Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) reduce incidence of cardiovascular events in type 2 diabetes (T2D) patients. Yet, the impact of GLP-1RAs on coronary lesion progression and cardiovascular outcomes after coronary stent implantation remains unclear. We aimed to investigate the effects of GLP-1RAs on coronary lesion progression and major adverse cardiovascular events (MACEs) after percutaneous coronary intervention (PCI). This prospective cohort study enrolled 1664 patients with T2D who underwent PCI from January 2020 to March 2024. Matched GLP-1RAs-treated and non-treated cohorts were formed using the propensity score matching method. The primary endpoint was the incidence of MACEs (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospitalization for heart failure). Secondary endpoints included in-stent restenosis and non-target lesion progression. Two 131-patient cohorts with balanced baseline characteristics were formed by propensity score matching. During the median follow-up period of 20 months (ranging from 6 to 48 months), the incidence of MACEs was significantly lower in the GLP-1RA group (7.63%)compared to the control group (19.85%) (HR 0.444; 95%CI, 0.215–0.918; P = 0.024). During a median follow-up period of 12 months, 79.39% (104/131) of patients in the control group and 82.44% (108/131) of patients in the GLP-1RA group successfully underwent coronary angiography follow-up. The incidence of in-stent restenosis was 2.78% (3/108) in the GLP-1RA group and 11.54% (12/104) in the control group (P = 0.028). Non-target lesion progression was found in 10.19% (11/108) patient of the GLP-1RA group and 22.12% (23/104) in the control group (P = 0.037). Notable disparities were observed between the two groups regarding improvements of BMI, SBP, HbA1c, LDL-C, CRP. GLP-1RAs significantly reduced the incidence of MACEs and coronary lesion progression in patients with T2D after coronary stent implantation. These findings suggest that GLP-1RAs may have beneficial effects on cardiovascular outcomes and coronary artery disease progression in this population.
Similar content being viewed by others
Introduction
T2D is a chronic metabolic disorder characterized by insulin resistance and impaired insulin secretion, leading to hyperglycemia and increased cardiovascular risk. Patients with T2D often have comorbid conditions such as hypertension, dyslipidemia, and obesity, which further exacerbate the risk of developing atherosclerotic cardiovascular disease (ASCVD).The management of these patients often involves percutaneous coronary intervention (PCI)with stent implantation1. However, despite advances in interventional techniques, patients with T2D still face a higher risk of adverse cardiovascular events and coronary lesion progression compared to non-diabetic patients2. This underscores the urgent need for novel therapeutic strategies to mitigate residual cardiovascular risk in this high-risk population.
Recent years have witnessed a paradigm shift in the management of patients with T2D, with increasing focus on therapies that not only improve glycemic control but also confer cardiovascular benefits. Glucagon-like peptide-1 receptor agonists (GLP-1RAs)have emerged as a promising class of antidiabetic agents with potential cardiovascular benefits3. Extensive preclinical and clinical researches have demonstrated that GLP-1RAs exert beneficial effects on various aspects of cardiovascular health. In experimental models of atherosclerosis, GLP-1RAs have been shown to reduce plaque burden and improve plaque stability through multiple mechanisms. These include attenuation of inflammatory responses by downregulating adhesion molecules and pro-inflammatory cytokines, inhibition of macrophage infiltration and foam cell formation, and modulation of lipid metabolism.
Previous studies have shown that GLP-1RAs can improve glycemic control, reduce body weight and lower blood pressure4,5. Moreover, several large-scale clinical trials6,7,8,9,10such as LEADER, SUSTAIN-6, and REWIND have demonstrated that GLP-1RAs can reduce the incidence of MACEs in patients with T2D and established cardiovascular disease or high cardiovascular risk.
