercialized by global brands and others are paving their way through research and innovation. The surging interest in biobased materials stems from the global urgency to transition towards more sustainable, renewable, and ecofriendly material solutions. A significant new material innovation is BC, which is derived from commonly used bioingredients such as tea, coffee, and sugar2 .
BC possesses a unique set of properties such as mechanical strength, permeability, porosity, and viscoelasticity3. BC is produced by certain strains of bacteria, notably the Acetobacter xylinum, which synthesizes cellulose chains and excretes them to form a three-dimensional (3D), nano-fibrillar network. The resultant BC boasts of exceptional purity compared to plant-derived cellulose, as it is devoid of lignin and hemicellulose. Moreover, BC is characterized by superior mechanical properties, excellent water-holding capacity, and a remarkable degree of crystallinity, making it a highly versatile material for a range of applications including fashion and textiles4,5,6. In the realm of material design, BC offers a sustainable alternative for fabricating textiles, packaging materials, and acoustical devices among others1.
Despite several advantages, the commercial application of BC-based products is limited due to scalability, cost and other challenges. Several earlier research publications on BC have focused on the fabrication of biobased materials using commercially available ingredients for nitrogen and carbon sources7,8. However, the number of studies using the local ingredients available in tropical countries is limited9. Hence, the major aim of this research is to fabricate BC utilizing indigenous food sources available in tropical countries such as Vietnam in addition to using the traditional methods of drying similar products such as rice wrap. This research not only presents a pathway to produce BC with localized resources but also opens avenues for understanding the interplay between different cultivation conditions and the resultant BC properties. Moreover, the endeavor to incorporate local resources and traditional drying techniques accentuates the cultural integration and the potential for fostering sustainable material production practices within local communities3,10. In tandem with the material characterization, a microbial analysis of the growing medium or SCOBY mother was conducted to identify the constituent bacteria and yeast strains, offering insight into the microbial dynamics integral to the BC production process.
Literature review
BC production and applications
Recently, researchers have been focusing on fabricating new biobased materials produced from plants, in which cellulose is a major component. The demand for plant-based cellulosic materials is rapidly increasing, leading to deforestation of natural forests and intensification of farming, with its potential environmental impacts. Generally, for producing plant-based cellulosic fibres (also known as regenerated cellulose fibres or RCFs), wood is utilized in large quantities, which leads to massive deforestation (Zhang et al., 2018). Some of the recent research has focused on using cellulosic textile waste to produce RCFs by chemical methods. Although the major source of natural cellulose is plant matter, several species of microorganisms (such as Acetobacter, Achromobacter, and Rhodobacter bacteria) can produce BC11,12. The demand for BC in fashion and textile applications is growing due to its sustainability benefits13. BC is a renewable, biocompatible, nontoxic and biodegradable material, which leads to its growing applications in several areas such as healthcare, food, fashion and textiles14,15. Further, the scope of BC to be applied in various fashion and textile applications as a sustainable material has led to increased research on BC.
BC is prepared from a culture medium of carbon and nitrogen sources and the SCOBY16. Numerous studies have sought to optimize the production process by altering parameters such as the culture medium (tea and sugar sources), type of bacterial species, and ambient conditions (temperature, growth period and pH levels)11,17. One such BC is Kombucha, which is widely used as a drink in several parts of the world including developing and developed countries. Kombucha is prepared using a range of nitrogen sources (such as black tea, white tea, and coffee), carbon sources (different types of sugar) in addition to different types of SCOBY15,18. Kombucha has been a major research area in the material science domain for the last two decades.
A sustainable technology for the synthesis and modification of BC derived from kombucha was presented by Kamiński et al.19. They fabricated stable hydrogel bacterial cellulose (HGBC) with desirable physicochemical and mechanical properties for textile applications. Pedroso-Roussado20 explored the potential of BC as a textile material, shedding light on the initiatives by Suzanne Lee from BioCouture10. This discourse extended to the development of BC fibers and yarns, broadening their applicability in the textile sector. The unique properties of BC, and its resemblance to natural leather, make it a potential candidate for several fashion and textile applications.
Provin et al.21 investigated the purification of BC membranes from kombucha for diverse applications, including textiles. Their work highlighted the potential use of BC membranes in crafting sustainable leather-like fabrics and biodegradable packaging, thus contributing to the narrative of sustainable textile materials. Wood et al.22 accentuated the reproducibility of BC nanofibers for developing novel textiles, emphasizing the superior properties of BC over conventional materials like cotton, especially in terms of enhanced purity, crystallinity, tensile strength, and water retention. These attributes render BC a suitable candidate for various textile applications.
Existing methods for characterizing BC
The exploration of BC for textile applications necessitates a thorough evaluation of its properties and characteristics, employing both traditional and standard testing methods. Literature has increasingly acknowledged the distinct nature of BC and other bio-based materials, which may not always align completely with standard testing methods employed for traditional textiles12. Hence, some modified test methods can be employed where the standard methods can’t be used.
