Abstract
A gut microbiome-targeted diet can potentially mitigate chronic diseases, including malnutrition. In a prospective 12-week intervention trial, we evaluated the effects of six different plant-based fermented pickles (~ 50 g/day) on clinical, inflammatory, and gut microbiota parameters in a cohort of women (n = 230) in a rural setting with a high prevalence of undernutrition. Blood was collected at two, whereas stool was collected at three timepoints. Among fecal biomarkers, myeloperoxidase (MPO), Lipocalin-2 (LCN2), and 16S rRNA gene sequencing were measured at baseline, 8th, and 12th weeks. Overall, the compliance rate was > 70%. Among radish group, WBCs (p = 0.002, two-tailed paired T-test) decreased, whereas neutrophils and platelets decreased among both radish (p = 0.016, p = 0.017, two-tailed paired T-test) and carrot (p = 0.005, p = 0.006, two-tailed paired T-test) groups after intervention. Similarly, in lemon-chilli group, platelets decreased while mean corpuscular hemoglobin (MCH) increased (p < 0.001, p = 0.022, two-tailed paired T-test). In onion and lemon-chilli groups, α- (р =0.001 and p = 0.0005, Kruskal-Wallis Test, respectively) and β-diversity indices (p = 9e-04 and p = 0.022, pairwise PERMANOVA, respectively) were significantly increased, post-intervention. Linear discriminant analysis (LDA) of lemon-chilli group identified 25 bacterial taxa markers in 8th and 12th week, which included Eggerthellaceae and Oscillospiraceae, Erysipelatoclostridiaceae and Subdoligranulum. Correlation analysis revealed six taxa negatively associated with inflammatory markers such as C-reactive protein (CRP), LCN2, and platelets. Our study provides preliminary evidence that the consumption of traditional fermented pickles leads to beneficial changes in women’s hematological and gut microbiota profiles.
Similar content being viewed by others
Introduction
Malnutrition is becoming a severe global issue, particularly in low and middle-income countries (LMICs), affecting more than 2 billion people1. In Pakistan, women of reproductive age (WRA) mainly suffer from malnutrition due to inaccessibility to food, contaminated drinking water, and limited choices of food. This often leads to disruption in gut homeostasis, dysbiosis of gut microbial species, and an increase in gut or systemic inflammation2,3. Gut microbial dysbiosis increases pathogenic bacteria, which leads to immune system activation and inflammation4,5. However, studies suggest that diet plays a significant role in shaping the gut microbiome, and both long-term and short-term dietary interventions can effectively modulate gut microbial communities and the host’s immune system6,7,8. Hence, promising diets should be explored through clinical trials in various populations to determine their effects on gut microbiome diversity, inflammation, and overall health.
Microbiome-targeted diets profoundly influence the gut microbiome and immune biomarkers9 such as Mediterranean, vegan, or ketogenic diets that are linked to gut microbiome modifications by activating both innate and adaptive immune system10,11,12,13. Similarly, numerous studies highlighted the beneficial effect of fermented food intervention on gut microbial species, increasing diversity in microbial taxa, and improving overall gut health6,14,15,16. However, fermented food trials are either limited to over-the-counter and non-traditional dairy fermented foods from the developed world6, or traditional fermented food from regions other than South Asia, like kimchi, sourdough, and sauerkraut17, which does not reflect the diet of a rural malnourished woman of Pakistan.
Plant-based fermented foods offer a promising solution of low-cost locally fermented products that are a rich source of both prebiotics, such as complex carbohydrates, and probiotics, such as Lactiplantibacillus brevis, Pediococcus pentosaceus, Lactiplantibacillus plantarum, and Lactiplantibacillus fermentum, which are favorable for gut and metabolic health7,18,19. The fermentation process converts complex carbohydrates into short-chain fatty acids (SCFA), such as acetate, propionate, butyrate, and proteins into bioactive peptides, improving the nutritional value and bioavailability of foods8. Currently, plant-based fermented pickles, including Kimchi, Jangajji, Sauerkraut, Torshi, Suancai, and traditional Chinese pickles, are reported for their potential health benefits20. South Asian countries have a long historical culture of consuming fermented and non-fermented pickles, locally known as “Achar”21. However, the scarcity of human clinical trials necessitates investigating the role of locally available fermented pickles in improving gut health and inflammatory biomarkers, particularly in women of resource-limited settings. In this intervention trial, the effect of daily consumption of 50g fermented pickle was investigated on clinical parameters such as complete blood count (CBC), CRP, inflammatory biomarkers MPO, LCN2, and stool microbiota of WRA living in a malnourished settings of rural Sindh, Pakistan 22.
Results
Participants achieved up to 70% compliance with assigned fermented pickle consumption during the intervention period
To examine the effect of pickle consumption on the gut microbiota and clinical parameters, 223 healthy WRA, ranging from 18 to 48 years, were recruited for an 8-week dietary trial (12-week complete protocol). Of 407 participants screened for the eligibility criteria, 223 were recruited and assigned to one of the 6 interventions and 1 control group. Each arm had 30 participants. After 13 loss-to-follow-ups, the final enrollment was 29–31 per group (Fig. 1a). The mean age of the recruited women was 30.2 ± 7.9 years (mean ± SD), with a mean body mass index (BMI) of 21.0 ± 4.1 kg/m² (mean ± SD). There was no difference in the baseline parameters, including age, BMI, and weight, between the groups. A majority of participants were under 25 years of age, and most of them were married (88%), and 8% were pregnant. The mean socioeconomic status among the groups was significantly different (p = 0.02, two-way ANOVA), highest in the mango-oil group (0.31 ± 0.7) and lowest in the carrot group (−0.3 ± 1.1). Among the baseline clinical parameters (BMI, weight, respiratory rate, temperature, blood pressure, heart rate), heart rate (p = 0.03, two-way ANOVA) and blood pressure (p = 0.003, two-way ANOVA) were significantly different. (Table 1).
During our study, the participants successfully completed an 8-week intervention protocol by consuming the assigned pickles, confirmed by measuring the weight of the pickle jar before handing it over to the participant, and in return, after every 3 days. The compliance with assigned pickle consumption was categorized into 100%, 99 − 90%, 89 − 80%, and 79 − 70%. Among all groups, 93% of participants in the carrot group had 100% compliance with pickle consumption, followed by radish (80%), mango-water (80%), mango-oil (71%), onion (47%), and lemon-chilli (26%) groups. However, even the least compliant participants had more than 70% compliance with the assigned intervention (Figure S1). A complete food recall log was maintained at every 2 weeks of follow-up, classified as cereals, fruits, vegetables, dairy products, meat, beverages, and pickles. The diet, other than pickles, was balanced among the groups except for meat, which was less among the onion and lemon-chilli participants (7% and 13% respectively) (Table S1a-c). The side effects noted with the Achar intervention were mostly abdominal discomfort and bloating. The majority of those were recorded for the radish group (28%), followed by lemon-chilli (19%), and carrot (15%) (Table S2).
