Abstract
Xanthium is represented in Europe by three species complexes: X. strumarium L., X. orientale L., and X. spinosum L. The former two complexes are similar, in both morphology and ecological requirements. Xanthium strumarium is native to the Old World, whereas X. orientale originates from America and was accidentally introduced into Europe about two centuries ago. Since then, it has colonized the whole continent, while the native congener has become increasingly rare.
Over two years, we conducted competition experiments to assess the impact of the introduced X. orientale on the fitness of the native X. strumarium. Germination time, dry biomass, number of burs (pistillate flower heads) and bur biomass were measured as proxies of fitness. Xanthium strumarium was grown alone (control), together with conspecifics (intraspecific competition) or with X. orientale plants (interspecific competition). We also evaluated the allelopathic effect of X. orientale over X. strumarium, by watering Xanthium seedlings with exudate of X. orientale dry leaves.
Growth and reproductive traits of X. strumarium were significantly lower in individuals growing in proximity of X. orientale compared to the control, whereas intraspecific competition has a lower but still significant effect. Xanthium orientale, although, germinates and grows faster than the Old-World congener, and under interspecific competition regime, X. strumarium produces significantly lower biomass, number of burs and bur biomass. Watering with exudates negatively influences the germination and the growth of the two species. We therefore believe that interspecific competition of the introduced congener may be one of the causes explaining the drastic decline of X. strumarium populations in Europe in the past century.
Similar content being viewed by others
Introduction
Invasive species are widely recognised as major contributors to biodiversity loss1. An invasive species can be defined as a non-native species (alien species) whose introduction or spread has been found to threaten or adversely impact biodiversity and related ecosystem services (EU Regulation No 1143/2014, Article 3, clause 2). Invasive species represent a significant conservation challenge, having been responsible for numerous extinctions2. Nevertheless, a significant proportion of the evidence supporting this argument is based on straightforward correlations between the prevalence of exotic species and the decline of native species in degraded ecosystems. However, it is important to recognise that direct causality is not the only possible interpretation of these findings1.
The concept of competition in plant ecology dates back centuries. Authors such as de Crescentiis in the fourteenth century (Ruralium Commodorum) and De Candolle3 discussed competitive interactions among plants. Darwin4, influenced by Malthus5, emphasised competition as a selective force shaping species evolution. Early ecologists regarded competition as an intrinsic part of nature6.
The principle of competitive exclusion, as defined by Hardin7, states that “complete competitors cannot coexist”. This implies that, in case two non-interbreeding populations occupy the same ecological niche and geographic territory, one will inevitably exclude the other. Grime8 identified several characteristics that contribute to the success and competitiveness of herbaceous plants. These include a tall stature, a growth form that allows for effective utilisation of the environment below and above ground, a high maximum potential relative growth rate, and a tendency to deposit a dense layer of litter on the ground surface.
Few studies explicitly compared co-occurring close relatives among plant species, estimating differences in both niche and competitive ability9. It is hypothesised that species that are part of the same genus and have the same life form can exhibit similarities in resource capture. Garcia-Serrano et al.10 conducted a comparative analysis of the competitive interactions between three species of the genus Senecio L.: one native and two invasive. Their findings revealed that in the majority of cases, one of the invasive species exhibited a higher competitive ability, leading to a greater population density. There are various studies that have proven the reduction of native populations due to competition with invasive congener, both in animal and plants (e.g. Senecio10; Podarcis11; Natrix12).
Xanthium L. is a genus of the subtribe Ambrosiinae (tribe Heliantheae), in the Asteraceae family13. The genus is characterised by the unusual pistillate inflorescences formed by two flowers enclosed completely in structures covered by hooked spines and beaks (catoclesium; hereafter also referred as bur for simplicity). Molecular marker-based studies have unequivocally demonstrated the existence of at least three distinct polymorphic species within the X. sect. Xanthium14: X. strumarium L., X. orientale L. and X. chinense Mill. The first species, X. strumarium, is native to the Old World, as evidenced by both fossil records15 and historical evidence (Dioscorides’ De Materia Medica, 1st Century BC). It presents small infructescences with straight apical beaks and few spines (Fig. 1b). Xanthium orientale, instead, is native to America but has become cosmopolitan as a result of human-assisted dispersal14. It is one of the most variable species complexes within the genus (Fig. 1a).
(a) Details of the burs of a Xanthium orientale subsp. italicum individual growing near Vers-Pont-du-Gard, Gard department, South France (photo: Natascha Wagner). (b) X. strumarium growing at the Old Botanical Garden of the Georg-August-University of Göttingen, Germany. (c) Seedlings of X. strumarium (small) and X. orientale subsp. italicum (tall) during the competition experiments carried out in 2022. Burs were sowed in the same day, and the picture was taken approximately two weeks after the first germination. (d) Two trays of the allelopathy experiments carried out in 2023. Seedlings belong to the same species, the tray on the left was watered with distilled water (control), the one on the right with the leachate of the leaves of X. orientale (treatment). (e) Competition experiments in summer 2022 in the Old Botanical Garden of the Georg-August-University of Göttingen. Plants in the front are X. strumarium (control and intraspecific competition pots), in the back pots containing also X. orientale subsp. italicum (tall plants; Interspecific competition).
