Abstract
Probiotics present a promising preventive strategy for addressing urinary tract infections (UTIs) and serve as a viable alternative to conventional antimicrobial treatments. The integration of Lactobacillus-derived efflux pump inhibitors (EPIs) with conventional antibiotics presents a promising strategy to enhance the efficacy of treatments against antibiotic-resistant superbugs. In this study, we isolated eight vaginal Lactobacillus spp. from 54 healthy Indian women to explore their probiotic properties, specifically the ability of cell-free supernatant (CFS) from Lactobacillus jensenii. to inhibit efflux in Multi Drug Resistant (MDR) E. coli and K. pneumoniae. From the CFS, we obtained seven fractions, among which compound 7 (C7) markedly reduced the MIC of erythromycin against the K. pneumoniae MTCC 432 strain by 32-fold from 64 to 2 µg and restored its sensitivity, indicating potent efflux inhibitory activity. Gas Chromatography-Mass Spectrometry (GC-MS) analysis identified one major compound from the C7 fraction as Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)-, also known as (-)-Terpinen-4-ol. In vivo studies using zebrafish infected with clinical strain of K. pneumoniae 1845 followed by treatment with (-)-Terpinen-4-ol and erythromycin caused a significant 7-log reduction in bacterial bioburden. Additionally, time-kill assay demonstrated that the combination of erythromycin with (-)-Terpinen-4-ol caused a remarkable 7-log reduction in both K. pneumoniae, and E. coli cell counts. Furthermore, (-)-Terpinen-4-ol -erythromycin combination exhibited a six-log reduction in bacterial burden in infected T24 bladder cell lines and was found to be non-toxic. This study underscores the efflux inhibitory potential of (-)-Terpinen-4-ol extracted from the CFS of human vaginal probiotics, paving the way for future pharmacological research and therapeutic applications.
Similar content being viewed by others
Introduction
Urinary tract infections (UTIs) are increasingly recognized as a significant health concern, particularly due to the rising prevalence of antibiotic-resistant pathogens. UTIs are among the most common infections, affecting a substantial portion of the population; approximately 60% of women and 10% of men experience at least one UTI in their lifetime1. The rise in antibiotic resistance significantly undermines the effectiveness of standard treatments for common bacterial infections. The recently released 2022 Global Antimicrobial Resistance and Use Surveillance System (GLASS) report underscores the gravity of the situation, revealing alarming resistance rates among prevalent bacterial pathogens. Of particular concern are the median reported rates in 76 countries, indicating 42% resistance to third-generation cephalosporins in E. coli and 35% resistance in methicillin-resistant Staphylococcus aureus2. In 2020, 20% of E. coli-mediated UTIs showed reduced susceptibility to key antibiotics. K. pneumoniae also exhibits increasing resistance, leading to higher reliance on last-resort antibiotics like carbapenems, for which resistance is spreading3. The OECD projects a doubling of resistance to these critical drugs by 2035, highlighting an urgent need for improved alternate strategies4. Antibiotic resistance remains a critical global health issue, with efflux pumps that extrude antibiotics from bacterial cells before they can reach their intracellular targets, plays a central role in drug resistance 5,6,7. To date, no efflux pump inhibitors or combinations with antibiotics have received regulatory approval for clinical use, likely due to concerns regarding potential toxicity in humans8,9,10.The identification of microbial small molecule efflux pump inhibitors (EPIs) has been facilitated by the natural selection of compounds specifically evolved to traverse the outer and inner membranes of Gram-negative bacteria11. To combat resistance mechanisms in target bacteria, antibiotic-producing bacteria have developed strategies to mount combined attack through the co-production of efflux pump inhibitors. These microbes represent a unique bioprospecting opportunity to screen for efflux pump inhibitors. Literature presents substantial evidence that bacteria produce inhibitors against efflux pumps12,13 and, nontoxic secondary metabolites capable of quenching quorum sensing-controlled activities in other species14.Screening of terrestrially derived microbial fermentations has led to the identification of two new natural product EPIs from Streptomyces, namely EA-371α and EA-371δ. These EPIs target the MexAB-OprM efflux pump in Pseudomonas aeruginosa and enhance the minimum inhibitory concentrations (MICs) of levofloxacin by fourfold and eightfold, respectively10. Furthermore, the microbial EPI MP-601205 dihydrochloride is effective in treating P. aeruginosa respiratory infections in cystic fibrosis patients15,16. A wide array of natural product chemical scaffolds, including polyphenols, flavones, flavanols, flavonolignans, flavonoids, diterpenes, triterpenoids, oligosaccharide-glycosides, and pyridines, have been validated as EPIs in Gram-positive bacteria such as Staphylococcus aureus. However, discovering natural EPIs targeting efflux pumps in Gram-negative bacteria presents a significant challenge.17.
Integrating probiotics into UTI management represents a promising avenue for addressing the limitations of conventional antimicrobial therapies. By producing bioactive compounds, Lactobacillus helps in maintaining a healthy vaginal and urinary microbiome, thus preventing the colonization of uropathogens18.Lactobacillus-derived EPIs represent an innovative strategy to enhance the potency of antibiotics against resistant strains. In this study,we focused on the ability of cell-free supernatant (CFS) from Lactobacillus jensenii to inhibit efflux pumps in clinical isolates of K. pneumoniae and E. coli. Bioassay guided fractionation resulted in identification of active fraction of CFS viz., compound 7 (C7) as a potent efflux pump inhibitor. Gas Chromatography-Mass Spectrometry (GC–MS) analysis helped in identifying C7 as Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)-, also known as (-)-Terpinen-4-ol. Herein, we report the isolation, characterization, and demonstration of the efflux pump inhibitory (EPI) functionality of (-)-Terpinen-4-ol. Additionally, in an in vivo infection study using zebrafish, we assessed the impact of (-)-Terpinen-4-ol in combination with erythromycin to reduce the bioburden of K. pneumoniae MTCC 432 strain . This study highlights the potential of Lactobacillus-derived (-)-Terpinen-4-ol as an effective efflux pump inhibitor and a valuable adjunct to traditional antibiotics. The findings pave the way for further research into probiotic-based therapeutic approaches for managing antibiotic-resistant infections.
Materials and methods
Sample collection of human vaginal probiotics for cell-free supernatant preparation
This study included 54 healthy South Indian women, aged 18 to 40 years, who visited the outpatient clinic at the Obstetrics and Gynaecology Department of Trichy SRM Medical College and Research Centre between June 2022 and July 2022. All participants had healthy vaginal mucosa and provided informed consent prior to enrolment. Vaginal swabs were collected from each participant, adhering to the guidelines set forth by the Human Ethical Committee (both institutional and national). The study protocol was approved by the ethics committee (Registration number: 696/TSRMMCH&RC/ME-1/2022-IEC No: 127).
Inclusion criteria for the study required participants to have no recent antibiotic, probiotic, immune suppressant, or hormone treatments. Exclusion criteria included pregnancy, breastfeeding, and any clinically evident sexually transmitted infections such as Human papillomavirus, Chlamydia spp., Syphilis, Gonorrhoea, and bacterial vaginosis (BV), as well as parasitic infections like Trichomonas vaginalis. Preclinical data was collected on co-morbid conditions, method of delivery, menstrual status, hormone therapy, antibiotic usage, contraceptive practices, and clinical history, including urinary tract infections (UTIs), diabetes, hypertension, and other medical conditions. The detailed identification and GenBank accession numbers for the isolated Lactobacillus strains are available in our previous study19.
Preparation of cell free supernatant (CFS) for characterization
The culture broth of vaginal probiotic Lactobacilli was subjected to centrifugation at 10,000 × g for 30 min at 4 °C. After centrifugation, the pH of the supernatant was adjusted to 7.0 using 1 M NaOH and filtered through a sterilized filter (HI Media-CC Syringe-Driven filters NYLON-66, Pore size: 0.22μ, India). The resulting filtrate, known as Cell-Free Supernatant (CFS), was preserved at − 80 °C for future tests. Before initiating each experiment, a preliminary check was conducted to ensure the absence of any colonies. This involved spreading the CFS on an MRS agar plate and incubating it for 24 h at 37 °C. The absence of visible colonies confirmed the purity of the Cell-Free Supernatant for subsequent analyses20.