Overview of the fermented pickle intervention study. (a) Consort flow diagram for participant assessment, enrollment, allocation, follow-up, and analysis in each group. (b) The recipe followed for fermented pickle preparation. (c) The 12-week study timeline overview, sample collection, and experimental analysis.
Fermented pickle consumption modulates clinical parameters among intervention group participants
The analyses of blood samples at week 0 and week 8 among the different intervention groups reported a significant modulation in a few clinical parameters. The radish group participants reported a significant decrease in mean WBCs count (Mean pre = 7.6 × 10E9/L; post = 6.7 × 10E9/L, p = 0.04, two-tailed paired T-test), platelets (Mean pre = 316 × 10E9/L; post = 283 × 10E9/L, p = 0.03, two-tailed paired T-test), and neutrophils (Mean pre = 57.6%; post = 53.9%, p = 0.05, two-tailed paired T-test) counts post-intervention. Similarly, the carrot group participants also reported decreased platelets (Mean pre = 335 × 10E9/L; post = 297 × 10E9/L, p = 0.005, two-tailed paired T-test) and neutrophils (Mean pre = 60.2%; post = 56.2%, p = 0.006, two-tailed paired T-test) counts post-intervention. However, there was a significant decrease in platelets (Mean pre = 298 × 10E9/L; post = 232 × 10E9/L, p < 0.001, two-tailed paired T-test) and an increase in mean corpuscular hemoglobin (MCH) (Mean pre = 26pg; post = 27pg, p = 0.02, two-tailed paired T-test) and mean corpuscular hemoglobin concentration (MCHC) (Mean pre = 30.8pg; post = 31.6pg, p < 0.001, two-tailed paired T-test) among the participants of the lemon-chilli group, post-intervention. Among inflammatory biomarkers, LCN-2 increased (Mean pre = 46.9ng/ml; post = 75.5ng/ml, p = 0.01, two-way ANOVA) only upon mango-water pickle intake (Fig. 2 and Table S3a).
Pre and post-intervention clinical markers: (a-f) Boxplot for week 0 and week 8 hematological markers. [significant p-values: (a) Radish participants: WBCs (p = 0.002, paired T-test), (b) Carrot participants: Platelets (p = 0.005, paired T-test), Radish participants: Platelets (p = 0.016, paired T-test), Lemon-chilli participant:s Platelets (p < 0.001, paired T-test), (d) Carrot participants: Neutrophils (p = 0.006, paired T-test), Radish participants: Neutrophils (p = 0.01, paired T-test), (e) Lemon-chilli participants: MCH (p = 0.022, paired T-test), Mango-water participants: MCH (p = 0.005, paired T-test) (f) Lemon-chilli participants: MCHC (p < 0.001, paired T-test), Carrot participants: MCHC (p = 0.013, paired T-test).] (g-h) Boxplot for Week 0, Week 8, and Week 12 stool inflammatory biomarkers. [(g) Mango-water participants: LCN2 (p < 0.01, two-way ANOVA).] (* represents a significant p-value by paired T-test or ANOVA.).
In the multivariate model where baseline parameter taken as reference and adjusted for dairy-based fermented food consumption, compliance, and taste, there was a decreasing trend in WBCs among all intervention groups, but a significant reduction was noted among the radish group participants (ß= −0.93, CI=−1.68, −0.17, р =0.01). Similarly, the platelets counts were decreased among all the intervention groups, but the reduction among the lemon-chilli group was significant (ß=−65.97, CI=−101.03, −30.90, р<0.0001) (Table S3b).
The presence of culturable bacterial strains in the consumed pickles
Microbiological analyses of MRS and BHI media showed the presence of culturable bacteria in the pickles (Figure S2). Bacterial sequencing of the selected gram-positive strains identified lactic acid bacteria (LAB), including Lactiplantibacillus pentosus, Lactiplantibacillus plantarum, Pediococcus pentosaceus, Pediococcus acidilactici, Levilactobacillus brevis, Weissella cibaria, and Staphylococcus hominis in the pickles (Table S4).
Fermented pickle consumption remodelled the stool microbiota
Overall, 631 stool samples were collected from the enrolled participants at three time-points. The average read count was 45,102 paired-end reads. 585 bacterial taxa were detected; however, with a relative abundance > 2.5%, only 11 taxa in the Intervention group and 13 in the control group were identified (Figure S3). The core baseline taxa of the intervention and control groups were consistent, with differences in the relative abundance of genera including Bifidobacterium, Prevotella, Collinsella, and Catenibacterium (Figure S4). Post-intervention α- and β-diversity indices were similar between the intervention and control groups (Figure S5−S8). The gut microbiome exhibits high inter-individual variability, which can mask group-level effects23. Therefore, our analysis focused on within-subject changes from baseline to the treatment time point, with each participant treated as their own control.
Pre- and post-intervention alpha diversity
The calculated “Observed” and “Shannon” α-diversity indices showed significant variations across the intervention groups. The Observed α-diversity among the mango-water (р<0.01, Kruskal-Wallis test), and carrot groups (p = 0.02, Kruskal-Wallis test) changed significantly among the 3 timepoints by decreasing at week 8. While the mango-water group showed a recovery in diversity index by week 12, the carrot group continued to decrease compared to baseline. Whereas the Observed α-diversity of the onion group significantly increased at week 8 and week 12 (р =0.001, Kruskal-Wallis test). Similarly, both Observed (р =0.0005, Kruskal-Wallis test) and Shannon (p = 0.04, Kruskal-Wallis test) diversity indices increased significantly among the lemon-chilli group at both week 8 (end-intervention) and week 12 (post-intervention) time points (Fig. 3a-f and Figure S9). The linear regression model, adjusted for dairy-based fermented food consumption, compliance, and taste, also showed significant increase in Observed diversity (ß= 28.10, CI = 12.00, 44.21, р =0.001) among onion group whereas in both Observed (ß= 35.38, CI = 16.61, 54.16, р<0.0001) and Shannon (ß= −0.27, CI = 0.04, 0.50), р =0.024) diversity for lemon-chilli group (Table S3b).
