Abstract
Fruit waste is an abundant and eco-friendly green resource for the synthesis of nanoparticles, offering a sustainable alternative to conventional physical and chemical methods. In this study, we prepared green iron oxide nanoparticles (Fe2O3-NPs) with excellent biological and catalytic properties. The materials are synthesized from polyphenol-rich extracts of Citrus limetta (sweet lemon) peels. After extensive characterization via UV, XRD, FTIR, SEM, TEM, EDX, and PL, the materials were tested for their biological properties and catalytic degradation of 2-NP and Methylene Orange (MO). We observed excellent antibacterial properties of Fe2O3-NPs, especially against gram-negative bacteria, with MICs of 0.625 mg/mL against E. coli and 1.125 mg/mL against P. aeruginosa. The eco-friendly materials also resulted in potential antiparasitic potential against L. tropica, the parasite that causes leishmaniasis. Furthermore, the NPs exhibit remarkable antioxidant properties and considerable enzyme inhibition ability against α-amylase, α-glucosidase, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE), indicating their potential usefulness in treating diabetes mellitus and Alzheimer’s disease. In addition to their outstanding biological properties, NPs have shown remarkable catalytic properties in the degradation of environmental pollutants such as 2-NP and methyl orange (MO). 98.32% degradation was observed for 2-NP in just 15 min, whereas 97.5% degradation was recorded for MO in 25 min. This excellent catalytic potential shows that the multifunctional materials synthesized from waste biomass can be utilized for important biological and environmental applications.
Similar content being viewed by others
Introduction
The uniqueness and credibility of green synthesized metallic nanoparticles (MNPs) have attracted considerable attention, as these nanomaterials have opened up the gateways to tremendous applications in health care, energy, and the environment1,2. Recently, ferric oxide nanoparticles (maghemite: Fe2O3-NPs and magnetite: Fe3O4-NPs) have vitalized the field of nanotechnology because of their small size and well-reported applications in the fields of metal ion sensing, catalysis, chemical and thermal stability, electrochemical sensing, magnetic resonance imaging (MRI), water splitting, and energy storage3,4,5,6,7,8,9. In addition to these applications, Fe2O3-NPs are well-reported nanoabsorbents that provide valuable materials in the field of environmental remediation10,11.
Over the last decade, considerable progress has been made in the medical and biomedical sectors for Fe2O3-NPs because of their biocompatible nature, large surface area-to-volume ratio, and magnetic behavior. The adaptability and compatibility of these NPs have led researchers to explore them as drug delivery vehicles for antibacterial, antileishmanial, antifungal, anticancer, and antidiabetic drugs12,13,14,15,16,17,18,19,20. Fe2O3-NPs have also been successfully explored for biosensing, bioimaging, cell labeling, cell tracking, targeted gene therapy, and tissue engineering21,22,23,24,25. Furthermore, Fe2O3-NPs can behave as nanoenzymes during oxidative stress management, as they mimic the activities of natural enzymes while being artificial materials26,27. Interestingly, Fe2O3-NPs have also been explored for their ability to pass through the blood‒brain barrier, which is a prominent challenge in delivering drugs to the brain28,29.
Several approaches (green, chemical, and physical) have been reported for the synthesis of Fe2O3-NPs, with each approach associated with certain advantages and disadvantages. The chemical approach involves the utilization of toxic chemicals, which can be hazardous if handled improperly, whereas the physical approach involves high pressure and temperature, which are costly and quite difficult to maintain30,31,32. Although such approaches produce uniformly sized and shaped NPs, the green method is preferred over these approaches33. The green approach involves the utilization of plant biomass and microbes, which are comparatively eco-friendly, inexpensive, and easy to handle. Several plant extracts have been documented for use in the synthesis of Fe2O3-NPs, such as Oscillatoria limnetica, Spondias dulcis, Olea europaea, Piper betel, Prosopis farcta, Azadirachta indica, Camellia sinensis, Rhus punjabensis, and Sida cordifolia34,35,36,37,38,39. These plant extracts not only function as reducing, stabilizing, and capping agents but also confer bioactive properties to the NPs. Similarly, new approaches have been recently introduced for the synthesis of metallic NPs (MNPs) by using agro-waste as a reducing and capping material. These agricultural by-products, such as fruit peels, leaves, shrubs, and seeds, contain phytochemicals (flavonoids, phenolics, and alkaloids) that are reducing in nature and provide a sustainable, eco-friendly, and cost-effective approach for MNP synthesis. By using waste for the production of valuable products, this approach also supports a circular economy and sustainable development40,41.
Citrus peel makes up approximately 40–50% of the total mass of fruit, but it is mostly ignored and considered waste. However, it is a source of various health-benefiting compounds, especially phenols and carotenoids. Phenolic compounds have proven to be valuable sources of antioxidant materials, as biomass is enriched with antioxidant compounds such as p-coumaric, ferulic, gallic, vanillic, and sinapic acids. Interestingly, these antioxidant phenolic compounds are more abundant in the peel than in the edible part. Therefore, the peel of citrus fruit can be used for various health-promoting activities, especially as a coating material for nanoparticles42,43.
In this study, we utilized an ecofriendly approach for Fe2O3-NPs synthesis using Citrus limetta peels (waste), thus supporting sustainability and circular economy principles. After synthesis, the optical, physicochemical, morphological, and biological capabilities of the NPs were comprehensively assessed via multiple characterization techniques and biological assays. The NPs were also utilized to evaluate their catalytic potential for the degradation of 2-Nitrphenol and methyl orange (MO). We thus demonstrated the multifunctional potential of citrus-waste-derived nanomaterials for both biomedical and environmental applications.
Materials and methods
Chemicals and reagents
Analytical or HPLC grade chemicals and reagents were used in this study. The chemicals used in this study were as follows: FeCl3. 6H2O (≥ 98%) from Samchun Pure Chemical Co., Ltd., and sodium borohydride (NaBH4, ≥ 98%), 2-nitrophenol (2-NP, 98%), and methyl orange (MO, 85%) from Sigma Aldrich were used. Levofloxacin (≥ 98.0- HPLC), Acarbose (≥ 95% HPLC), and Galantamine (≥ 94% HPLC), which were used as positive controls, were also purchased from Sigma Aldrich. Distilled water was utilized in all of the experiment where required to avoid any potential contamination.
Methodology
Biomass collection, processing, and extract formulation
Waste from C. limetta (Peels), also known as sweet lemon or Mosambi, was collected from a local juice shop in the localities of Islamabad, Pakistan. The peels were cleaned with tap water, shade-dried, and ground into a fine powder via a mortar and pestle. Briefly, 5 g of the powder was taken and stirred in 150 mL of distilled water to prepare the extract at 55 °C for 2 h. Later, the flask containing the extract was transferred to a shaking incubator for prolonged mixing at room temperature for 12 h. After prolonged shaking, the extract was filtered three times via muslin cloth. Finally, the extract was poured into Petri dishes, oven-dried (40 °C for 16 h), and subsequently collected in a Falcon tube. The dried extract was stored at 4 °C until further use.
Gas chromatography‒mass spectrometry (GC/MS) analysis of Peel extracts
GC‒MS analysis was performed via a gas chromatograph (GC) coupled with a mass spectrometer (MS) to identify the volatile compounds in the sample. The samples were extracted via hydrodistillation or solvent extraction before analysis. The system was equipped with a capillary column, and helium was used as the carrier gas at a constant flow rate. The oven temperature was programmed to start at 50 °C and was increased at a rate of 5 °C per min until it reached 250 °C. The sample was injected in split mode with a split ratio of 1:20, and ionization was conducted in electron ionization (EI) mode at 70 eV. The compounds were identified on the basis of their retention times (RTs) and mass fragmentation patterns, which were compared with the NIST or Wiley spectral library. The relative abundance of each compound was determined via total ion chromatogram (TIC) peak area percentages44.
Identification and quantification of phenolic and flavonoid compounds by HPLC
HPLC analysis was conducted to identify and quantify phenolic and flavonoid compounds in the sample. The separation was performed via a high-performance liquid chromatography (HPLC) system equipped with a UV‒Vis detector set at 280 nm. A C18 reversed-phase column was used for compound separation, with a mobile phase consisting of a mixture of solvent A (water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The gradient elution program was applied at a constant flow rate, and the injection volume was set appropriately. The sample was filtered before injection, and detection was carried out by monitoring the absorbance at 280 nm. Identification and quantification were achieved by comparing the retention times (RTs) and peak areas with those of authentic standards. The concentration of each compound was calculated on the basis of a standard calibration curve, ensuring accurate quantification45.
Citrus limetta (waste)-mediated synthesis of Fe2O3-NPs
In this study, the waste extract of C. limetta was used as a reducing and capping agent to prepare Fe2O3-NPs. Briefly, FeCl3. 6H2O (3.7 g) (0.125 M) was dissolved in 100 mL distilled water, and the temperature was increased to 80 °C, followed by the addition of peel extract (100 mg), which was separately prepared in 50 mL distilled water. The reaction was carried out for 4 h and continuously stirred at 80 °C. Finally, the resulting mixture was washed via centrifugation at 4500 rpm, sonicated for 5 min, poured into Petri plates, oven-dried at 75 °C for 12 h, and finally collected after complete drying. The collected product was ground into a fine powder by using a mortar and pestle and then calcined at 200 °C for 3 h. The NPs were then stored in an Eppendorf tube labeled with G-Fe2O3-NPs.
Yield recovery and characterization of the prepared materials
The green-synthesized Fe2O3-NPs were thoroughly characterized for their optical, physicochemical, and morphological characteristics via UV-Visible spectroscopy, Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), photoluminescence (PL), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy-dispersive X-ray (EDX) analysis. The yield of the prepared Fe2O3-NPs was evaluated via the following formula:
Here, “W” represents the weight of the precursor salt, whereas “w” represents the weight of the prepared NPs. Absorbance of citrus extract and prepared NPs was analyzed by Uv-vis spectroscope within wavelength ranging from 240 nm to 600 nm. Functional group analysis and capping of the phytochemicals were confirmed at wavelengths between 4000 and 400 and 500 cm− 1 via FTIR of Fe2O3-NPs and citrus extract respectively and, and the crystallinity of the NPs was evaluated via an X-ray diffractometer fitted with Cu as the radiation source and Ni as the filter. This characterization was carried out between 20° and 70°, and the crystallite size of the NPs was calculated via the Debye–Scherer equation46.