In the 1820’s, some of the first naturalised populations of X. orientale in Europe were detected near Pavia, in Italy, by Moretti, who described the taxon “X. italicum Moretti”16. Those populations were typically found near riverbanks and wastelands. Nowadays the species is spread throughout the Mediterranean basin, but also in other regions of the world, and is in fact considered a dangerous invasive alien weed species, causing considerable losses in agricultural crops (e.g.,17,18,19). In Europe, it is categorised as an invasive species in 20 countries, while naturalised in eight other territories20. Its diffusion is likely related to a high genetic diversity, high germination rates (above 90%), a long flowering period, wind pollination, zoochory and hydrochory21. It is not clear, however, whether the presence of X. orientale also affects native species and especially the native congener.
Apart from competition, allelopathy, is another important factor for plant-plant interactions. It is defined as any direct or indirect harmful or beneficial effect by one plant on another through the production of chemical compounds that escape into the environment22,23. The seedlings of Xanthium, the leaves as well as other plant parts contain poisonous alkaloids and glycosides (e.g. potassium carboxyatractyloside,24). Among these, many are considered allelopathic compounds, such as xanthatine, 1α, 5α-epoxyxynthatin, 4-epiisoxanthanol, 4-epixanthanol, loliolide, dehydrovomifoliol, phenolics and volatile oils25,26. It was proven that these chemicals do not only play an important role in plant-plant interactions (e.g.,27,28,29) but can also cause animal diseases or are even toxic for grazing animals, especially in the early growth stages of the plants 30.
A recent study 31 demonstrated a significant decline of X. strumarium populations in Europe following the introduction of its congeneric X. orientale. This was evidenced by occurrence data based on herbarium specimens of the two species dating from 1800 to the present day. A similar pattern has been observed in other parts of its original distribution range (e.g.32,33,34,). In Italy, the native species has almost disappeared from collections, with the last known collected specimen being a voucher sampled by Brullo in Sicily in 197331. In contrast, the invasive species has been increasingly collected over the last century, being present nowadays in all Italian regions35 and considered invasive in almost half of them36. The decline of the native species is sometimes considered correlated to changes in land use (intensification of agriculture) and disappearance of ruderal places37, especially in areas where the species is traditionally considered an archaeophyte (e.g., Central and Northern Europe;38,39,40). There, the decline of the native species seems to have occurred before the arrival and spreading of the non-native congener37. However, studies based on fossils and pollen record have demonstrated that X. strumarium was already growing in natural places (e.g., riverbanks), rather than synanthropic environments in Central Europe already in prehistoric times41. Moreover, the hypothesis of the change in land use alone would not explain the decline of the species in areas where it is surely native and where ruderal places are still relatively abundant (e.g., The Mediterranean and the Middle East).
The factors contributing to the decline and disappearance of X. strumarium populations remain to be fully elucidated. The goal of the present study is to ascertain whether the interspecific competition between the European X. strumarium and the American X. orientale or the allelopathic effects of X. orientale over X. strumarium contributed to the decline of the autochthonous species populations. We conducted competition experiments in common garden and controlled climate chamber conditions to test the effect of the presence of X. orientale on the germination, growth and fitness of X. strumarium plants. The study was conducted over two years, 2022 and 2023.
Materials and methods
Plant material
Xanthium species are annual long-day herbs. In natural conditions, achenes germinate in the late spring, and the deriving plants flower in the late summer and produce mature infructescences in autumn. The two species considered in this study show slightly different phenology. Xanthium strumarium flowers earlier than X. orientale, and therefore, produces mature infructescences already in summer14,42.
We used X. strumarium burs from the Old Botanical Garden of the Georg-August University of Göttingen, where the species has been cultivated for a long time, and in summer 2022, burs were collected from a natural population growing near Kobylí (Břeclav district, Czech Republic; N 48.947050; E 16.896475). Infructescences for X. orientale were collected in Sicily in winter 2019–2020, in “Quattro Finaite”, Santa Flavia, on the margin of a countryside street about 15 km southeast from Palermo (N 38.037222; E 13.498889). They were collected from plants belonging to the “X. italicum” morphotype, which is also referred to as X. orientale subsp. italicum (Moretti) Greuter by some authors. For the experiments of 2023 (see below), we used burs collected in 2022 from plants cultivated in the botanical garden and derived from those originally collected in Italy.
Competition experiments
We used whole infructescences (burs) and not achenes or seeds, since they are the dispersal unit of the plant and seeds germinate when they are still inside them. Burs house two fruits (achenes), each containing a single seed. In the first favourable season after ripening, only one seed germinates; the second one will germinate in the following year or afterwards43,44. In spring 2022, burs were sown in 3L pots containing a common well-drained soil mixture consisting of 10:3 (v/v) of a universal soil and sand, with the addition of 0.002% (v/w) Osmocote Pro fertilizer (ICL Europe B.V., Ludwigshafen am Rhein, Germany). We sowed 15 pots with only X. strumarium burs (control), 15 with two burs of the same species (intraspecific competition), and 20 pots containing one bur of X. strumarium and one of X. orientale (interspecific competition; Fig. 1c). Pots were placed in a YORK® climate chamber (Johnson Controls, Cork, Irland) until germination and for the first weeks after it, in order to have constant conditions (16 h of light at 25 °C, 8 h of darkness at 20 °C, 60% humidity). Germination time was registered as days after sowing. At the end of May, the pots were placed outside in the Old Botanical Garden of the University of Göttingen (Fig. 1e). Upon attaining maturity, the ripe infructescences were collected, and plants were harvested at the base of the stem and dried in a Universal Oven UF200 (Memmert GmbH, Büchenbach, Germany) for 24–36 h at 65 °C. As a proxy to measure fitness, we used the number of infructescences produced by a plant, the average weight of the infructescences and the plant dry biomass.