Pre- and post-intervention beta diversity
The overall β-diversity, a measure of within-sample variation, that changed in all intervention groups except the radish group across the 3 timepoints. However, β-diversity relative to baseline (week 0), remained unchanged among the participants of the mango-water, mango-oil, and radish groups, both at week 8 and week 12. Among the six interventions, a significant shift in β-diversity was observed when compared to the pre-intervention values at week 8 in the onion (p = 9e-04, pairwise PERMANOVA), and lemon-chilli (p = 0.02, pairwise PERMANOVA) groups. This significant variation persisted at week 12 among onion (p = 0.0265, pairwise PERMANOVA), and lemon-chilli (p = 0.018, pairwise PERMANOVA). In contrast, the participants in carrot group did not show a change at week 8 compared to baseline whereas a notable variation in β-diversity was exhibited at week 12 (p = 9e-04, pairwise PERMANOVA), which showed that gut adaptation among the carrot pickle participants took a longer period than the onion and lemon-chilli participants (Fig. 3g-l).
Pre and post-intervention gut microbiota diversity indices. (a-f) α-diversity of participants across three timepoints in intervention groups. [Pairwise significant p-values: Mango (water) 0–8 weeks (p = 0.018) and 8–12 weeks (p = 0.01), Mango (oil) 8–12 weeks (p = 0.02), Carrot 0–12 weeks (p = 0.007), Onion 0–8 weeks (p < 0.000) and 0–12 weeks (p = 0.004), Lemon-chilli 0–8 weeks (p < 0.000) and 0–12 weeks (p = 0.002)]. (g-l) β-diversity of the participants across three timepoints in intervention groups. [Pairwise significant p-values: Mango (water) 8–12 weeks (p = 0.003), Mango (oil) 8–12 weeks (p = 2e-04), Carrot 0–12 weeks (p = 9e-04) and 8–12 weeks (p = 3e-04), Onion 0–8 weeks (p = 9e-04) and 0–12 weeks (p = 0.026), Lemon-chilli 0–8 weeks (p = 0.022) and 0–12 weeks (p = 0.018)]. (*p represents overall significant p-value using Kruskal-Wallis or PERMANOVA among the 3 timepoints. * shows significant paired Kruskul-Wallis or PERMANOVA among 2 timepoints).
Pre- and post-intervention composition and relative abundance of bacteria
Based on the taxa rank counts, a relative abundance above 2.5% was plotted to analyze the change in bacterial communities over time in different groups. The Phylum-level abundance plot showed the presence of Firmicutes, Bacteroidota, and Actinobacteriota. Post-intervention, most of the intervention groups, such as carrot, lemon-chilli, mango-oil, and mango-water groups, showed an increase in Firmicutes, except for onion and radish groups, where Actinobacteriota increased compared to the baseline bacterial abundance (Fig. 4a). At the family level, the relative abundance above 2.5% belonged to Ruminococcacea, Lachnospiraceae, Prevotellaceae, Eggerthellaceae, Coriobacteriaceae, Bifidobacteriaceae, Atopobiaceae, and Erysipelatoclostridiaceae. The relative abundance of the genus from Lachnospiraceae, Bifidobacteriaceae, and Ruminococcaceae families increased after fermented pickles intervention (Fig. 4b). The Genus-level relative abundance plot above 2.5% included Prevotella, Collinsella, Fecalibacterium, Catenibacterium, Blautia, Bifidobacteria, and Agathobacter (Figure S10).
Pre- and post-intervention top-taxa remodelling
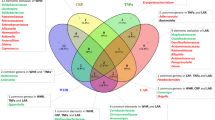
To identify unique bacterial taxa and persistence of bacterial taxa post-treatment, we plotted a Venn diagram to examine remodelling of the top 20 bacterial ASVs over time. The Venn diagram showed the shared and unique taxa at 3 time points. As the α- and β-diversity indices significantly changed in the lemon-chilli and onion group participants, we therefore focused on these two groups. The Venn diagram showed that approximately 70–75% of taxa were shared at 3 time points, while only 25–30% differed post-intervention in both groups. The lemon-chilli group had Fecalibacterium that appeared in week 12. However, the Subdoligranulum appeared in week 8 and persisted in week 12, even after the intervention was discontinued (Fig. 4c). Similarly, in the onion group participants, Olsenella and Prevotella persisted both at week 8 and week 12, whereas there were no unique taxa identified at week 8. Fecalibacterium appeared in week 12 when the intervention was discontinued (Fig. 4d).
Pre and post-intervention gut microbiota remodelling. (a) Relative abundance of phylum among different intervention groups. (b) Relative abundance of family among different intervention groups. (c) Venn-diagram for shared top 20 taxa at three time points in the lemon-chilli pickle intervention group participants. (d) Venn-diagram for shared top 20 taxa at three time points in the onion pickle intervention group participants.
Pre- and post-intervention linear discriminant analysis (LDA)
Linear Discriminant Analysis (LDA) was plotted to examine the significant post-intervention bacterial markers. The results of significant bacterial markers were ranked by LDA scores at 3 timepoints. In the lemon-chilli group, 25 bacterial taxa were significantly distinct, including Eggerthellaceae and Oscillospiraceae with the highest LDA score at week 8, whereas 10 bacteria, including Erysipelatoclostridiaceae and Subdoligranulum, showed the highest LDA score at week 12 (Fig. 5a). Similarly, among the participants of the onion group, only Actinobacteriota was a significant marker at week 8, while Olsenella, Turicibacter singuinis, and Intestinibacter were highly discriminant at week 12 (Fig. 5b). The cladograms were plotted to show the phylogenetic distances of the distinct taxa in both groups. The diameter of the circle represented the abundance of the taxon (Fig. 5c-d). In the mango-oil group, Agathobacter, Eubacterium, and Dorea were distinct in week 8 and week 12, respectively. Dorea was also highly distinct in the mango-water group in week 12 (Figure S11a-b). In the carrot group, Coriobacteriales and Collinsella were distinct in week 8 and Firmicutes, Clostridia, and Lachnospiraceae at week 12, while in the radish group, Firmicutes and Eggerthellaceae were discriminant taxa at week 12 (Figure S11c-d).