The electrical band structure of the Fe2O3-NPs was examined via photoluminescence (PL) analysis, and the NP size and shape were confirmed by scanning and transmission electron microscopy. Finally, the elemental composition of the NPs was evaluated via EDX spectroscopy.
pH-based dispersion studies
The dispersion capability of the green Fe2O3-NPs was observed at various pH values (pH 2, pH 7, and pH 12) as a function of storage time47. Specifically, 0.25 mg/mL of the Fe2O3-NPs were stirred and suspended in a glass vial containing 7 mL of pH media (distilled water with different pH values). The samples were subjected to ultrasonication to ensure proper dispersion and finally placed for visualization of their dispersive nature, as evidenced by images taken at varying time intervals up to 24 h.
Biological properties
Bactericidal properties of the Fe2O3-NPs evaluated against gram-positive and gram-negative strains
The bactericidal efficacy of phytochemical-capped Fe2O3-NPs was assessed via a typical well diffusion approach47. Concentration-dependent activity was evaluated against several gram-positive and negative strains, including Staphylococcus aureus (ATCC 6538), methicillin-resistant Staphylococcus aureus (ATCC 33591), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC-15442). Initially, nutrient media was prepared in Petri dishes, followed by swabbing with 100 µL of bacterial strain suspension, and later, the plates were allowed to dry for 5 min. The wells were subsequently prepared and tagged as A, B, C, D, -ve, and + ve. Serial dilutions of the prepared NPs were added to the tagged wells so that each tagged well contained a specific dilution of Fe2O3-NPs (Well-A: 40 mg/mL, Well-B: 20 mg/mL, Well-C: 10 mg/mL, and Well-D: 5 mg/mL). Levofloxacin was used as the positive control, while DMSO was used as the negative control for this study. The plates were incubated for 20 h at 37 °C, after which the zone of inhibition (ZOI) was measured in millimeters (mm) via a measurement scale. Samples that exhibited > 10 mm ZOIs at 5 mg/mL were further scrutinized for MICs via the micro broth dilution technique. After careful observation of visible growth, the lowest concentration at which the NPs demonstrated visible and notable growth inhibition of the strain was labeled the MIC of the test sample.
Anti-parasitic assay against Leishmania tropica
The antileishmanial potential of the NPs was evaluated via a method described by Khan et al. (2023) against the promastigote (flagellated) and amastigote (nonflagellated) stages of a well-known strain, Leishmania tropica-KWH2346,48. First, fresh cultivation of both parasitic strains was carried out for 12 h in M199 media supplemented with 10% fetal bovine serum. Subsequently, 180 µL of the fresh parasitic culture mixture and 10 µL of the NP sample (concentrations: 12.5–200 µg/mL) were mixed in the wells of a 96-well plate and incubated for 72 h at 28 ± 1 °C. Finally, a 4 h reincubation was carried out after the addition of 10 µL (0.5 mg/mL) of MTT solution. NPs dissolved in DMSO served as a negative control, while amphotericin-B was used as a positive control for this assay. The analysis of absorbance was carried out at 570 nm, and the results were denoted as % inhibition.
In this equation, Abp represents the absorbance of the sample (NPs), whereas Abn denotes the absorbance of the negative control.
Antioxidant studies of green-synthesized Fe2O3-NPs
Free radical scavenging assay (FRSA) against DPPH
The Fe2O3-NPs were observed via DPPH-FRSA via a protocol recently used by Ahmad et al. (2024)49,50,51. This assay comprises two steps. In the first step, 20 µL of NP solution (multiple concentrations: 50 µg/mL-400 µg/mL) was mixed with 180 µL of the DPPH reagent in the specified wells of a 96-well plate, which was led by a 1 h dark incubation at 37 ± 1 °C. Finally, in the second step, the absorbance of the sample was analyzed at 517 nm, and the free radical scavenging ability of the NPs was evaluated via an equation.
Here, DMSO and ascorbic acid served as negative and positive controls, respectively. In this equation, Ab denotes the absorbance of the sample (NPs with DPPH reagent).
2,2-AAzino-bis-3-ethylbenzothiazoline-6-sulfonic acid assay (ABTS-FRSA)
Each sample’s ABTS scavenging evaluation was carried out with certain minor adjustments utilizing the methodology previously described by Ihsan et al.52. Potassium persulfate (6 mM) and ABTS stock solutions (14 mM) were combined in a 1:1 ratio to create the ABTS solution, which was then stored at room temperature for 15 h in the dark. After combining 20 µL of the NP stock solutions (multiple concentrations: 50 µg/mL-400 µg/mL) from the sample with the ABTS solution (180 µL), the combination was allowed to settle at room temperature in the dark for 15 min. A microplate reader was used to measure the absorbance of the tested sample at a wavelength of 734 nm. The negative control was DMSO, whereas the positive control was ascorbic acid. The following equation was used to obtain the results of this assay.
Here, Abs represents the absorbance of the ABTS-NP sample, whereas Abc represents the absorbance of the ABTS solution.
Ferric/total reducing power assay (FRAP/TRP)
The test followed a modified protocol from Aziz et al. (2021)53,54. Initially, in an Eppendorf tube, a mixture of 100 µL of diluted Fe2O3-NP mixture (multiple concentrations: 50 µg/mL-400 µg/mL), 190 µL of phosphate buffer, and 100 µL of potassium ferricyanide was mixed, followed by the placement of the mixture in an Eppendorf tube in a water bath at 70 °C for 50 min. After incubation, 200 µL of 10% w/v trichloroacetic acid was added to the reaction mixture, which was subsequently centrifuged at 3500 rpm for 10 min. The supernatant (150 µL) was carefully placed in the tagged well of the plate, which contained 50 µL of ferric chloride solution. The absorbance of each sample was then measured at 630 nm, and the TRP activity was calculated as µg AAE/mg NPs.
Phosphomolybdate-based total antioxidant capacity (TAC)
A phosphomolybdate test was carried out to determine the antioxidant ability of the NPs52,55. In this study, 100 µL of the test sample (NP) and 900 µL of the TAC reagent were combined in an Eppendorf tube. The mixture was heated in a water bath at 65 °C for 45 min. After the incubation process, 200 µL from each Eppendorf tube was transferred to the corresponding wells of a 96-well plate. A microplate reader was used to detect the absorbance at 630 nm, and TAC activity was calculated as µg AAE/mg NPs.
Enzyme Inhibition studies with green-synthesized Fe2O3-NPs
α-amylase Inhibition assay
The enzyme inhibitory effect of the NPs was determined via a protocol described in earlier studies46. The methodology involved preparing a reaction mixture on a 96-well plate with a 15 µL test sample, 45 µL starch, and 30 µL α-amylase enzyme. The 96-well plate was then placed in an incubator for 25 min at 45 ± 1 °C. Each well received 20 µL of 1 M hydrochloric acid and 90 µL of iodine solution. After 15 min, the absorbance at 540 nm was analyzed. Acarbose was utilized as a positive control, and DMSO was used as a negative control. The data were examined as % inhibition of α-amylase, which was calculated via the following equation:
Here, Ats denotes the absorbance of the test sample (NP), Anc denotes the absorbance of the negative control, and Ab denotes the absorbance of the blank.
α-Glucosidase Inhibition
The α-glucosidase inhibitory effects of the NPs were evaluated via the well-established methodology of Faisal et al.56. For this assay, a reaction mixture was prepared containing 480 µL of phosphate buffer, 260 µL of p-nitrophenyl α-D-glucopyranoside (5 mM), and 15 µL of the NP sample in an Eppendorf tube. The reaction mixture was incubated for 30 min at 37 °C in the absence of a light source. Then, 250 µL of the α-glucosidase was added to each well of a 96-well plate after incubation for 25 min at 37 °C. Finally, 200 µL of Na2CO3 (200 mM) was added to the reaction mixture, and after the completion of the reaction mixture, 200 µL of the aliquot was taken and added to the specified wells of the 96-well plate. The absorption was measured at 540 nm, and similar controls were used as in the α-amylase inhibition studies.
Here, Ats denotes the absorbance of the prepared NPs, Anc denotes the absorbance of the negative control, and Ab denotes the absorbance of the blank.
Acetylcholinesterase and butyrylcholinesterase inhibition studies
Elman’s approach was used to assess the inhibitory effects of Fe2O3-NPs on butyrylcholinesterase (BChE) and acetylcholinesterase (AChE)57. The amounts of NPs used in the experiments ranged from 50 µg/mL to 400 µg/mL. In brief, sonication was used to disperse the NPs in phosphate-buffered saline (PBS) to obtain the relevant stock solutions. Phosphate buffer (pH 7.4) was used to dilute the enzyme solutions until final concentrations of 0.03 U/mL for AChE and 0.01 U/mL for BChE were reached. Using distilled water, substrate solutions containing 0.0005 M acetylcholine iodide (AChI), 0.0005 M butyrylcholine iodide (BChI), and 0.00022 M 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) were produced in a similar manner and kept at 8 ± 1 °C. The typical positive control was galantamine hydrobromide dissolved in methanol, and a negative control (Io) that included all of the reaction’s constituent parts except the NPs (Is) was used. A reaction mixture containing 430 µL of phosphate buffer, 20 µL of enzyme mixture, and 55 µL of the NPs was typically incubated for 20 min as part of the procedure. Next, 395 µL of DTNB and 100 µL of AChI/BChI were added, and the samples were incubated again for 35 min at the original room temperature. On the basis of the hydrolysis of AChI by AChE (enzyme) and BChI by BChE (enzyme), the 5-thio-2-nitrobenzoate anion is produced in this test. The absorbance was detected at 412 nm, and the % inhibition was evaluated as
Statistical analysis
Biological activities were performed in triplicate (n = 3), and Origin Pro software was used to write the results as the mean values with standard deviations (± SDs).