Given the established benefits of GLP-1RAs on cardiovascular outcomes and their favorable effects on atherosclerotic lesion progression, it is plausible to hypothesize that these agents may have a disease-modifying role in patients with T2D undergoing PCI. However, the specific impact of GLP-1RA therapy on coronary lesion progression and long-term cardiovascular outcomes in this subgroup of patients remains to be fully elucidated. The present prospective cohort study aims to address this knowledge gap by evaluating the effects of GLP-1RA treatment on coronary atherosclerosis progression and MACEs occurrence in patients with T2D following PCI.
Methods
Study design and population
This prospective cohort study was conducted from January 2020 to March 2024. We screened 3681 patients who underwent PCI during this period and enrolled 1796 patients with T2D. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) diagnosis of T2D; (3) undergoing coronary stent implantation due to chronic coronary syndrome or acute coronary syndrome. The exclusion criteria included: (1) severe liver or kidney dysfunction; (2) history of malignancy; (3) inability to follow up regularly (4) known intolerance or hypersensitivity to GLP-1RA therapy, including significant gastrointestinal adverse reactions (such as persistent nausea, vomiting, or diarrhea) or severe hypoglycemic episodes that would preclude continued use of the drug. The study strictly followed the ethical guidelines of the Declaration of Helsinki and received the approval of the local ethic committee (SH9H-2019-T160-2). Ethical approval of the study was obtained from Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine.All patients provided written informed consent. To reduce selection bias and ensure comparability between the treatment groups, we employed the propensity score matching method. The matching variables included demographic characteristics (age and sex), physical examination findings (body mass index, blood pressure), laboratory data results (hemoglobin A1c, fasting plasma glucose, creatinine, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides and cTroponin I), comorbidities (presence of hypertension, dyslipidemia, atrial fibrillation,heart failue, smokers and diabetes duration), and concomitant medications (beta-blockers, ACEI/ARB/ARNI, SGLT2-inhibitors,statins, antiplatelet drugs,and anticoagulant drugs). These variables were selected based on their associations with both GLP-1 receptor agonist treatment and major adverse cardiovascular events, as reported in the literature. Patients were matched in a 1:1 ratio using the nearest neighbor matching algorithm, with a caliper width of 0.2 standard deviations of the logit of the propensity score. This approach helped to balance the baseline characteristics between the groups, thereby enhancing the validity of our comparative analysis.
Data collection and follow-up
Baseline clinical characteristics, laboratory data, and medication use were recorded at enrollment. Follow-up visits were scheduled at 1, 3, 6, 12, 24, 36 and 48 months after stent implantation. The primary endpoint was the occurrence of MACEs, including non-fatal stroke, non-fatal myocardial infarction, hospitalization for heart failure, and cardiovascular death11. Secondary endpoints included in-stent restenosis12 and non-target lesion progression13,14. In this prospective observational study design, ISR was classified using Mehran’s classification: type I (focal) for ISR lesions ≤ 10 mm in-stent, type II (diffuse) for ISR lesions > 10 mm in-stent, type III (hyperplastic) for ISR lesions > 10 mm and beyond the stent margin, and type IV (occlusive) for complete occlusion. The N-TLP was defined as follows: 1. Initial coronary angiography revealing lumen diameter stenosis of 50% or greater, with an increase in stenosis degree exceeding 10%. 2. Initial coronary angiography showing lumen diameter stenosis < 50% with an increase in stenosis degree exceeding 30%. 3. Primary coronary angiography exhibiting normal vessels with new stenosis equal to or exceeding 30%. 4. Follow-up coronary angiography identifying any lesions progressing to complete occlusion of the coronary artery.
Coronary angiography and PCI
Multiple matched angiographic views were obtained for each patient following intracoronary nitroglycerin administration. PCI procedures were performed at the Catheter Laboratory of Cardiology, Shanghai Ninth People’s Hospital. Syngo AX software (Siemens Medical Solutions, AX, Berlin, Munich) was utilized for image acquisition and analysis. Two independent technicians, blinded to QCA analysis and clinical outcomes, conducted the analysis. The confidentiality of the collected data was strictly maintained for the purposes of this study. All patients underwent standard medical therapy post-PCI, incorporating antiplatelet agents, statins, and ACEI/ARB/ARNI, beta-blockers to reduce ischemic events and improve outcomes.