Pre- and post-intervention correlation analysis between gut-microbiota markers and clinical variables
The Spearman correlation analyses of distinct taxa identified at week 0 (pre-intervention) and week 8 (post-intervention) with clinical variables significant at the univariate level. In the lemon-chilli group, Fusicatenibacter identified at week 0 was negatively correlated (rho=−0.3; p = 0.01, Spearman correlation) with MPO, while the taxa identified post-intervention did not correlate with MPO. CRP was negatively correlated with Ruminococcaceae (rho=−0.3; p = 0.03, Spearman correlation), and Coprococcus (rho=−0.4; p = 0.00, Spearman correlation). LCN2 was negatively correlated with Eubacterium coprostanoligene (rho=−0.3; p = 0.02, Spearman correlation) and Eubacterium siraeum (rho=−0.3; p = 0.03, Spearman correlation). There was a negative correlation with platelets for the following group of taxa: Oscillospiraceae (UCG005) (rho=−0.3; p = 0.01, Spearman correlation), Eubacterium siraeum (rho=−0.3; p = 0.02, Spearman correlation), and Anaerovoracaceae (Family XIII AD3011) (rho=−0.2; p = 0.04, Spearman correlation). Ruminococcaceae (rho = 0.3; p = 0.04, Spearman correlation) was positively correlated with neutrophils. In the onion group, only post-intervention Actinobacteriota (rho = 0.3; p = 0.02, Spearman correlation) was positively correlated with LCN2 (Fig. 5e-f and Table S5a-b).
Pre and post-intervention LDA and correlation analyses. (a) LDA for the lemon-chilli pickle intervention group participants at three points. (b) LDA for the Onion pickle intervention group participants at three time points. (c) Cladogram of distinct taxa in the lemon-chilli pickle intervention group participants. (d) Cladogram of distinct taxa in the onion pickle intervention group participants. (e) Correlation analysis of pre (week-0) and post (week-8) distinct taxa with clinical biomarkers in the lemon-chilli pickle intervention group. (f) Correlation analysis of pre (week-0) and post (week-8) distinct taxa with clinical biomarkers in the onion pickle intervention group participants. The taxa belonging to the same group with the same relative abundance were considered one. In a heatmap, yellow squares indicate significant positive correlations (rho > 0.5, p < 0.05, Spearman correlation) and blue squares indicate significant negative correlations (rho < − 0.5, p < 0.05, Spearman correlation). (The letter before the taxa represents taxonomic rank: “p_” for phylum; “c_” for class; “o_” for order; “f_” for family; “g_” for genus; and “s_” for species. And “sp.” indicates an unidentified species name using the database.).
Discussion
Gut microbiota-targeted diets like fermented foods have the potential to improve the gut microbiome9. Our study highlights the first community-based intervention trial of plant-based fermented food in Pakistan. This study was designed to overcome the barriers to the intervention trials of fermented food, such as availability, standardization of doses, affordability, accessibility, social acceptability as a food item, and variable consumption of diverse fermented food available over the counter. These factors limit the generalizability of the results of fermented food trials.
Most well-designed fermented food intervention trials are limited to the Western world and are rare in highly malnourished settings, where such trials are most needed to tackle food insecurity and potentially play a beneficial role in gut homeostasis. Plant-based fermented foods offer the added advantage of extended shelf life without specific refrigeration requirements, making them suitable for underprivileged populations in rural areas. This diet is also appropriate for lactose-intolerant individuals and vegans, as well as those with religious restrictions on animal-based food consumption. It is convenient to either prepare these traditional fermented foods at home or scale them up as a local business to support women’s empowerment24.
This current trial was conducted to assess the impact of different traditional plant-based fermented foods intake, prepared by a local women entrepreneur, on the health of women of reproductive age living in high malnutrition areas. The main findings of this work highlight the significance of fermented food in ameliorating gut microbiota. The notable findings of our study are: (1) modulation of hematological parameters post-intervention; (2) identification of LABs in locally fermented achars, (3) change in α- and β-diversity indices post-treatment, and remodelling of gut microbiota at 8- and 12-weeks post-intervention, and (4) identifying distinct bacterial markers and their correlation with clinical biomarkers, post-intervention.
In this 12-week trial, the critical examination of multiple time point parameters of women revealed that different fermented pickles consumption uniquely modulated the gut microbiota and modestly affected the clinical parameters. This study showed successful compliance with 50g/day fermented pickle intake by the study participants. The minimum compliance for all intervention groups was 70%, which showed that allocating intervention based on choice was an effective strategy for better compliance. Most participants consumed the assigned pickles daily, especially mango-water and mango-oil pickles. Some participants shared reservations about the taste of carrot and lemon-chilli pickles. Daily consumption and taste inclination make mango-water the preferred pickle, followed by mango-oil and onion. Abdominal discomfort, heartburn, and diarrhea were the most frequently reported side effects, predominant among the radish, lemon-chilli, and carrot groups. Similar side effects were reported by another clinical trial conducted on fermented vegetable intervention in Western women16.
Upon assessing the impact of fermented pickle intervention on gut microbiota diversity indices, this study reported that among 6 intervention groups, there was a profound effect of lemon-chilli and onion pickle on modulating gut microbiota as evidenced by changes in α- and β- diversity indices. The results showed that upon regular intake of onion and lemon-chilli pickles for 8 weeks, the participants’ gut microbiota diversity was significantly increased. The increase in diversity was sustained after intervention discontinuation for at least 4 weeks, as reported in week-12 follow-ups. Fermented foods contain probiotics and related metabolites such as polyphenolic compounds and SCFA that potentially increase the diversity and functionality of the gut microbiome25. However, mango-water, mango-oil, radish, and carrot participants showed either non-significant or delayed changes in gut microbiota diversity. It could be due to a short intervention duration, providing insufficient time for gut microbiota remodelling26. Environmental factors could also hinder the gut microbiome diversity, including inadequate diet due to socioeconomic factors, continuous exposure to pathogens, lack of access to clean water, or lack of sanitation and hygienic lifestyle27,28. The increase in gut diversity upon pickle consumption aligns with the previous prospective cohort study conducted in the Chinese population, where pickle consumption significantly reduces the risk of diabetes by increasing the gut microbiota diversity29. In our study, mango-water and carrot pickle interventions reduced the α-diversity, and these results were consistent with another study conducted on the Pakistani population, which associated pickle consumption with low α-diversity30. Hence, our study concluded that the type and composition of fermented pickles showed variable effects on gut microbiota diversity.
One of the aims of this study was to explore the impact of fermented pickles intake on clinical and inflammatory markers. The data showed that in the participants who consumed lemon-chilli pickle, a significant reduction in platelets was observed in week 8. Platelets are known to interact with immune cells in inducing immune-mediated inflammatory diseases (IMIDs)31. Other inflammatory biomarkers, including WBCs, CRP, and MPO, though insignificant, were also decreased among the lemon-chilli group’s participants. Similarly, a significant reduction in WBCs was observed among the participants who consumed radish pickles. WBCs elevation is also a well-known inflammatory biomarker and a decrease in WBCs count represents a recovery trajectory in active inflammation patients32. However, among most of our other intervention groups, including mango-water, mango-oil, carrot, and onion, less modulations were observed in both clinical and inflammatory parameters, with the exception of LCN2 with mango-water.. LCN2 belongs to the family of proteins involved in inflammation and adaptive immune response, and an increase in LCN2 could be due to enhanced bioavailability of bioactive compounds like carotenoids and polyphenols, which are known to modulate inflammatory pathways33,34. The insignificant change in inflammatory parameters coincides with a 6-week clinical trial of fermented vegetable consumption in women, where the change in inflammatory biomarkers did not reach statistical significance16.