Gas chromatography–mass spectrometry (GC–MS)
Gas chromatography-mass spectrometry (GC–MS) analysis was performed using a GCMS-QP2010 SE (Shimadzu Corporation, Kyoto, Japan) with the following configurations. The carrier solvent utilized was IJC2 methanol. The column oven temperature was set at 50.0 °C, and the injection temperature was 280.0 °C conducted in split mode with a split ratio of 10.0. The flow control mode was set to linear velocity with a pressure of 53.5 kPa, a total flow of 14.0 mL/min, a column flow of 1.00 mL/min, and a linear velocity of 36.3 cm/sec. The purge flow was maintained at 3.0 mL/min. The oven temperature program started at 50.0 °C for 1.00 min, ramped at 6.00 °C/min to 220.0 °C (held for 5.00 min), and further increased at 6.00 °C/min to 280.0 °C (held for 15.00 min). The MS conditions included an ion source temperature of 230.0 °C and an interface temperature of 280.0 °C, with a solvent cut time of 5.00 min. The detector gain mode was set relative to the tuning result with a gain of 0.85 kV + 0.20 kV and a threshold of 100. MS acquisition parameters were configured to start at 5.00 min and end at 59.33 min in scan mode, with an event time of 0.40 s and a scan speed of 1666, covering an m/z range of 40.00 to 600.00. Samples were prepared and introduced into the GC–MS system under these specified conditions. The ready check parameters confirmed that the column oven, SPL1, and MS were all operational, and equilibrium time was set at 3.0 min.
Checkerboard assay and MIC reversal
The fold reduction in MIC (MIC reversal) of erythromycin when combined with (-)-Terpinen-4-ol was determined as reported earlier21. To elucidate the combinatorial effects, a combination of (-)-Terpinen-4-ol (5 µg) and antibiotics (256 µg) was employed using a checkerboard assay against both reference and clinical isolates, as reported previously22 The Fractional Inhibitory Concentration (FIC) index was computed, and interactions were categorized as synergistic if FIC values were < 0.5, additive within the range of 0.5–2.0, and antagonistic if > 2.0. The potential of Terpinen-4-ol to restore erythromycin sensitivity in erythromycin-resistant strains of K. pneumoniae and E. coli UTI89 was assessed. Sub-Minimum Inhibitory Concentration (sub-MIC) levels of (-)-Terpinen-4-ol were combined with varying concentrations of erythromycin and incubated for 18–24 h at 37 °C, and the reduction in MIC relative treatment with erythromycin alone was noted.
Real time efflux analysis
To assess the efflux pump inhibitory potential of (-)-Terpinen-4-ol against K. pneumoniae and E. coli UTI89, real-time efflux studies were conducted utilizing ethidium bromide as a substrate. The cells were first de-energized and then treated with (-)-Terpinen-4-ol (5 µg) for 1 h. The cells were then reenergized with glucose and residual fluorescence of EtBr was measured for a period of 0–20 min. Phenyl arginine beta naphthylamide (PAßN) and Carbonyl cyanide m-chlorophenyl hydrazine (CCCP) were maintained as positive controls. The resultant fluorescent intensity was quantified using excitation at 360 nm and emission at 590 nm (Microplate reader, Biotek), as previously documented. The rise in EtBr fluorescence relative to untreated cells indicated efflux inhibition by the tested compounds23.
Membrane permeability
To evaluate the membrane permeabilizing property of (-)-Terpinen-4-ol, we performed an NPN uptake assay. NPN (1-N-phenylnaphthylamine) is a non-polar fluorophore that fluoresces in a phospholipid environment. The intact outer membrane of Gram-negative bacteria is asymmetric and acts as a permeability barrier to even hydrophobic molecules like NPN. When the outer membrane is permeabilized, NPN can penetrate and exhibit fluorescence. Mid-log phase cells of K. pneumoniae and E. coli UTI89 were treated independently with (-)-Terpinen-4-ol, erythromycin and CTAB were deemed as a positive control. Post NPN addition to all samples, the fluorescence of NPN was measured after 3 min at an excitation wavelength of 375 nm and an emission wavelength of 420 nm . The NPN uptake factor was calculated as the ratio of the fluorescence value of the background-subtracted bacterial suspension to that of the buffer24.
Membrane potential studies
To determine whether (-)-Terpinen-4-ol inhibits efflux by disrupting membrane potential like the protonophore CCCP, membrane potential studies were conducted as previously described25. The assay utilized Disc3, a cationic membrane-permeabilizing dye. Mid-log phase cells of K. pneumoniae BC1415 were treated with Disc3, and fluorescence intensity (excitation at 622 nm and emission at 670 nm) was monitored until a plateau was reached. Following this, 0.5% glucose was added, and fluorescence was recorded continuously. Subsequently, (-)-Terpinen-4-ol or 10 μM CCCP were introduced, and changes in fluorescence intensity were documented. The experiment was performed in triplicate to ensure reproducibility.
Time kill assay
Early log-phase cells were exposed to the following treatments: erythromycin alone and a combination of erythromycin (7.2 μg/ml) with (-)-Terpinen-4-ol (5 μg/ml). An untreated culture was maintained as a growth control. Samples were withdrawn at various time intervals (0, 1, 2, 3, 4, 5, and 24 h), serially diluted, and plated on agar plates. Following incubation at 37 °C for 24 h, Colony Forming Units (CFU)/ml were calculated from plate counts, enabling the assessment of the bactericidal effect of the combination26.
Measurement of intracellular ATP concentration
The intracellular ATP levels were quantified using the ATP Assay Kit (Invitrogen, Life Technologies, United Kingdom). K. pneumoniae cells were exposed independently to treatments with (-)-Terpinen-4-ol at a concentration of 5 µg/ml, erythromycin, and CCCP at 2 µg/ml, the latter serving as a positive control. At designated time intervals (0, 4, and 6 hours), the cells were lysed according to the kit’s protocol. ATP content was subsequently measured using a Synergy HT multi-mode microplate reader, and the results were expressed as relative luminescence units (RLUs).
In vivo infection and toxicity studies
All experiments were conducted in accordance with the relevant national and/or institutional guidelines Institutional Animal Ethics Committee, SASTRA Deemed University (CPCSEA-510/SASTRA/IAEC/RPP) for the ethical treatment and use of animals, adhering to Animal Biosafety Level 2 standards. The experiments reported were performed in accordance with ARRIVE guidelines. Adult zebrafish (Danio rerio), of both male and female genders, measuring 4 to 5 cm in length and weighing approximately 300 mg, were procured from a local aquarium in Thanjavur, India.
Intramuscular infection of zebrafish (N = 3) was carried out using erythromycin-resistant K. pneumoniae, corresponding to an optical density (OD) of 0.4 (~ 1 × 10⁶ CFU/ml), following previously reported procedures with slight modifications. Two hours post-infection, compounds, namely (-)-Terpinen-4-ol or erythromycin alone, and the combination of (-)-Terpinen-4-ol with erythromycin, were administered via intramuscular injection as a single dose. Twenty-four hours post-treatment, the fish were euthanized, decapitated, and muscle tissue was dissected, homogenized, serially diluted, and plated onto LB agar to determine colony counts after 24 h of incubation. Based on the cell counts, a graph was plotted, and the ability of (-)-Terpinen-4-ol alone and in combination with erythromycin to reduce bacterial bioburden in infected muscle tissue was estimated.21.