Catalytic application
Catalytic degradation of 2-nitrophenylphenol via NaBH4 with green synthesized Fe2O3-NP NPs as the catalyst
Chemicals
During this assay, NaBH4 (Sigma Aldrich, 98%) was used as a reducing agent, and the nitro phenolic compound (2-nitrophenol) (2NP, 98%, Sigma Aldrich) was used as the organic contaminant. The whole assay was aided by a continuous supply of distilled water.
Catalytic activity
The catalytic activity of the catalyst against the reduction of nitroaromatic organic pollutants, for example, 2-NP, was investigated58. The initial process involved the preparation of an aqueous solution (250 ppm) of 2-NP, 0.3783 g NaBH4 in 50 mL distilled water, and 0.1 g, 0.5 g, and 0.025 g catalysts (Fe2O3-NPs) in 100 mL distilled water, followed by slight stirring at 200 rpm. A 100 µL aliquot was taken from the stirred solution at intervals of time and diluted with distilled water. The diluted solution was stirred to achieve homogeneity before being transferred to a quartz cuvette to monitor the progress of the reaction via UV‒Vis spectroscopy. The reduction rate of 2-NP was analyzed through the difference between the initial absorbance of the characteristic peak and the absorbance at different intervals of time. The reaction kinetics were assessed via the pseudo-first-order rate equation59.
Here, “Ct” represents the concentration of 2-NP at time t, whereas “Co” represents the concentration at the start of the reaction. “t” represents the time at which reaction kinetics are needed. Similarly, the % conversion at the end of the reaction was evaluated via the following equation59:
Here, “At” represents the concentration of dye at a specific time, whereas “Ao” represents the concentration of dye at the start of the reaction.
Photocatalytic degradation of Methyl orange using green-synthesized Fe2O3-NPs as a catalyst
Chemicals
For this assay, methyl orange dye (85%) was acquired from Sigma Aldrich. Fe2O3-NPs were used as catalyzing materials, and the whole experiment was aided by a continuous supply of distilled water when needed.
Photocatalytic activity
Methyl orange (MO) is an anionic dye used as a control in this assay. Initially, a stock solution was prepared by adding 250 mg of MO dye to 1 L of distilled water and maintaining it at 25 ± 1 °C59. A total of 200 mL of MO dye was taken from the stock solution, and different concentrations (0.1 g, 0.05 g, and 0.025 g) of NPs were added to them in an Erlenmeyer flask. The prepared solution was stirred at 300 rpm under direct sunlight. Sample aliquots were taken at various intervals and centrifuged at 8000 rpm for 6 min. The absorbance of the supernatant was analyzed via a UV‒Vis spectrometer. The reaction kinetics were assessed via the pseudo-first-order rate equation59.
Here, “Ct” represents the concentration of methyl orange at time t, whereas “Co” represents the concentration at the start of the reaction. “t” represents the time at which reaction kinetics are needed. Similarly, the % conversion at the end of the reaction was evaluated via the following Eq. 59:
Here, “At” represents the concentration of dye at a specific time, whereas “Ao” represents the concentration of dye at the start of the reaction.
Results and discussion
Gas chromatography‒mass spectrometry (GC/MS) analysis of C. limetta peels
GC‒MS analysis of the C. Limetta peels revealed that D-limonene (22.96%) was the dominant compound, confirming its characteristic presence in the peels (Table 1). Other notable monoterpenes identified include β-pinene (0.66%), γ-terpinene (0.60%), α-pinene (1.18%), and β-myrcene (0.58%), all of which are commonly found in citrus. Additionally, oxygenated terpenes such as linalool (0.70%) and terpinen-4-ol (1.32%) were detected, contributing to the potential antimicrobial and aromatic properties of the extract. These results indicate that the peel extract is rich in bioactive terpenes, supporting its potential applications in food preservation, pharmaceuticals, and natural antimicrobial formulations.
Identification and quantification of phenolic and flavonoid compounds by HPLC
HPLC analysis of Citrus limetta peel extract revealed the presence of several polyphenolic compounds that are commonly found in citrus peels, as summarized in Table 2. The identified compounds included gallic acid, catechol, p-hydroxybenzoic acid, catechin, chlorogenic acid, vanillic acid, syringic acid, p-coumaric acid, rutin, ferulic acid, o-coumaric acid, hesperidin, resveratrol, rosmarinic acid, myricetin, quercetin, apigenin, and kaempferol. Among these compounds, rutin (RT = 11.04 min, area = 3795.13) was the most abundant, followed by P-coumaric acid (RT = 9.69 min, area = 431.17) and apigenin (RT = 18.84 min, area = 498.16). Other notable compounds with relatively high contents included chlorogenic acid (RT = 7.12 min, area = 232.87), quercetin (RT = 17.97 min, area = 204.07), and O-coumaric acid (RT = 12.71 min, area = 211.84). The compounds detected at moderate concentrations were kaempferol (RT = 19.41 min, area = 146.12), P-hydroxybenzoic acid (RT = 5.85 min, area = 88.37), and ferulic acid (RT = 11.48 min, area = 28.88). Fewer detected compounds included gallic acid (RT = 2.99 min, area = 36.60), catechol (RT = 4.09 min, area = 39.74), and catechin (RT = 6.80 min, area = 39.10). The least abundant compounds were hesperidin (RT = 13.85 min, area = 11.31), resveratrol (RT = 15.62 min, area = 9.30), and myricetin (RT = 16.81 min, area = 15.00). These results confirm the presence of key flavonoids and phenolic acids, which are characteristic of citrus-derived extracts, potentially contributing to their reducing and capping potential along with their antioxidant and therapeutic properties.
Plausible synthesis mechanism of green Fe2O3-NPs via C. limetta peels extract
In the current plant-mediated synthesis of Fe2O3-NPs, the peel extract of C. limetta (waste) was added as a reducing and capping agent. The peel extract of citrus fruit is pharmacologically significant because of its high content of phenols and carotenoids, which are natural components of antioxidant and anticancer drugs43. Initially, a solution of FeCl3. 6H2O was prepared by adding 3.7 g of H2O to 100 mL of distilled water followed by a drop-by-drop addition of the citrus peel extract. The color shifted from yellowish to slightly reddish when an extract of citrus was added to the precursor salt. During the 4 h stirring time, the color continued to darken, and finally, reddish brown-colored NPs were obtained, which were subsequently ground to a fine powder, calcinated, and stored for further use. The whole synthesis procedure involves the ionic dissociation of FeCl3. 6H2O into Fe3+ and Cl−, which upon the addition of extract, turns Fe3+ into Fe2+ followed by further reduction into Feo, as the extract contains phenols and flavonoids, which are reduced in nature. The reduced Feo oxidizes to form Fe2O3-NPs during growth, stabilization, and nucleation reactions, while the remaining phytochemicals act as capping agents, preventing further aggregation or agglomerate formation, as illustrated in Fig. 169.
% Yield recovery of the C. limetta-functionalized Fe2O3-NPs
Various factors can influence the % yield of the produced metallic NPs, such as the type and amount of precursor salt, reducing agent, stabilizing and capping material, reaction methodology, reaction time, pH, and temperature of the reaction. In addition, plant extracts can strongly influence the overall yield of a product70. In this study, the % yield is documented in Table 3.
A yield of 19.32% was obtained after the NPs were ground in a mortar and pestle. In the context of phytosynthesis, this yield is considerable because green methods are often associated with a low yield of the final product, as the yield is highly influenced by the type of phytochemicals present in the plant extract, including flavonoids, alkaloids, and polyphenols, and their reducing and stabilizing potential. For example, an –OH bond at the aromatic ring of flavonoids assists in the stabilization of NPs, as the –OH groups prevent the NPs from agglomerating by reducing the number of free radicals in the reaction mixture. Moreover, other phytochemicals, such as alkaloids and polyphenols, also carry –OH groups, which provide enhanced stabilization to NPs, thus improving the overall % yield70,71.
Optical, physicochemical, and morphological characterization of the materials synthesized via a green approach
The UV–Visible absorption spectrum of the citrus extract, as depicted in the Fig. 2a, exhibits two prominent absorbance peaks at 270 nm and 313 nm. These peaks are characteristic of the presence of various bioactive phytochemicals commonly found in citrus species. The absorbance at 270 nm can be attributed to π→π* transitions of aromatic rings, which are typically associated with flavonoids and phenolic compounds, key antioxidants in plant extracts. The second peak, observed at 313 nm, suggests the presence of conjugated systems, often linked to flavones, flavanols, or other polyphenolic compounds72,73. Such spectral characteristics are consistent with the known composition of citrus extracts, which are rich in vitamin C, flavonoids (e.g., hesperidin, naringin), and limonoids, all of which play a vital role in antioxidant, anti-inflammatory, and antimicrobial activities72. The absorption peaks confirms the complex mixture of phytochemicals and supports the potential use of citrus extracts as natural reducing and stabilizing agents in the green synthesis of NPs74. As compared to the UV spectra of extract, the green synthesized Fe2O3-NPs showed different pattern. For instance, the spectrum reveals two characteristic peaks at approximately 280.5 nm and 337 nm, as shown in Fig. 2a. The absorption peak at 280.5 nm can be attributed to the π→π* transitions of phenolic compounds and flavonoids present in the citrus extract, which act as both reducing and stabilizing agents during the green synthesis process73. This peak indicates the presence of aromatic rings and conjugated systems, which play a crucial role in reducing Fe³⁺ ions to Fe⁰ or Fe2O3 NPs. The second peak at 337 nm is likely due to Surface Plasmon Resonance (SPR) of iron NPs75,76. This suggests successful capping of Fe2O3-NPs by phytochemicals from the extract, preventing agglomeration and enhancing stability. Unlike noble metal NPs, Fe2O3-NPs do not exhibit sharp surface plasmon resonance (SPR) bands in the visible region; instead, they show broad and gradual absorbance due to their semiconducting or superparamagnetic nature77.