We also assessed the transition of gut microbiota composition during the study among intervention groups. The baseline gut microbiota composition is a key determinant of the favorable outcome of nutritional interventions, mainly shaped by host factors such as genetics, as well as geographic, dietary, and cultural factors35. Our study was conducted in a specific region, presenting consistent core taxa across the groups. Hence, the microbial shifts observed following pickle consumption may reflect region and population-specific microbiome profiles. Future studies across diverse populations are needed to assess the generalizability of these findings. Previous research has shown that Westernized diets high in fat and sugar are associated with a low abundance of fiber-degrading taxa, such as Prevotellaceae, Ruminococcaceae, and Lachnospiraceae36. We hypothesized an increased abundance of these taxa as a result of the fermented pickles intervention. Consistent with the hypothesis,relative abundance data showed a notable shift in Lachnospiraceae, Bifidobacteriaceae, and Ruminococcaceae families that belong to the phylum Firmicutes and Actinobacteria. Most of these species, belonging to phylum Firmicutes and Actinobacteria, were also part of the core taxa of study participants as identified at baseline; however, it is uncertain to suggest if the abundance of the already existing species increased or intervention introduced new species6. As plant-derived microbes contributes to human gut microbiome diversity37, our study aimed to identify bacterial taxa present in pickles. The microbiological examination of pickles identified LAB, including L. pentosus, L. plantarum, Pediococcus pentosaceus, Pediococcus acidilactici, L. brevis, Weissella cibaria, and S. hominis. These taxa differed from those found dominant in gut microbiota of study participants, suggesting their limited colonization potential. However, our findings were based on a culture-dependent method, which may underestimate microbial diversity. The similar taxa were reported in a review conducted on LAB isolated from Asian fermented foods38.
Another goal of this study was to identify if the top-taxa remodelled post-intervention. Remodelling showed beneficial bacterial occurrence and persistence over time. The Venn diagram suggested that post-intervention, most of the top-taxa remained unchanged, with the exception of Subdoligranulum and Fecalibacterium in lemon-chilli, and Prevotella and Olsenella in onion participants. Since these taxa persisted in both post-intervention time points, this shows the capability of the gut microbiome to adapt and sustain dietary changes39. All these bacteria are SCFA producers, crucial for improved gut health and reduced inflammation40,41. The LDA further validates that different vegetable and fruit pickle consumption uniquely remodels the gut microbiota and presents distinct gut taxa among the participants. This could be attributed to different vegetables possessing dissimilar bioactive constituents42, even if fermented under the same process and containing similar bacteria. As reported by Kiczorowski et al., the fermentation of different vegetables showed the synthesis of diverse essential enzymes and other active constituents, including minerals, vitamins, antioxidants, phenols, and heavy metals43.
Finally, this study also correlated the pre- and post-intervention distinct taxa identified by LDA analyses with clinical parameters. The analyses revealed that post-intervention taxa negatively correlated with LCN2, CRP, and platelets, suggesting the anti-inflammatory role of the intervention and predicting potential beneficial modulation in systemic inflammation through dietary changes44. Conversely, the positive association of bacterial taxa with neutrophils suggests an enhanced immune response upon lemon-chilli intake. This dual relationship of gut microbiota with the immune system upon lemon-chilli consumption highlights the complex interaction of gut microbiome, diet, and host health45.
Rich distinct taxa and their correlation with clinical biomarkers in the lemon-chilli intervention group favor the hypothesis that combining the beneficial components of different vegetables synergistically enhances gut diversity and functionality. This could be potentially due to the combined effect of capsaicin, an active constituent of chilli which increases the abundance of Ruminococcaceae and Lachnospiraceae, both butyrate-producing bacteria, and pectin, an active dietary fiber of lemon, which leads to the production of SCFA via gut microbiome46. Given the distinct response by fermented lemon-chilli pickle, future exciting opportunities could be to analyze the mixed-vegetables pickle response in the gut microbiome compared to individual pickle intake. Furthermore, additional studies could be conducted to strategically assess the mechanistic role of fermented pickle intake in alleviating different health conditions, particularly malnutrition and gut-inflammatory diseases in murine models and large, diverse population cohorts.
Despite few limitations, this study revealed significant insights into the effect of consuming fermented pickles on the gut microbiota and clinical parameters This study had a modest number of participants (30/arm) from one district of Pakistan, which limits generalizability. The exclusion criteria were limited to pickle consumption rather than all fermented foods. Participants were not restricted to one type of fermented food; it is possible to have mixing of milk based fermented food as our goal was to exclusively study plant based fermented food as part of Grant Global Challenge Funds call (https://gcgh.grandchallenges.org/challenge/integrating-tradition-and-technology-fermented-foods-maternal-nutrition). Another limitation is inconsistent timepoints of blood (two timepoints) and stool (three timepoints) sampling due to operational constraints. Future studies with multiple sampling points would effectively capture the effects of dietary interventions on host inflammation and microbiome dynamics. The intervention duration was 8 weeks, which could be limited to assess the long-term effects Our previous work conducted in the Pakistani population suggested that inflammatory biomarkers can be used as proxy of gut-inflammation47,48. The outcome analyses of these inflammatory biomarkers and 16S rRNA gene profiling were valuable, but had certain limitations 49,50. Integrating metabolic and compositional profiling into analyses could offer a comprehensive view of the mechanistic and biological processes involved in host-diet-microbiome interactions. Future studies should also include comprehensive microbial profiling of the fermented pickles and stratify study participants by baseline microbiota to better understand variation in response. Lastly, this study was conducted in healthy women, and future studies should be performed in the context of environmental enteric dysfunction (EED).. Causality-related data can be generated using additional experimental models to explore the mechanistic underpinnings of such interventions.
Conclusion
In conclusion, the findings of this study emphasized that regular intake of different fermented pickles has a varied but beneficial impact on the gut microbiome and other significant clinical parameters, potentially enhancing overall health outcomes in WRA. Among the six intervention groups, lemon-chilli and onion pickles demonstrated a notable impact on gut microbiota, as evidenced by changes in α- and β-diversity indices. However, future investigations into a large population are needed to further explore the interactions of fermented pickles, particularly mixed varieties, on the gut microbiota and overall health outcomes. Moreover, the use of integrated multi-omics approaches and murine models of malnutrition and gut inflammatory diseases could offer valuable mechanistic insights into host-diet-gut microbiome interactions and their role in treating gut pathologies.