Histopathological analysis of zebrafish administered with (-)-Terpinen-4-ol was conducted to assess potential tissue alterations. Following treatment, the fish were sacrificed and fixed in 10% formalin. Tissue samples were then processed for embedding, sectioned into thin slices, and stained with haematoxylin and eosin. The stained sections were examined and imaged using a bright-field microscope (Nikon Eclipse Ni-U, Japan).
In vitro cell line infection and toxicity studies
For the present study, T24 cells an uroepithelial cell line derived from transitional bladder carcinoma, and Raw macrophages (NCCS, Pune) were chosen for their demonstrated similarity to primary human bladder epithelial cells. T24 cells were cultivated in McCoy’s 5A medium supplemented with 10% foetal bovine serum, 200 µL T24 cells were seeded in a 24-well plate with a concentration of 105 cells/ml. T24 bladder cells were nurtured at 37 °C for 16 h in an atmosphere of 5% CO2/95% air, maintaining constant humidity levels. Further, the wells were separated into (i) untreated, (ii) commercial (-)-Terpinen-4-ol (Tr), (iii) Lactobacillus-derived (-)-Terpinen-4-ol (Ltr), (iv) erythromycin (Ery), (v) Ery + Ltr, and (vi) Ery + Tr. The bacterial culture was mixed at MOI of 10 and was added to the 24-well plate and kept for 1 h of incubation at 37 °C with 5% CO2 for 24 h. After incubation, cells were washed with sterile PBS to remove the unbound bacteria and were subjected to gentamicin protection assay by treating with ~ 2 mg/ml of gentamicin(a very high concentration was employed as the strains used were resistant to gentamicin) for 1 h. Post gentamicin protection, the media was aspirated, cells were washed thrice with PBS and were incubated with different compounds as mentioned above and post treatment, the cells were maintained for 24 h at 37 °C with 5% CO2. Subsequent detachment of cells and is achieved through trypsinization cells were lysed to recover intracellular bacteria . Bacterial counts (CFU·mL − 1) are determined via the serial dilution and plating on Luria–Bertani agar plates. Each assay was performed in triplicates, with the entire experiment repeated twice for robust validation.
For cytotoxicity assessments in raw macrophage/T24 cells, 3-4,5-dimethylthiazol-2-yl-2,5 diphenyl tetrazolium bromide (MTT) assay is employed. Raw macrophage/T24 cell lines cells were grown till they reached 70–80% confluence in the T-flask, then the spent media was removed, followed by the addition of 1 ml of trypsin–EDTA, and incubated at 37 °C for 2 min. subsequently, 4 ml of DMEM/McCoy’s 5A containing 10% FBS was added to stop the trypsinization. The solution was transferred into a falcon tube and centrifuged at 1000 rpm for 5 min. After centrifugation, the supernatant was discarded and the pellet was resuspended in 1 ml of fresh media, made up to the required volume by adding 9 ml of fresh media. 100 µl of the diluted cells were aliquoted to all the wells in 96 well plates The different groups (treated and untreated) as mentioned above were maintained and incubated in a CO2 incubator for 24 h at 37 °C. Post incubation, the media was replaced with fresh media containing MTT (0.5 mg/mL) which was further incubated for 2 h in CO2 incubator. Following this the MTT was removed and 100 µl DMSO was added. The absorbance of the extracted MTT dye was measured at 595 nm27.
Gene expression study
The test culture, treated with antibiotic, (-)-Terpinen-4-ol, along with the untreated control plate and PABN treated samples underwent static incubation at 37 °C for 24 h. Bacterial RNA extraction from the treated and untreated culture cells was performed using the RNeasy mini kit (Qiagen Technologies, Hilden, Germany), following the manufacturer’s instructions. Subsequently, same quantity of high-quality total RNA from every sample was employed to synthesize cDNA using the High-capacity cDNA reverse transcription kit (Bio-Rad), adhering to the manufacturer’s protocols. The RNA and cDNA were spectrophotometrically quantified using Thermo Scientific’s Nano Drop-One. Quantitative real-time polymerase chain reactions (qRT-PCRs) were performed with iQTM SYBR® Green Supermix (Bio-Rad) on a QuantStudio™ 5 System (Applied Biosystems, by Thermo Fisher Scientific), targeting various gene sequences. Post-normalization with the housekeeping 16S rRNA gene as an internal control, the gene expression levels were assessed and presented as relative fold-differences. The details of the primers are available in Supplementary table (Table. S4).
Statistical analysis
All experiments were performed in triplicate, and statistical significance was determined using one-way ANOVA and Student’s t-test, where appropriate. Data are presented as mean ± standard deviation (SD), and a p-value of < 0.05 was considered statistically significant. GraphPad Prism (version 8.0) was used for all analyses.
Results
Screening and characterization of Lactobacillus metabolites in CFS to inhibit drug efflux in Klebsiella pneumoniae
The initial screening of the cell-free supernatant (CFS) from seven Lactobacillus strains (Lactobacillus jensenii, Limosilactobacillus fermentum (n = 2), Lactobacillus crispatus, Lactobacillus amylovorus, Lactobacillus fornicalis, , Ligilactobacillus salivarius), isolated from the vaginal microbiota of 54 healthy Indian women, demonstrated the potential of CFS from L. jensenii to reduce the ethidium bromide minimum inhibitory concentration (MIC) in K. pneumoniae MTCC 432 strain by 2–4fold. Additionally, we performed a drug resistance profile of clinical strains of K. pneumoniae (KP BC936, KP BC1415, KP 1876, KP 44350, KP 44357, KP 15410, KP 27736) and E. coli (U1007, UTI89, CFT073, F11). Erythromycin was identified as the antibiotic to which these strains exhibited resistance, prompting its use for the MIC reversal assays (Table. S1).
The CFS of L. jensenii significantly reduced the MIC of erythromycin by 32-fold. Bioactivity-guided fractionation of the CFS from L. jensenii resulted in separation of seven distinct compounds (C1-C7). To assess the potential of these compounds to inhibit efflux, a MIC reversal assay was performed using erythromycin against the K. pneumoniae MTCC 432 strain (Fig. S1A). The assay revealed a substantial 32-fold MIC reversal of erythromycin from 64 to 2 μg for C7, indicating potent efflux inhibitory activity. Notably, Compound 7 (C7) exhibited significant fluorescent properties under ultraviolet (UV) light (Fig. S1B). Gas Chromatography-Mass Spectrometry (GC–MS) analysis identified a prominent peak with an area of 1,529,209 units, a retention time of 12.261 min, and a 94% similarity match to Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)-, commonly known as (-)-Terpinen-4-ol, an isomer of terpineol in the NIST database (Fig. S2, Table, S2).
(-)-Terpinen-4-ol from the cell-free supernatant acts synergistically with erythromycin
(-)-Terpinen-4-ol did not exhibit any intrinsic antibacterial activity at a concentration of 5 μg/ml against clinical strains of K. pneumoniae and E. coli. At 5 μg/ml, (-)-Terpinen-4-ol caused 32-to-128-fold reduction in erythromycin MIC in diverse clinical isolates of K. pneumoniae and E. coli, indicating potent efflux inhibitory effect. In addition, checkerboard assay showed that (-)-Terpinen-4-ol exhibited synergy with erythromycin with a Fractional Inhibitory Concentration Index (FICI ≤ 0.5) (Table 1). Importantly, (-)-Terpinen-4-ol caused a 4–32-fold MIC reversal for a range of antimicrobials against diverse clinically relevant pathogens viz., Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, and MRSA (Table. S3). These observations highlight the strong efflux inhibitory potential of (-)-Terpinen-4-ol and advocate its development/use as a broad-range efflux pump inhibitor in diverse clinically relevant pathogens.
