Abstract
One of the most invasive freshwater fish in Europe is the stone moroko (Pseudorasbora parva), but its potential for food use remains largely unexplored. Fish were collected from ponds (Raszyn, Stare Kurowo) and a natural river (Wardynka). The study measured fish weight and length, calculated the Fulton coefficient, determined the chemical composition of the meat, energy value plus yield. Heavy metal content was determined after HNO3 digestion using GFAAS method. Health risk was assessed using EDI, THQ, HI, CR. The data were statistically analyzed. No differences in length were found. Individuals from Stare Kurowo and Wardynka had significantly higher body weights than those from Raszyn. Wardynka fish had the highest water content (74.83%), the lowest dry matter, protein, and fat levels. All samples contained low concentrations of heavy metals, with the lowest levels in Wardynka and the highest in Raszyn. Estimated daily intake (EDI) values ranged from 0.28% to 3.03% of the reference dose, remaining under 1% in most cases. Nutrient levels matched typical European freshwater fish. Differences among sites likely stemmed from food availability and competition. The low metal content and favourable meat quality indicate potential for food production, helping control this invasive species and protect native fish.
Similar content being viewed by others
Introduction
For many years, anthropogenic pressure on aquatic ecosystems – manifested through water pollution, river regulation, and dam construction – has led to the transformation of natural habitats for numerous hydrobiont species1,2,3,4. These changes disrupt ecosystems, affecting biodiversity and diminishing the environment’s capacity for self-regulation5. A major threat to the preservation of natural habitats is the presence of alien and invasive species, whose spread is further accelerated by climate change6. Alien species, introduced either intentionally or unintentionally –through transport, translocation, or other human activities – have dispersed widely7. Owing to their biological characteristics, including high fecundity, they readily adapt to new environmental conditions and rapidly colonize new water bodies8,9,10. At the same time, they negatively impact biodiversity by displacing native species and degrading their habitats11. According to Gozlan et al.12, as many as 625 freshwater fish species have been introduced into water bodies beyond their natural range.
In European waters, one of the most invasive fish species is the stone moroko (Pseudorasbora parva Temminck & Schlegel, 1846)9,12,13. This freshwater species from the family Cyprinidae is native to Asian waters, including China, Taiwan, Korea, and Japan, where it typically inhabits lakes and ponds rich in aquatic vegetation12,14,15. Its small body size – typically not exceeding 12.5 cm in length – and, more importantly, its high fecundity and prolonged, abortive spawning strategy contribute to its successful and often unnoticed invasion10,15,16.
The species was first recorded in Europe in 1961 at the Nucet fish farm in the Dâmbovița basin in southern Romania, and in subsequent years in Albania17,18 and Lithuania13,16. Today, its distribution covers nearly all of Europe19. In Poland, the species was first observed in 1990, introduced from Hungary along with stocking material of Asian herbivorous fish, mainly topmouth gudgeon (Hypophthalmichthys spp.)15. In the following years, as in other parts of Europe, it spread to most freshwater bodies in Poland, both flowing and standing20. The primary threats posed by the stone moroko are associated with the transmission of parasites and pathogens21, as well as competition for food and habitat12,22. The species frequently preys on the eggs and early larval stages of native fish and, in some cases, facultative parasitism has been observed19.
One method of combating invasive species is to eradicate them from aquatic environments and prevent their reintroduction23. According to EU Regulation24, the release of individuals of this species back into surface waters is prohibited in EU countries. Consequently, in European waters, captured stone moroko are typically discarded, although they could potentially be utilized in the food industry. This is particularly relevant given that the species is a traditional food source in its native range14,25. The highest consumption of the Pseudorasbora species occurs in China, Taiwan, and Japan, where it is valued for its tender, flavourful meat, relatively few bones, and low fat content26. In its native habitat, natural water catches do not meet market demand, leading to the species being farmed in ponds alongside other fish26. In China, traditional use of this species as an ingredient in local dishes, mainly in fried form, as well as feed for larger predatory fish in aquaculture systems. It is estimated that Pseudorasbora parva may constitute up to 5% of the biomass of small fish in local river catches, which, given the total freshwater fish consumption of approximately 56,000 tons annually in China, suggests a hypothetical annual catch level of this species ranging from 560 to 2,800 tons27,28. In Central Europe, although there is no tradition of consuming such small fish, the ongoing population increase and the potential use of stone moroko meat have sparked growing interest among food producers in processing this species29. However, the decline in native fish populations and the demand for highly nutritious meat have led food producers to seek new raw materials. In European countries, invasive species are increasingly being used for this purpose29, as their populations can be controlled through targeted fishing. Moreover, these fish, typically small in size, can be consumed whole due to their soft bones, thereby providing the body with a greater amount of micronutrients compared to larger fish species30. One such species is the Amur sleeper, which, like other small-sized fish, can be used in the food industry both as a consumable fish and for the production of animal feed31. Due to its high fecundity, rapid life cycle, and tolerance to diverse environmental conditions, Pseudorasbora parva may serve as an alternative feed resource in aquaculture systems. Small freshwater fish such as roach, bleak, ruffe, perch, and Amur goby are readily available but rarely used in the food industry. However, they are rich sources of protein, omega-3 fatty acids (EPA, DHA), vitamin B₁₂, and micronutrients like iodine and selenium32,33. They can be processed into mechanically deboned meat (MDM), used in pâtés, canned products, and fish snacks. MDM is an economical raw material, retains high nutritional value, and contains calcium from small bones34. In South and Southeast Asia, small fish are traditionally fermented to produce sauces and pastes such as prahok, shidhal, and ngari. Fermentation improves shelf life, increases nutrient bioavailability, and imparts an intense umami flavor35,36. The skin and bones of these fish are sources of type I collagen, used in supplements, functional beverages, and gelatin products. Fish collagen is characterized by good bioavailability and cultural acceptability37,38. Fish consumption is an important part of a healthy diet, recommended by the World Health Organization (WHO). Fish provide high-quality protein, unsaturated omega-3 fatty acids (EPA and DHA), vitamins (D, B12), and minerals such as iodine, selenium, and phosphorus. Regular fish consumption, especially of fatty marine species, is associated with a reduced risk of cardiovascular diseases39, improved brain and vision development particularly in children and pregnant women40, decreased risk of depression and mood disorders41, and enhanced immunity42. Although freshwater fish generally contain less omega-3 fatty acids than marine fish, they still represent a valuable dietary component. They provide good-quality protein, B vitamins, phosphorus, and selenium. Their consumption may support metabolic health and the immune system43,44.
Unfortunately, research to date on stone moroko in European waters has primarily focused on their dispersal12,45,46, population and biological traits – including age and length structure, growth, condition, and fecundity9,10,13,47 – as well as parasite and pathogen transmission15,19,21, and guidelines for population monitoring and control23. However, there has been no research on the nutritional and energy value, nor on the carcass yield, of stone moroko populations from European waters. Moreover, the accumulation of heavy metals in their tissues may represent the greatest limitation to their use as a food source48,49. Elevated levels of heavy metals in aquatic organisms may pose risks to human health and safety50,51,52. Among heavy metals, Co, Ni, Cd, and Pb are extremely toxic, and high levels of these elements in the human body can lead to severe toxic, neurotoxic, carcinogenic, mutagenic, and teratogenic effects, such as impairment of mental function and the central nervous system, as well as damage to the blood, lungs, kidneys, bones, liver, and other vital organs53. The content of cadmium and lead is very important because they belong to the most toxic heavy metals and are regulated by the European Union in relation to fish intended for human consumption54. Cobalt and nickel are important for the environment, reflecting both the natural geochemical background and potential anthropogenic pollution55. Selected heavy metals are widespread and represent one of the main causes of environmental contamination worldwide56, which is why their presence in food should be carefully monitored57,58.
Previous studies on heavy metal concentrations in the Pseudorasbora parva species have been conducted in their native distribution areas26,52, while in Europe, only the population inhabiting the Arno River in Italy has been examined59. These analyses revealed significant differences in metal content between native and non-native populations, highlighting the need to expand such studies to newly colonized areas – particularly with regard to food safety considerations.
The aim of this study was to determine and compare (i) carcass yield, (ii) nutrient content, (iii) energy value, and (iv) concentrations of lead (Pb), cadmium (Cd), cobalt (Co), and nickel (Ni) and (v) the risk to human health associated with the consumption of Pseudorasbora parva were also assessed.
Materials and methods
Ethics statement
The fish used in this study were obtained from legal commercial fishing conducted by third parties. They were specimens of the stone moroko (Pseudorasbora parva), an invasive species whose release back into the natural environment is prohibited under Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species, as well as the Act of 16 April 2004 on Nature Protection (Journal of Laws 2004 No. 92, item 880, as amended). Prior to being used for research purposes, the fish were sedated using the Propiscin preparation. The research team did not participate in their euthanasia or in the procurement of biological material. According to Polish law, research procedures conducted on dead specimens of invasive species, such as the stone moroko, do not require approval from the Animal Experimentation Ethics Committee. The study did not involve any experiments on live animals; therefore, the ARRIVE guidelines regarding procedures to minimize animal suffering were not applicable to the conducted research. The methods and the origin of the biological material were described in the manuscript with full transparency and in accordance with applicable ethical standards.