Materials and methods
Study design and site
A community-based multi-arm clinical trial was conducted in Matiari, a rural district of Sindh, Pakistan. 223 women of reproductive age (WRA) were recruited from Dec 2021 to Oct 2022 to explore the impact of a fermented pickle diet on the gut microbiota and immune parameters. Approval from the Ethical Review Committee of Aga Khan University was obtained (ERC-2022-6595-23253: Grand Challenges Fermented Food - Achars (fermented pickles) in Pakistan) (Approval: 02-10-2021). The trial was registered on Clinicaltrials.gov, identifier: NCT06748313 (Registration: 27-12-2024). Informed consent was taken from all the recruited participants. All study procedures were performed according to institutional guidelines and regulations, and following the Declaration of Helsinki.
Eligibility criteria
Participants under 18 or above 49 years of age, with any gastric/major illness history, frequently consumed pickles, or antibiotics/probiotics within 2 weeks of the interview date were excluded from the study.
Fermented pickle preparation
Fermented pickles were prepared by a local vendor using a traditional recipe. The fresh vegetables and fruits (5 kg) separately, were cut and mixed with turmeric (50 g), salt (100 g), mashed mustard seeds (50 g), chilli powder (60 g), spring garlic (250 g), mustard oil (50 ml), and water or oil (3 L) – depending on water-based or oil-based achar. The prepared mixture was then put under shade at 20–30℃ for 2–14 days, sun-dried for 3–5 days, and packed for consumption. These recipes were aligned with those reported by Bhera et al.51 with a few traditional changes (Fig. 1b).
Intervention
The participants were assigned to 6 different fermented pickles based on their preference; those who did not like pickles were assigned to the control group (30/per group, total N = 210). The total number of participants enrolled was 223, exceeding the required sample size to account for potential loss-to-follow-up. The intervention groups included the mango-water-based pickle group (N = 32), mango-oil-based pickle group (N = 31), carrot pickle group (N = 33), radish Pickle group (N = 32), onion Pickle group (N = 32), lemon-chilli pickle group (N = 32), and control group (N = 31). All participants except the control group consumed 50 g/day of fermented pickles for 8 weeks in addition to their regular meals. Refilling of the pickles was ensured every third day. The food recall questionnaire, side effects, clinical parameters, and compliance follow-up data were collected every 2 weeks (Fig. 1c).
Compliance, side effects, and food recall data
The food frequency recall questionnaire, side effects, and medication intake were logged by trained research staff every 2 weeks. Food was classified into six categories: cereals, fruits and vegetables, dairy products, meat, beverages, and pickles. To avoid sharing weighed pickles, an additional jar was provided to the participants for other house members, and compliance was validated by measuring the weight of the jars returned. The field staff were also trained to monitor participants’ compliance with the assigned task. As per protocol, the staff evaluated and recorded intervention compliance by measuring the weight of the pickle jar before handing it over to the participant, and in return, after every 3 days was calculated using the formula “Total achar consumed = weight of achar bottles provided – the weight of achar bottles remaining”. Percentage compliance was calculated using the following formula:
Microbiological analysis of food and bacterial sequencing
The pickle samples were cultured using De-mannose Ragosa Sharpe (MRS) Agar and Brain Heart Infusion (BHI) agar to calculate the number of live bacteria present in the pickle brine. The brine was serially diluted using 1X Phosphate Buffer Saline (PBS). Agar plates were incubated at 37℃ for 48–72 h under aerobic and anaerobic conditions. The colony forming units (CFU) per ml were counted from a countable plate and colonies were sub-cultured to isolate pure colonies, and stored in 20% glycerol at −80℃ for later use.
DNA was extracted from the selected gram-positive colonies isolated using a lysis buffer and Qiagen DNA extraction kit (Qiamp-51106) according to the manufacturer’s instructions. Lysis buffer was prepared by adding lysozyme to a lysis solution containing 1 M Tris-HCl (pH 8.0) (Sigma), 0.5 M EDTA (pH 8.0) (Invitrogen), and Triton X-100 (Sigma-Aldrich). DNA was subjected to shallow sequencing for taxonomic identification of pure bacterial strains. Kit used for library preparation (DNA prep). The samples were multiplexed using an indexing kit and pooled to generate a single library loaded onto the MiSeq flow cell (v3–600 cycles kit). For every sample, reads with Q > = 15 was used for taxonomic identification. The Bacterial and Viral Bioinformatics Resource Center (BV-BRC) platform was used to identify the taxonomy of bacteria using paired-end reads (https://www.bv-brc.org/).
Anthropometric measurements and clinical parameters
Anthropometric measurements, heart rate, temperature, respiratory pressure, and blood pressure were documented every at 2-week follow-up during the intervention.
Sample collection
Stool samples were collected at week 0 (pre-intervention), week 8 (end-intervention), and week 12 (post-intervention) by the field team within 30 mins of stool pass and snap-frozen using dry-ice for transport to the Matiari Research Lab (MRL) and stored at −80 ℃.
Similarly, blood samples were collected at week 0 (pre-intervention) and week 8 (end-intervention) in EDTA tubes and transported to the MRL at 4 ℃. An aliquot of blood was used to measure complete blood count (CBC) and C-reactive protein (CRP) levels.
Stool inflammatory cytokines
Commercial ELISA kits were used to measure intestinal inflammation using a myeloperoxidase (MPO) kit (Immunodiagnostic AG, Stubenwald-Allee, Bensheim), and lipocalin-2 (LCN2) (GenWay Biotech, San Diego, CA, USA). All protocols were performed per the manufacturer’s instructions. The final dilution was measured by determining the appropriate concentration of a biomarker using a linear range of the standard curve. LCN2 at the dilutions of 1:30 and 1:50, and MPO at 1:50 and 1:100 were considered. All plates were read on 450 nm using a BioRad iMark (Hercules, CA) plate reader.
Stool DNA extraction
DNA was extracted according to the manufacturer’s protocol, using a Qiagen DNeasy Power Soil Pro Kit (Qiagen, Germany) and quantified using Invitrogen Qubit 1X dsDNA HS assay kit (Thermo Scientific, USA) on a Qubit Fluorometer. The purity of DNA was assessed using a Nanodrop 2000 spectrophotometer (Thermo Scientific, USA) and stored at −80 °C ULT freezer, until further use.
