Abstract
Tuberculous pleural effusion (TPE) can be effectively diagnosed using adenosine deaminase (ADA); however, high ADA levels in pleural effusion (PE) have also been shown to be linked to other diseases. In this study, we aimed to develop and validate a prediction model and differentiate TPE in patients with high ADA levels. This retrospective analysis of patients with ADA levels ≥ 25 IU/L was conducted at our healthcare institution between January 2017 and December 2023. After collecting and analyzing clinical and laboratory data, we developed predictive models using 31 indicators from serum and PE. The model’s performance was assessed using the area under the receiver operating characteristics curve (AUC), accuracy, sensitivity, and specificity. Based on their significance in disease prediction, the top five variables were selected for use in constructing the prediction model. We externally validated the diagnostic model using a cohort from a different hospital. Among the 237 included patients with high ADA levels, 133 (56.1%) and 104 (43.9%) were diagnosed with TPE and non-TPE, respectively. The LightGBM model was superior to the other models, achieving an AUC of 0.926, with high accuracy, sensitivity, and specificity. The top five variables, including effusion lymphocyte percentage, effusion lactate dehydrogenase/ADA, age, effusion total protein, and peripheral blood platelet count, were essential for creating an accurate predictive model, which demonstrated strong performances on the training, test, internal validation, and external validation sets. The results were then validated with decision curve analysis and a calibration curve. This novel predictive model based on clinical and laboratory features in serum and PE showed strong diagnostic capability in detecting TPE in patients with high ADA levels.
Similar content being viewed by others
Introduction
Pleural effusion (PE) is a widespread medical problem with a diverse range of causes. The differential diagnosis of PE is often challenging. The most common exudative pleural effusions (PEs) are tuberculous pleurisy effusion (TPE), parapneumonic effusion (PPE), and malignant pleural effusion (MPE)1. Definitive diagnosis of TPE depends on the detection of Mycobacterium tuberculosis by microscopy and/or culture in PE or pleural biopsy tissue specimens. However, PE has a low positive rate of acid-fast staining, while conventional microscopic PE with homogeneous-Nelson (Ziehl-Nielsen) or gold amine (Auramine) staining exhibit a mycobacterium detection rate of < 10.0%2. Although the mycobacterial culture of pleural biopsy specimens is relatively sensitive (40–60%)3, it is time intensive, and waiting for culture results can delay treatment initiation. It also requires standardized laboratories, and the acquisition of pleura is an invasive procedure, through either transthoracic needle or thoracoscopic pleural biopsy4. Therefore, a reliable, simple, and safe diagnostic method is urgently required.
The enzyme adenosine deaminase (ADA) exists in many cells, with a particularly important role in T-cell activation and lymphocyte differentiation. A previous study demonstrated that when the ADA threshold is 23–45 U/L, the diagnostic sensitivity and specificity for TPE were about 90%5. When this threshold is 40 ± 4 U/L, the sensitivity and specificity of TPE diagnosis are 93.0% and 90.0%, respectively. Another study reported that an ADA level > 70 U/L was highly indicative of TPE6. However, ADA is nonspecific and is also elevated in numerous other diseases, including other infectious diseases such as empyema, as well as autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and malignant tumors. A recent study reported that extremely high ADA levels suggest a low probability of TPE, while also proposing that the possibility of hematologic malignant PE should be considered in diagnosis7.
Recently, various clinical prediction models have been suggested to accurately diagnose TPE; however, these models vary greatly among studies due to differences in study variables, model construction methods, and sources of research subjects8. The pleural lactate dehydrogenase (LDH)/ADA ratio was recently recognized as a significant marker in diagnosing TPE, with a significantly lower ratio observed in TPE than in non-TPE9,10. Therefore, in this study, we aimed to develop and validate a prediction model based on laboratory data, such as pleural ADA and the LDH/ADA ratio, that could differentiate TPE in patients with elevated levels of ADA.