Study area
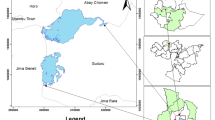
The fish stone moroko (Pseudorasbora parva) (Fig.1.) used in the study were obtained from legal angling activities conducted by third parties, the Figspecimens used originated from waste material of commercial fishing. In each of the three habitats, fish were sampled three times between September 12th and 15th, 2024. This was due to the fact that a single sampling was unreliable, and it was necessary to repeat the sampling. They had been previously sedated using the anesthetic agent Propiscin, in accordance with applicable regulations and animal welfare standards. Our team was not involved in the process of euthanizing the fish. Immediately after capture, the specimens were kept on ice and transported to the laboratory for analysis. The fish originated from three habitats with differing environmental conditions (Table 1.):
(a) the pond farm in Stare Kurowo – designated as ‘SK’ (133 individuals)
(b) natural waters of the Wardynka River – designated as ‘WR’ (132 individuals)
(c) ponds located in the Raszyńskie Ponds Nature Reserve in Falenty – designated as ‘RP’ (157 individuals) (Figure 2).
The Raszyńskie Ponds Nature Reserve, located near Warsaw, is a valuable protected area covering over 100 hectares, encompassing fishponds, reed beds, and meadows. This area has significant tourism potential, particularly for ornithology, due to the presence of numerous wetland bird species and the availability of educational trails. Fishing operations within the reserve (primarily carp farming) and the surrounding agriculture play a significant role in shaping the local landscape and natural environment. At the same time, the area is exposed to anthropogenic pressures. Pollution can come from both agricultural runoff (nitrogen and phosphorus compounds) and nearby traffic (dust containing heavy metals such as Pb, Zn, and Cu). The complex origins of these pollutants, both point-based and diffuse, indicate the need for constant monitoring of bottom sediments for heavy metals60. The Wardynka River is a small tributary of the Stobnica River, offering significant natural and recreational value, particularly for hiking and fishing. The surrounding agricultural areas are a major source of pollution, particularly nutrients and heavy metals (Pb, Zn, Cu), delivered through surface runoff and settlement activity. Although there is no heavy industry, anthropogenic pressure affects the quality of water and bottom sediments, as confirmed by ecological assessments (quality class IV). The river requires monitoring due to the risk of heavy metal accumulation61. The fish ponds in Stare Kurowo combine fish farming with tourism potential, particularly for anglers. Local agriculture provides nutrients but can also cause runoff of fertilizers and heavy metals (Pb, Zn, Cu) into the ponds. The lack of heavy industry limits major sources of pollution, but point emissions from transportation and development impact water quality, requiring environmental monitoringown data,62.
Size and condition of fish
The fish were immediately placed individually into bags after being caught and preserved on ice, then transported to the laboratory for further analysis. Then, the fish were weighed using an Axis 2000 electronic scale (±0.1 g), and their total length (TL) was measured with an electronic calliper (±0.1 mm). Additionally, Fulton’s condition factor (CF) was calculated using the formula: CF = 100 × (W/TL3)63. This coefficient is widely used to determine the nutritional status of fish and even to assess the environment in which they live. Fish in “better” condition (nutritional and health status), as indicated by a higher CF value, are more robust and therefore heavier at a given length63.
Nutritional and energy value
Immediately after catching, the fish were filleted and grouped by origin: “RP” (141 individuals), “SK” (125 individuals), and “WR” (126 individuals). The fish from each group were then ground separately using a Braun Multiquick 7 MQ7045 hand blender to obtain homogeneous samples. The basic chemical composition of the fish flesh was analysed following the procedures recommended by the Association of Official Analytical Chemists64.
Water content (moisture content) and dry weight
Approximately 500 mg of flesh from each fish group was placed in a pre-dried and pre-weighed weighing dish and dried to constant weight at 105 °C for approximately 6 hours using a Binder 9010-0079 dryer (WTB Binder, Germany). After drying, the samples were weighed, and moisture content was calculated based on the difference between initial and final weight65. All analyses were performed in triplicate for each of the three fish groups.
Total protein content
Total protein content was determined using the Kjeldahl method66 with a Kjeltec KT200 distillation unit (Foss, Denmark). Approximately 500 mg of flesh from each fish group was analysed in triplicate. The samples were wet-digested in the presence of 10 cm3 of concentrated sulphuric acid, after which the released ammonia was distilled using the Kjeltec apparatus. The excess hydrochloric (or sulphuric) acid remaining after distillation was titrated with a standard sodium hydroxide (NaOH) solution. A protein conversion factor of 6.25 was applied in accordance with AOAC Method No. 973.4864.
Fat content
The extraction-weighing method was used to determine total fat content. Lipids were extracted using a chloroform: methanol mixture (2:1, v/v) following the methodology described by Folch67, which is currently widely applied for lipid extraction from the muscle tissue of various fish species68,69,70. Fat content was determined gravimetrically by evaporating the solvent and weighing the lipid residue remaining after evaporation, in accordance with AOAC Method No. 960.3964.
Ash content
Total mineral content (ash) was determined using the dry ashing method in a muffle furnace at 550 °C, in accordance with AOAC Method No. 999.1164.
Carbohydrate content
For this purpose, the difference was calculated using the formula: 100% – (water + protein + ash + fat), and the results are expressed as percentages71.
Energy value
Calculations were performed in accordance with EU Regulation No. 1169/2011 (Official Journal of the EU L 304 of 21.11.2011, as amended)72. The following conversion factors were applied: 4 kcal and 14 kJ per 1 g of protein and carbohydrates, and 9 kcal and 37 kJ per 1 g of fat.
Carcass yield
To determine carcass yield, individual specimens were weighed to obtain total body weight (g) and carcass weight after evisceration, using an Axis 2000 electronic balance (±0.1 g). Carcass yield was calculated as the ratio of carcass weight to total body weight, following the method described by Żmijewski et al.73.
Heavy metal content
Digestion of fish samples for heavy metal analysis
The contents of lead (Pb), nickel (Ni), cadmium (Cd), and cobalt (Co) were determined in a total of 48 individuals from three populations (16 individuals per population). The heavy metal data obtained are wet weight based. Flesh samples weighing 1.00 ± 0.01 g were collected from the dorsal part of each carcass and placed in 5 mL of concentrated HNO3 for digestion using a high-pressure microwave digestion system (Speedwave Xpert, Berghof, Eningen, Germany). After digestion, the samples were diluted to a final volume of 10 mL. Ultra-pure concentrated nitric acid HNO3 (69%, RCI Labscan Limited) (Merck, Germany) and Milli-Q water (18.2 MΩ) were used throughout the procedure74.
Elemental analysis
The elemental analysis was carried out using a Hitachi Zeeman ZA3000 Series polarized atomic absorption spectrometer (Hitachi High-Technologies Corporation, Tokyo, Japan).The concentrations of Cd, Pb, Co, and Ni were measured using graphite furnace atomic absorption spectroscopy (GFAAS). Calibration curves were prepared using certified standard solutions: 1000 mg/L standards for Cd, Ni, and Pb from Merck (Germany), and for Co from CPAchem Ltd. (Bulgaria). Each sample was analysed in at least three replicates. The limits of detection (LOD, in mg/L) were 0.0002 for Cd and Pb, and 0.0005 for Co and Ni74. The analytical method was tested using the reference material Fish muscle ERM-BB422 (European Reference Materials, Geel, Belgium). The recovery of elements was 95% –108%, the accuracy for the reference material was 96% for Cd, 104% for Co, 95% for Pb and 108% for Ni.
Human health risk
To estimate the daily intake (EDI; in mg·kg⁻1·day⁻1) of heavy metals through fish muscle consumption, the following equation was applied75,76:
where: Ci is the concentration of the element in fish muscle (mg·kg⁻1 wet weight); IR is the fish muscle intake rate (0.03 kg·day ⁻1). Due to the recent introduction of the stone maroko to topwater European waters, it is not widely consumed. Consequently, the daily food ration of this species is lower than that of native species and similar to the daily ration of other invasive fish species, such as the round goby. Therefore, the daily human food ration for the topwater was assumed to be 0.03 kg/day (or 30 g/day), based on Nędzarek and Czerniejewski77; and BW is the mean body weight (70 kg for adults)77.
The resulting EDI values were compared with the reference (safe) oral dose of the element (RfD)78,79. According to NYS DOH80, if the ratio of the elemental EDI value to the RfD is equal to or less than 1, the risk is considered minimal; if it is between 1-5 times the RfD, the risk is low; if it is between 5-10 times the RfD, the risk is moderate; and if it exceeds 10 times the RfD, the risk is considered high.