Statistical analysis
Statistical analysis in this study was performed using SPSS Statistical Software (version 22.0, SPSS Inc., Chicago, IL, USA) and R Statistical Software (version 3.0.1, the R Foundation for Statistical Computing). Quantitative variables were calculated for arithmetic means ± standard deviations while qualitative variables were given as frequency and percentage (%). Moreover, t test and one way ANOVA test were used appropriately to analyze quantitative variables. Qualitative variables were compared using a two-sided chi square test. To identify potential predictors for the occurrence of MACEs, univariate and multivariate Cox regression analysis were performed. Predictors with a significance of P < 0.10 in the univariate analysis were entered into the multivariate model, as well as mandatory inclusion variables that were considered as important predictors of clinical endpoints. Relative risks are expressed as hazard ratios (HR) with 95% confidence intervals (CIs). Freedom from MACEs at 48 months was analyzed with Kaplan–Meier statistics, with difference between the groups assessed using the log-rank test. All values were two-tailed, and a P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A flowchart of patient enrollment was shown in Fig. 1. We screened 3681 patients who underwent PCI from January 2020 to March 2024 and enrolled 1796 patients with T2D. During the screening process, 32 patients were excluded because of severe comorbidities,25 patients with history of malignancy were excluded and another 30 were excluded due to incomplete follow-up data. Meanwhile,45 patients were excluded because of intolerance or hypersensitivity to GLP-1RA therapy or severe hypoglycemic episodes. Thus, the final study cohort consisted of 1664 patients. After propensity score matching,the cohort included 262 participants, of which 131 were administered GLP-1RA, and the remaining 131 were allocated to the control group to receive standard glucose-lowering therapy. Patients’ demographic and health characteristics are shown in Table 1 (before propensity score matching) and Table 2 (after propensity score matching). Before matching, patients in the GLP-1RA group were younger than those in the control group (61.28 ± 10.39 vs. 72.70 ± 10.83 years, p < 0.001). While BMI (26.44 ± 5.20 vs 24.74 ± 7.75 kg/m2, p = 0.011) and baseline HbA1c of patients in the GLP-1RA group were higher than those in the control group (8.61% ± 1.67vs7.84% ± 1.56, p < 0.001). The GLP-1 RA group had more male and smoker than the control cohort. Additionally, patients in the GLP-1RA group tend to have higher levels of glucose, total cholesterol, LDL cholesterol, troponin and lower levels of Creatinine.
Study medications and dosing regimens
Among the GLP-1RAs prescribed, liraglutide and semaglutide were the most commonly used agents. The specific types and dosages of these medications are detailed as follows: Initially, 46.6% (n = 55) of the patients were prescribed liraglutide. Of these, 17.8% (n = 21) switched to semaglutide during the study period. The distribution of liraglutide dosages among those who remained on liraglutide was as follows: 0.6 mg/day (11.8%), 1.2 mg/day (52.9%), and 1.8 mg/day (35.3%). The mean duration of liraglutide treatment before switching was 9.8 months.The final proportion of patients using semaglutide was 71.1% (n = 84), including both those who initially started with semaglutide and those who switched from liraglutide. Among the patients using semaglutide on the subcutaneous formulation, with dosages of 0.25 mg/week (27.4%), 0.5 mg/week (51.2%), and 1.0 mg/week (21.4%). The mean duration of semaglutide treatment was 28 months.
Primary endpoint
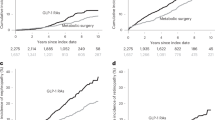
During the median follow-up of 20 months(ranging from 6 to 48 months), the incidence of MACEs was significantly lower in the GLP-1RA group (7.63%) compared to the control group (19.85%) (HR 0.444; 95%CI, 0.215–0.918; P = 0.024). Among these events, which include non-fatal stroke, non-fatal myocardial infarction, hospitalization for heart failure, and cardiovascular death, there was no significant difference in the incidence rates between the two groups.