In 2023, we used seedlings from the control of the allelopathy experiments (see below), which were transplanted, as for the previous year, in 3L pots, and experiments followed the same scheme and conditions as in 2022.
Allelopathy experiments
The dry X. orientale plants that resulted from the experiments of 2022 were used to produce leaf exudate. Dry leaves were ground and soaked in distilled water for a few days. The leachate was produced on a weekly basis. We used 100 g of leaves in 2 L of distilled water (5% w/v). The resulting exudate was then filtered and used for the allelopathy experiments.
Burs of X. strumarium and X. orientale were sown in eight trays, containing 54 0.18 L pots each. Four trays were used for X. strumarium, four for X. orientale. For each species, a total of 108 burs (two trays) were treated with leaf exudate, while 108 burs were watered with distilled water and used as a control (Fig. 1d). The pots were watered singularly every day with spray bottles. Seventeen days after the first germination, the number of seedlings was registered, and individual plants were photographed on a white background and a ruler in a HerbScan TM 008 with a Canon EOS 5SD R camera (50.6 MegaPixel; Canon Inc., Ōta, Japan). The total leaf area was extracted using the software program ImageJ v. 1.53 (45, available at https://imagej.net/ij/).
Statistical analyses
After checking ANOVA assumptions, a two-way ANOVAs, followed by Tukey’s Honest Significant Difference (HSD) test for post hoc pairwise comparisons was performed to test the significance of the differences in dry biomass, numbers of infructescences produced and average weight of the infructescences of X. strumarium plants under a control, interspecific and intraspecific competition regime. For the intraspecific competition, the two plants growing in the same pot were both analysed separately as focal against the other. The analyses were done for both years separately. Two-ways ANOVAs and pairwise Tukey tests were also performed to test the significance of the differences in germination time and leaf area between Xanthium plants treated with X. orientale dry leaves exudate and those watered with distilled water in the allelopathy experiments conducted in 2023. All analyses were done in R v.4.4.246, using the R Studio software (v.2022.02.2-485;47 available at http://www.rstudio.com/). Boxplots and graphics were also produced in R, using ggplot248.
Results
Germination
In the first year we tested the germination times of the two species. In general, X. orientale germinated sooner than X. strumarium (7.57 and 10.09 days after sowing on average, respectively). This difference was found to be highly significant in the ANOVA test (p < 0.001) (Fig. 2; Table 1).
Competition
The dry biomass of X. strumarium was measured from control samples and those growing in an intra- and inter-specific competition regimes. Results from the ANOVA analyses showed a significant effect of competition on biomass (Table 2) and fruit number (Table 3) of the species for the experiments carried out during the first year (p < 0.001 for both treatments) and the second (p < 0.001 for both treatments), while for the bur weight (Table 4) only in 2022 there was a significant effect (p < 0.001). In general, the Tukey-test (Table S1a–f) showed a significant effect of interspecific competition (p < 0.001) in all cases except for the bur weight in 2023, whereas for the intraspecific competition it was highly significant (p < 0.001) for the plant biomass in both years and for bur number in 2022, significant (p < 0.01) for the bur number and only marginally significant (p < 0.05) for the bur weight in 2022 (Fig. 3). The Tukey test analysis (Table S1a, f) also revealed a significant difference in plant biomass between the treatments (intraspecific versus interspecific competition) in both years. In 2022, a significant difference was also observed in the infructescence weight (Fig. 3; Table S1c).
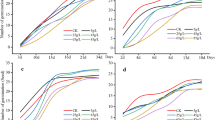
Effect of inter- (green, first box) and intra-specific competition (pink, third box) on the dry biomass, number of burs and mean bur weight of X. strumarium. In the two lines are shown results from the experiments of the two different years. (***) for P value < 0.001, (**) P value < 0.01, (*) P value < 0.05, (.) P value < 0.1 based on the Tukey-test.
Going more into detail, the plant biomass had a decrease of around 70.5% in 2022 and 69.7% in 2023 under the interspecific competition (p < 0.001) and of 34.9% and 30.8% under the intraspecific one (p < 0.001) for first and second year respectively. The production of infructescences was significantly influenced by the treatment, with a decrease under interspecific competition regime of 31.5% and 52.4% in 2022 and 2023 (p < 0.001) and of 19.8 and 57.6% under intraspecific competition (p < 0.01 and p < 0.001 for 2022 and 2023, respectively). The average bur weight, instead, was significantly affected by the treatment only in the first year, with a decrease in mass of 35.6% for interspecific treatment (p < 0.001) and of 12.1% for intraspecific one (p = 0.02). In 2023, even if not significantly, the burs mass had a decrease of 5.6 and 11.1% for the inter- and intraspecific treatments, respectively (Fig. 3; Tables 2, 3 and 4 and S1a-f).