The risk assessment of heavy metal intake through fish muscle consumption was conducted following the methodology described by Tan et al.58 and the US EPA79,81. The non-carcinogenic target hazard quotient (THQ) was calculated using the following equation:
where: EF is the exposure frequency (365 days·year⁻1); ED is the exposure duration, equivalent to the average human lifespan (70 years); IR is the fish muscle ingestion rate (0.03 kg·day ⁻1).); Ci is the concentration of the element in fish muscle (mg·kg⁻1); RfD is the oral reference dose for the contaminant (mg·kg⁻1·day⁻1); BW is the average body weight (70 kg for adults); and AT is the averaging time for non-carcinogens (365 days·year⁻1 × ED).
THQ represents the ratio of the estimated exposure to a substance over a specified period to the reference dose (RfD) for that substance; a THQ ≥ 1 indicates a potential health risk associated with consumption.
To assess the potential risk from combined exposure to multiple elements, a hazard index (HI) was calculated as the sum of the individual THQs:
Carcinogenic risk (CR) was calculated for Cr, Ni and Pb using the formula:
where: the parameters were defined as in Equation 6, except for CSF, which represents the cancer slope factor established by the US EPA79. CSF for each studies heavy metals are 6.3E+00 mg·kg−1·day−1 for Cd, 1.7E+00 mg·kg−1·day−1 for Ni, 8.5E-03 mg·kg−1·day−1 for Pb. According to the US EPA79, a lifetime cancer risk below 1E-06 is considered negligible, a risk above 1E-04 is considered unacceptable, and a risk within the range of 1E-06 to 1E-04 is deemed acceptable. For the EDI and THQ calculations, the average elemental concentrations from each of the analysed fish muscle samples were used.
Statistical analysis
In order to ascertain whether the distribution of the obtained results is normal and exhibits a constant variance, the test of homogeneity of variance and Levene’s test were employed. Subsequent to this, an analysis of variance (ANOVA) was conducted, with the Tukey test serving as a post hoc test. Pearson’s correlation coefficient (r) was used to assess the relationship between heavy metal content and fish length and weight. Statistical analyses were conducted using Statistica 13.3 software (Dell Inc., USA).
Results
Size and condition of fish
Fish sizes and Fulton’s Condition Factor (CF) values for the tested populations of stone moroko are presented in Table 2.. Overall, the total length of fish ranged from 57.10 to 97.50 mm, with no statistically significant differences observed among the populations (ANOVA, p > 0.05). Similarly, unit weight ranged from 2.63 to 7.99 g across populations. However, based on mean values, statistical analysis revealed that fish from the SK and WR rearing ponds had significantly higher mean unit weights compared to those from the RP ponds (ANOVA, p < 0.05). The CF values ranged from 0.68 to 1.42, with fish from the RP exhibiting a significantly lower mean CF (p < 0.05).
Nutritional and energy value
Fish from the WR exhibited the highest water content and the lowest dry matter in muscle tissue. The average water content in this population was 74.83%, significantly higher than in the other groups (ANOVA, p < 0.05). Conversely, fish from this habitat showed the lowest protein and fat content compared to those from farmed environments (Table 3.). The mean ash content did not differ significantly among the studied populations (ANOVA, p > 0.05), while the highest carbohydrate content was recorded in fish from the RP.
Energy value
The results of the comparative analysis of the energy value of stone moroko meat from the different study sites are presented in Table 4.. The analysis revealed a distinctly higher energy value in the meat of fish from farmed ponds compared to that of the wild population.
Carcass yield
The stone moroko was characterized by a carcass yield ranging from 70% to 83%. Mean values of this parameter for individuals from the different populations are presented in Table 5.. The highest carcass yield was recorded in fish from the SK (mean: 82.90%), while significantly lower values were observed in fish from the other sites (ANOVA, p < 0.05).
Heavy metal content
Clear differences were observed in the concentrations of individual metals in the meat of stone moroko (Table 6.). In all studied populations, the elemental concentrations in fish meat followed the order: Co < Cd < Pb < Ni.
Pearson linear regression analysis revealed a positive correlation between the concentrations of Cd, Pb, and Co, and a negative correlation between Ni concentration and fish length and weight. However, statistically significant correlations were observed only between Pb concentration and both fish length and weight (p < 0.05) (Table 7.).
In general, the heavy metal content in fish was influenced by their site of origin. The lowest concentrations were recorded in fish from the WR, while the highest were observed in individuals from pond farming systems, particularly from the RP. Notably high metal levels were found in fish from the RP site – for example, the concentrations of the elements with the highest levels in the flesh, Ni and Pb, were 0.215189 mg·kg−1 and 0.01010 mg·kg−1, respectively. In contrast, the corresponding values in fish from the WR were 0.186706 mg·kg−1 for Ni and 0.00773 mg·kg−1 for Pb.
Human health risk assessment
All results of the health risk analysis are presented in Table 8.. The estimated daily intake (EDI, in mg·kg⁻1·day⁻1) of heavy metals resulting from the consumption of 0.03 kg·day⁻1 (30 g) of fish muscle ranged from 1.01E-06 (for Co, fish from SK) to 9.22E-05 (for Ni, fish from RP). The lowest EDI values for all metals were recorded in fish muscle from the WR (Table 8.). EDI values ranged from 0.28% to 3.03% of the reference dose (RfD), with most values remaining below 1% RfD in the tested variants. The lowest EDI values were observed for Co (0.28–0.41% RfD), followed by Cd (1.17–1.57% RfD), and the highest for Pb (2.31–3.03% RfD). The target hazard quotient (THQ) ranged from 9.63E-04 (for Cd, fish from SK) to 1.10E-03 (for Cd, fish from RP), with the lowest THQ values recorded for fish from the WR.
The THQ index did not exceed reference dose limits in any of the fish samples analysed. The hazard index (HI) ranged from 2.92E-03 to 3.83E-03, with the lowest value observed in fish from the WR. Carcinogenic risk (CR) varied depending on the heavy metal and the sampling site. The lowest CR value was recorded for Pb (1.97E-09 in fish from the WR), while the highest was noted for Ni (1.10E-05 in fish from the RP). Based on the cumulative carcinogenic risk (∑CR), the highest value was found in fish from the RP (1.17E-05), and the lowest in fish from the WR (1.00E-05).
Discussion
The stone moroko is an invasive species known for its resistance to adverse environmental conditions and its ability to readily adapt to new habitats47, contributing to its rapid spread in European waters12,15,16,20. This species is characterized by a small body size, short life cycle, early maturation, abortive spawning, and an extended reproductive season10,12. In the populations examined in this study, fish length and weight ranged from 57.10 to 97.50 mm and 2.63 to 7.99 g, respectively, aligning with previous findings on the population structure of this species in European waters9,13,15,47. According to recent studies, individuals in some European waters reach lengths of 60–100 mm and weights of 8–10 g. In contrast, significantly smaller individuals (40.56–58.60 mm and 0.74–2.53 g) are found in small watercourses with limited food resources47,82,83,84. The condition of the fish captured in this study was relatively poor, with a mean Fulton’s condition factor of 1.04 (range: 0.68–1.42), indicating poorer overall condition compared to populations from other rivers and lakes in Central Europe15,85. For example, in the Oder River basin, the average value of this parameter for the Amur sleeper was 1.44 ± 0.2486., in the Ros River it ranged from 1.46 to 2.4887, and in the Kremenchug Reservoir it ranged from 1.87 to 2.5288. However, in some water bodies with less favorable environmental and feeding conditions for this species, the Fulton’s condition factor can drop to values even below 1.0089.
Fish is considered a nutritionally valuable component of the human diet, primarily due to its content of long-chain n-3 polyunsaturated fatty acids90,91. Additionally, fish is rich in vitamins such as A, D, B6, and B12, and contains significant levels of essential micronutrients including iron, potassium, and selenium92. The composition of essential nutrients and the calorific value of fish meat vary considerably between species (Table 9.). On average, fish meat contains: water (66–81%), protein (15–21%), minerals (1.2–1.5%), fat (0.2–25%), and carbohydrates (0.0–0.5%)93. Overall, the nutrient composition of the stone moroko analysed in this study falls within the typical ranges reported for the most commonly occurring freshwater fish species in Europe94,95,96,97,98.
However, comparing the nutritional composition of the studied populations with other populations in European waters is difficult, as no data on the nutritional value of the stone moroko from this region were found in the available literature. This is likely due to the species not previously being considered a potential raw material for food processing. In its native range, however, the reported nutritional composition includes protein content of 12.80–16.41%, fat content of 2.82–9.82%, water content of 77.29–80.20%, and ash content of 1.05–3.33%25,107. In general, the protein and fat contents in individuals from the three European locations examined in this study (14.07–15.25% and 2.27–3.75%, respectively) fall within the ranges reported for native populations. However, compared to fish from China, the studied specimens exhibited higher ash content (2.88–2.91%) and lower water content (72.19–74.83%). According to the classification by Stansby and Olcott108 as cited by Ahmed et al.93, based on fat and protein content, the stone moroko can be categorized as class E fish – low-fat (less than 5.0%) and low-protein (less than 15%) species. The classification of fish is based on their fat and protein content, distinguishing five main groups with different nutritional profiles and uses in human nutrition. Fish from classes A and B are characterized by high protein content and a favorable composition of omega-3 fatty acids, which are important for heart and nervous system health. Class D fish, with very low fat and high protein content, are recommended for weight-loss and high-protein diets, supporting muscle mass. In contrast, class E fish, which are easily digestible and low in calories, represent a valuable and economical component of special diets, particularly in regions with limited access to high-quality protein42,43,109.