A Kaplan–Meier (KM) curve was used to follow up the survival status of the two patient groups, as shown in Fig. 2, indicating that the GLP-1RA group had a lower risk of MACEs during the follow-up period compared to the control group. we also examined the incidence of individual components of MACEs, including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and hospitalization for heart failure. The results are summarized in Table S1.
Additionally, we constructed both univariate and multivariate Cox proportional hazards models to further explore the risk factors influencing the occurrence of MACEs, as shown in Table 3. The forest plots (Figs. S1 and S2) provide a visual representation of the hazard ratios and their 95% confidence intervals for each variable in both the univariate and multivariate analyses.
The results of the univariate Cox proportional hazards model showed that age and duration of diabetes were positively associated with the risk of MACES, while the use of GLP-1RA was negatively associated with the risk of MACES. Other variables, such as gender, hypertension, dyslipidemia, and smoking did not show significant associations with the risk of MACES in this model. At multivariate Cox regression analysis, diabetes duration (HR 1.08, 95% CI 1.02–1.14, P = 0.008) was the independent risk factor, and GLP-1 RAs therapy (HR 0.42, 95% CI: 0.20–0.88, P = 0.022) was the independent protective factor for the occurrence of MACEs in this population.
Secondary endpoints
The incidence of in-stent restenosis was 2.78%in the GLP-1RA group and 11.54%in the control group (P = 0.028). The incidence of non-target lesion progression was 10.19%in the GLP-1RA group and 22.12%in the control group (P = 0.037). These results indicate that GLP-1RAs reduce the risk of both in-stent restenosis and non-target lesion progression.
Changes in clinical parameters
Significant differences were observed between the two groups in changes in several clinical parameters (BMI, SBP, HbA1c, LDL-C, CRP) during the follow-up period (Fig. 3). These results suggest that GLP-1RAs may have a multifaceted beneficial impact on cardiovascular risk factors and overall metabolic control in T2D patients after coronary stent implantation.
Adverse drug reactions
During the follow-up period, no significant adverse events related to GLP-1RAs treatment were reported in our study cohort. Specifically, there were no cases of severe hypoglycemia, pancreatitis or other serious adverse effects commonly associated with GLP-1 RAs. Mild gastrointestinal side effects, such as nausea and vomiting, are commonly reported with GLP-1RA therapy. However, in our study, these side effects were minimal and did not lead to treatment discontinuation. The incidence of gastrointestinal adverse events was comparable between the GLP-1RA group (4/131) and the control group (2/131), suggesting that the gastrointestinal tolerability of GLP-1RAs was acceptable in this patient population and did not result in treatment discontinuation.
Discussion
Our study evaluated the effects of GLP-1RA on MACEs and coronary lesion progression in T2D patients post-PCI. We found that GLP-1RA significantly reduces MACEs incidence and inhibits coronary lesion progression. Additionally, GLP-1RA improves BMI, SBP, HbA1c, LDL-C, and CRP levels.
GLP-1RA has significant clinical implications for type2 diabetes patients with ASCVD. It not only effectively controls blood glucose but also provides comprehensive cardiovascular protection. Multiple large-scale RCTs have demonstrated that GLP-1RA can significantly reduce the risk of MACES in type2 diabetes patients. For example, the LEADER trial showed that liraglutide reduced MACEs risk by 13%, while the SUSTAIN-6 trial indicated a 26% reduction with semaglutide. These findings have been further confirmed in real-world studies, highlighting the robustness of GLP-1RA’s cardiovascular benefits. Our study, a prospective cohort study conducted in a real-world clinical setting, reflecting actual clinical practice and patient management, demonstrates that the use of GLP-1RAs in patients with T2D undergoing coronary stent implantation is associated with a significant reduction in major adverse cardiovascular events (MACEs)and coronary lesion progression. GLP-1RA’s role extends beyond glycemic control; it addresses multiple cardiovascular risk factors, including dyslipidemia, hypertension, and obesity, making it a cornerstone in the comprehensive management of T2D patients with ASCVD. Current clinical guidelines strongly recommend GLP-1RA for T2D patients with established ASCVD or high cardiovascular risk, underscoring its importance in clinical practice.