The electrical band structure of the Fe2O3-NPs was examined via photoluminescence analysis, which enabled us to comprehend the dynamics of charge carriers in evaluating the optoelectronic and photocatalytic capabilities of the NPs. The intensity of the peak in the PL absorption spectra indicates the transfer capability of the photogenerated electrons. The higher the peak intensity is, the higher the transfer rate and the lower the photocatalytic behavior of the NPs78. The PL spectra of the Fe2O3-NPs synthesized via the green approach displayed a strong peak centered between 438 and 450 nm because of the excitation emission, as depicted in Fig. 2b. The transition between the valence band (VB), which is mostly made up of Fe (4 s) states, and the conduction band (CB), which is primarily made up of O (2p) states, is responsible for the excitation state in Fe2O3-NPs. Electrons are transferred from the VB to the CB upon activation with 350 nm irradiation, producing electron‒hole pairs79. The PL spectrum shows an optical transition within the Fe2O3-NPs, representing the recombination of excitation, with a peak centered between 438 and 455 nm.
The FTIR spectrum of the citrus aqueous extract exhibited prominent absorption bands at 3388 cm⁻¹, corresponding to O–H stretching vibrations of hydroxyl groups in phenolic compounds and carbohydrates, along with possible N–H stretching from proteins80. A band at 2935 cm⁻¹ is attributed to C–H stretching of aliphatic groups present in terpenoids and fatty acids, as presented in Fig. 3a81. The peak at 1685 cm⁻¹ is characteristic of C = O stretching vibrations in carbonyl groups from aldehydes, ketones, and flavonoids82. The absorption at 1519 cm⁻¹ can be assigned to aromatic C = C stretching in polyphenolic structures. The band at 1380 cm⁻¹ corresponds to -CH3 bending vibrations from methyl groups83. The strong peak at 1035 cm⁻¹ indicates C–O–C stretching in polysaccharides and glycosidic linkages. A smaller peak at 904 cm⁻¹ is associated with β-glycosidic linkages or C–H bending of substituted alkenes, while the absorption at 716 cm⁻¹ can be attributed to aromatic C–H bending in phenolic compounds83. These functional groups confirm the presence of diverse phytoconstituents, including polyphenols, terpenoids, and carbohydrates, which can act as reducing and stabilizing agents during nanoparticle synthesis. Furthermore, FTIR was employed to identify the capped functional groups of citrus fruit and produced Fe2O3-NPs. The citrus extract is rich in flavonoids, carotenoids, furo-coumarins, and organic acids43. Figure 3b shows the FTIR spectrum of the Fe2O3-NPs, with absorption peaks at 3352, 2918, 1601, 1365, 1028, and 558 cm− 1. The broad absorption peak in the range of 3352 cm − 1 is due to the phenolic or alcoholic hydroxyl group (-OH) stretching of organic acids and flavonoids80. This broad nature of the peak is due to the formation of inter- and intramolecular hydrogen bonds84. The broad peak ranging from 3400 to 3100 cm− 1 is also a clear indication of the presence of -OH groups on the Fe2O3-NP surface80,85. Another band at 2918 cm− 1 is associated with the C–H widening vibrations of methoxy or methyl groups81. Other absorption peaks observed at 1601, 1365, and 1028 cm− 1 correspond to C = C (alkene), CH3 bending, and C-OH stretching, which clearly indicate the possible reduction of carotenoids, organic acids, and flavonoids86. A very blunt peak near 558 cm− 1 corresponds to the occurrence of the Fe‒O bond87. A similar pattern was observed in other studies, confirming the purity and homogeneity of our research88,89. For example, Liang et al. (2015) observed a broad peak ranging from 443 to 558 cm− 1 attributed to the Fe-O bond, whereas Kumar et al. (2020) observed –OH stretching, CH3 bending, and C-OH stretching attributed to phytochemicals present in citrus waste, confirming the successful capping of these functional groups on the surface of Fe2O3-NPs86.
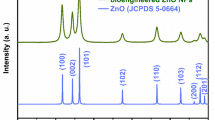
The crystallinity, crystal shape and size, and phase identification of the green Fe2O3-NPs were evaluated via X-ray diffraction. The graph was plotted within the 2θ range from 20–70°, as depicted in Fig. 3c. The spectra showed a cubic spinel shape [JCPDS No: 39-1346] with diffraction peaks at 26.23°, 30.36°, 35.52°, 43.38°, 53.6°, 57.24°, and 63.19°, corresponding to the 220, 311, 222, 400, 422, 511, and 440 diffraction planes, respectively90. No peak for impurities was detected throughout the visible spectra, confirming the synthesis of pure Fe2O3-NPs. Moreover, the approximate size, calculated via the Debye–Scherer formula, was approximately 35 nm. Similar peaks were observed in various other studies, confirming the purity and homogeneity of our research91,92,93. For example, Zhu et al. (2007) observed similar phase diffraction peaks at approximately similar wavelengths, with similar JCPDS card numbers of 39-1346, indicating a cubic spinel shape and confirming the successful synthesis of Fe2O3-NPs93.
Furthermore, the structural morphology and size of the Fe2O3-NPs were comprehensively analyzed by scanning and transmission electron microscopy (SEM and TEM). The SEM micrograph revealed a narrow size distribution, with the NP size calculated by ImageJ to be 62 ± 8.0 nm. The SEM micrograph revealed the cubic spinel shape of the NPs with very slight agglomeration, which may have occurred because of the intensive coating of phytochemicals on the surface of the NPs, as shown in Fig. 3d. The TEM micrograph confirmed the shape of the Fe2O3-NPs, with an average size of approximately 56 ± 13.5 nm, as shown via the size distribution, as shown in Fig. 3e and f. Moreover, energy-dispersive X-ray spectroscopy was employed to assess the elemental composition of the mentioned NPs, as shown in Fig. 3g. The EDX spectra confirmed the presence of the elements Fe and O in the sample, with weight percentages as follows: Fe: 46.82% and O: 53.18%, while the atomic percentages were as follows: Fe: 20.14% and O: 79.86%.
pH-based dispersion studies
Nanoparticles (NPs) have unique chemical, physical, and biological properties, making them valuable in medicine, electronics, catalysis, and nanotechnology. However, proper dispersion is essential to maintain their stability, functionality, and effectiveness. Poor dispersion may not only reduce their performance but also affect the biological properties of NPs, as it dictates the behavior of the particles with surrounding living systems and cells. Moreover, inadequate dispersion may cause safety concerns and toxicity profiling of the materials in question94. In this study, C. limetta-functionalized Fe2O3-NPs were observed for their dispersion ability at various pH values, including pH 2, pH 7, and pH 12. The NPs were completely dispersed after sonication at basic, neutral, and basic pH values. However, the NPs at pH 7 started to sediment after 30 min of sonication, as shown in Fig. 4. In contrast, the NPs at pH 2 and pH 12 remained mostly dispersed after 30 min. However, at pH 12, the materials started slow sedimentation after 1 h and continued until 6 h, when the NPs were completely sediment. On the other hand, the particles at pH 7 were completely sedimented after 2 h, and interestingly, the NPs at pH 2 remained partly dispersed even after 24 h, which confirmed their superior dispersion ability in acidic media. Our study thus revealed that C. limetta functionalization of Fe2O3-NPs resulted in different responses in neutral, acidic, and basic environments but exhibited excellent dispersion stability, especially in acidic environments.
Pictorial visualization of pH-based dispersion studies of phytochemical-capped Fe2O3-NPs in neutral, basic, and acidic media as a function of storage time. The NPs could be visualized to have distinct pH-responsive behavior, with the basic pH resulting in a comparatively better medium for dispersion.
Biological applications
G-Fe2O3-NPs displayed excellent bactericidal ability against gram-negative strains
Global health is facing unique challenges due to the overuse or misuse of antibiotics, such as antibiotic resistance, enhanced allergic reactions, damage to the gut flora, increased risk of secondary infections, and potential toxicity not only to patients but also to the environment. In this context, nanoparticles (NPs) are emerging as promising alternatives to traditional antibiotics because of their unique properties, such as broad-spectrum antimicrobial activity, lower resistance development, targeted delivery potential, biofilm penetration ability, and synergistic effects with existing antibiotics to achieve the same results95. The bactericidal ability of the prepared G-Fe2O3-NPs was explored via various concentrations of MNPs (5, 10, 20, and 40 mg/mL). Several gram-positive and gram-negative strains were used in the assay, and a concentration-dependent agar well diffusion analysis of the bactericidal capacity of the NPs was performed. In general, the green-synthesized Fe2O3-NPs showed excellent antibacterial ability against gram-negative strains, as shown in Fig. 5a and b; Table 4. For example, the NPs caused a zone of inhibition (ZOI) of 27 ± 1.2 mm and 25 ± 1.8 mm against E. coli and P. aeruginosa at the maximum concentration, and it decreased to 21 ± 1.4 mm and 20 ± 1.6 mm at a minimum concentration of 5 mg/mL. However, at lower concentrations, the NPs were more effective against E. coli, as the MIC was 0.625 mg/mL, and the MBC was 1.125 mg/mL. A 2.0 ratio of MBC to MIC was recorded, indicating that the green-synthesized Fe2O3-NPs were bactericidal against E. coli. P. aeruginosa was the most sensitive strain against the synthesized NPs, with an MIC of 1.125 mg/mL and an MBC of 2.5 mg/mL, as observed via the micro broth dilution method. The MBC-to-MIC ratio was calculated as 2.2, indicating their bactericidal potential. Similarly, the green-synthesized NPs also had a very clear ZOI of 26.5 ± 1.2 mm against S. aureus (gram-positive), with an MIC of 2.5 mg/mL, an MBC of 5 mg/mL and an MBC/MIC of 2.0, indicating their bactericidal potential. The findings show that the positive strains are also sensitive to the NPs but are not as sensitive as the gram-negative strains are. Interestingly, MRSA showed no sensitivity to any concentration of the MNPs, which showed resistance even against the prepared metallic NPs. Our study confirms the bactericidal ability of Fe2O3-NPs, especially against gram-negative strains; thus, the NPs have the potential to be further investigated for antimicrobial studies in vitro and in vivo.