(-)-Terpinen-4-ol inhibits EtBr efflux in K. pneumoniae and E. coli UTI89
To verify the ability of (-)-Terpinen-4-ol to inhibit efflux pumps, both commercially procured (-)-Terpinen-4-ol ( procured from TCI chemicals (Tokyo Chemical Industry (India) Pvt Ltd., Chennai) and Lactobacillus-derived (-)-Terpinen-4-ol (5 μg/ mL) were used in a real-time efflux (RTE) assay using K. pneumoniae MTCC 432, K. pneumoniae BC1415 (clinical strain), and E. coli UTI89. De-energized cells were treated with both (-)-Terpinen-4-ol for 1 h and then re-energized with glucose. Subsequently, ethidium bromide (EtBr) fluorescence was measured over 20 min. The results demonstrated that (-)-Terpinen-4-ol significantly enhanced the inhibition of EtBr efflux relative to the positive controls PABN and CCCP in all the three strains tested viz., K. pneumoniae MTCC 432 (Fig. 1a), K. pneumoniae BC1415 (Fig. 1b), and E. coli UTI89 (Fig. 1c), Notably, dye accumulation in the presence of both (-)-Terpinen-4-ol was higher than the accumulation induced by either PABN, (a known inhibitor of AcrAB and MexAB efflux pumps) and CCCP, a standard protonophore that inhibits multiple efflux pumps by disrupting the proton motive force.
(-)-Terpinen-4-ol inhibits EtBr efflux in MDR K. pneumoniae and E. coli. Deenergized cells of (a) K. pneumoniae MTCC 432, (b) K. pneumoniae BC1415 and (c) E. coli UTI89 were treated with Ltr- Lactobacillus derived (-)-Terpinen-4-ol and Tr- Commercially procured (-)-Terpinen-4-ol for 1 h. Phenyl arginine beta naphthylamide (PABN) and Carbonyl cyanide m-chlorophenyl hydrazine (CCCP) were maintained as positive controls. The results presented correspond to the mean of three independent readings ± SD (n = 3).
To evaluate the mechanism of efflux inhibition mediated by (-)-Terpinen-4-ol and whether it interacts directly with efflux pumps or functions by disrupting the proton motive force (PMF), membrane potential studies were performed using DiSC₃, both prior to and following re-energization with glucose. The results (Fig. 2) indicated that both extracted and procured (-)-Terpinen-4-ol exhibited a fluorescence trend like that of CCCP. Specifically, after the stabilization of DiSC₃ fluorescence, glucose addition led to a slight decline. However, the subsequent addition of (-)-Terpinen-4-ol caused a pronounced decrease in DiSC3 fluorescence, suggesting that the PMF reestablished by glucose was disrupted by (-)-Terpinen-4-ol, leading to dye re-partitioning into the lipid bilayer, The trend observed was similar to the standard protonophore CCCP that disrupts PMF. These findings imply that (-)-Terpinen-4-ol effectively reduced the MIC of erythromycin/other antimicrobials, possibly by indirectly depleting the energy (proton motive force) required for the efflux pumps to extrude erythromycin in K. pneumoniae BC1415. The dissipation of proton motive force might explain the broad-spectrum efflux inhibitory effect wielded by (-)-Terpinen-4-ol against diverse clinically relevant pathogens for different antimicrobials.
(-)-Terpinen-4-ol perturbs membrane potential similar to CCCP. DiSC3 was added to mid log cells of K. pneumoniae BC1415 fluorescence (Ex 622 nm and Em 670 nm) was recorded until plateau. 0.5% glucose was added, and fluorescence was again monitored. (-)-Terpinen-4-ol and CCCP were added, which led to sudden decline in the fluorescence intensity. Ltr- Lactobacillus derived (-)-Terpinen-4-ol and Tr- Commercially procured (-)-Terpinen-4-ol. The experiment was performed in triplicates and the error bar represents standard error of the mean (n = 3).
(-)-Terpinen-4-ol enhances the outer membrane permeability in E. coli and K. pneumoniae
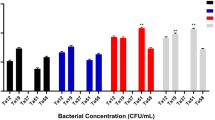
The outer membrane acts as a permeability barrier in Gram-negative bacteria, and synergy with antibiotics can be attributed not only to efflux inhibition but also to outer membrane permeabilization 28. To assess the impact of (-)-Terpinen-4-ol on outer membrane permeability, we measured N-phenyl-1-naphthylamine (NPN) fluorescence in K. pneumoniae MTCC 432/BC1415 and E. coli UTI89. An increase in NPN fluorescence indicates outer membrane permeabilization due to the hydrophobic periplasmic environment. In K. pneumoniae MTCC432 /BC1415, erythromycin treatment caused a slight increase in membrane permeability compared to the untreated control. However, (-)-Terpinen-4-ol alone induced ~ 1.2-fold increase in membrane permeability. Notably, when both commercial and Lactobacillus-derived (-)-Terpinen-4-ol were combined with erythromycin, a significant fourfold increase in membrane permeability was observed. For E. coli UTI89, erythromycin treatment led to ~ 2.5-fold increase in membrane permeability, while (-)-Terpinen-4-ol alone resulted in a ~ 1.5-fold increase. The combination of erythromycin with (-)-Terpinen-4-ol caused a remarkable 6 -fold increase in membrane permeability (Fig. 3). These results demonstrate that (-)-Terpinen-4-ol synergistically enhances the membrane permeabilizing effect of erythromycin in both K. pneumoniae MTCC 432/BC1415 and E. coli UTI89 strains. By increasing membrane permeability, (-)-Terpinen-4-ol facilitates greater access of erythromycin to its target, potentiating its bactericidal activity.
Membrane permeability of MDRK. pneumoniae and E. coli UTI89 is enhanced by (-)-Terpinen-4-ol treatment. Mid-log K. pneumoniae MTCC 432/BC1415 and E. coli UTI89 were treated with either erythromycin (Ery) or (-)-Terpinen-4-ol (Ltr- Lactobacillus derived and Tr- Commercially procured) or in combination (Ltr/Tr + Ery) along with fluorescent dye N-Phenyl 1-Naphthylamine (NPN) and fluorescence intensity was measured using a spectrofluorometer. NPN uptake factor was calculated as the ratio of background subtracted fluorescence of different groups to that of buffer. CTAB (cetyltrimethylammonium bromide) is used as a positive control (Unpaired t-test, n = 3, 95% CI, p*** < 0.0001).
(-)-Terpinen-4-ol synergistically potentiates bactericidal effect of erythromycin in time-dependent killing assays
The bactericidal effect of erythromycin, in combination with commercial (-)-Terpinen-4-ol (Tr) and Lactobacillus-derived (-)-Terpinen-4-ol (Ltr), was evaluated using a time-kill assay against K. pneumoniae MTCC 432/BC1415 and E. coli UTI89. Early-log phase cultures of these strains were subjected to various treatments: (i) untreated, (ii) Ltr, (iii) Tr, (iv) erythromycin (Ery), (v) Ery + Ltr, and (vi) Ery + Tr. The results (Fig. 4) demonstrated that treatments with erythromycin alone, as well as with either Tr or Ltr alone, did not result in a significant reduction in cell counts after 24 h. In contrast, combinations of erythromycin with either Ltr or Tr led to a substantial ~ 10 log fold reduction in cell counts relative to the initial founder population. Usually, a decline of 3 log fold implies synergy between the compound and antibiotic combinations of erythromycin with either Ltr or Tr. As the combination caused > 9 log reduction, these observations imply a strong synergistic effect between (-)-Terpinen-4-ol and erythromycin, consistent with the results observed in the checkerboard assay.
Bactericidal effects of erythromycin (Ery) in combination with commercial (-)-Terpinen-4-ol (Tr) and Lactobacillus-derived (-)-Terpinen-4-ol (Ltr) against MDR K. pneumoniae (Kp) and E. coli. (a) Effect on K. pneumoniae MTCC 432 (b) Effect on K. pneumoniae BC1415 (c) Effect on E. coli UTI89, with untreated and erythromycin use as controls. The error bars represent the standard deviation among the three replicates (n = 3).