Methods
Study population
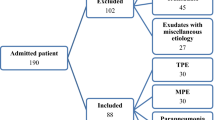
In this retrospective observational study, the data of patients with PE who were admitted to Sanmen People’s Hospital of Zhejiang Province from January 2017 to December 2023 were retrospectively analyzed. All patients met the following criteria: (1) PE diagnosis verified through chest X-ray, computed tomography (CT) scan or ultrasound; (2) patients undergoing diagnostic thoracentesis; and (3) PE ADA levels ≥ 25 IU/L (the range was determined using the reference range provided as part of the reagent guidelines). In the event that a patient had multiple procedures, only the results of the initial thoracentesis were examined. The exclusion criteria were as follows: (1) Patients with PE of unknown etiology; (2) Patients with incomplete clinical or laboratory data. The patients were classified into two groups: TPE and non-TPE (MPE and PPE). The external validation set comprised a cohort of 100 patients with PE in the First Affiliated Hospital Zhejiang University School of Medicine from March 2022 to July 2024.
This study was approved by the Ethics Committee of Sanmen People’s Hospital (No. 2025-0013) and the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2025-0835). Due to the retrospective nature of the study, the Ethics Committee of Sanmen People’s Hospital and the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine waived the need of obtaining informed consent. All methods were performed in accordance with the Declaration of Helsinki.
Data collection
All patient data were obtained from the Hospital Information System (HIS) and included the following: (1) Clinical information, including sex, age, and clinical diagnoses; (2) blood laboratory data, including white blood cell (WBC) count, neutrophil count (Neu.#), lymphocyte count (Lym.#), monocyte count (Mon.#), platelet count (PLT), total bilirubin (TB), total protein (TP), albumin (ALB), glucose (GLU), total cholesterol (TC), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (Ca125), D-dimer, activated partial thromboplastin time (APTT), fibrinogen (FBG), and LDH; and (3) laboratory data of PE, including nucleated cell count, Neu.%, Lym.%, Mac.%, effusion TP, effusion GLU, effusion ADA, and effusion LDH.
Derived inflammatory indices
The following inflammatory indices were derived from the collected laboratory data:
-
1.
Neutrophil to lymphocyte ratio (NLR): blood neutrophil count/lymphocyte count.
-
2.
Lymphocyte to monocyte ratio (LMR): blood lymphocyte count/monocyte count.
-
3.
Platelet to lymphocyte ratio (PLR): blood platelet count/lymphocyte count.
-
4.
Platelet to neutrophil ratio (PNR): blood platelet count/neutrophil count.
-
5.
LDH/ADA ratio (LAR): Effusion LDH/ADA.
Diagnostic criteria
TPE was diagnosed when the patient fulfilled at least one of the following criteria: PE positive Mycobacterium tuberculosis culture or nucleic acid amplification test; granulomatous inflammation in pleural biopsy specimens, excluding other granulomatous diseases; and resolution of the clinical manifestations and imaging findings of PE after empirical antituberculosis treatment.
MPE was diagnosed when the patient fulfilled at least one of the following criteria: identification of malignant cells in PE; and confirmation of a primary tumor and detection of atypical cells in PE, coupled with clinical evidence of tumor dissemination, after ruling out other potential causes of PE.
PPE was diagnosed when the patient fulfilled at least one of the following criteria: associated with bacterial pneumonia, pulmonary abscess, or bronchiectasis with infection; and resolution of PE due to antibacterial treatments. The MPE and PPE groups were defined as the non-TB group.
Statistical analysis and model development
The Extreme Smart Beckman Coulter DxAI platform (https://www.xsmartanalysis.com) as well as R software version 4.2.3 were used to analyze all statistical data. Frequencies are used to express categorical variables, while the mean ± standard deviation (SD) or median with inter-quartile range (IQR) are used to express continuous variables. Variables with > 20% missing data were excluded. The predictive median was used to match to impute numeric variables if missing values were < 20%. Clinical and laboratory features were compared using the chi-square test, Mann–Whitney test, or Student’s t-test. Spearman correlation analysis was used to determine the variables associated with TPE, with P-value < 0.05 considered statistically significant.