(a) Graphical depiction of the bactericidal ability of C. limetta-synthesized green Fe2O3-NPs and (b) pictorial representation of the observed antibacterial results. The alphabets A, B, C, and D show serial dilutions corresponding to 40 mg/mL, 20 mg/mL, 10 mg/mL, and 5 mg/mL, respectively. The symbols “–” refer to the negative control, whereas “+” or “PC” symbolize the positive control, which was Levofloxacin. The assay was carried out three times (n = 3), and the values are presented as the means ± SDs.
An elaborate literature review of Fe2O3-NPs demonstrated that when metallic NPs (MNPs) bind to the surface of bacterial cells, they quickly penetrate the membrane while disrupting the lipid bilayer and porin structures, leading to blockage of channels for passive diffusion. Upon entering the cell, the Fe2O3-NPs undergo ionic dissolution, releasing Fe2+ and Fe3+ ions, which play a role in metal ion toxicity by disturbing essential enzymatic pathways, as described in Fig. 6. These released ions then participate in Fenton and Harber-Weiss reactions and, as a result, produce ROS such as (•OH). In turn, ROS induce oxidative stress, which affects the macromolecular structure, especially when Fe2O3-NPs interact with ribosomes, leading to disruption of translation and protein destabilization. Additionally, the direct interaction of metal ions with DNA causes mutations, DNA strand breaks, and alterations in gene expression, which lead to microbial cell death96. The phytochemicals present in C. limetta, such as phenols and flavonoids, capped on the surface of the NPs can also accelerate the bactericidal process because of the intrinsic antibacterial properties of the capped phyto-constituents, such as naringin, poncirin, rhoifolin, marmesin, tangeretin, nobiletin, glyflavanone, and lemairone97,98.
Green-synthesized Fe2O3-NPs were highly active against the flagellated and non-flagellated forms of Leishmania tropica
Leishmania tropica, the causal agent of cutaneous leishmaniasis, proceeds through two stages: the promastigote stage and the amastigote stage. Promastigotes from sandflies (vectors) reach mammalian or animal hosts and switch into amastigotes within macrophages of the host organism. Amastigotes multiply within host cells, causing tissue damage, skin lesions, and ulceration, which are prominent signs of the disease. Understanding the pathogenicity of both phases is essential for developing effective treatment options for leishmaniasis. The inhibition or targeting of both the amastigote and promastigote forms of the parasite can prevent both the transmission and infection of the parasite55,57,99. In our study, a concentration-dependent antileishmanial study was performed against Leishmania tropica-KWH23. In general, the green-synthesized Fe2O3-NPs displayed effective antileishmanial efficacy, which increased with increasing concentration (25 < 50 < 100 < 200 µg/mL). At the highest tested concentration, the Fe2O3-NPs resulted in 54.8 ± 2.2% and 51.2 ± 2.6% inhibition for promastigotes and amastigotes, respectively, as displayed in Fig. 7. A sudden decrease in % inhibition occurred at 50 µg/mL, where the interaction of NPs with parasites resulted in decreases of 29.2 ± 1.6% and 25.7 ± 1.2% for promastigotes and amastigotes, respectively. At the lowest concentration, the % inhibition decreased to 14.8 ± 1.0 and 10.4 ± 1.4% for promastigotes and amastigotes, respectively. Moreover, the half-maximal inhibitory concentrations (IC50s) for promastigotes and amastigotes were measured as 144.8 µg/mL and 158.3 µg/mL, respectively. Compared with Amphotericin B, the positive control, C. limetta waste-synthesized green Fe2O3-NPs, resulted in significant antiparasitic potential against L. tropica and have the potential to be further explored for antileishmanial studies in pristine forms or as drug delivery vehicles for antileishmanial drugs.
Comprehensive literature suggests that the antileishmanial mechanism of Fe2O3-NPs involves a combination of oxidative stress, cellular toxicity, and immune modulation. When interacting with the parasite membrane, the NPs pass through and produce reactive oxygen species (ROS) that cause oxidative stress (OS). ROS may result in peroxidation of lipids (membrane damage and leakage), oxidation of proteins (enzyme denaturation), and DNA fragmentation, leading to cell cycle arrest and ultimately leading to cell death, as demonstrated in Fig. 8. The NPs not only exert direct antileishmanial effects but also enhance host immune responses to eliminate intracellular amastigotes inside macrophages. Their immune modulation properties work by activating macrophages, increasing the production of cytokines (TNF-α, IL-12, and IFN-γ), and increasing oxidative stress. Fe2O3-NPs have also been shown to alter the iron metabolism that the parasite requires for its survival and thus aid in the mortality of the parasite100.
Green-synthesized Fe2O3-NPs have outstanding antioxidant properties
Assessing the antioxidant ability of nanoparticles is important for understanding how they can be used to prevent oxidative stress and cell damage and thus may play a vital role in medicine by protecting against chronic diseases, aging, and inflammation101. Free radicals generally cause oxidative stress within the biological system, disrupting the activity of macromolecules such as carbohydrates, lipids, proteins, and nucleic acids. The body has an internal defense system to cope with the production of excessive free radicals but may depend upon external antioxidant-rich formulations, especially during disease stages. Recently, MNPs, synthesized via secondary metabolites and phenolic compounds, have emerged as a potential alternative to commercialized drugs because of their biocompatibility, durability, and excellent antioxidant potential102. This study investigated the antioxidant properties of C. limetta peel extracts synthesized from Fe2O3-NPs via various approaches, including 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity (DPPH-FRSA), 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS-FRSA), total antioxidant capacity, and total reducing power. Various concentrations (50, 100, 200, and 400 µg/mL) of NPs were used in the assays. The results are summarized in Fig. 9. Initially, the free radical scavenging ability (FRSA) of the NPs was examined via DPPH-FRSA and ABTS-FRSA assays. The DPPH assay converts purple DPPH radical solution to light yellow when antioxidant particles are present, whereas the ABTS test transforms green‒blue ABTS into a colorless solution because of the reducing power of antioxidant materials46,103,104. At the highest concentration, the green-synthesized Fe2O3-NPs inhibited 57.21 ± 2.20% of the DPPH activity, which was reduced to 24.4 ± 2.2% at the lowest concentration, whereas 53.3 ± 1.20% of the maximum tested concentration of ABTS was inhibited, which was reduced to 15.47 ± 2.26% at the lowest concentration. To further assess the antioxidant potential of the Fe2O3-NPs, a TAC assay was conducted in which the NPs displayed excellent antioxidant ability, which was calculated as 77.3 ± 2.84 µg AAE/mg at the highest concentration and was reduced to 24.24 ± 2.64 µg AAE/mg at the lowest NP concentration. The test is based on the production of a green phosphate/MoV complex as a result of the reduction of phosphomolybdate ions in the presence of an antioxidant105. TRP presented a relatively low antioxidant potential, which was calculated as 73.36 ± 2.44 µg AAE/mg and was reduced to 20.42 ± 2.44 µg AAE/mg at the highest to lowest concentrations. The whole process of this assay consists of simple transformation steps where potassium ferricyanide (Fe3+) initially interacts with the antioxidant material and then transforms into potassium ferrocyanide (Fe2+), which finally transforms into a ferric-ferrous complex due to constant interaction with ferric chloride47,106. The excellent antioxidant activity of the Fe2O3-NPs is due to the inherent ability of the NPs to reduce the amount of oxidized free radicals and phytochemicals (p-coumaric, ferulic, and sinapic acids) that are functionalized over the surface of the NPs. These phytochemicals are excellent free radical neutralizing agents in nature and therefore enhance the ability of Fe2O3-NPs to scavenge these oxidative stress-producing elements42,43. This study thus revealed that C. limetta-synthesized Fe2O3-NPs are excellent antioxidant materials and can be assessed further for various in vitro and in vivo applications.
Phytochemically capped Fe2O3-NPs displayed outstanding enzyme Inhibition capacity
The interaction between proteins and NPs is now well documented. This contact causes the creation of a “protein corona,” which influences the physicochemical and biological properties of nanoparticles. Multiple factors influence the interaction between proteins and NPs, such as NP shape and size, type, surface capping, and surface charge. Similarly, NPs can interact with enzymes in various ways, either by enhancing or inhibiting their activity, and depend on both the properties of the NPs and their enzyme characteristics, such as their stability, structure, and active site accessibility46,107. In the present study, the antidiabetic efficacy of Fe2O3-NPs was analyzed against α-amylase, α-glucosidase, acetylcholinesterase, and butyrylcholinesterase enzymes at concentrations ranging from 50 to 400 µg/mL. All the enzymes showed concentration-dependent inhibition, as demonstrated in Fig. 10. α-amylase and α-glucosidase are the key enzymes involved in the hydrolysis of complex carbohydrates into simple glucose. α-amylase is involved in the breakdown of starch into oligosaccharides, while α-glucosidase further hydrolyzes it to convert it into simple glucose, which is ultimately absorbed into the bloodstream, causing hyperglycemia. Inhibiting the pathway of these enzymes can help diabetic individuals control their blood glucose levels108. For α-amylase, Fe2O3-NPs displayed a % Inhibition of 56.77 ± 2.88 at 400 µg/mL which was then decreased to 14.88 ± 2.24% at the lowest used concentration. However, for α-glucosidase, NPs displayed an inhibition percentage of 52.81 ± 2.64% at the highest concentration which upon reaching the lowest concentration, reduced to 6.21 ± 2.2% %. The IC50 for α-amylase and α-glucosidase were calculated as ≥ 378 µg/mL and ≥ 392 µg/mL. Comparatively, NPs displayed better antidiabetic efficacy against α-amylase at all the concentrations. Later on, the antialzheimer’s efficacy was also evaluated against Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) enzymes. These enzymes are responsible for the hydrolysis of acetylcholine which is a key component in Alzheimer’s disease. Inhibition of these enzymes was always vital during the discovery and development of the anti-Alzheimer drugs and is still a favorable target for novel therapies109,110,111. In our study, the green synthesized Fe2O3-NPs inhibited the Acetylcholinesterase enzyme up to 41.28 ± 3.8% at the top concentration which reduced to 2.99 ± 0.22%. Similarly, the NPs employed against Butyrylcholinesterase, at the higher concentration, displayed 53.17 ± 3.82% inhibition which upon using the lowest concentration, reduced to 4.10 ± 0.24%. The IC50 for acetylcholinesterase and butyrylcholinesterase were calculated as ≥ 400 and 390 µg/mL, respectively. Our study thus demonstrated that the synthesized NPs interact differently with different enzymes and has the potential to target key enzymes involved in the pathogenicity of diabetes and Alzheimer’s disease.