Allelopathy
We compared the germination times of the two species under treatment and control conditions. In general, X. orientale seemed to have higher germination rates. For the control individuals, 84.3% of the burs germinated for X. orientale, for a total of 91 seedlings, whereas 76.9% of burs germinated for X. strumarium, with a total of 82 seedlings.
The treatment seemed to have influenced germination of the two species, with a decrease of circa 16% of germinations for X. orientale (67.6% of germinated burs, for a total of 72 seedlings) and of 12% for X. strumarium (64.8%, for a total of 70 seedlings). However, the germination time was significantly influenced by the treatment only for X. orientale (p < 0.001 in the Tukey-test) and marginally X. strumarium (p < 0.05 in the Tukey-test; Table S1g).
The leaf area was significantly affected by the treatment (p < 0.001; Table 5). Specifically, under treatment with X. orientale leaves exudate, there was a reduction in growth of about the 60% in both species (p < 0.001; Fig. 4; Table S1h).
Effect of treatment with leachate of dry leaves of X. orientale on the germination and growth (leaves area 17 days after the first germination) of X. strumarium (green, third box: control; yellow, fourth box: treatment) and X. orientale subsp. italicum (purple, first box: control; caerulean, second box: treatment). (***) for P value < 0.001, (**) P value < 0.01, (*) P value < 0.05, (.) P value < 0.1.
Discussion
In the present study, garden experiments were conducted over a period of two consecutive years in order to ascertain whether the presence of the introduced X. orientale could be considered a contributing factor to the observed decline of X. strumarium populations in the Old World.
Invasive alien species can cause the displacement and local extinction of native congeners through various mechanisms, with the most significant being habitat destruction, competitive exclusion, reproductive interference, and hybridisation. We tested competitiveness of the American X. orientale, nowadays widespread in different parts of the world, with the one of the congeneric X. strumarium, native to the Old World and declining in different parts of its distribution range. In both years, the competition with X. orientale was observed to have a detrimental effect on the growth and reproduction of X. strumarium. The reduction in biomass, and thus growth, of the plants growing under interspecific competition, along with the lower number of ripened fruits, may suggest that the competition of the congener is one of the factors causing the decrease of X. strumarium populations over the years with, consequently, the almost complete disappearance of the species in its native range. Alien plant species are considered to be a contributing cause of about 25% of plant extinctions49,50. Trophic competition has been often indicated as cause of exclusion of native species by introduced invaders, even though evidence supporting a general and primary role of the invasive aliens in the displacement of the native species remains limited51. In most studies on plants reviewed by Gurevitch and Padilla51, competition and indirect habitat effects of alien plants were believed to be the main threats causing population declines of native species. The deleterious effect of competition for the co-existence of native and alien species is supposed to be more important in closely related species (e.g., congeners). This principle, also known as “competition-relatedness hypothesis”52 was first formulated by Darwin4 and later discussed by several researchers53,54,55. However, only a few studies have corroborated these expectations on experimental ground, both in plants 10,81 and in animals12.
It is noteworthy that, although patterns are generally consistent, differences are registered in the mean biomass, number and mean weight of the infructescences between the two years. In the first year, plants of X. strumarium were on average smaller, and produced fewer and smaller (lighter) burs. Summer 2022 was extraordinarily dry in Western and Central Europe, which is probably the reason for these differences. This is also reflected in the effect of the competition of X. orientale on X. strumarium, as observed in the difference of infructescence weight between controls and plants growing under interspecific competition, which was significant only in 2022. Probably, the better climatic conditions of 2023 mitigated competition, suggesting that the effect of nter- and intra-specific competition can differ considerably under different environmental conditions, as observed already in other plant groups56.
Another important factor influencing plant interactions is allelopathy. It can act in combination with resource competition influencing growth and fitness of plant species growing in sympatry57, as demonstrated by empirical studies on tree species58. In our experiments, a lesser number of seedlings germinated under treatment with the leachate of the leaves of X. orientale compared to the control, both in X. strumarium and in X. orientale. The leachate negatively influenced the growth of the seedlings of both species. If, on the one hand, these results confirmed the allelopathic effect of X. orientale on the germination and growth of other species, as already observed in numerous other studies (e.g.,27,28,29), on the other hand, they evidenced an allelopathic effect of a species over itself, which is something rarely tested in other studies. The fact that we found a significant effect of the leachate of X. orientale on the germination and growth of seedlings of the same species opens questions on the significance of such experiments and on the biological meaning of the results. In our particular case, it could be hypothesized that burs of X. orientale germinate earlier, and seedlings grow faster than those of the congener (see Fig. 1) and start inhibiting via allelopathy and later on via resource competition the growth and reproduction of those plants germinating later (i.e., X. strumarium).