For feature screening, significance tests and correlation analysis were employed. Significance tests were performed to identify variables that were significantly different between the TPE and non-TPE groups. Chi-square tests, Mann–Whitney tests, or Student’s t-tests was used for statistical difference analysis, with P-values < 0.05 being considered statistically significant. The correlation coefficient was calculated to identify highly correlated pairs among all features. To avoid multicollinearity, paired with a correlation coefficient value > 0.9, one feature was randomly discarded.
The Extreme Smart Beckman Coulter DxAI platform was used to create the predictive model. Utilizing the selected features, a total of six predictive models were created: Logistic Regression, XGBoost, LightGBM, Random Forest, Adaboost, and DecisionTree. A refinement step was then used to boost their performance; this involved evaluating the features based on their significance and choosing the top five most important features from the model with the best performance. This approach guarantees that the models were constructed using the most impactful features, which may enhance their predictive accuracy.
Thirty-two cases (15%) were randomly selected from the total sample (test set), while the remainder of the samples (training set) were used for five-fold cross-validation. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the performance of the model. Then, decision curve analysis (DCA) was used to evaluate the prediction model’s clinical applicability. Calibration curves were subsequently generated to judge the consistency between the predicted probability of the model and the actual probability of occurrence.
Results
Participants
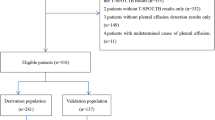
During the study period, 397 patients with PE and ADA levels > 25 IU/L were identified. Among these patients, 81 were excluded due to multiple PE samplings, and 79 were excluded due to meeting the exclusion criteria, including 26 patients with PE of unknown etiology and 53 patients whose clinical or laboratory data were incomplete. Ultimately, 237 patients were included in the final analysis. The included patients were categorized into TPE and non-TPE groups (n = 133 and 104, respectively) based on clinical features. Among 133 patients in the TPE group, 86 patients had fever, 26 patients had night sweats, and 6 patients had weight loss, the median duration of symptoms was 7 days. According to the Technical Specifications for Tuberculosis Prevention and Control Work in China (2020 Edition), the time point for observing the effectiveness of anti-tuberculosis treatment is 2 months after the initiation of treatment, which is evaluated based on the results of sputum smear for acid-fast bacilli, sputum culture for Mycobacterium tuberculosis, chest CT scans, and blood tests. Among the 133 patients with tuberculous pleural effusion, 119 were followed up. The median follow-up time was 12 months, with the 25th and 75th percentiles being 8 months and 24.5 months, respectively. Importantly, no patients experienced relapse during the follow-up period. The flowchart of the study can be found in Fig. 1.
Comparison of clinical features
Patients in the TPE group were considerably younger than those in the non-TPE group (P < 0.001). In the TPE group, effusion TP, GLU, lymphocyte percentage, serum TP, ALB, TC, Ca125, peripheral blood platelet count, PNR, plasma D-dimer, and APTT were all elevated in contrast to those in the non-TPE group (P < 0.05). By contrast, the TPE group displayed significantly lower effusion LDH levels, LDH/ADA ratio, neutrophil percentage, peripheral blood WBC count, neutrophil count, NLR and serum TB, LDH, and CEA levels compared with the non-TPE group (P-values < 0.001). There were no significant differences in sex, effusion ADA levels, nucleated cells, macrophage percentage, peripheral blood lymphocyte count, monocyte count, PLR, LMR, FBG, and serum GLU between the two groups. The demographic and laboratory characteristics of the included patients are shown in Table 1.
Correlation analysis with PE
The correlation between PE and various indicators was analyzed using Spearman’s method. As a result, we identified significant positive correlations between effusion LDH and effusion LDH/ADA, as well as between peripheral blood NLR and peripheral blood neutrophil and lymphocyte count, with correlation coefficients of r = 0.942, r = 0.954, and r = 0.996, respectively (Fig. 2).