Differences in nutrient content between stone moroko populations may be influenced by factors such as food availability, food quality, resource diversity, and most notably, the species’ varied diet across different aquatic environments110,111,112. These factors likely explain the nutritional differences observed in the stone moroko from riverine versus pond-reared populations in our study. Wild fish from the WR exhibited significantly lower fat and protein content and higher water content compared to fish from farmed ponds. Similar trends have been reported in China, where nutritional comparisons between wild and farmed stone moroko showed protein contents of 16.34% and 16.41%, fat contents of 2.82% and 4.65%, water contents of 79.20% and 77.29%, and ash contents of 1.45% and 1.05%, respectively107.
It should also be noted that the basic nutritional composition of fish can be influenced by a wide range of factors, including size and sex113,114, age, seasonal variations93, as well as maturity and diet type93,110. Furthermore, as highlighted by Marenkov et al.115, the presence of heavy metals in the aquatic environment may also have a significant impact on the nutritional quality of fish.
The nutritional value of the stone moroko suggests that it may be suitable for inclusion in diets requiring low-calorie and low-fat foods. However, further research is needed to explore the technological aspects of its processing and to conduct sensory evaluations of food products developed from this species. Due to its small size, stone moroko could be used similarly to small fish in Asian cuisine – for example, as fried snacks, ingredients in fish pastes or preserves, or additions to soups. In many Asian countries, small fish are also commonly used in dried or fermented forms, which may offer additional possibilities for incorporating this species into human diets116,117,118.
A potential limitation to the use of the stone moroko in the food industry is its heavy metal content, which poses both environmental and human health risks119,120. Heavy metals are highly stable and non-biodegradable, making them persistent in the environment, particularly in sediments, where they can accumulate over long periods and negatively impact the physiology of aquatic organisms121. In fish, heavy metals accumulate through the gills, body surface, and gastrointestinal tract, leading to metabolic, physiological, and histological changes, including the alteration of enzyme activity and metabolite levels122. When consumed through fish flesh, these metals may pose health risks to humans48. The levels of heavy metals detected in the tissues of the studied stone moroko were similar to those reported for other fish species115,123,124,125,126. The relatively low levels of heavy metal content in stone moroko muscle tissue obtained in our study are consistent with the findings of other authors. For example, Cordeli et al.124 reported Pb concentrations in fish from the Danube ranging from 0.006 to 0.014 mg·kg⁻1, which corresponds to the range of 0.0077–0.0101 mg·kg⁻1 in our material. Similarly, Cd values in the study by Habib et al.125 for freshwater fish (0.002–0.004 mg·kg⁻1) were comparable to our results (0.0027–0.0037 mg·kg⁻1), and Ni concentrations (0.18–0.22 mg·kg⁻1) were within the range reported by Jarf et al.126 for European perch (0.16–0.25 mg·kg⁻1). However, comparing elemental concentrations between different stone moroko populations remains difficult due to the limited availability of such data, despite the species being frequently studied for its invasive nature12,46,127. Moreover, variation in fish length and age structures across populations9,13,47 may influence elemental content due to bioaccumulation processes128. Despite these limitations, Table 10. presents the concentrations of the analysed elements in muscle tissue from different regions.
Ni and Pb were the predominant elements found in the muscle tissue of the stone moroko, while Cd and Co levels were significantly lower own data,59.In our study, Pb content was nearly four times higher than that of Co, and Ni content was approximately 70 times greater than Cd content. Overall, the muscle tissue of stone moroko from the three studied populations was characterized by relatively low concentrations of Pb, Cd, Co, and Ni compared to both the native population from Chinese waters26 and the non-native population from the Arno River in Italy59. Specifically, the Ni content in stone moroko from Polish waters was about 40% lower than in specimens from the polluted Arno River59. Pb content was nearly twice as low as in fish from Lake Nanyi and crab ponds in China26, and more than 45 times lower than in individuals from the Arno River59. In contrast, the lowest Pb and Cd concentrations have been recorded in fish from unpolluted Iranian waters129. These differences in heavy metal content across stone moroko populations likely reflect the varying levels of environmental contamination in their respective habitats130, as similar trends in Pb and Ni concentrations were also observed for Cd and Co.
Research by Rajkowska and Protasowicki128 indicates that heavy metals tend to bioaccumulate in organisms over time, resulting in positive correlations between their concentrations and fish size. However, a study by Rakocevic et al.131 suggests that such significant relationships are primarily observed for essential elements, while no clear correlations were found for non-essential elements. Similarly, the study by Balzani et al.59 on stone moroko, as well as our findings regarding Cd, Co, and Ni concentrations, did not reveal significant relationships between metal levels and fish size. This may be attributed to the short lifespan of the species,9,13,47,75, which limits the duration of exposure to heavy metals present in the aquatic environment and food chain.
Heavy metals are characterised by their high stability and resistance to biodegradation. They readily accumulate in the environment, including sediments, where they persist for long periods—particularly in aquatic ecosystems, which serve as the ultimate sinks for these pollutants and where they can negatively impact the physiology of living organisms121. In fish, heavy metals accumulate through the gills, skin, and gastrointestinal tract, and their presence in tissues can lead to metabolic, physiological, and histological changes, including alterations in enzyme activity and metabolite levels122.
The estimated daily intake (EDI) of most heavy metals analysed in this study was below the reference (safe) oral dose (RfD) established by the U.S. Environmental Protection Agency51. Based on the results, it can be concluded that with a daily intake of 30 g of fish muscle – corresponding to the average annual fish consumption in Poland, estimated at approximately 12 kg per person – the consumer ingests approximately 1.5% of the RfD for Cd, 2–3% for Pb, and 0.2–0.4% for Co and Ni across all fish samples analysed.
Based on the standards set by European Commission Regulation (EC) No 1881/2006132, which defines maximum permissible levels for certain contaminants in foodstuffs—0.30 mg·kg⁻1 for Pb and 0.05 mg·kg⁻1 for Cd—it can be concluded that the concentrations of Pb and Cd in the meat of the stone moroko did not exceed the established safety limits. For food safety reasons, provisional tolerable daily intakes (PTDI) have been established for Cd and Pb, amounting to 0.83 and 1.5 μg kg⁻1 day⁻1, respectively133,134. In our study, the EDI values were lower than the PTDI for these metals, which also suggests that the meat of the analyzed fish should not pose a health risk.
For the meat of the stone moroko examined from different locations, the THQ values were below 1.0, indicating no non-carcinogenic risk to humans from consuming the studied fish. Similar findings were reported by Tkachenko et al.135 for farmed carp, where THQ values for Cd, Pb, Hg, and As were 2×10⁻5, 1.29×10⁻3, 1.46×10⁻4, and 1×10⁻4, respectively. These results support the conclusion that the meat of the stone moroko analysed is safe for human consumption.
Conclusions
In this study, we demonstrated that populations of the stone moroko from different habitats may vary not only in morphometric traits but also in nutritional value and heavy metal content, depending on environmental conditions. Fish from the RP exhibited lower body weight compared to individuals from other habitats, while differences in total length were negligible. These variations may be influenced by environmental factors such as food availability or competition with other species present in the ecosystem. Similarly, differences in nutrient composition among populations are likely shaped by the specific habitat conditions of each water body.
Nutritional analyses showed that the nutrient levels in fish from all habitats remained within the ranges typically reported for common freshwater fish species in Europe. Wild specimens from the WR contained lower levels of fat and protein, likely due to increased energy expenditure required for food acquisition and a comparatively limited food base, as opposed to the more controlled conditions of pond farming. Relatively low concentrations of the tested heavy metals were recorded in the meat of stone moroko across all three populations, with values several times lower than those reported by other researchers. Particularly low lead levels indicate that the meat of these fish may be largely free from such contaminants, enhancing its suitability for human consumption. The estimated daily intake (EDI) values for most heavy metals were well below established safety thresholds, with negligible health risk to consumers.
The findings of our study suggest that the stone moroko, as an invasive species, has potential for use in the food industry. Its targeted harvesting and utilization as a food source could help control its population and mitigate its negative impact on native aquatic fauna. Introducing this species into the human diet may also reduce pressure on more vulnerable or overexploited fish species. Given its rapid population growth in many water bodies and the lack of natural predators, the exploitation of stone moroko is unlikely to harm aquatic ecosystems and may in fact support efforts to restore ecological balance.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Danet, A., Giam, X., Olden, J. D. & Comte, L. Past and recent anthropogenic pressures drive rapid changes in riverine fish communities. Nat. Ecol. Evol. 8(3), 442–453. https://doi.org/10.1038/s41559-023-02271-x (2024).