Catalytic applications
Degradation of 2-Nitrophenol via NaBH4 and G-Fe203-NPs as catalyst
2-Nitrophenol is an organic molecule with substantial negative environmental effects owing to its reactivity and volatility. As a typical product of manufacturing processes such as dye, pesticide, and pharmaceutical production, it is continually dumped into water reservoirs, which result in contamination. This chemical resists biodegradation, aggravating its environmental effect by polluting water and soil. The persistent presence of 2-nitrophenol in the environment poses implications since it has the potential to damage aquatic ecosystems and endanger human health via bioaccumulation112,113. In our study, the C. limetta synthesized green Fe2O3-NPs were explored as catalyst for the degradation 2-NP, as a waste. UV-Vis spectroscope was employed to measure the reduction in absorbance peak at various time intervals, to confirm the catalytic behavior of prepared NPs. The two most common techniques for reducing nitroaromatic compounds are catalytic hydrogenation and stoichiometric reduction. As shown in Fig. 11, it is thermodynamically possible to convert nitroaromatic compounds into amines (2-aminophenol) when NaBH4 is present in the system. The enormous kinetic barrier between the electron donor (BH− 4) and acceptor (nitroaromatic chemicals), however, prevents this conversion. The catalyst addition actively breaks through this barrier, creating a different and low activation energy pathway for electron transfer to acceptor species114,115.
2-NP was catalytically converted to 2-AP using the catalyst (Fe2O3-NPs). A steady decrease in absorption spectra at 410 nm was observed, as seen in Fig. 12. When the conversion process was conducted without the catalyst, a very little drop in peak intensity was observed, and it persisted even after 180 min. On the other hand, 0.1 g of the catalyst took only 15 min to completely convert (98.32%) the given 2-NP solution to 2-AP, whereas 0.05 and 0.025 g of the catalyst took only 20 and 40 min to completely reduce 2-NP into 2-AP, respectively, as shown in Fig. 12. The reaction time, pseudo-first-order rate constant, and % conversion of 2-NP into 2-AP at the end of the reaction are presented in Table 5.
The decrease in Kapp with decreasing catalyst concentration correlates with the reduced availability of active sites to ROS species on the 2-NP surface, and a proportional relationship between the catalyst concentration and the reaction rate is shown.
Graphical demonstration of the degradation of 2-NP in the presence of a catalyst (Fe2O3-NPs) and NaBH4. Here, (a) represents the UV spectra for the reduction of 2-NP in the absence of Fe2O3-NPs, (b–d) represent the UV spectra for the reduction of 2-NP in the presence of 0.1 g, 0.05 g, and 0.025 g of Fe2O3-NPs, respectively.
Photocatalytic degradation of methyl orange dye via the use of NPs as a catalyst
Methyl orange is a synthetic anionic dye that is commonly used in the textile industry as a dyeing material and as a pH indicator in laboratories. It is non-biodegradable and highly water soluble, making it an environmental pollutant. When MO dye is released in water bodies, it reduces light penetration in water and disturbs aquatic ecosystems. This dye impairs the growth and reproduction of various aquatic organisms and has carcinogenic effects on humans. The photocatalytic ability of MNPs is essential for understanding their ability in fields such as electrochemical sensing and environmental remediation116. Citrus limetta (waste)-mediated Fe2O3-NPs were used as a catalyzing agent for the degradation of MO dye. According to the literature, MNPs interact with dye molecules, and this interaction allows the dye to adsorb on the surface of MNPs, as presented in Fig. 13. Under a direct light source, Fe2O3-NPs absorb energy, and electrons are excited from the valence band to the conduction band. This phenomenon creates electron‒hole pairs and leads to the production of ROS such as •OH and O2−. These ROS initiate oxidative reactions and thus destroy the molecular structure of dye molecules. Interestingly, MNPs can accelerate the catalysis process by providing additional binding sites to ROS species on dye molecules117.
In this study, Fe2O3-NPs synthesized via C. limetta (waste) were used to degrade MO dye. For the MO dye, the absorption peak was observed at 464 nm, and a decrease in the absorption spectra was observed with time, as presented in Fig. 14. When the degradation process was analyzed for MO dye without a catalyst, a very slight decrease in the absorbance peak intensity was observed, and it persisted even after 180 min. On the other hand, 0.1 g of catalyst took 25 min to degrade the MO dye to the maximum extent, whereas 0.05 and 0.025 g of catalyst took 75 and 125 min to degrade the MO dye, respectively. The reaction time, pseudo-first-order rate constant, and % degradation of MO dye at the end of the reaction are presented in Table 6.
The decrease in Kapp with decreasing catalyst concentration is correlated with the reduced availability of active sites for ROS species on the MO dye surface, and a proportional relationship between the catalyst concentration and the reaction rate is shown.
Graphical demonstrations of the degradation of MO dye in the presence of a catalyst (Fe2O3-NPs). Here, (a) represents the UV spectra for the reduction MO dye in the absence of Fe2O3-NPs, (b–d) represent the UV spectra for the reduction of MO dye in the presence of 0.1 g, 0.05 g, and 0.025 g of Fe2O3-NPs, respectively.
Conclusion
In this study, iron oxide nanoparticles (Fe2O3-NPs) were successfully synthesized using C. limetta (sweet lime) peel extract, offering a sustainable and eco-friendly route for nanoparticle production from fruit waste. The synthesized NPs were comprehensively characterized, confirming their structural integrity, stability, and phytochemical functionalization. The NPs exhibited outstanding biological activities, including potent antibacterial effects—particularly against Gram-negative strains, antiparasitic potential against Leishmania tropica, strong antioxidant capacity, and notable enzyme inhibition relevant to diabetes mellitus and Alzheimer’s disease management. Additionally, the nanoparticles exhibited remarkable catalytic efficiency in degrading environmental pollutants such as 2-nitrophenol and methyl orange, highlighting their role in environmental remediation. These findings underscore the multifunctional potential of green synthesized Fe2O3-NPs and validate the valorization of agro-waste for biomedical and industrial applications. Future work should focus on scaling up and optimization of the green synthesis process, in vivo validation, detailed toxicity profiling, and mechanistic studies to confirm therapeutic and environmental safety.
Data availability
The raw datasets can be provided by the corresponding author upon reasonable request.
References
Haseeb, H. A. et al. Polygonum Bistorta linn. As a green source for synthesis of biocompatible selenium nanoparticles with potent antimicrobial and antioxidant properties. BioMetals https://doi.org/10.1007/s10534-024-00622-0 (2024).
Zahid, M. U. et al. Molecular perspectives on acacia gum as a functional polysaccharide for biomedical and industrial applications: A review. Int. J. Biol. Macromol. 319, 145412 (2025).
Sridevi, H., Bhat, M. R., Kumar, P. S., Kumar, N. M. & Selvaraj, R. Structural characterization of cuboidal α-Fe2O3 nanoparticles synthesized by a facile approach. Appl. Nanosci. 13, 5605–5613 (2023).
Paulson, E. & Jothibas, M. Significance of thermal interfacing in hematite (α-Fe2O3) nanoparticles synthesized by sol-gel method and its characteristics properties. Surf. Interfaces 26, 101432 (2021).
Rajyashree, L., Manoharan, C., Loganathan, A. & Venkateshwarlu, M. Electrochemical and sensing properties of α-Fe2O3 nanoparticles synthesized using hydrothermal method at low reaction temperature. J. Mater. Sci.: Mater. Electron. 34, 462 (2023).
Zhou, Y. et al. Recent progress in γ-Fe2O3-based catalysts: An overview of the synthesis and applications in environmental remediation. Chem. Eng. J. 475, 146198 (2023).
Kumar, P., Kumar, S., Tapwal, A. & Thakur, N. Chemical/green synthesized cobalt/copper-doped α-Fe2O3 nanoparticles: Potential for environmental remediation. J. Mater. Res. 39, 836–849 (2024).
Hanumanthappa, M. et al. Almond gum assisted synthesis of Mg doped Fe2O3 nps: Structural analysis, electrochemical sensing, and optical applications. ChemPhysMater 1, 330–337 (2022).
Amali, R. K. A., Lim, H. N., Ibrahim, I., Zainal, Z. & Ahmad, S. A. A. A copper-based metal–organic framework decorated with electrodeposited Fe2O3 nanoparticles for electrochemical nitrite sensing. Microchim. Acta 189, 356 (2022).
Venkateshaiah, A., Černík, M. & Padil, V. V. T. Metal oxide nanoparticles for environmental remediation. Nanatechnol. Environ. Remediation (Wiley). 183–213. https://doi.org/10.1002/9783527834143.ch11 (2022).
Quang, N. D. et al. Fe2O3 hierarchical tubular structure decorated with Cobalt phosphide (CoP) nanoparticles for efficient photoelectrochemical water splitting. Chem. Eng. J. 417, 129278 (2021).
Surendra, D. M. et al. Synthesis, characterization and assessment of anticancer potency of oxcarbazepine with folic acid conjugated Fe2O3 nanostructures as nano-drugs. J. Mol. Struct. 1306, 137842 (2024).