(-)-Terpinen-4-ol was nontoxic and drastically reduced bioburden in combination with erythromycin in zebrafish infection model
Zebrafish were independently infected with approximately 1 × 10⁶ CFU/ml (corresponding to an OD of 0.4) of the K. pneumoniae BC1415 strain. Two hours post-infection, the fish were subjected to various treatments: (i) untreated, (ii) commercial (-)-Terpinen-4-ol (Tr), (iii) Lactobacillus-derived (-)-Terpinen-4-ol (Ltr), (iv) erythromycin (Ery), (v) Ery + Ltr, and (vi) Ery + Tr. Twenty-four hours post-treatment, the fish were euthanized, muscle tissue was dissected, homogenized, serially diluted, and plated onto LB agar plates. After 24–48 h of incubation, colony counts were determined. The untreated (infected) group exhibited ~ 10 log CFU/ml. No significant decline in cell counts was noted for the groups treated with erythromycin (Ery), Ltr, or Tr alone, as erythromycin was used at its MIC (7.5 µg/ml) and (-)-Terpinen-4-ol was used at its MIC (5 µg/ml). However, the combination treatments (Ery + Ltr and Ery + Tr) resulted in a highly significant 8–9 log reduction in bioburden (p* < 0.0001) relative to the untreated control, erythromycin alone, Ltr alone, or Tr alone, as determined by the student’s t-test (Fig. 5A). Histopathological examination of liver and muscle tissue sections from fish injected with (-)-Terpinen-4-ol revealed no observable signs of inflammation, neutrophil accumulation, or tissue damage. Untreated muscle tissue displays normal zebrafish muscle fibers with clear myosepta and no signs of inflammation. In contrast, erythromycin-treated muscle shows some muscle fiber disarray. Muscle tissue from zebrafish treated with Ltr + Erythromycin and Tr + Erythromycin displays improved muscle fiber organization compared to erythromycin-treated samples, with Tr + Erythromycin appearing closer to normal. Tr-treated muscle appears normal (Fig. 5B Top panel). As for the liver tissues, untreated and Tr-treated samples show normal zebrafish liver architecture, with hepatocytes arranged in a typical cord-like pattern. Erythromycin-treated liver samples exhibit hepatocyte disorganization, potentially indicating inflammation. Ltr + Erythromycin and Tr + Erythromycin liver tissues show reduced hepatocyte disorganization compared to the erythromycin-treated sample, suggesting lack of deleterious effect in both treatments (Fig. 5B bottom panel). These findings suggest that (-)-Terpinen-4-ol does not induce adverse histopathological changes in the liver or muscle tissues of the fish, supporting its potential safety for further applications.
(-)-Terpinen-4-ol synergistically potentiates bactericidal effect of erythromycin in infected zebrafish. (A) Different treatment combinations (Erythromycin (Ery), (-)-Terpinen-4-ol (Ltr- Lactobacillus derived and Tr- Commercially procured) were administered 2–3 h post-infection. The colony counts were scored after 24 h and represented as log CFU/ml. Experiment was performed in triplicates and statistical analyses were performed using Student’s t-test. (p< ***0.0001) (B) Histopathology analysis of Muscle and Liver tissues at 40X maginification (N = 3).
(-)-Terpinen-4-ol in combination with erythromycin reduces intracellular bioburden of K. pneumoniae
The cytotoxicity of (-)-Terpinen-4-ol was assessed on T24 bladder cells and Raw macrophages, revealing that (-)-Terpinen-4-ol is non-toxic at the concentrations evaluated.. In addition, its efflux inhibitory efficacy was evaluated during intracellular infection of T24 bladder cells with K. pneumoniae BC1415. Treatment with (-)-Terpinen-4-ol, along with erythromycin resulted in a significant reduction in the bacterial bioburden, declining from an initial 9 log CFU/ml to 3 log CFU. Furthermore, even at a higher concentration of 10 µg/ml, (-)-Terpinen-4-ol continued to exhibit a similar trend of bioburden reduction (Fig. 6), while still being non-toxic to both T24 bladder cells and Raw macrophages (Fig. S3). These findings underscore the potential of (-)-Terpinen-4-ol as an effective efflux inhibitory agent against K. pneumoniae, capable of significantly reducing bacterial load in combination with erythromycin.
Terpinen-4-ol in combination with erythromycin significantly reduces bacterial bioburden in human urinary bladder cancer (T24 ) cells. upon incubation with (-)-Terpinen-4-ol (Commercial (Tr) and Lactobacillus extracted (Ltr)) and in combination with erythromycin (E) (n = 3) Unpaired t-test, n = 3, 95% CI, p*** < 0.0001.
(-)-Terpinen-4-ol reduces intracellular ATP levels
To understand the impact of (-)-Terpinen-4-ol on intracellular ATP levels, K. pneumoniae BC1415 was exposed to (-)-Terpinen-4-ol at a concentration of 5 µg/ml, and ATP levels were monitored over a period of 6 h. After 4 h of exposure, ATP levels increased in the untreated control and erythromycin-treated groups. In contrast, the ATP levels in the (-)-Terpinen-4-ol alone treatment group showed only a minimal increase. Furthermore, a significant impairment in ATP production was observed in K. pneumoniae cells treated with the combination of (-)-Terpinen-4-ol and erythromycin (Fig. 7) . This impairment was comparable to that observed for the protonophore CCCP, at 2 μg/mL, which is known to disrupt ATP production.
(-)-Terpinen-4-ol in combination with erythromycin reduces ATP levels in MDR K. pneumoniae. K. pneumoniae BC1415 was exposed to (-)-Terpinen-4-ol during 6 h. The ATP levels were quantified using a luciferin-luciferase bioluminescence detection assay. CCCP (2 µg/mL) was included for comparison. The results presented correspond to the mean of three independent readings ± SD. Results were considered highly significant where ***P < 0.001.
We extended our investigation to assess the impact of (-)-Terpinen-4-ol on the expression of key RND efflux pump genes in K. pneumoniae. Specifically, we analysed the expression of oqxA, kexC, kexE and AcrA genes, , which contributes to multidrug resistance by expelling a range of antibiotics, including fluoroquinolones and beta-lactams. The expression of the oqxA gene is strongly associated with resistance phenotypes in clinical isolates of K. pneumoniae. Additionally, KexCand KexE are part of the RND efflux pump family and are involved in reducing intracellular concentrations of antimicrobial agents, thereby contributing to resistance. AcrA is a component of the AcrAB-TolC efflux pump system in K. pneumoniae, which plays a major role in multidrug resistance The treatments included erythromycin, commercial (-)-Terpinen-4-ol, purified Terpinen-4-ol and the positive control PAβN. Our results indicated that erythromycin significantly upregulated the expression of kexC,which was remarkably downregulated in both commercially procured (-)-Terpinen-4-ol and purified (-)-Terpinen-4-ol, which implies that the erythromycin efflux could be mediated by RND pump KexC. Importantly even PaβN treatment resulted in threefold down regulation of KexC relative to erythromycin treated cells, The notable alteration in the expression of KexE and AcrA in commercial and purified Terpinen-4-ol could be accounted by the other spent media components in partially pure Terpinen-4-ol (Fig. 8). These findings suggest that (-)-Terpinen-4-ol inhibits KexC, the component of the RND efflux pump system, thereby enhancing the efficacy of erythromycin against K. pneumoniae. The observed reduction in gene expression, corroborated by the positive control, validates the effectiveness of the treatments.
(-)-Terpinen-4-ol’s inhibition of RND efflux pumps in K. pneumoniae. Representative gene expression analysis of MDR K. pneumoniae BC1415 cells subjected to 1 h treatment with the following viz., erythromycin, commercial Terp-4-ol, Purified Terpinen − 4-ol and the positive control PAβN. RNA was extracted post treatment and equimolar concentration of RNA was converted to cDNA, and qRTPCR was performed. After normalisation with the housekeeping gene rpoB the gene expression levels were evaluated using ∆∆ct method and expressed as a relative fold-difference in Gene expression Commercial and Lactobacillus extracted (-)-Terpinen-4-ol is mentioned as Terp-4-ol and fractionated Terinen-4-ol as purified Terp respectively.