However, it must be noticed that both species grow in relatively open environments (seashores, riverbanks, ruderal places), where the effect of competition might be less important in determining population dynamics of both species. It is therefore questionable if competition and allelopathy alone are enough to explain the pattern of displacements observed in X. strumarium in the Old World. Another important mechanism that can produce the displacement of one species by a congener is Reproductive Interference (RI; i.e., the negative reproductive interaction among closely related species). RI can often impede coexistence of close relatives59,60. In plants, RI can be generated by various mechanisms, e.g., loss of ovules through seed set failure, pollen allelopathy, stigma clogging caused by interspecific pollen transfer, and the production of unviable hybrids (see61 and reference therein). The presence of asymmetric RI was already found in the genus Xanthium, in empirical studies involving X. chinense and X. orientale (X. orientale subsp. italiucum) in Japan32. However, it must be noticed that, differently from the two above-mentioned species, X. strumarium has a different phenology than X. orientale, which precludes any overlap of the flowering time between the two species14. In our garden experiments, burs of X. strumarium ripened well before X. orientale plants began flowering. Moreover, self-pollination can mitigate the negative effects of mutual reproductive interference between coexisting congeners62, and Xanthium is very well known to have a mating system promoting selfing63, as also corroborated by studies based on isozyme data64. Therefore, it seems unlikely that RI plays an important role in this setup, although experimental studies, using ecotypes of X. orientale more adapted to cold environments (and therefore flowering earlier;65,66), may help addressing the issue.
An alternative way in which a close relative invader can cause local extinction in native plant species is via hybridiszation, in cases where the formed hybrids are viable and can backcross with the parental species67. Hybridiszation with invasive congeners can threaten rare native taxa to extinction via genetic swamping, where the rare form is progressively replaced by hybrids, or by demographic swamping due to wasteful production of maladaptive hybrids (the former being more frequent;68). In such cases, extinction can occur within a few generations69 and numerous examples have been observed in different plant species70,71,72,73. Although most studies focused on hybrid extinction of rare species74,75, simulation studies demonstrated that even common species are at risk when exposed to highly competitive and invasive congeners, especially if hybrids are also competitive and highly fertile76. However, in our specific case, the aforementioned difference in flowering time should preclude the hybrid formation. Phenological differences hindering hybridiszation between different lineages in X. sect. Xanthium was already reported in other studies65. McMillan 82 was able to produce artificial hybrids between X. strumarium and X. orientale, which however were not highly fertile. The relative infertility of the experimental hybrids was interpreted to be a strong enough barrier to prevent the production of hybrid swarms and therefore maintain the genetic identity of X. strumarium77,78. In phylogenomic analyses including several samples of the two species79, no evidence of hybridiszation was found, although putative hybrids between the two species were included in the analyses (e.g., the original material of “X. sallentii Sennen” and “X. widderii Sennen”;80).
In conclusion, the presence of X. orientale, invasive to a vast part of the Old World, negatively influences the growth of the native congener X. strumarium via resources competition and allelopathy. This could have contributed to the decline and disappearance of X. strumarium observed in a great part of its distribution range. However, other potential causes, such as the disappearance of some of the suitable habitats (ruderal and waste places, extensively cultivated fields), the dispersal agents (e.g., the decline of transhumant sheep cattle in part of Europe), may have also played an important role on the decline of X. strumarium, at least in part of its former distribution range. The extent to which those processes contributed, in alternative or in combination with the biotic factors taken into account in our study, needs to be further investigated.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
06 December 2025
The original online version of this Article was revised: The Acknowledgements section was missing. The correct information now accompanies the original Article.
References
Didham, R. K., Tylianakis, J. M., Hutchinson, M. A., Ewers, R. M. & Gemmell, N. J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 20, 9. https://doi.org/10.1016/j.tree.2005.07.006 (2005).
Simberloff, D. Invasive species. In Conservation Biology for All (eds Sodhi, N. S. & Ehrlich, P. R.) (Oxford Academic, Oxford, 2010). https://doi.org/10.1093/acprof:oso/9780199554232.003.0008.
De Canadolle, A. P. Prodromus Systematis Naturalis Regni Vegetabilis 1824–1873 (Treuttel et Würtz, 1824).
Darwin, C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life. (J. Murray, 1859)
Malthus, T.R. An essay on the Principle of Population, as it affects the future improvement of Society. With remarks on the Speculations of Mr. Godwin, M. Condorcet, and Other Writers. (J. Johnson, 1798)
Grace, J. B. & Tilman, D. Perspectives on Plant competition (Academic Press Inc, 1990).
Hardin, G. Competitive exclusion principle. Science 131, 1292–1297 (1960).
Grime, J. Competitive exclusion in herbaceous vegetation. Nature 242, 344–347. https://doi.org/10.1038/242344a0 (1973).
Eckhart, V. M. et al. Contrasting soil-texture niches facilitate coexistence of two congeneric plants that differ in competitive ability. AoB Plants 9, plx066. https://doi.org/10.1093/aobpla/plx066 (2017).
Garcia-Serrano, H., Sans, F. X. & Escarré, J. Interspecific competition between alien and native congeneric species. Acta Oecol. 31, 96–78. https://doi.org/10.1016/j.actao.2006.09.005 (2007).