Impact of coronary lesion progression on MACEs in T2D patients
Coronary lesion progression, including in-stent restenosis (ISR) and non-target lesion progression (NTLP), significantly impacts major adverse cardiovascular events (MACEs) in type 2 diabetes (T2D) patients post-PCI. ISR results from abnormal proliferation of vascular smooth muscle cells and excessive extracellular matrix synthesis within the stent, leading to re-narrowing of the coronary artery lumen and increasing the risk of recurrent ischemic events. NTLP, often due to systemic atherosclerosis, can lead to new ischemic events. Both ISR and NTLP contribute to higher MACEs rates, emphasizing the need for effective therapeutic strategies to inhibit coronary lesion progression.
Impact of GLP-1RA on atherosclerotic plaques in T2D patients
GLP-1 receptor agonists (GLP-1RAs) may mitigate these risks by inhibiting coronary lesion progression through anti-inflammatory and anti-proliferative effects, stabilizing the vascular environment, and addressing systemic atherosclerosis. However, their impact on atherosclerotic plaque progression remains under investigation. A double-blind trial15 showed that exenatide improved glycemic control but did not significantly alter carotid plaque volume or composition over 18 months. In contrast, liraglutide was associated with increased coronary artery fibrous plaque volume in asymptomatic T2D patients without prior coronary artery disease, suggesting mixed outcomes16. Ongoing trials, such as the T-Plaque trial17 (evaluating tirzepatide) and the STRIDE trial18 (evaluating semaglutide), aim to provide further insights into the cardiovascular benefits of GLP-1RAs.
Potential mechanisms
The mechanisms underlying the cardiovascular benefits of GLP-1RAs are likely multifactorial19. Improved glycemic control, as evidenced by the significant reduction in HbA1c in the GLP-1RA group, is an important factor. Chronic hyperglycemia is a well-known risk factor for cardiovascular disease, and better glycemic control can reduce the risk of atherosclerosis and its complications20. Additionally, the observed weight loss and reduction in systolic blood pressure in the GLP-1RA group may also contribute to the cardiovascular benefits21,22. Weight reduction can improve insulin sensitivity and reduce the metabolic burden on the cardiovascular system, while lower blood pressure can reduce the risk of myocardial infarction and stroke. The reduction in LDL-C and CRP levels in the GLP-1RA group suggests that these agents may also have anti-inflammatory and lipid-lowering effects. Inflammation plays a crucial role in the pathogenesis of atherosclerosis23,24, and the observed reduction in CRP levels indicates that GLP-1RAs may reduce systemic inflammation, thereby stabilizing atherosclerotic plaques and reducing the risk of plaque rupture. The reduction in LDL-C levels, although modest, may also contribute to the overall cardiovascular benefits by reducing the accumulation of cholesterol in the arterial wall25.
Limitations
Our study has several limitations. First, it was a single-center study with a relatively small sample size, which may limit the generalizability of our findings. Second, the follow-up duration was relatively short, and longer-term studies are needed to fully assess the long-term cardiovascular benefits of GLP-1RAs in this patient population. Third, we did not perform detailed mechanistic studies to elucidate the exact pathways through which GLP-1RAs exert their cardiovascular benefits. Future studies should focus on exploring the underlying mechanisms and validating our findings in larger multicenter cohorts.