Our collective observations imply that (-)-Terpinen-4-ol has multifaceted effects, all of which contribute to potent inhibition of efflux transporters in MDR pathogens, thereby restoring antimicrobial sensitivity. (-)-Terpinen-4-ol dissipates membrane potential, which curbs the energy needed for the efflux transport proteins, reduced PMF also results in decreased ATP production. In addition, (-)-Terpinen-4-ol also downregulates genes encoding RND efflux transporters. A combination of all these effects, along with the enhanced permeabilization of antimicrobials contributes to a broad spectrum and potent efflux inhibition observed with Terpinen-4-ol on diverse pathogens (Tabe S3). Although the antibiofilm effects of (-)-Terpinen-4-ol derived from other sources are well documented, we are reporting for the first time that, (-)-Terpinen-4-ol derived from CFS of human vaginal derived lactobacilli strains exerts potent efflux inhibitory effect in both in vitro cultured T24 cells and in vivo in zebrafish infection model leading to 6–9 log reduction in bacterial bioburden in combination with erythromycin. Thus our observations underscore the need to evaluate nontoxic Terpinen-4-ol as a strong efflux inhibitory agent against MDR pathogens in higher animal models.
Discussion
Efflux pumps significantly contribute to multidrug resistance by enabling bacteria to evade the effects of numerous antibiotic classes and other substrates7,29. Understanding the diverse mechanisms and substrate ranges of efflux pumps is essential for developing effective strategies to combat bacterial (Multi Drug Resistance) MDR and enhance the efficacy of antimicrobial therapies30.
As microbial derived efflux pump inhibitors (EPIs) are quite rare, our study focused on screening cell-free supernatants (CFS) of human vaginal probiotics, particularly Lactobacillus species. We identified that the CFS of Lactobacillus jensenii possesses efflux inhibitory properties. To our knowledge, this is the first study to investigate the efflux inhibitory potential of CFS from human vaginal derived probiotic isolates. Through the bioassay guided fractionation and Gas Chromatography–Mass Spectrometry (GC–MS) based characterization indicated that the bioactive metabolite is (-)-Terpinen-4-ol, a monoterpene. Studies have highlighted the therapeutic potential of terpenes in combating antibiotic resistance, particularly their role as antibiofilm agents/inhibitors10. Furthermore, research has demonstrated that monoterpenes can impact the structure and function of bacterial membranes by interacting with membrane components such as polysaccharides, fatty acids, phospholipids, and proteins. This interaction facilitates the intracellular action of antibiotics, enhancing their efficacy against resistant bacterial strains31.
In this study, Lactobacillus derived (-)-Terpinen-4-ol from the cell free supernatant, despite showing absence of clinically relevant intrinsic antibacterial effects at the tested concentration, has demonstrated synergistic effects (Table 1) when used in conjunction with conventional antibiotics, thereby enhancing antibiotic activity. Real time efflux assay demonstrated that (-)-Terpinen-4-ol significantly enhanced the inhibition of EtBr efflux compared to the positive controls, PAßN and CCCP (Fig. 1). Our findings indicate that as an adjuvant, (-)-Terpinen-4-ol effectively reduces the MIC of erythromycin by 64–128-fold (Table 1), which is a substantial reversal. According to earlier reports32, a decrease in MICs by at least fourfold relative to their original values in the presence of an efflux pump inhibitor (EPI) is considered a strong indication of efflux inhibition. These findings indicate that (-)-Terpinen-4-ol not only acts as a potent efflux pump inhibitor but also synergizes with conventional antibiotics against efflux pump-overexpressing bacterial strains, thereby improving their efficacy.
Bacterial efflux pumps (EPs) are recognized as either primary active transporters that utilize ATP as an energy source, or secondary active transporters driven by the electrochemical potential difference generated by pumping out Na + and H + ions across the membrane33. Our observations indicate that (-)-Terpinen-4-ol effectively reduces the minimum inhibitory concentration of erythromycin, which can be attributed to its ability to disrupt the proton motive force (PMF). This disruption impedes the membrane potential, thereby indirectly depleting the energy necessary for the efflux pumps to expel erythromycin (Fig. 2). Furthermore, our studies demonstrated a significant impairment in ATP production in Klebsiella pneumoniae cells treated with the combination of (-)-Terpinen-4-ol and erythromycin (Fig. 7). This suggests that the combined treatment not only inhibits the function of secondary active transporters but also depletes the energy reserves required for primary active transporters, leading to an overall reduction in efflux activity and enhanced antibiotic susceptibility. Enhancing outer membrane permeability and inhibiting efflux pumps are promising approaches to restore the efficacy of existing antibiotics against resistant strains34,35. (-)-Terpinen-4-ol enhances outer membrane permeability, facilitating greater intracellular concentrations of antibiotics and thereby improving their efficacy (Fig. 3). (-)-Terpinen-4-ol has been shown to disrupt bacterial membrane integrity through several mechanisms. The compound’s lipophilic nature allows it to penetrate through cytoplasmic membranes and cell walls, leading to membrane disruption36 some compounds disrupt the outer membrane, such as polymyxin B nano peptide (PMBN), can enhance the uptake of antibiotics and synergize with them to overcome resistance37.Furthermore, (-)-Terpinen-4-ol exhibited a synergistic effect in time-dependent killing assays, where the combination of (-)-Terpinen-4-ol and erythromycin resulted in significantly enhanced bacterial killing compared to either agent alone (Fig. 4). This synergism likely arises from (-)-Terpinen-4-ol’s dual action: disrupting the PMF and enhancing outer membrane permeability, both of which compromise bacterial defense and potentiate the activity of erythromycin.
The search for efflux pump inhibitors (EPIs) has identified a variety of natural products with the potential to be used in antibacterial drug development. However, the clinical development of many of these compounds is limited mainly due to their significant toxicity38. In contrast, (-)-Terpinen-4-ol, derived from human vaginal Lactobacillus, shows promise as a relatively safe EPI. At the concentration tested it was non-toxic to human bladder T24 cells and macrophages (Fig. S3) In addition, toxicity studies in a zebrafish model showed no apparent hepatotoxicity or myotoxicity (Fig. 5B).
In studies involving Pseudomonas aeruginosa, terpinen-4-ol was found to inhibit quorum sensing (QS) by interacting with QS receptors and downregulating critical QS genes 39. Additionally, (-)-Terpinen-4-ol exhibited significant effects against Bacillus cereus by preventing biofilm formation and spore germination, reducing the production of the extracellular matrix, particularly exopolysaccharides, inhibiting swarming motility, and reducing protease activity 40. In our study, we showed that (-)-Terpinen-4-ol at a concentration of 5 μg/ml significantly reduced (p < 0.05) the formation of mature biofilms of clinically isolated K. pneumonaie and E. coli strains. in combination with erythromycin (Fig. S4). This finding underscores the potential of (-)-terpinen-4-ol as a potent postbiotic agent that can enhance the efficacy of existing antibiotics and prevent the formation of resilient biofilms.
RND (Resistance-Nodulation-Division) transport proteins are a prominent and versatile efflux pump class found in Gram-positive and Gram-negative bacteria. The tripartite structure—comprising an inner membrane component (IMC), a periplasmic adaptor protein (PAP), and an outer membrane channel (OMC)—is unique to Gram-negative bacteria. RND pumps export a wide range of substrates, including antibiotics, antiseptics, dyes, and detergents.Our results demonstrate that (-)-Terpinen-4-ol induces downregulation of gene expression associated with these RND efflux pumps. This downregulation suggests that (-)-Terpinen-4-ol may effectively inhibit the activity of RND efflux systems, thereby potentially reducing antibiotic resistance (Fig. 8C).