Villalba-Briones, R., Calle, P., Montiel, M., González-Narváez, M. & Vitvar, T. Hydrochemical and microbiological evaluation of groundwater in an agricultural area of Ecuador. J. Water Land Dev. 62, 17–28. https://doi.org/10.24425/jwld.2024.150277 (2024).
Więcaszek, B. et al. Recent data of the biology of the twaite shad, Alosa fallax returning for spawning to the Szczecin Lagoon (southern Baltic Sea). J. Water Land Dev. 62, 97–105. https://doi.org/10.24425/jwld.2024.151557 (2024).
Dudgeon, D. & Strayer, D. L. Bending the curve of global freshwater biodiversity loss: what are the prospects?. Biol. Rev. 100(1), 205–226. https://doi.org/10.1111/brv.13137 (2025).
Lin, G. et al. Length-Weight Relationships of Pseudorasbora parva (Temminck and Schlegel, 1842) Around the World. Pak. J. Zool. 56(4), 2022. https://doi.org/10.17582/journal.pjz/20221012141044 (1895).
Robinson, T. B., Martin, N., Loureiro, T. G., Matikinca, P. & Robertson, M. P. Double trouble: the implications of climate change for biological invasions. NeoBiota. 62, 463–487. https://doi.org/10.3897/neobiota.62.55729 (2020).
Peller, T. & Altermatt, F. Invasive species drive cross-ecosystem effects worldwide. Nat. Ecol. Evol. 8(6), 1087–1097. https://doi.org/10.1038/s41559-024-02380-1 (2024).
Czerniejewski, P. Adaptacje biologiczne inwazyjnych gatunków krabów Eriocheir sinensis (H. Milne Edwards, 1853) i Rhithropanopeus harrisii (Gould, 1841) w środowisku estuarium Odry. 2013.Wyd. ZAPOL.
Piria, M., Mrkonjić, F. M., Gavrilović, A., Shiming, W., Li, L., Tanuwidjaja, I., Tang, R., Zhao, H., Yang, Q., Li, D. Length-weight relationships and condition between invasive and native populations of topmouth gudgeon Pseudorasbora parva. 55th Croatian and 15th International Symposium on Agriculture. Vodice, 16-21 2020.
Kirczuk, L., Dziewulska, K., Czerniejewski, P., Brysiewicz, A. & Rząd, I. Reproductive potential of stone moroko (pseudorasbora parva, temminck et schlegel, 1846) (teleostei: cypriniformes: gobionidae) inhabiting central europe. Animals. 11(9), 2627. https://doi.org/10.3390/ani11092627 (2021).
Ruland, F. & Jeschke, J. M. How biological invasions affect animal behaviour: a global, cross-taxonomic analysis. J. Anim. Ecol. 89(11), 2531–2541. https://doi.org/10.1111/1365-2656.13306 (2020).
Gozlan, R. E. et al. Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions: Pan-continental invasion of topmouth gudgeon. Fish Fish. 11(4), 315–340. https://doi.org/10.1111/j.1467-2979.2010.00361.x (2010).
Rakauskas, V., Virbickas, T. & Steponėnas, A. Several decades of two invasive fish species (Perccottus glenii, Pseudorasbora parva) of European concern in Lithuanian inland waters; from first appearance to current state. J. Vertebr. Biol. 70(4), 21048–1. https://doi.org/10.25225/jvb.21048 (2021).
Zhu, D. M., Yang, K., Sun, N., Wang, W. M. & Zhou, X. Y. Embryonic and larval development of the topmouth gudgeon, Pseudorasbora parva (Teleostei: Cyprinidae). Zoologia. 35, 8. https://doi.org/10.3897/zoologia.35.e22162 (2018).
Czerniejewski, P. et al. Population structure and parasite fauna of stone moroko, Pseudorasbora parva (Temminck et Schlegel, 1846) in a watercourse of the Oder catchment area (‘Central Plains’ European Ecoregion). Eur. Zool. J. 90(2), 568–583. https://doi.org/10.1080/24750263.2023.2226686 (2023).
Artaev, O. Prediction of current and future suitable habitats for three invasive freshwater fish species in Europe. Water. 15(11), 2091. https://doi.org/10.3390/w15112091 (2023).
Knežević, B. Pseudorasbora parva (Schlegel), (Pisces, Cyprinidae), a new genus and species in the Lake Skadar. Glasnik Republickog Zavoda za Zastitu Prirode Prirodnjackog Muzeja Titograd. 14, 79–84 (1981).
Banarescu, P. Pseudorasbora parva (Temminck et schlegel 1846). Freshwater Fishes Europe. 5, 207–224 (1999).
Špelić, I. & Piria, M. Occurrence of topmouth gudgeon, Pseudorasbora parva, in relation to environmental factors in lowland streams and canals. Vertebr. Biol. 72, 23049. https://doi.org/10.25225/jvb.23049 (2023).
Kakareko, T., Grabowska, J., Mazurska, K. Analiza stopnia inwazyjności gatunków obcych w Polsce wraz ze wskazaniem gatunków istotnie zagrażających rodzimej florze i faunie oraz propozycją działań strategicznych w zakresie możliwości ich zwalczania oraz analiza dróg niezamierzonego wprowadzania lub rozprzestrzeniania się inwazyjnych gatunków obcych wraz z opracowaniem planów działań dla dróg priorytetowych. 2018.
Spikmans, F. et al. Impact of the invasive alien topmouth gudgeon (Pseudorasbora parva) and its associated parasite Sphaerothecum destruens on native fish species. Biol. Invasions. 22, 587–601. https://doi.org/10.1007/s10530-019-02114-6 (2020).
Britton, J. R., Davies, G. D. & Harrod, C. Trophic interactions and consequent impacts of the invasive fish Pseudorasbora parva in a native aquatic foodweb: a field investigation in the UK. Biol. Invasions. 12, 1533–1542. https://doi.org/10.1007/s10530-009-9566-5 (2010).
Britton, J. R., Brazier, M., Davies, G. D. & Chare, S. I. Case studies on eradicating the Asiatic cyprinid Pseudorasbora parva from fishing lakes in England to prevent their riverine dispersal. Aquat. Conserv.: Mar. Freshw. Ecosyst. 18(6), 867–876. https://doi.org/10.1002/aqc.919 (2008).
Rozporządzenie Parlamentu Europejskiego I Rady (UE) NR 1143/2014 z dnia 22 października r. w sprawie działań zapobiegawczych i zaradczych w odniesieniu do wprowadzania i rozprzestrzeniania inwazyjnych gatunków obcych. 2014
Liang, Y. J. et al. Evaluation of nutritive quality and nutritional components in body of Pseudorasbora parva. J. Anhui Agric. Univ. 38(4), 1869–1871. https://doi.org/10.7671/j.issn.1001-411X.2010.03.017 (2010).
Guoqing, D. et al. Enrichment of heavy metals in Pseudorasbora parva from crab pond. Chin. Agric. Sci. Bull. 33(1), 146–150 (2017).
Gozlan, R. E. et al. Disease threat to European fish. Nature 435(7045), 1046. https://doi.org/10.1038/4351046a (2005).
Liang, X.-F., Shen, D. & Liu, J. Food habits and seasonal variation in diet composition of Pseudorasbora parva in a shallow lake. China. J. Freshw. Ecol. 25(4), 507–514. https://doi.org/10.1080/02705060.2010.9664405 (2010).
Czerniejewski, P., Bienkiewicz, G. & Tokarczyk, G. Nutritional quality and fatty acids composition of invasive chinese mitten crab from odra estuary (Baltic Basin). Foods 12(16), 3088. https://doi.org/10.3390/foods12163088 (2023).
White, J. M. et al. Micronutrient gaps during the complementary feeding period in 6 countries in Eastern and Southern Africa: A comprehensive nutrient gap assessment. Nut. Rev. 79(Supp. 1), 16–25. https://doi.org/10.1093/nutrit/nuaa142 (2021).
Bavinck, M., Ahern, M., Hapke, H.M., Johnson, D.S., Kjellevold, M., Kolding, J., Overå, R., Schut, T. & Franz, N., eds. Small fish for food security and nutrition. FAO Fisheries and Aquaculture Technical Paper No. 694. Rome, FAO. https://doi.org/10.4060/cc6229en 2023
Boyd, C. E., McNevin, A. A. & Davis, R. P. The contribution of fisheries and aquaculture to the global protein supply. Food Sec. 14, 805–827. https://doi.org/10.1007/s12571-021-01246-9 (2022).
Bogard, J. R. et al. Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal. 42, 120–133. https://doi.org/10.1016/j.jfca.2015.03.002 (2015).
Bedrníček, J., Buchtová, H., Kročová, Z. & Szydlowska, K. Onion peel powder as an antioxidant-rich material for sausages prepared from mechanically separated fish meat. Antioxidants 9(10), 974. https://doi.org/10.3390/antiox9100974 (2020).