Noteworthy contributions in the field have been made by researchers exploring the diverse aspects of BC testing and applications. Emam23 investigated organic antimicrobial reagents in cellulosic textile finishing, highlighting the importance of achieving a balance between comfort, easy care, health, and durability in antimicrobial textiles. This work provided a foundation for understanding the interplay of antimicrobial agents with cellulosic materials like BC, aiding in the development of health-centric textile products. Provin et al.12 tackled the challenge of wettability associated with BC in textile applications. Their comprehensive review gathers various studies and methods aimed at minimizing BC’s hydrophilicity, thereby expanding its applicability in textiles. This discourse is crucial for addressing one of the inherent challenges in BC textile applications, paving the way for research focused on enhancing BC’s compatibility with textile processes. Nayak et al.24 has recently summarized different sources and characterizations of BC for fashion and textile applications. A range of standard test methods for evaluating chemical, mechanical, surface, thermal and biological properties has been discussed in this paper.
Feiguerias et al.25 reviewed various technologies used to produce cellulose-based textiles, including BC. Their discussion on surface modification techniques unveils the potential of tailoring BC’s surface properties to meet specific applications. Moreover, the exploration of sustainable cellulose sources and life cycle assessment (LCA) of cellulose fiber production methods adds a sustainability dimension to the production and application of BC. The work by Kamiński et al.19 showcases a novel and ecofriendly technology for the synthesis and modification of BC for textile applications. By elucidating the manufacturing process of HGBC from a yeast/bacteria culture, they highlighted a pathway for developing BC with desired mechanical and physicochemical properties suitable for textile applications. Costa et al.26 ventured into evaluating BC films as an alternative textile surface for developing clothing prototypes. Their exploration encompassed the production and characterization of BC films using different bacterial strains and assessing their compatibility with traditional sewing techniques. This work extends the discourse on BC’s potential as a textile material, especially in the realm of clothing design and fabrication.
Interweaving with these significant contributions, established methods like SEM for morphological analysis; tensile strength and bursting strength for mechanical properties; chemical modification techniques and several other techniques continue to play crucial roles in analyzing BC24. However, the distinct nature of BC, especially its microbial composition and growth dynamics, calls for a blend of traditional textile testing and innovative biological testing approaches for fashion and textile applications.
This research acknowledges the limitation in existing textile testing methods in relation to BC. This stems from the fact that unlike traditional textile manufacturing processes, where the ‘farming’ of fibre is part of a distinct primary production phase (with its own agricultural testing processes), while fibre processing into yarns and textile manufacturing are further and separate stages of manufacturing (with their own specific testing regimes). While growing BC, the textile material can be produced directly by the organism itself, merging separate parts of a supply chain into a continuous and integrated process, requiring a more integrated, multidisciplinary testing regime.
Ghalachyan et al.27 have identified and evaluated the sensory nature of new materials like BC, which is essential to their adoption in various products. While some traditional textile evaluation methods are employed, this study involved the analysis of bacteria and yeast strains integral to BC production, as well as exploring the influence of parameters such as growing duration, washing, and drying methods on the properties of BC. These dimensions, although not standard in textile testing, are pivotal for a holistic understanding of BC and its applicability. The diversification in testing throughout the textile production process is also reflected in the evaluation of different aspects of BC cultivation, like the pH levels of the growth medium, which can significantly impact the properties of the final material. Additionally, the assessment of BC’s compatibility with traditional sewing techniques by Costa et al.26 underscores the necessity of bridging traditional textile practices with the novel attributes of BC.
The discourse on BC testing and analysis hints at a broader need for the textile industry to evolve its testing paradigms to accommodate the unique attributes of biobased materials24. Developing new methods or standards for testing and analyzing BC may not only enhance the understanding of this biomaterial but also pave the way for its standardized and broader application in the rapidly growing biobased textile industry. The existing methods for testing and analyzing BC, coupled with the innovative approaches seen in the recent research form a robust framework for comprehending BC’s properties and potential applications. The amalgamation of traditional testing methods with novel analytical approaches reflects the evolving narrative of BC testing, resonating with the broader transition towards biobased materials in textile applications. This study contributes to the emerging interdisciplinary field of biobased materials, identifying and bridging the divisions between scientific and aesthetic modes of evaluation, and between industrial and biological production processes.
Materials and methods
Sample library creation
The creation of a rich and diverse sample library was the critical starting point in this research, laying the foundation for extensive recording, testing and analysis. Utilizing locally sourced ingredients from Vietnam, over 150 samples were meticulously cultivated, documented, and stored in the sample library. Out of these samples, six samples were selected for further investigation of various properties.
Ingredients and additives
The primary ingredients used to fabricate BC included various types of carbon and nitrogen sources that were indigenous and easily available in Vietnam. Nitrogen sources included teas such as black tea, oolong tea and green tea; and carbon sources included sugars such as white sugar, brown sugar, and sugar cane juice to fabricate BC. The above-mentioned ingredients were used for the creation of a specimen library in addition to other ingredients such as banana, mango, watermelon and dragon fruit. The local ingredients used for fabricating BC for characterization in this study are listed in Table 1.