Capula, M. Competitive exclusion between Podarcis lizards from Tyrrhenian islands: Inference from comparative species distributions. pp. 89–93. Korsós, Z. and Kiss, I. (eds). Proc. Sixth Ord. Gen. Meet. S. E. H., Budapest (1992).
Metzger, C., Ursenbacher, S. & Christe, P. Testing the competitive exclusion principle using various niche parameters in a native (Natrix maura) and an introduced (N. tessellata) colubrid. Amphibia Reptilia 30, 523–531 (2009).
Tomasello, S., Stuessy, T. F., Oberprieler, C. & Heubl, G. Ragweeds and relatives: Molecular phylogenetics of Ambrosiinae (Asteraceae). Mol. Phylogenetics Evol. 130, 104–114. https://doi.org/10.1016/j.ympev.2018.10.005 (2019).
Tomasello, S. How many names for a beloved genus? Coalescent-based species delimitation in Xanthium L (Ambrosiinae, Asteraceae). Mol. Phylogenet. Evol. 127, 135–145. https://doi.org/10.1016/j.ympev.2018.05.024 (2018).
Löve, D. The genus Acanthoxanthium (DC.) Fourr. revived. Lagascalia 5, 55–71 (1975)
Moretti, G. D. Quibusdam plantis Italiae (Decas Quinta, Pavia, 1822).
Regnier, E. E., Stoller, E. W. & Nafziger, E. D. Common Cocklebur (Xanthium strumarium) root and shoot interference in soybeans (Glycine max). Weed Sci. 37(3), 308–313 (1989).
Bozsa, R. C. & Oliver, L. R. Shoot and root interference of common Cocklebur (Xanthium strumarium) and soybean (Glycine max). Weed Sci. 41(1), 34–37 (1993).
Katinczi, G., Torma, M., Béres, I. & Horváth, J. Competition between Xanthium italicum and crops under field conditions. Cereal. Res. Commun. 37, 77–80 (2009).
Kalusová, V. et al. Alien plants of Europe: An overview of national and regional inventories. Preslia 96, 149–182. https://doi.org/10.23855/preslia.2024.149 (2024).
Tang, J. S. & Ma, M. Genetic diversity and genetic differentiation of invasive weed Xanthium italicum in China. CR Biol. 343(1), 63–72 (2020).
Molisch, H. D. Einfluss Einer Pflanze Auf Die Andere-allelopathie (Fischer, 1937).
Rice, E. L. Allelopathy 2nd edn. (Academic Press, 1984).
Cutler, H. G. & Cole, R. J. Carboxyatractyloside: A compound from Xanthium strumarium and Atractylis gummifera with plant growth inibiting properties. The probable inhibitor A. J. Nat. Prod. 46(5), 609–613 (1983).
Shao, H., Zhang, Y. M., Nan, P., Huang, X. L. & Zhang, C. Chemical composition and phytotoxic activity of the volatile oil of invasive Xanthium italicum Moretti from Xinjiang China. J. Arid. Land. 5, 324–330. https://doi.org/10.1007/s40333-013-0170-2 (2013).
Yuan, Z. et al. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 23(11), 2840. https://doi.org/10.3390/molecules23112840 (2018).
Borbély, M. & Dávid, I. Changeability of allelopathy depending on several factors. Cereal Res. Comm. 36, 1383–1386 (2008).
Tanveer, A. et al. Allelopathic effects of Xanthium strumarium L. on seed germination and seedling growth of crops. Allelopathy J. 21(2), 317–328 (2008).
Benyas, E., Hassanpouraghdam, M. B., Zehtab Salmasi, S. & Khatamian Oskooei, O. S. Allelopathic effects of Xanthium striumarium L. shoot aqueous extract on germination, seedling growth and chlorophyll content of lentil (Lens culinaris Medic.). Rom. Biotech. Lett. 15 (3), 5223–5228 (2010).
Nasir, F. & Kahn, M. S. Validation of Some of the Ethnopharmacological Uses of Xanthium strumarium and Duchesnea indica. Pak. J. Bot. 44(4), 1199–1201 (2022).
Müller-Kiefer, J. & Tomasello, S. Invasive or threatened? The decline of the old-world Xanthium strumarium (Asteraceae) in Italy based on herbarium records. Fl Medit. 32, 17–24. https://doi.org/10.7320/FlMedit32.017 (2022).
Takakura, K. & Fujii, S. Reproductive interference and salinity tolerance differentiate habitat use between two alien cockleburs: Xanthium occidentale and X. italicum (Compositae). Plant. Ecol. 206, 309–319 (2010).
Danihelka, J., Chrtek, J. Jr. & Kaplan, Z. Checklist of vascular plants of the Czech Republic. Preslia 84, 647–811. https://doi.org/10.23855/preslia.2021.255 (2012).
Zehm, A. et al. Rückgang seltenster Pflanzenarten ist ungebremst—Freilanduntersuchungen zur Bestandsentwicklung vom Aussterben bedrohter Gefäßpflanzenarten Bayerns. Berichte der Bayerischen Botanischen Gesellschaft 90, 5–42 (2020).
Galasso, G. et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 152(3), 556–592. https://doi.org/10.1080/11263504.2018.1441197 (2018).
Celesti-Grapow, L. et al. (eds) Flora vascolare alloctona e invasiva delle regioni d’Italia (Casa Editrice Università La Sapienza, 2010).