Lactobacillus species are not known to synthesize terpinen-4-ol, prompting us to explore several hypotheses regarding its potential origin. One possibility involves a non-canonical biosynthetic pathway, where horizontal gene transfer (HGT) from environmental microbes may enable Lactobacillus to acquire terpene synthase (TPS) genes, thereby facilitating the production of terpenes. Additionally, it is plausible that Lactobacillus could enzymatically modify dietary precursors or produce terpinen-4-ol as a stress-induced metabolic byproduct in response to oxidative stress. Under such conditions, reactive oxygen species (ROS) may non-enzymatically rearrange media components into terpenoids, a phenomenon that has been documented in other bacterial species41,42 To validate these hypotheses, we are planning further investigations, including GC–MS analysis of growth media and genomic screening for TPS genes in our upcoming studies. Future studies employing metagenomic and transcriptomic analyses are needed to confirm the biosynthetic pathway. The precise mechanism by which Lactobacillus species produce (-)-Terpinen-4-ol remains unclear and we acknowledge this as a limitation of our study and a deeper understanding of the biosynthesis of (-)-Terpinen-4-ol within Lactobacillus spp. would strengthen the evidence for its probiotic-derived origin and enhance its potential application as a therapeutic efflux pump inhibitor. The vaginal microbiome consists of a complex microbial community. Investigating potential interactions between Lactobacillus strains and other microbes may reveal cooperative pathways involved in terpene production.
The precise mechanism by which (-)-Terpinen-4-ol inhibits efflux pumps in Klebsiella pneumoniae remains to be fully elucidated. While our study demonstrates a clear reduction in erythromycin MIC in the presence of (-)-Terpinen-4-ol, the molecular details of this interaction require further investigation. Some studies suggest that (-)-Terpinen-4-ol may disrupt bacterial membrane integrity36,43, potentially affecting efflux pump function. Alternatively, it is possible that (-)-Terpinen-4-ol directly interacts with efflux pump proteins, inhibiting their activity. Further research, such as molecular docking studies and site-directed mutagenesis, is needed to determine the specific binding site and mechanism of action of (-)-Terpinen-4-ol as an efflux pump inhibitor.
This study pioneers a novel strategy to combat antibiotic resistance by identifying (-)-Terpinen-4-ol—a terpene derived from Lactobacillus species (human vaginal probiotics)—as a potent efflux pump inhibitor (EPI) that synergizes with antibiotics like erythromycin to enhance their efficacy against resistant bacteria. Unlike prior research on synthetic or plant-based EPIs, this work highlights Lactobacillus spp. as a natural, safer source of EPIs, with (-)-Terpinen-4-ol specifically disrupting bacterial efflux pumps (e.g., MexAB-OprM in Pseudomonas) and modulating biofilm formation through altered hydrophobicity and quorum sensing. The findings diverge from earlier terpene studies44,45, which focused on plant-derived compounds or different mechanisms, positioning probiotic-derived metabolites as a promising, low-toxicity alternative for overcoming multidrug resistance and advancing microbiome-inspired antimicrobial therapies.
Overall, the demonstrated efficacy of Lactobacillus derived (-)-Terpinen-4-ol in enhancing the activity of erythromycin and its synergistic effects with conventional antibiotics underscore its potential as a valuable tool in overcoming antibiotic resistance. It represents a significant advancement in the search for effective efflux pump inhibitors and could play a crucial role in restoring the efficacy of existing antibiotics, ultimately contributing to the fight against multidrug-resistant bacterial infections.
Conclusion
In this study, we have identified and characterized (-)-Terpinen-4-ol, a monoterpene derived from the cell-free supernatant of Lactobacillus jensenii, as a promising efflux pump inhibitor. Our findings highlight that (-)-Terpinen-4-ol significantly enhances the efficacy of conventional antibiotics, such as erythromycin, by inhibiting bacterial efflux pumps, specifically the RND family of efflux systems, which are crucial in multidrug resistance. The potent efflux inhibition observed with (-)-Terpinen-4-ol is attributed to its ability to disrupt the proton motive force and enhance outer membrane permeability. This dual mechanism increases the intracellular concentration of antibiotics and impedes the energy-dependent efflux processes. The observed synergistic effects in time-dependent killing assays further validate the potential of (-)-Terpinen-4-ol to combat antibiotic resistance effectively. Moreover, (-)-Terpinen-4-ol exhibits a favourable safety profile, showing negligible toxicity in human cell lines or in a zebrafish model at the concentration tested, distinguishing it from other efflux pump inhibitors that often face limitations due to toxicity concerns. Our study underscores the value of exploring microbial-derived compounds for the development of new and effective efflux pump inhibitors. (-)-Terpinen-4-ol represents a novel and safe candidate that could contribute to restoring the efficacy of existing antibiotics and addressing the urgent challenge of multidrug-resistant bacterial infections.
Data availability
The 16srRNA sequence datasets generated and/or analysed during the current study are available in the NCBI GenBank repository, The organism’s name and the accession number are as follows: Lactobacillus jensenii strain NANDAN (Accession No: OP648111), Limosilactobacillus fermentum strain NANDAN (Accession No: OP648129). All relevant data has been comprehensively presented in the main text and supplementary materials. Raw data is available upon request at sai@scbt.sastra.edu.
References
Reza Mortazavi-Tabatabaei, S. et al. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int. J. Prev. Med. 10, 169 (2019).
Lin, W.-P., Huang, Y.-S., Wang, J.-T., Chen, Y.-C. & Chang, S.-C. Prevalence of and risk factor for community-onset third-generation cephalosporin-resistant Escherichia coli bacteremia at a medical center in Taiwan. BMC Infect. Dis. 19, 245 (2019).
Band, V. I. et al. Carbapenem-resistant Klebsiella pneumoniae exhibiting clinically undetected Colistin Heteroresistance leads to treatment failure in a murine model of infection. MBio 9, 10–1128 (2018).
Embracing a One Health Framework to Fight Antimicrobial Resistance. (OECD, 2023). https://doi.org/10.1787/ce44c755-en.
Webber, M. A. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 51, 9–11 (2003).
Sharma, A., Gupta, V. & Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 149, 129 (2019).
Soto, S. M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4, 223–229 (2013).
Zhang, L. et al. Bacterial efflux pump inhibitors reduce antibiotic resistance. Pharmaceutics 16, 170 (2024).
Ferrer-Espada, R. et al. A permeability-increasing drug synergizes with bacterial efflux pump inhibitors and restores susceptibility to antibiotics in multi-drug resistant Pseudomonas aeruginosa strains. Sci. Rep. 9, 3452 (2019).
Stavri, M., Piddock, L. J. V. & Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 59, 1247–1260 (2007).
Wright, G. D. Something old, something new: revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 60, 147–154 (2014).
Whalen, K. E., Poulson-Ellestad, K. L., Deering, R. W., Rowley, D. C. & Mincer, T. J. Enhancement of antibiotic activity against multidrug-resistant bacteria by the efflux pump inhibitor 3,4-dibromopyrrole-2,5-dione Isolated from a Pseudoalteromonas sp. J. Nat. Prod. 78, 402–412 (2015).
Lee, M. D. et al. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Il Farmaco 56, 81–85 (2001).
Teasdale, M. E., Liu, J., Wallace, J., Akhlaghi, F. & Rowley, D. C. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl. Environ. Microbiol. 75, 567–572 (2009).
Kunz Coyne, A. J., El Ghali, A., Holger, D., Rebold, N. & Rybak, M. J. Therapeutic strategies for emerging multidrug-resistant Pseudomonas aeruginosa. Infect. Dis. Ther. 11, 661–682 (2022).
Tegos, P. et al. Microbial efflux pump inhibition: Tactics and strategies. Curr. Pharm. Des. 17, 1291–1302 (2011).
Kourtesi, C. et al. Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol. J. 7, 34–52 (2013).