Cultivation
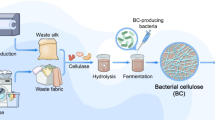
The starter liquids were derived from three distinct SCOBY mothers, each fed with different ingredient combinations, namely black tea with white sugar, black tea with brown sugar, and dragon fruit with black tea and no added sugar. The suspension proportions were standardized to 3 L of water, 30 g of tea, 300 g of sugar, and 300 ml of starter liquid. Figure 1 (a) shows the steps used for fabricating BC samples and Fig. 1 (b) is the schematic representation of the steps. The culture medium was prepared using the standardized proportions of ingredients. At first the nitrogen and carbon sources were mixed with boiling water. The mixture was then cooled to room temperature (25 °C). Then, the starter culture from the mothers was added to the mixture and allowed to inoculate for the desired number of days as mentioned in Table 1.
The cultivation process involved the preparation of a kombucha tea starter culture, comprising a blend of teas and sucrose, inoculated with a bacterial and yeast culture. The mixture could incubate at room temperature up to 18 days, with periodic analyses to assess its chemical and microbiological properties. Bacterial cellulose samples grow on the surface of the culture medium due to fermentation as a floating layer, and the process is done in a static condition or static route.
Washing and drying
Once the growing period was reached, the BC samples were taken off the top of the culture medium and washed in running tap water to remove the residual deposits from its surface. They were then soaked in fresh water for between 1 and 3 days followed by final washing and then drying directly in sunlight using bamboo-frames. Figure 2 shows the samples after washing (a), during drying in bamboo mats (b) and dried samples ready for investigation (c).
Growth monitoring
The growth of BC was monitored meticulously, recording parameters like growth duration, thickness of BC, pH levels at the start and end of cultivation, and visual observations of the BC’s appearance. The growing duration ranged between 4 and 44 days, with an average of 6 to 18 days, influenced by factors including temperature, pH28, type of ingredients, and light exposure.
Drying and coating
Drying methods devised in this research was inspired by the local Vietnamese practices in drying rice wraps, with bamboo racks directly under sunlight. Post drying, the BC samples were coated with a mixture of coconut oil (Premium pure organic coconut oil from Vietcoco brand) and beeswax (100% natural beeswax from Mintiml brand) by hand, allowing them to rest for two days for complete soaking before documentation, testing and evaluation. The objective of coating with coconut oil or wax was to prevent rapid absorption and desorption of moisture that can lead to quick deterioration of the BC samples.
Documentation
A BC sample library was created with the use of local ingredients. Each sample was assigned a unique identifiable code, with an index card attached following the drying and coating process. This provided a detailed record of all the relevant parameters and observations. The library of BC samples fabricated in this project are shown in Fig. 3.
Characterization
Once the BC samples were fabricated, six of them were subjected to a range of characterization techniques to evaluate their properties. It is essential to evaluate a range of properties to understand the behavior of a material for specific applications. In this research characterization was performed by SEM (for surface morphology), EDX (for elemental analysis), FTIR (for chemical structure), DSC (for thermal analysis), and mass spectrometer analysis (for bacterial species identification).
Scanning electron microscopy
The SEM is a versatile imaging technique used to collect high-resolution surface images to understand surface morphology. SEM uses a beam of electrons focused on the sample surface to produce images, which are much higher resolution compared to optical microscopy (OM). The SEM imaging technique can collect images ranging from less than a nanometer up to several nanometers (nm). In this research, field emission scanning electron microscopy (FESEM) was performed on the BC samples using the Philips XL30 FESEM. The images were captured with a spot size of 4.0 μm and an accelerating voltage of 20 kV. The BC samples were placed on a stub and sputter coated by irradiating with gold using a high-resolution ion beam sputtering system. Coating thickness of 100 angstroms (Å) was achieved by applying a current of 50 mA for 20s.
Energy dispersive X-ray
Generally, the EDX technique is used to determine elemental composition of various materials. This technique transports X-rays on the sample’s surface to predict the elemental composition of biomaterials such as BC. EDX analysis is performed in conjunction with the SEM for collecting information from near surface elements of a sample by positional mapping in it. In this research EDX microanalysis was used to investigate the composition of BC by analyzing various elements of the BC molecule, such as carbon (C), and Oxygen (O). The Philips XL30 FESEM, used for surface morphology, was also used for the EDX. All the specimens were sputter-coated with carbon for 30s before performing EDX. An accelerating voltage of 30 kV was used for all the specimens. To determine the distribution of the additives on the fibre, X-ray net counts were obtained at random locations on the stub with a collection time of 3000 s.