Narzary, Y. et al. Fermented fish products in South and Southeast Asian cuisine: Indigenous technology processes, nutrient composition, and cultural significance. J. Eth. Foods 8, 20. https://doi.org/10.1186/s42779-021-00109-0 (2021).
Beddows, C. G. (1998). Fermented fish and fish products. W: B. J. B. Wood (red.), Microbiology of Fermented Foods (t. 2, s. 416–440). Springer. https://doi.org/10.1007/978-1-4613-0309-1_13.
Barzkar, N., Sukhikh, S., Babich, O., Maran, B. A. V. & Jahromi, S. T. Marine collagen: Purification, properties and application. Fron. Mar. Scien. 10, 1245077. https://doi.org/10.3389/fmars.2023.1245077 (2023).
Benjakul, S., Sukhothai, N., Kishimura, H. Characteristics and applications of fish-derived collagen and gelatin. W: Y. H. Hui (red.), Handbook of Food Science and Technology (s. 351–374). Wiley-Blackwell. (2022)
Mozaffarian, D. & Rimm, E. B. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296(15), 1885–1899. https://doi.org/10.1001/jama.296.15.1885 (2006).
Swanson, D., Block, R. & Mousa, S. A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv. Nutr. 3(1), 1–7. https://doi.org/10.3945/an.111.000893 (2012).
Grosso, G. et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid. Med. Cell. Long. 2014, 313570. https://doi.org/10.1155/2014/313570 (2014).
World Health Organization (WHO). Healthy diet. 2020 [cited 2020 Oct 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/healthy-diet. 2020
Kris-Etherton, P. M., Harris, W. S. & Appel, L. J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106(21), 2747–2757. https://doi.org/10.1161/01.CIR.0000038493.65177.94 (2002).
Yang, Y. et al. Effect of ratio of oil to sample on the quality of fried fish (Pseudorasbora parva). J. Food Process. Preserv. 45(9), e15712. https://doi.org/10.1111/jfpp.15712 (2021).
Bianco, P. G. Occurrence of the Asiatic gobionid Pseudorasbora parva (Temminck and Schlegel) in south-eastern Europe. J. Fish Biol. 32, 973–974. https://doi.org/10.1111/j.1095-8649.1988.tb05440.x (1988).
Gozlan, R. E., Pinder, A. C. & Shelley, J. Occurrence of the Asiatic cyprinid Pseudorasbora parva in England. J. Fish Biol. 61, 298–300. https://doi.org/10.1111/j.1095-8649.2002.tb01755.x (2002).
Czerniejewski, P., Rybczyk, A., Linowska, A. & Sobecka, E. New location, food composition, and parasitic fauna of the invasive fish Pseudorasbora parva (Temminck & Schlegel, 1846) (Cyprinidae) in Poland. Turk. J. Zool. 43(1), 94–105 (2019).
Wu, X. et al. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci. Pollut. Res. 23(8244), 8259. https://doi.org/10.1007/s11356-016-6333-x (2016).
Wen, C. et al. Biofilm-mediated heavy metal bioaccumulation and trophic transfer in a mining-contaminated river. Water Res. 267, 122487. https://doi.org/10.1016/j.watres.2024.122487 (2024).
Chen, D. et al. Epidemiological investigation of clonorchis sinensis infection in freshwater fishes in the Pearl River Delta. Parasitol. Res. 107, 835–839. https://doi.org/10.1007/s00436-010-1936-5 (2010).
Ma, S. et al. Selenium accumulation and the effects on the liver of topmouth gudgeon Pseudorasbora parva exposed to dissolved inorganic selenium. Ecotoxicol. Environ. Saf. 160, 240–248. https://doi.org/10.1016/j.ecoenv.2018.05.047 (2018).
Zhang, H. et al. Assessment of heavy metal contamination in the surface sediments, seawater and organisms of the pearl river Estuary South China Sea. Sci. Total Environ. 950, 175266. https://doi.org/10.1016/j.scitotenv.2024.175266 (2024).
Jomova, K., Alomar, S. Y., Nepovimova, E., Kuca, K. & Valko, M. Heavy metals: toxicity and human health effects. Arch. Toxicol. 99(1), 153–209. https://doi.org/10.1007/s00204-024-03903-2 (2025).
Lehel, J., Pleva, D., Nagy, A. L., Süth, M. & Kocsner, T. Potential metal contamination in foods of animal origin food safety aspects. Appl. Sci. 15, 8468. https://doi.org/10.3390/app15158468 (2025).
Andreji, J., Dvořák, P. & Fik, M. Distribution of heavy metals (Ni Co, Pb, Cd, Hg) in tissues of European Chub (Squalius cephalus L.) from the middle course of the Nitra River Slovakia. Adv. Res. Life Sci. 2(1), 16–21. https://doi.org/10.1515/arls-2018-0022 (2018).
Kumar, M. et al. A review on heavy metal-induced toxicity in fishes: Bioaccumulation, antioxidant defense system, histopathological manifestations, and transcriptional profiling of genes. J. Trace Elem. Med. Biol. 83, 127377. https://doi.org/10.1016/j.jtemb.2023.127377 (2024).
N.Y.S. D.O.H. Nev York State Department of Health. Health advisory: alert to New York State healthcare facilities regarding the global emergence of invasive infections caused by the multidrug-resistant yeast Candida auris [cited 2017 Nov 24]. https://www.health.ny.gov/diseases/communicable/c_auris/docs/2016-08_candida_auris_advisory.pdf. 2017
Tan, Y. et al. Human health risk assessment of toxic heavy metal and metalloid intake via consumption of red swamp crayfish (Procambarus clarkii) from rice-crayfish co-culture fields in China. Food Control. 128, 108181. https://doi.org/10.1016/j.foodcont.2021.108181 (2021).
Balzani, P., Kouba, A., Tricarico, E., Kourantidou, M. & Haubrock, P. J. Metal accumulation in relation to size and body condition in an all-alien species community. Environ. Sci. Pollut. Res. Int. 29, 25848–25857. https://doi.org/10.1007/s11356-021-17621-0 (2022).
Nowak, A. & Kowalski, P. Environmental pressures on protected wetland areas near urban centers: a case study of stawy raszyńskie nature reserve. J.E.P. 12(3), 145–156. https://doi.org/10.1234/jep.2021.0123 (2021).
Tytła, M., Kostecki, M. Contamination of small urban watercourses on the example of a stream in Krakow (Poland). Environmental Earth Sciences. (2019).
Sojka, M., Jaskuła, J., Barabach, J. & Ptak, M. Heavy metals in lake surface sediments in protected areas in Poland: Concentration, pollution, ecological risk, sources and spatial distribution. Sci. Rep. 12, 15006 (2022).
Nash, R., Valencia, A. H. & Geffen, A. J. Origin of fulton’s condition factor—setting the record straight. Fisheries. 31, 236–238 (2006).
AOAC. Association of Official Agricultural Chemists, Official Methods of Analysis of AOAC International. 20th ed. Rockville, USA: AOAC International. (2016).
PN-ISO 1442:2000. Mięso i przetwory mięsne. Oznaczanie zawartości wody. 2000
PN-A-04018:1975/Az3:2002. Produkty rolniczo-żywnościowe. Oznaczanie azotu metodą Kjeldahla i przeliczanie na białko. 2002
Folch, J., Lees, M. & Stanley, G. S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509. https://doi.org/10.1016/S0021-9258(18)64849-5 (1957).
Zhang, X. et al. Fatty acid composition analyses of commercially important fish species from the pearl river estuary China. PLoS One. 15, e0228276. https://doi.org/10.1371/journal.pone.0228276 (2020).
Nava, V. et al. Determination of fatty acid profile in processed fish and shellfish foods. Foods. 12(3), 2631. https://doi.org/10.3390/foods12132631 (2023).
Nguyen, N., Pham, H., Hai, P. & Hung, L. Improved growth, body composition, and fatty acid composition in striped catfish juveniles, Pangasianodon hypophthalmus, fed with diets containing different oil sources. J. World Aquac. Soc. 55(3), e13064. https://doi.org/10.1111/jwas.13064 (2024).
Aberoumand, A. & Aminimehr, A. The effects of additives as a marinade producer on nutritional quality parameters of Oncorhynchus mykiss fish during storage at 4°C. Food Sci. Nutr. 12(1), 162–171. https://doi.org/10.1002/fsn3.3745 (2023).
Rozporządzenie UE nr 1169/2011 z dnia 25 października 2011 r. w sprawie przekazywania konsumentom informacji na temat żywności, zmiany rozporządzeń Parlamentu Europejskiego i Rady (WE) nr 1924/2006 i (WE) nr 1925/2006 oraz uchylenia dyrektywy Komisji 87/250/EWG, dyrektywy Rady 90/496/EWG, dyrektywy Komisji 1999/10/WE, dyrektywy 2000/13/WE Parlamentu Europejskiego i Rady, dyrektyw Komisji 2002/67/WE i 2008/5/WE oraz rozporządzenia Komisji (WE) nr 608/2004 (Dz. Urz. UE L 304 z 21.11.2011, z późn.zm.).