16S amplicon sequencing and statistical analyses
The DNA samples were shipped to the University of Minnesota Genomic Center (UMGC) for amplification of the V4 region of the 16S ribosomal RNA (rRNA) subunit gene6. On average, 45,102 reads per sample were generated. The quality of the raw fastq files was assessed using MultiQC and were further processed using the DADA2 pipeline to denoise (with the following parameters used in the filterAndTrim-step: maxN = 0, maxEE = c2, truncQ = 2, rm.phix = TRUE, compress = TRUE), dereplicate reads, merge pair-end reads, and remove chimeras52. Amplicon sequence variants (ASVs) were assigned using the naive Bayesian classifier via assignTaxonomy command and aligned against the SILVA rRNA gene database (https://benjjneb.github.io/dada2/training.html). A phylogenetic tree was constructed using the QIIME2“q2-phylogeny” plugin based on the ASV sequences. The data objects were combined into a Phyloseq object for downstream analysis. After pre-processing, the 585 ASVs were normalized by scaling the sample counts using a compositional transformation. This transformation ensured uniform representation of ASV counts across all samples to facilitate effective comparison. The relative abundance of ASVs was calculated, and those with relative abundance > 2.5% were considered as the most abundant within the bacterial community for each group. To highlight the dominant microbial phyla or families, we applied a 2.5% cutoff based on the median relative abundance across samples. Phyla or families with a median abundance greater than 2.5% were retained and displayed individually, while those below this threshold were grouped under the label “<2.5%”. This approach streamlines data presentation and highlights the most abundant, biologically relevant taxa.
α-diversity metrics (Observed and Shannon) were measured using the plot richness function in Phyloseq, with the significance calculated using the Kruskal-Wallis test. β-diversity was calculated using the Bray–Curtis index with 9999 permutations (Vegan package) and visualized as principal component analysis (PCoA) ordination plots. A pairwise PERMANOVA test was conducted to identify significant differences among groups. Intergroup comparison among the top 20 highly abundant taxa was used to identify the common and unique taxa.
Linear discriminant analysis (LDA) effect size (LEfSe) analysis was used to determine the taxa contributing to the differences among the groups at 0, 8, and 12 weeks, with significance tested by Kruskal-Wallis and Wilcoxon rank-sum test (p ≤ 0.05), followed by LDA to estimate effect size at log10 values. The LDA cut-off was set to 0.0 across all the groups. For the LEfSe analysis, the phyloseq object generated from the DADA2 pipeline contained filtered absolute counts at the amplicon sequence variant (ASV) level for each intervention group. The cladograms were plotted using ggdendro package in R. The correlation of taxa markers from Lefse (0 week and 8 week) with blood (WBCs, MCH, MCHC, neutrophils) and inflammatory (CRP, MPO, LCN2) parameters was calculated using Spearman’s correlation. All the microbiota data analysis was performed using R v.4.4.1 software.
Variables other than microbiota data were analyzed using Stata/SE 17.0 for Windows. Continuous variables were summarized as means with standard deviations (mean ± SD), while categorical variables were reported as frequencies and percentages. A paired t-test for two timepoints and repeated measure Analysis of variance (ANOVA) for three timepoints were utilized to compare mean values across groups, with post-hoc tests applied for specific group comparisons. The chi-square test was used to assess differences in categorical variables between groups. Additionally, multivariate regression analysis was conducted to control for potential confounding variables.
Data availability
The 16S rRNA gene sequencing data have been deposited into NCBI Sequence Read Archive (SRA) database under accession number PRJNA1274831. For further information, please contact the corresponding author, NTI, at [najeeha.iqbal@aku.edu](mailto: najeeha.iqbal@aku.edu) for data requests.
References
Popkin, B. M., Corvalan, C. & Grummer-Strawn, L. M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395 (10217), 65–74 (2020).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 (1), 55–71 (2021).
Manor, O. et al. Health and disease markers correlate with gut Microbiome composition across thousands of people. Nat. Commun. 11 (1), 5206 (2020).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30 (6), 492–506 (2020).
van den Munckhof, I. C. et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes. Rev. 19 (12), 1719–1734 (2018).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184 (16), 4137–4153 (2021). e14.
Marco, M. L. et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat. Reviews Gastroenterol. Hepatol. 18 (3), 196–208 (2021).
Mukherjee, A., Breselge, S., Dimidi, E., Marco, M. L. & Cotter, P. D. Fermented foods and Gastrointestinal health: underlying mechanisms. Nat. Reviews Gastroenterol. Hepatol. 21 (4), 248–266 (2024).
Ross, F. C. et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat. Rev. Microbiol. 1–16. (2024).
Rinott, E. et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut Microbiome modifications: A randomized controlled trial. Genome Med. 14 (1), 29 (2022).
Meslier, V. et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut Microbiome and metabolome independently of energy intake. Gut 69 (7), 1258–1268 (2020).
Ang, Q. Y. et al. Ketogenic diets alter the gut Microbiome resulting in decreased intestinal Th17 cells. Cell 181 (6), 1263–1275 (2020). e16.
Link, V. M. et al. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat. Med. 30 (2), 560–572 (2024).
Lee, W. et al. Effects of Kimchi consumption on body fat and intestinal microbiota in overweight participants: A randomized, double-blind, placebo-controlled, single-center clinical trial. J. Funct. Foods. 121, 106401 (2024).
Schropp, N. et al. The impact of regular sauerkraut consumption on the human gut microbiota: a crossover intervention trial. Microbiome 13, 52 (2025).
Galena, A. E. et al. The effects of fermented vegetable consumption on the composition of the intestinal microbiota and levels of inflammatory markers in women: a pilot and feasibility study. PLoS One. 17 (10), e0275275 (2022).
Valentino, V. et al. Fermented foods, their Microbiome and its potential in boosting human health. Microb. Biotechnol. 17 (2), e14428 (2024).
Coker, J. K., Moyne, O., Rodionov, D. A. & Zengler, K. Carbohydrates great and small, from dietary fiber to Sialic acids: how glycans influence the gut Microbiome and affect human health. Gut Microbes. 13 (1), 1869502 (2021).
Surve, S., Shinde, D. B. & Kulkarni, R. Isolation, characterization and comparative genomics of potentially probiotic Lactiplantibacillus plantarum strains from Indian foods. Sci. Rep. 12 (1), 1940 (2022).
Torres, S., Verón, H., Contreras, L. & Isla, M. I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness. 9 (2), 112–123 (2020).
Banik, A. et al. Characterization of lactic acid bacteria isolated from street pickles of Dhaka, Bangladesh. Heliyon 9(6) (2023).