Fourier transform infrared spectroscopy
The FTIR spectroscopy was used to determine the chemical composition of BC samples. A spectrophotometer (PerkinElmer Spectrum-400) was used to collect data on the absorbance of the samples to determine the functional groups, which was then analyzed to identify the chemical structure. The chemical structure of BC is crucial as it influences the chemical properties and reactivity of BC to chemicals. A complete understanding of chemical properties is essential to determine the potential application areas. A total of 16 scans per sample with the wave number range of 4000–650 cm-1 was used for scanning. The absorbance of the samples varies according to the functional groups, which is indicated in the spectra as a function of wave number.
Differential scanning calorimetry
The DSC was used to investigate the thermal behavior of BC samples29. The DSC can explain physical properties, chemical reactions, degradation temperature, glass transition temperature (Tg) and crystallization behaviour of fibrous materials. In DSC testing, a particular mass of the sample and the standard substance (which remains unchanged in its phase during the temperature range being examined) are heated uniformly within two distinct chambers that have the connections of a differential thermocouple. The change in temperature of the sample is then compared to that of the standard substance as the temperature increases. In this research the thermal properties were analyzed by a DSC equipment (Mettler Toledo, DSC821e) fitted with a sample robot.
Samples were kept within 40 µL aluminum crucibles, which also served as the control reference. Approximately 5–10 mg of the sample was gradually heated from the initial room temperature (30 °C) to 250 °C, incrementally increasing at a rate of 10 °C/min30. These samples were run in the non-isothermal mode of the equipment. The heating rate of 10 °C/min was selected as it has been reported to be an optimum rate that does not influence the shape and character of the endotherm of the BC samples31.
Identification of bacterial species
There are several bacterial species that can be used for the fabrication of BC. Identification of the bacterial species can help to understand the structure and properties of the BC samples. The bacterial species were identified using the Bruker Daltonik MALDI test at the Center for Bioscience and Biotechnology, Ho Chi Minh City, Vietnam. The mass spectrometer was used for identifying the bacterium species, which is more efficient than the traditional assays or sequencing method. The spectrometer used Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) technology to identify the bacteria accurately from a DNA (Deoxyribonucleic acid) analysis. For the bacterial count, the standard agar plate method was used to count the number of colony forming units per milliliter (CFUs/ml).
Results and discussion
Observations during growth
The static formation route was used to fabricate BC in this study. The BC structure was developed by the intertwining of the cellulosic fibrillar structures forming uneven surfaces32. It was observed that the growth of BC was quite slow at the beginning despite the high concentration of ingredients due to a lesser number of bacteria. As time elapsed, the bacterial quantity increased, and the growth process was faster. As time elapsed the concentration of ingredients started to decrease, which led to slower growth of BC and eventually to a stagnant phase. The BC samples grew as a floating membrane at the surface, which separated the air-liquid interface. Figure 4 shows the cultivation and harvesting of BC samples.
Surface morphology (SEM results)
The surface morphology of BC samples is shown in Fig. 5. It can be observed that the morphological structures of the six samples are different due to the use of different ingredients. Sample S1 displays a rough surface with irregular particle formations and visible fibrous structure33. Sample S2 appears smoother than S1, with fewer visible particles. However, some dispersed particles are present, which may suggest less microbial activity compared to S1. Sample S3 shows a rough texture, but with more scattered, smaller fragments and an overall compacted appearance compared to S1. The presence of distinct particulate matter, of size 5 to 6 microns, was observed in the samples S4 and S5. This particulate matter can be attributed to the use of sugar (such as dragon fruit) and tea type (such as Cape Jasmine fruit) used for BC preparation. In samples S1, S2, S3 and S6, the particulate matters were not distinctly visible and were smaller in size as the nitrogen and carbon sources were well mixed. The distinct cellulosic structure was not visible in the SEM images due to insufficient purification process used during the washing of the BC to remove fermentation residues26.
Sample S4 exhibits a dense clustering of round particles, indicating a high concentration of spherical or rod-like structures that are more uniform in size and shape compared to other samples, which are the organized fibril network of BC. A similar observation was made by Abdelraof et al.33 with a polydispersity index (PDI) of 0 to 0.3, indicating high homogeneity of the BC. Sample S5 has a unique angular particle structure not observed in other samples. This may indicate the presence of crystalline deposits on the surface, possibly due to specific microbial chemical interactions33. Sample S6 has a relatively smooth surface with minimal particles, and a few small bumps or undulations. The smoothness could be due to a lower degree of material deposition, or it may represent a clean or minimally processed stage. Overall, these images indicate distinct differences in surface morphology among the samples, possibly due to variations in bacterial growth or substrate interaction during the production process.
Energy dispersive X-ray results
The energy dispersive X-ray results of BC samples are shown in Fig. 6; and Table 2. Figure 6 (a-f) indicates the EDX spectra with elemental distribution (as peaks in the y-axis). Figure 6 (g) shows the chemical structure of BC, which is cellulose (C6H10O5). It can be observed from the peak height of the EDX spectra that the elements present in the BC samples were found to be carbon (C) followed by oxygen (O). As BC is a biomaterial that comprises hydrogen (H), carbon, and oxygen, the elements carbon and oxygen were detected in the EDX spectra. As such hydrogen can’t be uniquely detected by the EDX technique. Further, the percentage of elemental analysis in Table 2 indicated higher amounts of carbon than oxygen due to larger peak heights. The higher percentage of carbon can be attributed to the carbon coating before EDX although oxygen has higher molecular weight. As the samples were coated with carbon on the surface, the X-ray beam detected more carbon compared to oxygen.