Żmijewski, T., Kujawa, R., Jankowska, B., Kwiatkowska, A. & Mamcarz, A. Slaughter yield, proximate and fatty acid composition and sensory properties of rapfen (Aspius aspius L.) with tissue of bream (Abramis brama L.) and pike (Esox lucius L.). J. Food Compos. Anal. 19(2–3), 176–181. https://doi.org/10.1016/j.jfca.2005.03.006 (2006).
Nędzarek, A. & Czerniejewski, P. The edible tissues of the major European population of the invasive Chinese mitten crab (Eriocheir sinensis) in the Elbe River, Germany, as a valuable and safe complement in essential elements to the human diet. J. Food Compos. Anal. 96, 103713. https://doi.org/10.1016/j.jfca.2020.103713 (2021).
Nędzarek, A. & Czerniejewski, P. Impact of polyaluminum chloride on the bioaccumulation of selected elements in the tissues of invasive spiny-cheek crayfish (Faxonius limosus)–Potential risks to consumers. Sci. Total Environ. 828, 154435. https://doi.org/10.1016/j.scitotenv.2022.154435 (2022).
Zhou, M. et al. Enrichment of trace elements in red swamp crayfish: Influences of region and production method, and human health risk assessment. Aquaculture. 535, 736366. https://doi.org/10.1016/j.aquaculture.2021.736366 (2021).
Nędzarek, A. & Czerniejewski, P. Assessment of heavy metal concentrations and bioaccumulation in the invasive round goby (Neogobius melanostomus) and associated risks to human health. Eur. Zool. J. 91, 440–456. https://doi.org/10.3390/foods13111779 (2024).
Li, Z., Ma, Z., van der Kuijp, T. J., Yuan, Z. & Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 468, 843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090 (2014).
U.S. E.P.A. U.S. Environmental Protection Agency. Regional Screening Level (RSL) summary table (TR=1E-06,HQ=1) November 2023. https://semspub.epa.gov/work/HQ/400431.pdf. Accessed Jan 2024 10.
N.Y.S. D.O.H. Nev York State Department of Health, 2007. Hopewell precision area contamination: Appendix C – NYSDOH. In: Procedure for evaluating potential health risk for contaminants of concern. http://www.health.ny.gov/environmental/investigations/hopewell/appendc.html. Accessed Jan 2024 9.
U.S. E.P.A. U.S. Environmental Protection Agency. 2000. Guidance for assessing chemical contaminant data for use in fish advisories, vol 2. Risk assessment and fish consumption limits, 3rd Edition. http://www.epa.gov/waterscience/fish/guidance.html. Accessed Jan 2024 9.
Kapusta, A., Bogacka-Kapusta, E. & Czarnecki, B. The significance of stone moroko Pseudorasbora parva (Temminck and Schlegel) in the small-sized fian assemblages in the littoral zone of the heated Lake Lichenskie. Arch. Pol. Fisheries. 16, 49–62. https://doi.org/10.2478/s10086-008-0004-6 (2008).
Záhorská, E. & Kováč, V. Reproductive parameters of invasive topmouth gudgeon Pseudorasbora parva (Temminck and Schlegel, 1846) from Slovakia. J. Appl. Ichthyol. 25(4), 466–469. https://doi.org/10.1111/j.1439-0426.2009.01190.x (2009).
Záhorská, E., Kováč, V. & Katina, S. Age and growth in a newly-established invasive population of topmouth gudgeon. Cent. Eur. J. Biol. 5(2), 256–261. https://doi.org/10.2478/s11535-010-0002-8 (2010).
Wróblewski, P., Maciaszek, R. & Kasprzak, R. First record of stone moroko (Pseudorasbora parva), an invasive alien fish species, in water bodies of urban green areas in Poland. Fish. Aquat. Life. 32, 122–127. https://doi.org/10.2478/aopf-2024-0011 (2024).
Czerniejewski, P., Bienkiewicz, G. & Tokarczyk, G. Nutritional quality and fatty acids composition of invasive chinese mitten crab from odra estuary (Baltic Basin). Foods 12(16), 3088. https://doi.org/10.3390/foods12163088 (2023).
Rykov, Yu. V., Samoilenko, D. A. & Kulik, P. V. Charac teristic of stone moroco Pseudorasbora parva (Cyprini formes, Cyprinidae) in estuary pond of Mokraya Belosaraika river in Northern Azov basin. Ryb. Khoz. 67, 182–187 (2009).
Kotovskaya, A. A. & Khristenko, D. S. Distribution and some peculiarities of biology of Pseudorasbora parva (Temminck et Schlegel, 1846) in the littoral of the Kremenchug reservoir. Russ J. Biol. Invasions. 4, 156–160. https://doi.org/10.1134/S2075111713030065 (2013).
Stavrescu-Bedivan, M. M. et al. A biometric analysis and fishermen’s perception on Pseudorasbora parva (Temminck & Schlegel, 1846): a case study from romania. AgroLife Scien. Jour. 13(2), 243–250. https://doi.org/10.17930/AGL2024222 (2024).
Khalili, T. S. & Sampels, S. Nutritional value of fish: lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquac. 26(2), 243–253. https://doi.org/10.1080/23308249.2017.1399104 (2017).
Wang, Y. et al. Dietary fish and omega-3 polyunsaturated fatty acids intake and cancer survival: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 63(23), 6235–6251. https://doi.org/10.1080/10408398.2022.2029826 (2023).
Matos, J. et al. Influence of bioaccessibility of total mercury, methyl-mercury and selenium on the risk/benefit associated to the consumption of raw and cooked blue shark (Prionace glauca). Environ. Res. 143, 123–129. https://doi.org/10.1016/j.envres.2015.09.015 (2015).
Ahmed, I., Jan, K., Fatma, S. & Dawood, M. A. O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. (Berl.) 106(3), 690–719. https://doi.org/10.1111/jpn.13711 (2022).
Skałecki, P., Florek, M., Litwińczuk, A., Staszowska, A. & Kaliniak, A. Wartość użytkowa i skład chemiczny mięsa karpi (Cyprinus carpio L.) i pstrągów tęczowych (Oncorhynchus mykiss Walb.) pozyskanych z gospodarstw rybackich regionu lubelskiego. Rocz. Nauk. Pol. Tow. Zootech 9(2), 57–62 (2013).
Teame, T., Natarajan, P. & Tesfay, Z. Proximate and mineralcomposition of some commercially important fish species of tekezereservoir and lake Hashenge Ethiopia. Int. J. Fish. Aquat. Stud. 4(1), 160–164 (2016).
Mohanty, B. P. et al. DHA and EPAcontent and fatty acid profile of 39 food fishes from India. Biomed Res. Int. 1, 4027437. https://doi.org/10.1155/2016/4027437 (2016).
Geremew, H., Abdisa, M. & Goshu, G. Proximate composition of commercially important fish species in southern Gulf of Lake Tana. Ethiopia. Ethiop. J. Sci. Technol. 13(1), 53–63. https://doi.org/10.4314/ejst.v13i1.4 (2020).
Ayanda, I. O., Ekhator, U. I. & Bello, O. A. Determination of selected heavy metal and analysis of proximate composition in some fish species from Ogun River. Southwestern Nigeria. Heliyon. 5(10), e02512. https://doi.org/10.1016/j.heliyon.2019.e02512 (2019).
Linhartová, Z. et al. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquac. Int. 26, 695–711. https://doi.org/10.1007/s10499-018-0243-5 (2018).
Fallah, A. A., Saei-Dehkordi, S. S. & Nematollahi, A. Comparative assessment of proximate composition, physicochemical parameters, fatty acid profile and mineral content in farmed and wild rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Technol. 46(4), 767–773. https://doi.org/10.1111/j.1365-2621.2011.02554.x (2011).
Popelka, P., Marcinčák, S., Maskal’ová, I., Guothová, L. & Čertík, M. Comparison of the chemical composition and nutritional values of fresh and frozen rainbow trout. Slov. Vet. Res. 51(2), 73–80 (2014).
Ghassem, M. et al. Proximate composition, fatty acid and amino acid profiles of selected Malaysian freshwater fish. M. F. J. 8(1), 7–16 (2009).
Olopade, O. A., Taiwo, I. O., Lamidi, A. A. & Awonaike, O. A. Proximate composition of nile tilapia (Oreochromis niloticus)(Linnaeus, 1758) and tilapia hybrid (red tilapia) from Oyan Lake, Nigeria. Bulletin UASVM. Food Sci. Technol. Int. 73(1), 19–23. https://doi.org/10.15835/buasvmcn-fst:11973 (2016).
Saâ, N. I., Bakar, M. F. A. & Malik, N. H. Proximate composition of selected freshwater fish from Kampung Peta, Johor Malaysia. J. Sustain. Nat. Res. 2(2), 31–38. https://doi.org/10.30880/jsunr.2021.02.02.004 (2021).
Jankowska, B., Zakęś, Z., Żmijewski, T. & Szczepkowski, M. Fatty acid profile of muscles, liver and mesenteric fat in wild and reared perch. Food Chem. 118(3), 764–768. https://doi.org/10.1016/j.foodchem.2009.05.055 (2010).