Future directions
Future research should focus on elucidating the precise mechanisms through which GLP-1RAs exert their cardiovascular benefits. This may include studies on the effects of GLP-1RAs on endothelial function, plaque stability, and inflammation at the molecular level. Additionally, large-scale randomized controlled trials with longer follow-up periods are needed to confirm the cardiovascular benefits of GLP-1RAs in patients with T2D undergoing coronary stent implantation. Furthermore, studies should explore the optimal dosing and duration of GLP-1RA therapy to maximize cardiovascular benefits while minimizing potential adverse effects.
Conclusion
In conclusion, our study demonstrates that the use of GLP-1RAs in patients with T2D undergoing coronary stent implantation is associated with a significant reduction in major adverse cardiovascular events and coronary lesion progression. These findings highlight the potential cardiovascular benefits of GLP-1RAs beyond their glucose-lowering effects and suggest that these agents may be a valuable addition to the management of patients with T2D and coronary artery disease. Future studies are needed to further elucidate the underlying mechanisms and validate our findings in larger multicenter cohorts.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Włodarczak, A. et al. Mid-term safety and efficacy of magnesium bioresorbable vascular scaffolds-magmaris in diabetic population. 2-Years outcome in acute coronary syndrome cohort. Diab. Vasc. Dis. Res. 20(4), 14791641231188704. https://doi.org/10.1177/14791641231188705 (2023).
Ma, Y., Gao, H. & Wu, H. Comparison of adverse cardiovascular event endpoints between patients with diabetes and patients without diabetes based on coronary artery plaques: A systematic review and meta-analysis. J. Cardiothorac. Surg. 19(1), 672. https://doi.org/10.1186/s13019-024-03157-0 (2024).
Yagyu, H. & Shimano, H. Treatment of diabetes mellitus has borne much fruit in the prevention of cardiovascular disease. J. Diabetes Investig. 13(9), 1472–1488. https://doi.org/10.1111/jdi.13859 (2022).
Sivakumar, P. M., Premkumar, B., Prabhawathi, V. & Prabhakar, P. K. Role of GLP-1 analogs in the management of diabetes and its secondary complication. Mini Rev. Med. Chem. 21(20), 3166–3182. https://doi.org/10.2174/1389557521666210422114909 (2021).
Taha, M. B. et al. Glucagon-like peptide 1 receptor agonists: A medication for obesity management. Curr. Atheroscler. Rep. 24(8), 643–654. https://doi.org/10.1007/s11883-022-01041-7 (2022).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in T2D. N. Engl. J. Med. 375, 311–322 (2016).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with T2D. N. Engl. J. Med. 375, 1834–1844 (2016).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with T2D and cardiovascular disease (Harmony outcomes): A double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529 (2018).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in T2D. N. Engl. J. Med. 385, 896–907 (2021).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in T2D (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
McMurray, J. J. V. et al. Meta-analysis of risk of major adverse cardiovascular events in adults with T2D treated with bexagliflozin. Diabetes Obes. Metab. 26(3), 971–979. https://doi.org/10.1111/dom.15394 (2024).
Giustino, G. et al. Coronary in-stent restenosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 80(4), 348–372 (2022).
Ma, J. et al. Association between stent implantation and progression of nontarget lesions in a rabbit model of atherosclerosis. Circ. Cardiovasc. Interv. 14(11), e010764 (2021).
Quan, W. et al. Influence of LDL-cholesterol lowering on coronary plaque progression of non-target lesions in patients undergoing percutaneous coronary intervention: Findings from a retrospective study. J. Clin. Med. 12(3), 785 (2023).
Koska, J., Migrino, R. Q., Chan, K. C., Cooper-Cox, K. & Reaven, P. D. The effect of exenatide once weekly on carotid atherosclerosis in individuals with T2D: An 18-month randomized placebo-controlled study. Diabetes Care 44(6), 1385–1392. https://doi.org/10.2337/dc20-2014 (2021).