The presence of other elements such as sodium (Na) and (Cl) can be attributed to salts that might have been present in the fruits or other ingredients in very low amounts. The presence of some other elements such as phosphor (P), silicon (Si) and molybdenum (Mo) might be due to the instrumental error, which ranged from 9 to 13%. From Table 2 it can be observed that the percentage of carbon ranges from 52 to 63%, whereas oxygen ranges from 36 to 47% in the samples S1-S6. Carbon and oxygen together constitute about 99% of the total elements in all the six BC samples. The remaining elements add up to 1% of the composition. From the EDX analysis it can be concluded that the BC samples are purely organic materials made from hydrogen, carbon and oxygen, representing the cellulose structure as shown in Fig. 6 (g).
FTIR spectroscopy
FTIR spectroscopy was used to understand the chemical composition of BC fabric. The transmittance peak analyses of the spectra were performed to check the appearance, disappearance and shifting of peaks to find the functional groups34. The FTIR spectrographs of the BC samples are shown in Fig. 7. Figure 7 (a) represents the standard results while Fig. 7 (b) represents the normalized results. It can be observed from these figures that the major component of BC sample is cellulose, which is indicated by the broad peaks around 3240–3340 cm⁻¹. This region typically corresponds to O-H stretching vibrations35, which indicate the presence of O-H groups (such as in water or alcohol). Samples S1, S2, S3, and S6 show moderate absorption, while sample S4 and S5 show a very low absorption, suggesting lower hydroxyl content.
Sharp peaks are observed around 2850–2920 cm⁻¹. This region usually represents C-H stretching vibrations, associated with aliphatic hydrocarbons. Samples S1, S2, S3, S4, and S6 exhibit these peaks, while S5 has relatively lower intensity in this region, suggesting fewer hydrocarbon chains or different chemical environments. Significant peaks around 1730 cm⁻¹ are often attributed to C = O stretching, indicating the presence of carbonyl groups, which could be from ketones, aldehydes, or carboxylic acids. Sample, S5 shows a strong peak here, suggesting a higher carbonyl content, while S4 has a broader and more intense signal, implying complex or diverse carbonyl-containing structures.
Fingerprint region (1500–500 cm⁻¹) is highly characteristic of the specific molecular structure. Variations here can indicate different bonding patterns, such as C-O, C-N, or C-S stretching. Sample S6 shows significant deviations in this region, especially below 1000 cm⁻¹, which could suggest unique structural features or compounds not present in other samples. The cellulosic peak for C-O vibrations was observed in the region of 980–1060 cm− 1. The presence of carboxylic acids was identified from the C = O vibrations present at 1630 and 1730 cm− 1. The C-H vibrations of carboxylic acid were also identified from the peak vibrational peak present in the region 2850–2920 cm− 1. Some of the spectrographs show lower intensity peaks, which might be due to the presence of some fruit residues in the BC structure. The characteristic peaks of all the six BC samples are identical, indicating the cellulosic structure across the BC specimens. Table 3 shows the functional groups present in the BC samples.
DSC results
DSC was employed to analyze the thermal behaviors of the BC samples (such as glass transition temperature, phase transition temperature, and degradation or crystallization), which were fabricated from Vietnamese ingredients. Generally, BC is more crystalline than cellulose, and it does not exhibit a melting point, even though it has a strong crystalline structure. As shown in Fig. 8, the peaks at specific temperatures (above 150 °C) likely indicate phase transitions or other thermal changes, which may be associated with dehydration (free or crystalized water) or structural rearrangement (phase transition). The difference in position and intensity of the peaks may reflect variation in the crystalline structure or the interaction between water and hydroxyl groups in the bacterial cellulose structure. In the other hand, such change indicates the reorganization of the polymeric cellulose structure.
The transition temperature of each sample is marked by the sharp endothermic peaks (upward peaks) in the DSC curves. These peaks correspond to the temperatures at which the samples absorb heat to transition from the crystalline state to the amorphous state. Specifically, the peaks in the range of 151.6 °C to 169.1 °C across the samples suggest that these are likely the phase transition temperatures. The slight variation in temperatures among samples (e.g., 151.6 °C for S3 to 169.1 °C for S4, respectively) might be due to differences in sample composition, molecular weight, crystallinity, or purity. The peak with the highest enthalpy (energy change) at the dehydration temperature, showed an enthalpy of 4.679 mW/mg, indicating that this sample may have higher crystallinity or a more stable structure, requiring more energy.