Gerami, S. E. et al. Preparation of pH-sensitive chitosan/polyvinylpyrrolidone/α-Fe2O3 nanocomposite for drug delivery application: Emphasis on ameliorating restrictions. Int. J. Biol. Macromol. 173, 409–420 (2021).
Zoghi, M. et al. Synthesis and characterization of chitosan/carbon quantum dots/Fe2O3 nanocomposite comprising Curcumin for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 249, 125788 (2023).
Sharma, V. et al. Chloramphenicol and gentamycin-encapsulated iron oxide nanoparticles as a nanocarrier for antibacterial efficacy via targeted drug delivery. Nano Biomed. Eng. 15, 170–178 (2023).
Sandhu, Z. A. et al. Advances in the optimization of Fe nanoparticles: Unlocking antifungal properties for biomedical applications. Pharmaceutics 16, 645 (2024).
Ansari, M. A. & Asiri, S. M. M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. Int. J. Biol. Macromol. 171, 44–58 (2021).
Lari, H., Morsali, A. & Heravi, M. M. Quantum mechanical study of γ-Fe 2 O 3 nanoparticle as a nanocarrier for anticancer drug delivery. Z. Für Phys. Chem. 232, 579–592 (2018).
Islam, A. et al. Reactive oxygen species generating photosynthesized ferromagnetic iron oxide nanorods as promising antileishmanial agent. Nanomedicine 15, 755–771 (2020).
Khalil, A. T. et al. Biosynthesis of iron oxide (Fe 2 O 3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 10, 186–201 (2017).
Kannan, S. et al. Novel nanocarrier platform for effective treatment of visceral leishmaniasis. Bioconjug. Chem. 32, 2327–2341 (2021).
Alijani, H. Q. et al. Biosynthesis of core–shell α-Fe2O3@Au nanotruffles and their biomedical applications. Biomass Convers. Biorefinery 14, 15785–15799 (2024).
Pourmadadi, M. et al. Role of iron oxide (Fe2O3) nanocomposites in advanced biomedical applications: A state-of-the-art review. Nanomaterials 12, 3873 (2022).
Wang, X. et al. Cancer stem cell labeling using poly(l-lysine)-modified iron oxide nanoparticles. Biomaterials 33, 3719–3732 (2012).
Ngadiman, N. H. A. et al. γ-Fe2O3 nanoparticles filled Polyvinyl alcohol as potential biomaterial for tissue engineering scaffold. J. Mech. Behav. Biomed. Mater. 49, 90–104 (2015).
Sajjad, A. et al. Fabrication of hematite (α-Fe2O3) nanoparticles under different spectral lights transforms physio chemical, biological, and nanozymatic properties. Nano Trends 2, 100010 (2023).
Jabbar, H. S. Pseudo-water-soluble Fe2O3 as nanozyme catalyzed chemiluminescent reaction for detection of brilliant blue in gelatin and beverages. Food Chem. 453, 139678 (2024).
Hasannejad-Asl, B. et al. Nanoparticles as powerful tools for crossing the Blood-brain barrier. CNS Neurol. Disord. Drug Targets 22, 18–26 (2023).
Gárate-Vélez, L. et al. Anthropogenic iron oxide nanoparticles induce damage to brain microvascular endothelial cells forming the Blood-Brain barrier. J. Alzheimer’s Dis. 76, 1527–1539 (2020).
Darezereshki, E. Synthesis of maghemite (γ-Fe2O3) nanoparticles by wet chemical method at room temperature. Mater. Lett. 64, 1471–1472 (2010).
Lassoued, A., Dkhil, B., Gadri, A. & Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 7, 3007–3015 (2017).
Ramalingam, V., Harshavardhan, M. & Dinesh Kumar, S. Malathi devi, S. Wet chemical mediated hematite α-Fe2O3 nanoparticles synthesis: Preparation, characterization and anticancer activity against human metastatic ovarian cancer. J. Alloys Compd. 834, 155118 (2020).
Hachem, K. et al. Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: A review. BioNanoScience 12, 1032–1057 (2022).
Haris, M. et al. Oscillatoria limnetica mediated green synthesis of iron oxide (Fe2O3) nanoparticles and their diverse in vitro bioactivities. Molecules 28, 2091 (2023).
Vinayagam, R. et al. Structural characterization of green synthesized α-Fe2O3 nanoparticles using the leaf extract of spondias dulcis. Surf. Interfaces 20, 100618 (2020).
Yoonus, J., Resmi, R. & Beena, B. Evaluation of antibacterial and anticancer activity of green synthesized iron oxide (α-Fe2O3) nanoparticles. Mater. Today: Proc. 46, 2969–2974 (2021).
Ahmmad, B. et al. Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv. Powder Technol. 24, 160–167 (2013).
Naz, S. et al. Green synthesis of hematite (α-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J. Mol. Struct. 1185, 1–7 (2019).
Pallela, P. N. V. K. et al. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 5, e02765 (2019).
Adelere, I. A. & Lateef, A. A novel approach to the green synthesis of metallic nanoparticles: The use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 5 (2016).
Krishnani, K. K. et al. Metallic and non-metallic nanoparticles from plant, animal, and fisheries wastes: Potential and valorization for application in agriculture. Environ. Sci. Pollut. Res. 29, 81130–81165 (2022).
Singh, B., Singh, J. P., Kaur, A. & Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus Peel. Food Res. Int. 132, 109114 (2020).
Rafiq, S. et al. Citrus Peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 17, 351–358 (2018).
Cain, C. N., Schöneich, S. & Synovec, R. E. Development of an enhanced total ion current chromatogram algorithm to improve untargeted peak detection. Anal. Chem. 92, 11365–11373 (2020).
Thabti, I., Elfalleh, W., Hannachi, H., Ferchichi, A. & Campos, M. D. G. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC–MS. J. Funct. Foods 4, 367–374 (2012).
Khan, M. A. et al. A comparative study of green and chemical cerium oxide nanoparticles (CeO2-NPs): From Synthesis, characterization, and electrochemical analysis to multifaceted biomedical applications. BioNanoScience 13, 667–685 (2023).
Zahid, M. U. et al. A comparative study of PEGylated cobalt oxide nanoparticles (Co3O4-NPs) and cobalt sulfide nanoparticles (Co9S8-NPs) for biological and photocatalytic applications. BioNanoScience. https://doi.org/10.1007/s12668-024-01322-2 (2024).
Gillani, S. A. et al. The distinct biological properties of polyethylene glycol (PEG) and cetyltrimethylammonium bromide (CTAB)-Capped cerium oxide nanoparticles (CeO2-NPs). Plasmonics https://doi.org/10.1007/s11468-024-02757-9 (2025).
Ahmad, U. et al. Effect of surface capping on the biological properties of calcium oxide nanoparticles (CaO-NPs). Chem. Pap. https://doi.org/10.1007/s11696-024-03446-x (2024).
Jatoi, N. R. et al. A comparative study of Fagonia Arabica fabricated silver sulfide nanoparticles (Ag 2 S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties. Green Process. Synthesis 13 (2024).
Rehman, Z. et al. A comparative study of green and chemically prepared PEGylated zinc sulfide nanoparticles (ZnS-NPs) for biological applications. ChemistrySelect 9 (2024).
Ihsan, J. et al. Synthesis, characterization, and biological screening of metal nanoparticles loaded gum acacia microgels. Microsc. Res. Tech. 84, 1673–1684 (2021).
Aziz, S., Abdullah, S., Anwar, H., Latif, F. & Mustfa, W. Effect of engineered nickel oxide nanoparticles on antioxidant enzymes in freshwater fish, Labeo Rohita. Pak. Vet. J. 41, 424–428 (2021).
Hussain, A., Rasheed, H., Khan, M. A. & Bokhari, S. A. I. Potentials of TPC and TFC as free radical scavengers and microbial growth inhibitors in Himalayan endemic Artemisia sieversiana ehrhl ex willd. (Asteraceae) plant from northeastern, Pakistan. Anti-Infective Agents 22, (2024).
Khan, A. U. et al. Antimicrobial, antioxidant, and antileishmanial activity of Tavernier glabra mediated ZnO NPs and Fe2O3 NPs. Inorg. Chem. Commun. 148, 110297 (2023).
Faisal, S. et al. Edible mushroom (Flammulina velutipes) as biosource for silver nanoparticles: From synthesis to diverse biomedical and environmental applications. Nanotechnology 32, 065101 (2021).
Khalil, A. T. et al. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase Cobalt oxide (Co3O4) nanoparticles via sageretia thea (Osbeck). Arab. J. Chem. 13, 606–619 (2020).
Bae, S., Gim, S., Kim, H. & Hanna, K. Effect of NaBH 4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p -nitrophenol. Appl. Catal. B. 182, 541–549 (2016).
Gul Khatab, M. et al. Facile preparation of copper nanoparticles-decorated reduced graphene oxide for the catalytic reduction of water toxins. J. Mater. Sci. 59, 17860–17876 (2024).
Keyvani-Ghamsari, S., Rahimi, M. & Khorsandi, K. An update on the potential mechanism of Gallic acid as an antibacterial and anticancer agent. Food Sci. Nutr. 11, 5856–5872 (2023).
Zanwar, A. A., Badole, S. L., Shende, P. S., Hegde, M. V. & Bodhankar, S. L. Antioxidant role of Catechin in health and disease. Polyphenols Hum. Health Disease (Elsevier), 267–271. https://doi.org/10.1016/B978-0-12-398456-2.00021-9 (2014).
Liang, N. & Kitts, D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 8, 16 (2015).
Liu, J., Du, C., Beaman, H. T. & Monroe, M. B. B. Characterization of phenolic acid antimicrobial and antioxidant structure–property relationships. Pharmaceutics 12, 419 (2020).
Choi, S. S., Park, H. R. & Lee, K. A. A comparative study of Rutin and Rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 10, 1696 (2021).
Zduńska, K., Dana, A., Kolodziejczak, A. & Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 31, 332–336 (2018).