Kaplan, Z. et al. Distributions of vascular plants in the Czech Republic. Part 10. Preslia 93, 255–304. https://doi.org/10.23855/preslia.2021.255 (2021).
Laus, H. Mährens Ackerunkräuter und Ruderalpflanzen: zugleich ein Beitrag zur Phytogeographie des Landes (Verl. der Kommission zur naturwiss, Durchforschung Mährens, 1908).
Opravil, E. Xanthium strumarium L.—An European archaeophyte?. Flora 173, 71–79 (1983).
Isermann, M., Rabitsch, W. & Nehring, S. BfN-Schriften 710—In Deutschland wild lebende Archäobiota und deren Status im Naturschutz. (Bundesamt für Naturschutz, 2024) https://doi.org/10.19217/skr710.
Brinkkemper, O. & Kuijper, W.J. Zum Vorkommen der Spitzklette (Xanthium strumarium L.) in Europa. In: 7000 Jahre bäuerliche Landschaft. Entstehung, Erforschung, Erhaltung. Zwanzig Aufsätze zu Ehren von Karl-Heinz Knörzer. (eds. Kalis and Meurers-Balke) pp. 81–88 (Habelt-Verlag, 1993)
Miller, P. The Gardeners Dictionary, eighth ed. (Printed by the Author, 1768)
Arthur, J. C. Delayed germination in the cocklebur and other paired seeds. Proc. Soc. Prom. Agric. Sci. 16, 70–79 (1895).
Crocker, W. Role of seed coats in delayed germination. Contributions from the hull botanical laboratory. Bot. Gaz. 42, 265–291 (1906).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imagej: 25 years of image analysis. Nat. Methods 9(7), 671–675. https://doi.org/10.1038/nmeth.2089 (2012).
R Core Team R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2023)
RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, http://www.rstudio.com/ (2022)
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, New York, 2016).
Bellard, C., Cassey, P. & Blackburn, T. M. Alien species as a driver of recent extinctions. Biol. Lett. 12, 20150623. https://doi.org/10.1098/rsbl.2015.0623 (2016).
Blackburn, T. M., Bellard, C. & Ricciardi, A. Alien versus native species as drivers of recent extinctions. Front. Ecol. Environ. 17(4), 203–207. https://doi.org/10.1002/fee.2020 (2019).
Gurevitch, J. & Padilla, D. K. Are invasive species a major cause of extinctions?. Trends Ecol. Evol. 19(9), 470–474. https://doi.org/10.1016/j.tree.2004.07.005 (2004).
Cahill, J. F. Jr., Kembela, S. W., Lamba, E. G. & Keddy, P. A. Does phylogenetic relatedness influence the strength of competition among vascular plants?. Perspect. Plant. Ecol. 10, 41–50. https://doi.org/10.1016/j.ppees.2007.10.001 (2008).
Elton, C. S. Competition and the structure of ecological communities. J. Anim. Ecol. 15, 54–68 (1946).
Park, T. Experimental studies of interspecies competition. I. Competition between populations of the flour beetles, Tribolium confusum Duvall and Tribolium castaneum Herbst. Ecol. Monogr. 18, 267–307 (1948).
Dayan, T. & Simberloff, D. Ecological and communitywide character displacement: The next generation. Ecol. Lett. 8, 875–894 (2005).
Skálová, H., Jarošik, V., Dvořáčková, S. & Pyšek, P. Effect of intra- and interspecific competition on the performance of native and invasive species of impatiens under varying levels of shade and moisture. PLoS ONE 8(5), e62842. https://doi.org/10.1371/journal.pone.0062842 (2013).
San Emeterio, L., Damgaard, C. & Canals, R. M. Modelling the combined effect of chemical interference and resource competition on the individual growth of two herbaceous populations. Plant Soil 292, 95–103. https://doi.org/10.1007/s11104-007-9205-9 (2007).
Fernandez, C. et al. The Impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front. Plant Sci. 7, 594. https://doi.org/10.3389/fpls.2016.00594 (2016).
Takakura, K. I. & Fujii, S. Island biogeography as a test of reproductive interference. Popul. Ecol. 57, 307–319 (2015).
Weber, M. G. & Strauss, S. Y. Coexistence in close relatives: Beyond competition and reproductive isolation in sister taxa. Annu. Rev. Ecol. Evol. Syst. 47, 359–381 (2016).
Watanabe, S. & Maesako, Y. Co-occurrence pattern of congeneric tree species provides conflicting evidence for competition relatedness hypothesis. PeerJ 9, e12150. https://doi.org/10.7717/peerj.12150 (2021).
Katsuhara, K. R. & Ushimaru, A. Prior selfing can mitigate the negative effects of mutual reproductive interference between coexisting congeners. Funct. Ecol. 33(8), 1504–1513. https://doi.org/10.1111/1365-2435.13344 (2019).
Löve, D. & Dansereau, L. Biosystematic studies on Xanthium: Taxonomic appraisal and ecological status. Can. J. Bot. 37, 173–208 (1959).