The degradation temperature is typically indicated by an exothermic event (downward peak) at higher temperatures, corresponding to decomposition or oxidation reactions, representing the breakdown of the sample. However, the DSC spectrum is observed that no significant exothermic peaks are observed at temperatures below 250 °C, suggesting that thermal degradation has not occurred within this temperature range. This implies that the samples are stable up to 250 °C and that their degradation temperature is likely higher than this maximum experimental temperature. In general, since dehydration temperatures are relatively close across samples but differ slightly, it may indicate that the samples have similar base materials but with minor structural or compositional differences affecting their structural change behavior.
The absence of degradation peaks suggests that these materials may be thermally stable up to 250 °C, making them suitable for applications involving heating up to this temperature without decomposition. The DSC spectrum indicates that the samples have distinct dehydration temperatures ranging from 151.6 °C to 169.1 °C, with no indication of thermal degradation up to 250 °C (Table 4) since the degradation temperature of cellulose is known to be higher than 300 °C36. Hence, from the DSC results it can be concluded that the fashion and textile products manufactured from the BC samples can be thermally stable at high processing temperatures that is applied to cotton and other natural cellulosic fibres.
Microbial analysis results
Agar plates used for testing the bacterial species’ analysis are shown in Fig. 9. These plates were used for counting the number of CFUs of the bacterial species, which shows the growth of bacteria. Further, the results obtained from Bruker Daltonik MALDI tests of BC samples are shown in Table 5. It can be observed from the table that Acetobacter Indonesiensis, a gram-negative bacterium, is found in all the three samples tested for bacterial analysis. The bacteria species Acetobacter Indonesiensis has also been used in several other research for growing BC37,38,39. The bacteria of the genus Acetobacter are rod-shaped and elongated and belong to the group of acetic acid bacteria. This genus is the most widely used commercial bacteria and provides maximum growth of BC during the fermentation process. The bacteria of genus Acetobacter are purple, non-photosynthetic bacteria that can convert various sugar sources such as glucose, fructose, glycerol, and other organic substances into BC.
The other bacterial group found in the samples included Bacillus subtilis, Saccharomyces cerevisiae, Agrobacterium rubi, and Staphylococcus hominis. Bacillus subtilis is a rod-shaped and gram-positive bacteria, which was found in some BC40; Saccharomyces cerevisiae is a single-celled yeast, widely used in baking, brewing and wine making41; Agrobacterium rubi is a mesophilic plant pathogen; and Staphylococcus hominis is a gram positive bacteria with round-shape42. Hence, various types of bacteria and yeast were present in the samples, which originated from the three parent SCOBY used for fabrication.
The total bacterial count (CFU/ml), as obtained from the agar plate samples, has been shown in Table 6. It can be observed that the number of CFUs in sample S6 was the highest and S4 was the lowest, with values of 2.16*107 CFUs and 3.65*106 CFUs, respectively. From the results it can be concluded that the thickness of the samples does not depend on the bacterial count, rather the type of carbon and nitrogen sources. Despite the highest CFU values for S6, the thickness was not the highest due to weaker carbon and nitrogen sources available for bacterial growth.
Conclusions
Significant findings
This research investigated properties of BC samples fabricated from various indigenous ingredients available in Vietnam. The drying methods used in this research followed the traditional process of drying directly under the sunlight, which is used to dry food products such as paper rice in Vietnam. The unique drying process can produce patterned effects in the BC samples in addition to adequate drying and saving electricity. The BC samples were tested for a range of properties to understand their suitability for textile applications. The morphological structures of BC samples were different due to different sources of sugar and nitrogen in addition to fruit sources used for fabrication. The presence of distinct particulate matter in the SEM images was ascribed to the type of feedstock used to produce BC. EDX results showed carbon and oxygen as the major components of BC, which form the cellulose building block in BC.
FTIR spectroscopy showed various functional groups relating to the cellulosic structure present in BC. Functional groups such as O-H (broad peak from alcohol or water), C-H (medium to strong peak from cellulose and carboxylic acid), C = O (weak to strong peak from carboxylic acid), C = O (weak peak from carboxylic acid) and C-O (from cellulose) confirmed the cellulosic structure of BC. DSC results revealed the degradation temperature of BC samples ranged from 151.6 to 169.1 °C, which agrees with the earlier findings. The BC samples were tested by Bruker Daltonik MALDI test for identifying the type of bacteria present in the scoby. It was found that Acetobacter indonesiensis is the main bacteria present in SCOBY. Other bacterial species that were present included Bacillus subtilis, Saccharomyces cerevisiae, Agrobacterium rubi and Staphylococcus hominis.
Limitations and future directions
This study investigated the potential of some local ingredient indigenously available in Vietnam to fabricate BC. There are a wide range of local nitrogen and sugar sources, which are indigenously available in tropical countries like Vietnam. Future research can investigate these resources, such as oolong tea, white tea, coffee as nitrogen sources; and grapefruit, orange, pineapple, banana, and papaya as carbon sources to fabricate BC samples. Another limitation was associated with the range of testing, for example, the BC samples were not characterized for crystalline properties to understand the applicability of textile products at high temperature and establishing the wash care instructions. Hence, future research should focus on these additional characterization techniques. Further, the influence of purification process with different amounts of caustic soda (NaOH) can also be investigated in future studies. Finally, future studies can address garment fabrication and scaling issues that could be used in fashion manufacturing using BC. In future the authors will focus on fabricating pre-shaped garment panels to explore different ways of joining them to make final garments.