Buzdağlı, Y., Eyipınar, C. D., Kacı, F. N. & Tekin, A. Effects of hesperidin on anti-inflammatory and antioxidant response in healthy people: A meta-analysis and meta-regression. Int. J. Environ. Health Res. 33, 1390–1405 (2023).
Azeem, M. et al. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 80, 241–262 (2023).
Imran, M. et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 24, 2277 (2019).
Ajinkya, N. et al. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: Present and future. Materials 13, 4644 (2020).
Verma, D. K., Patel, S. & Kushwah, K. S. Green biosynthesis of silver nanoparticles and impact on growth, chlorophyll, yield and phytotoxicity of phaseolus vulgaris L. Vegetos 33, 648–657 (2020).
Bhatt, S. & Saraswat, S. A review on phytochemical mediated synthesis of nanoparticles through fruits and vegetables extract and their potential applications. Nanatechnol. Environ. Eng. 9, 359–374 (2024).
Mare, R. et al. Assessment of mediterranean citrus Peel flavonoids and their antioxidant capacity using an innovative UV-Vis spectrophotometric approach. Plants 12, 4046 (2023).
Kumar, S. & Pandey, A. K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013 (2013).
Sierra, J. A. et al. Biogenic approaches using citrus extracts for the synthesis of metal nanoparticles: The role of flavonoids in gold reduction and stabilization. New J. Chem. 40, 1420–1429 (2016).
Behera, S. S., Patra, J. K., Pramanik, K., Panda, N. & Thatoi, H. Characterization and evaluation of antibacterial activities of chemically synthesized iron oxide nanoparticles. World J. Nano Sci. Eng. 02, 196–200 (2012).
Vikram, S., Vasanthakumari, R., Tsuzuki, T. & Rangarajan, M. Investigations of suspension stability of iron oxide nanoparticles using time-resolved UV–visible spectroscopy. J. Nanopart. Res. 18, 272 (2016).
Laurent, S. et al. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008).
Liu, Q., Shen, J., Yang, X., Zhang, T. & Tang, H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B 232, 562–573 (2018).
Lassoued, A., Lassoued, M. S., Dkhil, B., Ammar, S. & Gadri, A. Synthesis, photoluminescence and magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys. E: Low-dimens. Syst. Nanostruct. 101, 212–219 (2018).
Ernawita et al. Vitro lipophilic antioxidant capacity, antidiabetic and antibacterial activity of citrus fruits extracts from Aceh. Indonesia Antioxidants 6, 11 (2017).
Song, S. Y., Kim, C. H., Im, S. J. & Kim, I. J. Discrimination of citrus fruits using FT-IR fingerprinting by quantitative prediction of bioactive compounds. Food Sci. Biotechnol. https://doi.org/10.1007/s10068-017-0263-3 (2017).
Velgosova, O., Dolinská, S., Podolská, H., Mačák, L. & Čižmárová, E. Impact of plant extract phytochemicals on the synthesis of silver nanoparticles. Materials 17, 2252 (2024).
Nortjie, E., Basitere, M., Moyo, D. & Nyamukamba, P. Assessing the efficiency of antimicrobial plant extracts from Artemisia Afra and Eucalyptus globulus as coatings for textiles. Plants 13, 514 (2024).
Bhuiyan, M. S. H. et al. Green synthesis of iron oxide nanoparticle using carica Papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 6, e04603 (2020).
Buazar, F. et al. Facile one-pot phytosynthesis of magnetic nanoparticles using potato extract and their catalytic activity. Starch - Stärke 68, 796–804 (2016).
Kumar, B. et al. Characterization and application of biosynthesized iron oxide nanoparticles using citrus paradisi peel: A sustainable approach. Inorg. Chem. Commun. 119, 108116 (2020).
Showmiya, K. & Ananthi, T. Phytochemical screening and FTIR analysis of Citrusmaxima linn. Leaves. Int. J. Pharma Res. Health Sci. 6, 2565–2569 (2018).
Fard, G. C., Mirjalili, M. & Najafi, F. Preparation of nano-cellulose/Α-Fe2O3 hybrid nanofiber for the cationic dyes removal: Optimization characterization, kinetic, isotherm and error analysis. Bulgarian Chem. Commun. 50 (2018).
Liang, C., Liu, H., Zhou, J., Peng, X. & Zhang, H. One-Step synthesis of spherical γ -Fe 2 O 3 nanopowders and the evaluation of their photocatalytic activity for orange I degradation. J. Chem. 2015, 1–8 (2015).
Fouda, A., Hassan, S. E. D., Saied, E. & Azab, M. S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by penicillium expansum strain (K-w). J. Environ. Chem. Eng. 9, 104693 (2021).
Bhosale, M. A., Ummineni, D., Sasaki, T., Nishio-Hamane, D. & Bhanage, B. M. Magnetically separable γ-Fe2O3 nanoparticles: An efficient catalyst for acylation of alcohols, phenols, and amines using sonication energy under solvent free condition. J. Mol. Catal. A: Chem. 404–405, 8–17 (2015).
Saifi, S. et al. Iron oxide-coated MWCNTs nanohybrid field emitters: A potential cold cathode for next-generation electron sources. J. Mater. Sci.: Mater. Electron. 31, 17482–17490 (2020).
Zhu, H. et al. A facile two-step hydrothermal route for the synthesis of γ-Fe2O3 nanocrystals and their magnetic properties. J. Mater. Sci. 42, 9205–9209 (2007).
Balasubramanian, R. et al. Dispersion and stability studies of resorcinarene-encapsulated gold nanoparticles. Langmuir 18, 3676–3681 (2002).
Tortella, G. et al. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: Advances and perspectives. Antibiotics 10, 783 (2021).
Wydra, R. J., Oliver, C. E., Anderson, K. W., Dziubla, T. D. & Hilt, J. Z. Accelerated generation of free radicals by iron oxide nanoparticles in the presence of an alternating magnetic field. RSC Adv. 5, 18888–18893 (2015).
Damián-Reyna, A. A., González-Hernández, J. C., Maya-Yescas, R. & de Jesús Cortés-Penagos, C. Del Carmen Chávez-Parga, M. Polyphenolic content and bactericidal effect of Mexican Citrus limetta and citrus reticulata. J. Food Sci. Technol. 54, 531–537 (2017).
Alam, F., Mohammadin, K., Shafique, Z., Amjad, S. T. & Asad, M. H. H. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 36, 1417–1441 (2022).
Clos, J., Grünebast, J. & Holm, M. Promastigote-to-Amastigote conversion in leishmania spp.—A molecular view. Pathogens 11, 1052 (2022).
Palomino-Cano, C., Moreno, E., Irache, J. M. & Espuelas, S. Targeting and activation of macrophages in leishmaniasis. A focus on iron oxide nanoparticles. Front. Immunol. 15, (2024).
Valgimigli, L., Baschieri, A. & Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 6, 2036–2051 (2018).
Kumar, H. et al. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 10, 1334 (2020).
Pyrzynska, K. & Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 5, 4288 (2013).
Ilyasov, I. R., Beloborodov, V. L., Selivanova, I. A. & Terekhov, R. P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 21, 1131 (2020).
Jan, S., Khan, M. R., Rashid, U. & Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of monotheca buxifolia fruit. Osong Public. Health Res. Perspect. 4, 246–254 (2013).
Bhalodia, N., Nariya, P., Shukla, V. & Acharya, R. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. AYU (An Int. Q. J. Res. Ayurveda) 34, 209 (2013).
Qadeer, B. et al. PEGylation of silver nanoparticles via berginia ciliata aqueous extract for biological applications. Emergent Mater. https://doi.org/10.1007/s42247-024-00727-9 (2024).
Wang, Y., Yang, Z. & Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 47, 534–539 (2010).
Balkrishna, A. et al. Anti-Acetylcholinesterase activities of Mono-Herbal extracts and exhibited synergistic effects of the phytoconstituents: A biochemical and computational study. Molecules 24, 4175 (2019).
Hampel, H. Revisiting the cholinergic hypothesis in Alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prevent. Alzheimer’s Dis. 1–14 (2018). https://doi.org/10.14283/jpad.2018.43
Faraone, I. et al. Antioxidant, antidiabetic, and anticholinesterase activities and phytochemical profile of Azorella glabra Wedd. Plants 8, 265 (2019).
Tchieno, F. M. M. & Tonle, I. K. P -Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 37, (2018).
Chen, Y., Feng, L. & Sadeghzadeh, S. M. Reduction of 4-nitrophenol and 2-nitroaniline using immobilized CoMn 2 O 4 NPs on lignin supported on FPS. RSC Adv. 10, 19553–19561 (2020).
Yuan, S., Tian, M., Cui, Y., Lin, L. & Lu, X. Treatment of nitrophenols by cathode reduction and electro-Fenton methods. J. Hazard. Mater. 137, 573–580 (2006).
Baysal, G., Uzun, D. & Hasdemir, E. The fabrication of a new modified pencil graphite electrode for the electrocatalytic reduction of 2-nitrophenol in water samples. J. Electroanal. Chem. 860, 113893 (2020).
Wu, L. et al. Study on the adsorption properties of Methyl orange by natural one-dimensional nano-mineral materials with different structures. Sci. Rep. 11, 10640 (2021).
Meena, J. et al. Copper oxide nanoparticles fabricated by green chemistry using tribulus terrestris seed natural extract-photocatalyst and green electrodes for energy storage device. Sci. Rep. 13, 22499 (2023).
Acknowledgements
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025).
Author information
Authors and Affiliations
Contributions
M.A.K., perceived the idea, carried out the experimental work, and wrote and reviewed the manuscript M.A., M.U.Z., S.H.A.H., and B.F.H. helped in investigation, data analysis and wrote the manuscript. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, M.A., Ahmed, M., Abu-Hussien, S.H. et al. Green synthesis of iron oxide nanoparticles (Fe2O3-NPs) from Citrus Limetta agrowaste for biological and photocatalytic applications. Sci Rep 15, 33107 (2025). https://doi.org/10.1038/s41598-025-17750-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17750-3