Dinelli, G., Bonetti, A. & Viggiani, P. Genetic structure and mating system of Italian Xanthium strumarium complex. Weed Sci. 51, 69–77. https://doi.org/10.1614/0043-1745(2003)051[0069:GSAMSO]2.0.CO;2 (2003).
Schull, C. A. Physiological isolation of types in the genus Xanthium. Bot. Gaz. 59, 474–483 (1915).
Ray, P. M. & Alexander, W. Photoperiodic adaptation to latitude in Xanthium strumarium. Am. J. Bot. 53(8), 806–816. https://doi.org/10.1002/j.1537-2197.1966.tb06837.x (1966).
Quilodrán, C. S., Currat, M. & Montoya-Burgos, J. I. Effect of hybridization with genome exclusion on extinction risk. Conserv. Biol. 32, 1139–1149. https://doi.org/10.1111/cobi.13120 (2018).
Todesco, M. et al. Hybridization and extinction. Evol. Appl. 9, 892–908. https://doi.org/10.1111/eva.12367 (2016).
Huxel, G. R. Rapid displacement of native species by invasive species: Effects of hybridization. Biol. Conserv. 89, 143–152 (1999).
Prentis, P. J., White, E. M., Radford, I. J., Lowe, A. J. & Clarke, A. R. Can hybridization cause local extinction: A case for demographic swamping of the Australian native Senecio pinnatifolius by the invasive Senecio madagascariensis?. New Phytol. 176, 902–912. https://doi.org/10.1111/j.1469-8137.2007.02217.x (2007).
Balao, F., Casimiro-Soriguer, R., García-Castaño, J. L., Terrab, A. & Talavera, S. Big thistle eats the little thistle: Does unidirectional introgressive hybridization endanger the conservation of Onopordum hinojense?. New Phytol. 206, 448–458. https://doi.org/10.1111/nph.13156 (2015).
Plume, O., Raimondo, F. M. & Troia, A. Hybridization and competition between the endangered sea marigold (Calendula maritima Asteraceae) and a more common congener. Plant Biosyst. 149(1), 68–77. https://doi.org/10.1080/11263504.2013.810182 (2015).
Pasta, S., Garfì, G., Carimi, F. & Marcenò, C. Human disturbance, habitat degradation and niche shift: The case of the endemic Calendula maritima Guss. (W Sicily, Italy). Rend. Fis. Acc. Lincei 28, 415–424. https://doi.org/10.1007/s12210-017-0611-5 (2017).
Levin, D. A., Francisco-Ortega, J. & Jansen, R. K. Hybridization and the extinction of rare plant species. Conserv. Biol. 10, 10–16. https://doi.org/10.1046/j.1523-1739.1996.10010010.x (1996).
Buerkle, C. A., Wolf, D. E. & Rieseberg, L. H. The Origin and Extinction of Species Through Hybridization. In Population Viability in Plants: Conservation (eds Brigham, C. A. & Schwartz, M. W.) 117–141 (Springer Verlag, 2003).
Wolf, D. E., Takebayashi, N. & Rieseberg, L. H. Predicting the risk of extinction through hybridization. Conserv. Biol. 15(4), 1039–1053. https://doi.org/10.1046/j.1523-1739.2001.0150041039.x (2002).
McMillan, C. Partial fertility of artificial hybrids between Asian and American Cockleburs (Xanthium strumarium L.). Nature 246, 151–153 (1973).
McMillan, C., Mabry, T. J. & Chavez, P. I. Experimental hybridization of Xanthium strumarium (compositae) from Asia and America. II. Sesquiterpene lactones of F1 hybrids. Am. J. Bot. 63, 317–323 (1976).
Manzo, E. & Tomasello, S. Recent long-distance-dispersal explains the range disjunction of the Old-Word Cockleburs (Xanthium strumarium). J. Biogeogr. 52, e15104. https://doi.org/10.1111/jbi.15104 (2025).
Widder, F. J. Die Arten der Gattung Xanthium: Beiträge zu einer Monographie. Repert. Spec. Nov. Regn. Veget. 20, 1–223 (1923).
Verlinden, M. & Nijs, I. Alien plant species favoured over congeneric natives under experimental climate warming in temperate Belgian climate. Biol. Invasions. 12, 2777–2787. https://doi.org/10.1007/s10530-009-9683-1 (2010).
McMillan, C. Experimental hybridization of Xanthium strumarium (Compositae) from Asia and America. I. Responses of F1 hybrids to photoperiod and temperature. Am. J. Bot. 62, 41–47 (1975).
Acknowledgements
The study was financed by the Deutsche Forschungsgemeinschaft (DFG), project TO1400/1-1, as part of the priority program 1991 “Taxon-Omics”. We gratefully acknowledge Professor Jiři Danihelka for his help and insightful discussions related to this research.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.T. designed research and sampled plant materials. E.M., C-S.E., J.F.P. and S.T. performed the garden, and climatic chamber experiments. E.M. and S.T. analysed the data. E.M. wrote the manuscript with the contribution of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manzo, E., Epifanio, CS., Pahl, J.F. et al. Interspecific competition with the American Xanthium orientale L. as a possible cause of the decline of the Old-World X. strumarium L.. Sci Rep 15, 32224 (2025). https://doi.org/10.1038/s41598-025-17814-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17814-4