Data availability
Data availability statementThe datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
bacterial cellulose
- CFU:
-
colony forming units
- DNA:
-
Deoxyribonucleic acid
- DSC:
-
differential scanning calorimetry
- EDX:
-
energy dispersive X-ray
- FESEM:
-
field emission scanning electron microscopy
- FTIR:
-
fourier transformation infrared
- HGBC:
-
hydrogel bacterial cellulose
- LCA:
-
life cycle assessment
- OM:
-
optical microscopy
- RCF:
-
regenerated cellulose fibres
- SCOBY:
-
symbiotic culture of bacteria and yeast
- SEM:
-
scanning electron microscopy
- 3D:
-
three-dimensional
References
Gorgieva, S. & Trček, J. Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials 9, 1–20 (2019).
Nguyen, H. T. et al. Kombucha-derived bacterial cellulose from diverse wastes: A prudent leather alternative. Cellulose 28, 9335–9353 (2021).
Klemm, D., Schumann, D., Udhardt, U. & Marsch, S. Bacterial synthesized cellulose-artificial blood vessels for microsurgery. Prog. Polym. Sci. 26, 1561–1603 (2001).
Portela, R., Leal, C. R., Almeida, P. L. & Sobral, R. G. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb. Biotechnol. 12, 586–610 (2019).
Sulaeva, I., Henniges, U., Rosenau, T. & Potthast, A. Bacterial cellulose as a material for wound treatment: properties and modifications. A review. Biotechnol. Adv. 33, 1547–1571 (2015).
Rosenau, T. et al. Chromophores in cellulosics, XI: isolation and identification of residual chromophores from bacterial cellulose. Cellulose 21, 2271–2283 (2014).
Güzel, M. Valorisation of bread wastes via the bacterial cellulose production. Biomass Convers. Biorefinery, 1–14 (2024).
Kumar, M., Kumar, V. & Saran, S. Current research and advances in the molecular aspect of bacterial cellulose synthesis with special emphasis on the production of bacterial cellulose from waste and its food applications. Cellulose 31, 3323–3351 (2024).
Nayak, R., Cleveland, D. & Joseph, F. Characterization of sustainable bacterial cellulose fabricated with Vietnamese ingredients for potential textile applications: tensile and handle properties. Results Eng. 1–10. https://doi.org/10.1016/j.rineng.2025.104030 (2025).
Lee, S., Du Preez, W. & Thornton-Jones, N. Fashioning the future: tomorrow’s wardrobe (Thames and Hudson, 2005).
Nayak, R., Cleveland, D., Tran, G. & Joseph, F. Potential of bacterial cellulose for sustainable fashion and textile applications: A review. J. Mater. Sci., 1–26 (2024).
Provin, A. P. et al. Use of bacterial cellulose in the textile industry and the wettability challenge-a review. Cellulose 28, 8255–8274 (2021).
Nayak, R., Cleveland, D. & Joseph, F. Characterization of sustainable bacterial cellulose fabricated with Vietnamese ingredients for potential textile applications: tensile and handle properties. Results Eng. 25, 1–10. https://doi.org/10.1016/j.rineng.2025.104030 (2025).
Fernandes, M., Souto, A. P., Dourado, F. & Gama, M. Application of bacterial cellulose in the textile and shoe industry: development of biocomposites. Polysaccharides 2, 566–581 (2021).
Liu, J., Bacher, M., Rosenau, T., Willför, S. & Mihranyan, A. Potentially Immunogenic contaminants in wood-based and bacterial nanocellulose: assessment of endotoxin and (1, 3)-β-d-glucan levels. Biomacromolecules 19, 150–157 (2018).
Qiu, K. & Netravali, A. N. A review of fabrication and applications of bacterial cellulose based nanocomposites. Polym. Rev. 54, 598–626 (2014).
Andriani, D., Apriyana, A. Y. & Karina, M. The optimization of bacterial cellulose production and its applications: a review. Cellulose 27, 6747–6766 (2020).
Domskiene, J., Sederaviciute, F. & Simonaityte, J. Kombucha bacterial cellulose for sustainable fashion. Int. J. Cloth. Sci. Technol. 31, 644–652 (2019).
Kamiński, K. et al. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 27, 5353–5365 (2020).
Pedroso-Roussado, C. The fashion industry needs microbiology: opportunities and challenges. Msphere 8, e00681–e00622 (2023).
Provin, A. P., Cubas, A. L. V., Dutra, A. R., d., A. & Schulte, N. K. Textile industry and environment: can the use of bacterial cellulose in the manufacture of biotextiles contribute to the sector? Clean Technol. Environ. Policy. 23, 2813–2825 (2021).