Çelik, M., Diler, A. & Küçükgülmez, A. A comparison of the proximate compositions and fatty acid profiles of zander (Sander lucioperca) from two different regions and climatic conditions. Food Chem. 92(4), 637–641. https://doi.org/10.1016/j.foodchem.2004.08.026 (2005).
Hu, Y. T. et al. Comparison of nutritional components between wild and pond-cultured Pseudorasbora parva. J. Anhui Agric. Univ. 45, 95–98 (2017).
Stansby, M. E. & Olcott, H. S. Composition of Fish 339–349 (In Stansby Industrial Fishery Technology. Reinhold Publishing Co., 1963).
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the tolerable upper intake level of EPA, DHA and DPA. EFSA Journal, 10(7) 2815. https://doi.org/10.2903/j.efsa.2012.2815 (2012).
Karki, S., Chowdhury, S., Nath, S., Murmu, P. & Dora, K. C. Seasonal changes in proximate composition and textural attributes of farm raised chocolate mahseer (Neolissochilus hexagonolepis). J. Entomol. Zool. Stud. 7(4), 696–701 (2019).
Ding, H. et al. Growth and feeding habits of invasive Pseudorasbora parva in the Chabalang Wetland (Lhasa, China) and its trophic impacts on native fish. J. Ocean. Limnol. 37, 628–639. https://doi.org/10.1007/s00343-019-8004-5 (2019).
Nocita, A., La Sala, G., Busatto, T., Santini, G. & Balzani, P. Population structure and dietary plasticity of four invasive populations of the topmouth gudgeon Pseudorasbora parva. Int. Rev. Hydrobiol. 107(5–6), 167–178. https://doi.org/10.1002/iroh.202302142 (2023).
Yousaf, M., Salam, A. & Naeem, M. Body composition of freshwater Wallago attu in relation to body size, condition factor and sex from southern Punjab. Pakistan. Afr. J. Biotechnol. 10(20), 4265–4268 (2011).
Naeem, M. & Ishtiaq, A. Proximate composition of Mystus bleekeri in relation to body size and condition factor from Nala Daik, Sialkot. Pakistan. Afr. J. Biotechnol. 10(52), 10765–10773 (2011).
Marenkov, O. M. et al. Influence of heavy metals on physiological and biochemical parameters of Pseudorasbora parva (Cypriniformes, Cyprinidae). Regul. Mech. Biosyst. 12(4), 745–752. https://doi.org/10.15421/0221103 (2021).
Chamnan, C., Thilsted, H. S. , Roitana, B., Lieng, S., Gerpaci, R. V, Nanna, R. The role of fisheries resources in rural Cambodia: combating micronutrient deficiencies in women and children. Phnom Penh: Department of Fisheries Post-harvest Technologies and Quality control, Fisheries Administration, Ministry of Agriculture, Forestry and Fisheries, Cambodia. 2009.
Szulecka, O. Farsze rybne – możliwości wykorzystania ryb małocennych. In: Działalność Podmiotów Rybackich i Wędkarskich w 2021 Roku. Uwarunkowania Gospodarcze, Ekonomiczne, Środowiskowe i Klimatyczne; Cejko A, Wołos A. red.; Urząd Skarbowy: Olsztyn, Polska. 209–219. (2022).
Krzywiński, T., Domiszewski, Z., Tokarczyk, G. & Bienkiewicz, G. Ocena przydatności mięsa ryb małocennych do produkcji żywności przekąskowej. Żywn Nauka Technol Jakość. 5(111), 123. https://doi.org/10.15193/ZNTJ/2014/96/111-123 (2014).
Liu, Y., Liu, G., Yuan, Z., Liu, H. & Lam, P. K. Heavy metals (As, Hg and V) and stable isotope ratios (d13C and d15N) in fish from Yellow River Estuary China. Sci. Total. Environ. 613, 462e471. https://doi.org/10.1016/j.scitotenv.2017.09.088 (2018).
Liu, J., Liu, R., Yang, Z. & Kuikka, S. Quantifying and predicting ecological and human health risks for binary heavy metal pollution accidents at the watershed scale using Bayesian Networks. Environ. Pollut. 269, 116125 (2019).
Shahjahan, M. et al. Effects of heavy metals on fish physiology–a review. Chemosphere. 300, 134519. https://doi.org/10.1016/j.chemosphere.2022.134519 (2022).
Zaynab, M. et al. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 34(1), 101653. https://doi.org/10.1016/j.jksus.2021.101653 (2022).
Chen, J., Jayachandran, M., Bai, W. & Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 369, 130874. https://doi.org/10.1016/j.foodchem.2021.130874 (2022).
Cordeli, A. N. et al. Bioaccumulation of metals in some fish species from the Romanian Danube River: A Review. Fishes 8(8), 387. https://doi.org/10.3390/fishes8080387 (2023).
Habib, S. S., Batool, A. I., Rehman, M. F. U. & Naz, S. Assessment and bioaccumulation of heavy metals in fish feeds, water, and some tissues of Cyprinus carpio cultured in different environments (Biofloc Technology and Earthen Pond System). Biol. Trace Elem. Res. 201(7), 3474–3486. https://doi.org/10.1007/s12011-022-03415-z (2023).
Jarf, M. P., Kamali, A., Khara, H., Pourang, N. & Shekarabi, S. P. H. Microplastic pollution and heavy metal risk assessment in Perca fluviatilis from Anzali wetland: Implications for environmental health and human consumption. Sci. Total Environ. 907, 167978. https://doi.org/10.1016/j.scitotenv.2023.167978 (2024).
Vivanco, M. M. & Taboada, O. M. Thermodynamic behavior of fish meal during adsorption. Dry. tech. 16(9–10), 1827–1842. https://doi.org/10.1080/07373939808917498 (1998).
Rajkowska, M. & Protasowicki, M. Distribution of metals (Fe, Mn, Zn, Cu) in fish tissues in two lakes of different trophy in Northwestern Poland. Environ Monit Assess. 185(3493), 3502 (2023).
Mansouri, B., Davari, B., Azadi, N. & Pordel, M. A. Bioaccumulation of metals in silver carp and stone moroko from Zarivar Wetland in Kurdistan Province. Iran. J. Adv. Environ. Health Res. 4(4), 227–233. https://doi.org/10.22102/JAEHR.2016.46242 (2016).
Subotić, S. et al. Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotoxicol. Environ. Saf. 98, 196–202. https://doi.org/10.1016/j.ecoenv.2013.08.020 (2013).
Rakocevic, J., Sukovic, D. & Maric, D. Distribution and relationships of eleven trace elements in muscle of six fish species from Skadar Lake (Montenegro). Tur J. Fish Aquat. Sci. 18(647), 657. https://doi.org/10.4194/1303-2712-v18_5_01 (2018).
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. 2006
EFSA. Scientific opinion on lead in food (request N. EFSA-Q-2007-137) (adopted on 18 March 2010) EFSA panel on contaminants in the food chain (CONTAM). EFSA J 8:1570. (2010).
FAO W. Safety evaluation of certain contaminants in food. WHO food additive series. 63. 2011.
Tkachenko, H., Kurhaluk, N., Kasiyan, O. & Kamiński, P. Dietary nutrients and health risks from exposure to some heavy metals through the consumption of the farmed common carp (Cyprinus carpio). J. Environ. Health Sci. Eng. 19, 793–804. https://doi.org/10.1007/s40201-021-00647-4 (2021).
Acknowledgement
The authors would like to express their sincere thanks to Konrad Żarkowski, manager of the breeding ponds in the “Stawy Falenckie” Nature Reserve, as well as to Mr. Janusz and Mr. Przemysław Margan for their support in obtaining research material and for providing fish from the Fish Farm in Stary Kurów. We also extend our gratitude to Jarosław Dąbrowski and Nadhira Benhadji for their valuable assistance with fish dissection during the laboratory analyses, also Bartosz Kierasiński for preparing and developing the map.
Funding
Open Access funding enabled and organized by research carried out and financed as part of the internal competition of the Institute of Technology and Life Sciences - National Research Institute "Raszyńskie Ponds in Science 2024", project no. 01/2024 entitled ”Biological analysis of different populations of the invasive species of the topmouth gudgeon, together with an assessment of the slaughter value and bioaccumulation of selected elements from the Raszyn Pond Farm and natural waters”.
Author information
Authors and Affiliations
Contributions
Study design: A.K and A.B. Sample preparation: A.K and P.C. Methodology: A.K, A.B, P.C and D.P. Data analysis: A.K, P.C and D.P. Manuscript writing: A.K, A.B, P.C and D.P. Conclusion: A.B. Literature check: A.K and D.P. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kozioł, A., Brysiewicz, A., Czerniejewski, P. et al. Nutritional quality and heavy metal in invasive stone moroko (Pseudorasbora parva) from natural waters and aquaculture ponds. Sci Rep 15, 34385 (2025). https://doi.org/10.1038/s41598-025-17243-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17243-3