Heinsen, L. J. et al. Liraglutide treatment is associated with progression of coronary artery fibrous plaque: A prospective 1-year follow-up study in asymptomatic patients with T2D. BMC Cardiovasc. Disord. 23(1), 214. https://doi.org/10.1186/s12872-023-03228-5 (2023).
Hamidi, H. et al. Effect of tirzepatide on the progression of coronary atherosclerosis using MDCT: Rationale and design of the tirzepatide treatment on coronary atherosclerosis progression: The (T-Plaque) randomized-controlled trial design. Am. Heart J. 278, 24–32. https://doi.org/10.1016/j.ahj.2024.08.015 (2024).
Bonaca, M. P. et al. Design and baseline characteristics of the STRIDE trial: Evaluating semaglutide in people with symptomatic peripheral artery disease and T2D. Eur. Heart J. Cardiovasc. Pharmacother. 10(8), 728–737. https://doi.org/10.1093/ehjcvp/pvae071 (2025).
Alnima, T., Smits, M. M. & Hanssen, N. M. J. Are the lipid-lowering effects of incretin-based therapies relevant for cardiovascular benefit?. Curr. Opin. Lipidol. 35(6), 259–267. https://doi.org/10.1097/MOL.0000000000000949 (2024).
Antoniou, S. et al. Effect of glycemic control on markers of subclinical atherosclerosis in patients with T2D mellitus: A review. World J. Diabetes. 12(11), 1856–1874. https://doi.org/10.4239/wjd.v12.i11.1856 (2021).
Rodriguez-Valadez, J. M. et al. Potential mediators for treatment effects of novel diabetes medications on cardiovascular and renal outcomes: A Meta-regression analysis. J. Am. Heart Assoc. 13(4), e032463. https://doi.org/10.1161/JAHA.123.032463 (2024).
Krisanapan, P. et al. Safety and efficacy of GLP-1 receptor agonists in T2D mellitus with advanced and end-stage kidney disease: A systematic review and meta-analysis. Diseases 12(1), 14. https://doi.org/10.3390/diseases12010014 (2024).
Libby, P. Inflammation and the pathogenesis of atherosclerosis. Vascul. Pharmacol. 154, 107255. https://doi.org/10.1016/j.vph.2023.107255 (2023).
Higashi, Y. Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxidants (Basel). 11(10), 1958. https://doi.org/10.3390/antiox11101958 (2022).
Ference, B. A., Braunwald, E. & Catapano, A. L. The LDL cumulative exposure hypothesis: Evidence and practical applications. Nat. Rev. Cardiol. 21(10), 701–716. https://doi.org/10.1038/s41569-024-01039-5 (2024).
Funding
This work was supported by the 15th Batch of Emerging Frontier Project Topics of Shanghai Shenkang (to Changqian Wang, #SHDC12023123), the Clinical Research Program of Shanghai Ninth People’s Hospital (to Jun Gu, #JYLJ202209), Shanghai Municipal Health Commission (to Jun Gu, #202240149), the National Natural Science Foundation of China (to LinGao,#82100462) and the Science and Technology Planning Project of Yunnan Province of China (to YangZhuo,#202402AA310073).
Author information
Authors and Affiliations
Contributions
Conception and design: P Yin, J Gu, C.Q.Wang. Analysis and interpretation of the data: H.S.Zeng, J.C.Hu. Drafting of the article: H.S.Zeng, J.C.Hu. Critical revision of the article for important intellectual content: All authors. Final approval of the article: All authors. Provision of study materials or patients: Y.Zhuo,M.Wang. Statistical expertise: L.Gao,Z.D.Tang, Administrative, technical, or logistic support: J.F.Zhang. Collection and assembly of data: H.S.Zeng, J.C.Hu.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, H., Hu, J., Zhuo, Y. et al. Effect of GLP-1RA on coronary progression and cardiovascular outcomes in type 2 diabetic patients after PCI: a prospective cohort study. Sci Rep 15, 31824 (2025). https://doi.org/10.1038/s41598-025-17574-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17574-1