Wood, J., Verran, J. & Redfern, J. Bacterial cellulose grown from kombucha: assessment of textile performance properties using fashion apparel tests. Text. Res. J. 93, 3094–3108 (2023).
E Emam, H. Antimicrobial cellulosic textiles based on organic compounds. 3Biotech 9, 1–14 (2019).
Nayak, R. C., Tran, D., Joseph, G. & Little, V. F. In Sustinable biomaterials (ed Springer) Ch. 12, 1–18 (2024).
Felgueiras, C., Azoia, N. G., Gonçalves, C., Gama, M. & Dourado, F. Trends on the cellulose-based textiles: Raw materials and technologies. Front. Bioeng. Biotechnol. 9, 1–20 (2021).
Costa, A. F. D. S. et al. Bacterial cellulose: characterization of a biomaterial for apparel products application. Res. J. Text. Appar. 26, 532–545 (2021).
Ghalachyan, A. Evaluation of Consumer Perceptions and Acceptance of Sustainable Fashion Products Made of Bacterial Cellulose (Iowa State University, 2018).
Hasanin, M. S., Abdelraof, M., Hashem, A. H. & El Saied, H. Sustainable bacterial cellulose production by achromobacter using Mango Peel waste. Microb. Cell. Fact. 22, 1–24 (2023).
Ke, B. Characterization of polyolefins by differential thermal analysis. J. Polym. Sci. 42, 15–23 (1960).
Krupa, I. & Luyt, A. Thermal properties of isotactic polypropylene degraded with gamma irradiation. Polym. Degrad. Stab. 72, 505–508 (2001).
Wochowicz, A. & Eder, M. Distribution of lamella thicknesses in isothermally crystallized polypropylene and polyethylene by differential scanning calorimetry. Polymer 25, 1268–1270 (1984).
Wu, Z. et al. Top-down peeling bacterial cellulose to high strength ultrathin films and multifunctional fibers. Chem. Eng. J. 391, 1–9. https://doi.org/10.1016/j.cej.2019.123527https://doi.org/https://doi.org/ (2020).
Abdelraof, M., Hasanin, M. S. & El-Saied, H. Ecofriendly green conversion of potato Peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 211, 75–83 (2019).
Berthomieu, C. & Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 101, 157–170 (2009).
Abdelraof, M., El Saied, H. & Hasanin, M. S. Green immobilization of glucanobacter Xylinum onto natural polymers to sustainable bacterial cellulose production. Waste Biomass Valoriz. 13, 2053–2069 (2022).
Anusuya, R. et al. Characterization and optimization of bacterial cellulose produced by acetobacter spp. J. Environ. Biol. 41, 207–215 (2020).
Jie, T. Y. et al. Isolation and identification of acetobacter tropicalis from selected Malaysian local fruits for potential BC production. Malaysian Appl. Biol.. 52, 133–143 (2023).
Tran, T. et al. Shedding light on the formation and structure of Kombucha biofilm using two-photon fluorescence microscopy. Front. Microbiol. 12, 1–13 (2021).
Yetiman, A. E. & Kesmen, Z. Identification of acetic acid bacteria in traditionally produced vinegar and mother of vinegar by using different molecular techniques. Int. J. Food Microbiol. 204, 9–16 (2015).
Savitskaya, I., Shokatayeva, D., Kistaubayeva, A., Ignatova, L. & Digel, I. Antimicrobial and wound healing properties of a bacterial cellulose based material containing B. subtilis cells. Heliyon 5, 1–10 (2019).
Farid, F., Sideeq, O., Khan, F. & Niaz, K. In Nonvitamin and Nonmineral Nutritional Supplements 501–508, Elsevier (2019).
Khusro, A., Aarti, C., Paray, B. A. & Agastian, P. Effect of mentha Piperita L. stress at sub-inhibitory dose on some functional properties of coagulase-negative Staphylococcus hominis. J. King Saud University-Science. 32, 2293–2300 (2020).
Acknowledgements
We wish to acknowledge and thank Prof. Rajiv Padhye (RMIT Australia), Dr. Nauman Choudhry (RMIT Australia), and Prof. Lalit Jajpura (NIT Jalandhar) for their support during testing and evaluation of samples.
Funding
This research was supported by a RMIT Vietnam’s Tier 1 Research Grant (IRG 2022–1).
Author information
Authors and Affiliations
Contributions
Donna Cleveland: Experimentation, result analysis and writing. Rajkishore Nayak: Experimentation, result analysis and writing. Frances Joseph: Editing, technical feedback and proof-read. Tuan-Anh Nguyen: Editing, technical feedback and proof-read.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors have obtained ethics approval from RMIT University’s Ethics Committee. The authors also have obtained the Consent to participate and consent to publish.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cleveland, D., Nayak, R., Joseph, F. et al. Characterization of sustainable bacterial cellulose from Indigenous Vietnamese biomass for potential textile applications. Sci Rep 15, 35267 (2025). https://doi.org/10.1038/s41598-025-16965-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16965-8