Watson, R. A. Enlisting probiotics to combat recurrent urinary tract infections in women—a military strategy for meeting the challenge. Antibiotics 12, 167 (2023).
Nair, V. G. et al. Biogenic amine tryptamine in human vaginal probiotic isolates mediates matrix inhibition and thwarts uropathogenic E. coli biofilm. Sci. Rep. 14, 15387 (2024).
Liu, H. et al. Antifungal activity of cell-free supernatants from Lactobacillus pentosus 86 against Alternaria gaisen. Horticulturae 9, 911 (2023).
Sundaramoorthy, N. S., Suresh, P., Selva Ganesan, S., GaneshPrasad, A. & Nagarajan, S. Restoring colistin sensitivity in colistin-resistant E. coli: Combinatorial use of MarR inhibitor with efflux pump inhibitor. Sci. Rep. 9, 19845 (2019).
Christena, L. R. et al. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 5, 12899–12909 (2015).
Sundaramoorthy, N. S. et al. Ferulic acid derivative inhibits NorA efflux and in combination with ciprofloxacin curtails growth of MRSA in vitro and in vivo. Microb. Pathog. 124, 54–62 (2018).
Helander, I. M. & Mattila-Sandholm, T. Fluorometric assessment of Gram-negative bacterial permeabilization. J. Appl. Microbiol. 88, 213–219 (2000).
Ogunniyi, A. D. et al. Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent. PLoS ONE 12, e0183457 (2017).
Grillon, A., Schramm, F., Kleinberg, M. & Jehl, F. Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies. PLoS ONE 11, e0156690 (2016).
Peng, C.-C., Chen, K.-C., Peng, R. Y., Su, C.-H. & Hsieh-Li, H. M. Human urinary bladder cancer T24 cells are susceptible to the Antrodia camphorata extracts. Cancer Lett. 243, 109–119 (2006).
Opperman, T. J. & Nguyen, S. T. Recent advances toward a molecular mechanism of efflux pump inhibition. Front. Microbiol. 6, 421 (2015).
Askoura, M., Mattawa, W., Abujamel, T. & Taher, I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J. Med. 6, 5870 (2011).
Piddock, L. J. V. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19, 382–402 (2006).
Sikkema, J., de Bont, J. A. & Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269, 8022–8028 (1994).
Couto, I., Costa, S. S., Viveiros, M., Martins, M. & Amaral, L. Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J. Antimicrob. Chemother. 62, 504–513 (2008).
Dwivedi, G. R. et al. Antibiotics potentiating potential of catharanthine against superbug Pseudomonas aeruginosa. J. Biomol. Struct. Dyn. 36, 4270–4284 (2018).
Ghai, I. & Ghai, S. Understanding antibiotic resistance via outer membrane permeability. Infect. Drug Resist. 11, 523–530 (2018).
Delcour, A. H. Outer membrane permeability and antibiotic resistance. Biochim. et Biophysica Acta (BBA) - Proteins Proteomics 1794, 808–816 (2009).
Bordini, E. A. F. et al. Antimicrobial effects of terpinen-4-ol against oral pathogens and its capacity for the modulation of gene expression. Biofouling 34, 815–825 (2018).
Muheim, C. et al. Increasing the permeability of Escherichia coli using MAC13243. Sci. Rep. 7, 17629 (2017).
Prasch, S. & Bucar, F. Plant derived inhibitors of bacterial efflux pumps: an update. Phytochem. Rev. 14, 961–974 (2015).
Bose, S. K., Chauhan, M., Dhingra, N., Chhibber, S. & Harjai, K. terpinen-4-ol attenuates quorum sensing regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Future Microbiol. 15, 127–142 (2020).
Zhao, L. et al. (+)-Terpinen-4-ol inhibits bacillus cereus biofilm formation by upregulating the interspecies quorum sensing signals diketopiperazines and diffusing signaling factors. J. Agric. Food Chem. 69, 3496–3510 (2021).
Jia, Q. et al. Terpene synthase genes originated from bacteria through horizontal gene transfer contribute to Terpenoid diversity in fungi. Sci. Rep. 9, 9223 (2019).
Ma, M. et al. The microbial biosynthesis of noncanonical terpenoids. Appl. Microbiol. Biotechnol. 108, 226 (2024).
Johansen, B., Duval, R. & Sergere, J.-C. First evidence of a combination of terpinen-4-ol and α-terpineol as a promising tool against ESKAPE pathogens. Molecules 27, 7472 (2022).
Cordeiro, L. et al. Terpinen-4-ol as an antibacterial and antibiofilm agent against Staphylococcus aureus. Int. J. Mol. Sci. 21, 4531 (2020).
Limaverde, P. W. et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem Toxicol 109, 957–961 (2017).
Acknowledgements
The authors acknowledge SASTRA Deemed University for the infrastructural support. We would like to express our gratitude to the Centre for Advanced Research in Indian Systems of Medicine (CARISM), supported by the FIST Biochemical Engineering-II grant (SR/FST/ET-1/2020/614), for their generous support. ICMR’s Funding to SN (EMDR/SG/14/2024/01-04170) for this study is gratefully acknowledged. Additionally, we extend our sincere appreciation to Ms. Sudha V for her indispensable assistance, notably in conducting GC-MS analysis. The authors would like to thank Trichy SRM Medical college and Research Centre for the collection and isolation vaginal probiotics. VGN acknowledges the support provided by the Women Scientist-B program, Department of Science and Technology, Government of India, under grant number DST/WOS-B/HN-32/2021 for conducting this study.
Funding
This research was funded by the Women Scientist-B program of the Department of Science and Technology, Government of India, under grant number DST/WOS-B/HN-32/2021 and ICMR’s Funding (EMDR/SG/14/2024/01-04170) to facilitate the execution of this study.
Author information
Authors and Affiliations
Contributions
VGN- Performed experiments, compiled results and manuscript writing, VU- Performed experiments, NM- Performed experiments for revision, BN- Performed experiments RCL- Conceived the work Y.B.R.D.R.- Purification, separation, and characterization of metabolites from CFS, Manuscript review. DN- Sample collection, ethical approval, Manuscript review, AA- Sample collection, Isolation, Manuscript review, PN-Sample collection, ethical approval, strain isolation, manuscript review, SN - work design, interpretation, compilation, and Manuscript writing & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors have filed a patent describing a formulation containing (-)-Terpinen-4-ol and two other postbiotics derived from Lactobacilli to treat induced UTI in mice model (Patent Application Number: 202441057663; Reference Number: TEMP/E-1/67129/2024-CHE). The collection of human vaginal samples was approved by the Human Ethical Committee (Registration number: 696/TSRMMCH&RC/ME-1/2022-IEC No: 127), with adherence to all relevant international and national guidelines. Informed consent was obtained from all participants before enrolment. Zebrafish studies were conducted in line with national and institutional guidelines (CPCSEA-510/SASTRA/IAEC/RPP) for the ethical treatment and use of animals, following Animal Biosafety Level 2 standards. To maintain the integrity of the histopathology study, the observer was blinded to the control and treatment groups.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nair, V.G., Unnikrishnan, V.K., Muralidharan, N. et al. Human vaginal Lactobacillus Jensenii -derived (-)-Terpinen-4-ol restores antibiotic sensitivity by inhibiting efflux pumps in drug resistant E. coli and K. pneumoniae. Sci Rep 15, 31823 (2025). https://doi.org/10.1038/s41598-025-17404-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17404-4
Keywords
This article is cited by
-
Synergistic antipersister, efflux inhibitory & antibiofilm activities of vaginal Lactobacillus-derived postbiotics against UPEC: toward a novel therapeutic for utis
Scientific Reports (2026)
-
Bile Salt Hydrolase Activity in the Food-Derived Strain Levilactobacillus brevis M3R3: Genomic and Functional Characterization
Probiotics and Antimicrobial Proteins (2025)