Kabir, F. et al. Impact of enteropathogens on faltering growth in a resource-limited setting. Front. Nutr. 9, 1081833 (2023).
Stiemsma, L. T., Nakamura, R. E., Nguyen, J. G. & Michels, K. B. Does consumption of fermented foods modify the human gut microbiota? J. Nutr. 150 (7), 1680–1692 (2020).
Reid, G. et al. Empowering women through probiotic fermented food in East Africa. J. Global Health 10(1) (2020).
Pyo, Y., Kwon, K. H. & Jung, Y. J. Probiotic functions in fermented foods: anti-viral, immunomodulatory, and anti-cancer benefits. Foods 13(15). (2024).
Fragiadakis, G. K. et al. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 111 (6), 1127–1136 (2020).
Moran-Ramos, S. et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes. 11 (4), 900–917 (2020).
Mills, M. et al. Household environment and animal fecal contamination are critical modifiers of the gut Microbiome and resistome in young children from rural Nicaragua. Microbiome 11 (1), 207 (2023).
Cai, Y. et al. Regular consumption of pickled vegetables and fermented bean curd reduces the risk of diabetes: a prospective cohort study. Front. Public. Health. 11, 1155989 (2023).
Gul, F. et al. Gut microbial ecology and exposome of a healthy Pakistani cohort. Gut Pathogens. 16 (1), 5 (2024).
Scherlinger, M., Richez, C., Tsokos, G. C., Boilard, E. & Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 23 (8), 495–510 (2023).
Foy, B. H., Sundt, T. M., Carlson, J. C., Aguirre, A. D. & Higgins, J. M. Human acute inflammatory recovery is defined by co-regulatory dynamics of white blood cell and platelet populations. Nat. Commun. 13 (1), 4705 (2022).
Zollner, A. et al. Faecal biomarkers in inflammatory bowel diseases: calprotectin versus lipocalin-2—a comparative study. J. Crohn’s Colitis. 15 (1), 43–54 (2021).
Sakaguchi, C. A. et al. Influence of 2 weeks of Mango ingestion on inflammation resolution after vigorous exercise. Nutrients 16 (1), 36 (2023).
Parizadeh, M. & Arrieta, M-C. The global human gut microbiome: genes, lifestyles, and diet. Trends Mol. Med. 29 (10), 789–801 (2023).
Moy, M., Diakiw, L. & Amato, K. R. Human-influenced diets affect the gut Microbiome of wild baboons. Sci. Rep. 13 (1), 11886 (2023).
Wicaksono, W. A. et al. The edible plant microbiome: evidence for the occurrence of fruit and vegetable bacteria in the human gut. Gut Microbes. 15 (2), 2258565 (2023).
Doo, H. et al. Lactic acid bacteria in Asian fermented foods and their beneficial roles in human health. Food Sci. Biotechnol. 1–13. (2024).
Leshem, A., Segal, E. & Elinav, E. The gut microbiome and individual-specific responses to diet. Msystems 5(5), https://doi.org/10.1128/msystems.00665-20 (2020).
Houtman, T. A., Eckermann, H. A., Smidt, H. & de Weerth, C. Gut microbiota and BMI throughout childhood: the role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 12 (1), 3140 (2022).
Zhang, Z. et al. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell. Communication Signal. 20 (1), 64 (2022).
Wen, J-J. et al. Different dietary fibers unequally remodel gut microbiota and charge up anti-obesity effects. Food Hydrocoll. 140, 108617 (2023).
Kiczorowski, P., Kiczorowska, B., Samolińska, W., Szmigielski, M. & Winiarska-Mieczan, A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 12 (1), 13422 (2022).
Guo, X. et al. Gut microbiota is a potential biomarker in inflammatory bowel disease. Front. Nutr. 8, 818902 (2022).
Schluter, J. et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 588 (7837), 303–307 (2020).
Yang, J., Li, W. & Wang, Y. Capsaicin reduces obesity by reducing chronic Low-Grade inflammation. Int. J. Mol. Sci. 25 (16), 8979 (2024).
Haberman, Y. et al. Mucosal genomics implicate lymphocyte activation and lipid metabolism in refractory environmental enteric dysfunction. Gastroenterology 160(6), 2055-2071.e0 (2021).
Iqbal, N. T. et al. A shared group of bacterial taxa in the duodenal microbiota of undernourished Pakistani children with environmental enteric dysfunction. Msphere 9 (6), e00196–e00124 (2024).
Hart, M. J., Torres, S. J., McNaughton, S. A. & Milte, C. M. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr. J. 20, 1–14 (2021).
Dreier, M. et al. High-throughput qPCR and 16S rRNA gene amplicon sequencing as complementary methods for the investigation of the cheese microbiota. BMC Microbiol. 22 (1), 48 (2022).
Behera, S. S., El Sheikha, A. F., Hammami, R. & Kumar, A. Traditionally fermented pickles: how the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods. 70, 103971 (2020).
Palmnäs-Bédard, M., de Santa Izabel, A., Dicksved, J. & Landberg, R. Characterization of the bacterial composition of 47 fermented foods in Sweden. Foods 12(20) (2023).
Acknowledgements
We thank our research and field teams at Matiari and the study participants for complying with our study protocol.We acknowledge the partial support of research from the National Institute of Health’s Fogarty International Center (5D43TW007585-13) in conducting this research as part of author SHH Ph.D. training.
Funding
This study was funded by the Bill & Melinda Gates Foundation (BMGF) through a grant: Global Grand Challenges: Integrating Tradition and Technology for Fermented Foods for Maternal Nutrition (INV-033567)https://gcgh.grandchallenges.org/grant/achars-pickles-reduce-inflammation-and-improve-microbiome-rural-pakistani-women (Funding: 13-07-2021). SH received research training support from the National Institute of Health’s Fogarty International Center (5D43TW007585-13). The funders had no role in the design, data collection, analysis of the study, or the decision to publish or prepare this manuscript.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: NTI, JI, SA, and SAA: designed research; SHH, SA, KA, AH, and FK: conducted research; AK and SHH: analyzed data; SHH: wrote the paper; JI, SAA and NTI: had primary responsibility for final content; FS, AKQ, and FU: provided data management; SHH and AK: provided data interpretation; NTI, SRM and SAA: provided substantial manuscript editing. All the authors contributed to the manuscript revision and confirmed their approval for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hafeez, S.H., Khalid, A., Ahmed, S. et al. Fermented pickles improve gut microbiota and immune profile in women in a community trial in rural Pakistan. Sci Rep 15, 34522 (2025). https://doi.org/10.1038/s41598-025-17721-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17721-8