Prediction model and performance
First, we identified 21 variables that showed significant differences between the TPE and non-TPE groups. Subsequently, feature correlation analysis was conducted on these indicators, and those with a correlation coefficient of more than 0.9 were removed; the selected features were then used for further model training. We attempted six common machine learning models, namely Logistic Regression, XGBoost, LightGBM, Random Forest, Adaboost, and Decision Tree, and evaluated their performance using the AUC. As a result, with an AUC of 0.926, the LightGBM model showed superior performance. Logistic Regression, XGBoost, Random Forest, Adaboost, and Decision Tree had AUCs of 0.915, 0.916, 0.904, 0.905, and 0.816, respectively (Fig. 3).
The feature weights were ordered from high to low. On this basis, the top five feature weights were selected to build the LightGBM model, including effusion Lym.%, effusion LDH/ADA, age, effusion TP, and peripheral blood PLT, with effusion Lym.% yielding the greatest weight (Fig. 4).
Fifteen percent of cases were randomly selected from the total sample (test set) for data validation, and the remainder of the samples (i.e., the training set) were used for five-fold cross-validation. The model achieved AUCs of 1.000, 0.911, and 0.970 on the training, validation, and test sets. Table 2 and Fig. 5a–c present the performance of this model.
External validation (61 TPE patients and 39 non-TPE patients) showed that the predictive model was highly effective, with the ROC curve analysis revealing an AUC of 0.869 (Fig. 5d).
We assessed the potential benefits and clinical applicability of the prediction model through DCA, which revealed notable benefits (Fig. 6a, b). Furthermore, as illustrated by the calibration curve in Fig. 7a, b, our model demonstrates a strong calibration effect. An online tool available through the Extreme Smart Beckman Coulter DxAI platform (http://www.xsmartanalysis.com/model/list/predict/model/html?mid=19630&symbol=2173pKXm268155JgfL33) can be used to generate predictive models using the current algorithm, and it was applied to estimate a patient’s risk of TPE by entering the parameters, as illustrated in Fig. 8.
Discussion
Although ADA is a well-known biomarker for TPE, its levels are also elevated in other inflammatory and malignant conditions. The ability to accurately differentiate TPE and non-TPE patients with high ADA levels is important for timely and appropriate treatment. In the current study, we developed and validated a predictive model to differentiate TPE and non-TPE in patients with PE ADA levels ≥ 25 IU/L. Our findings demonstrate that the LightGBM model, a predictive model based on clinical and laboratory features including effusion lymphocyte percentage, effusion LDH/ADA ratio, age, effusion total protein, and peripheral blood platelet count, achieved high diagnostic accuracy in identifying TPE, with an AUC of 0.926 in the training set and consistent validation across internal and external cohorts. The LightGBM model exhibited high accuracy, sensitivity and specificity, highlighting its potential value as a diagnostic tool in clinical practice.
In patients with TPE, the PE typically exhibits a high lymphocyte count, which is a hallmark of this condition. Studies have shown that TPE is characterized by a Th1 immune response, which is associated with increased levels of certain cytokines and immune cells, including lymphocytes, in the pleural fluid11. However, the presence of a high percentage of lymphocytes in PE is not exclusive to TPE, and the integration of lymphocyte percentage with cytokine profiles and other biochemical markers can provide a more comprehensive diagnostic approach, enhancing the accuracy of TPE identification in clinical practice12,13.
A previous study14 reported the diagnostic value of the pleural fluid LDH/ADA ratio, which has a high diagnostic power in differentiating TPE from other types of PE. This LDH/ADA ratio is particularly useful in cases with high ADA levels, as it provides additional specificity in distinguishing TPE from other conditions such as PPE and MPE. A previous systematic review suggested that the LDH/ADA ratio may be useful for differentiating TPE from PPE, recommending a cutoff value < 15 for this purpose15. Our findings are consistent with these results, revealing that the LDH/ADA ratios were lower in the TPE group than they were in the non-TB group (8.9 vs. 43.9, respectively).
Our results revealed that age is a significant factor in differentiating TPE and non-TPE. An earlier study conducted in the United States found that nearly half of TPE patients were younger than 45 years16. Age was independently predicted and negatively associated with TPE diagnosis. Furthermore, a prediction model that can evaluate the probability of TPE in patients with unexplained PE incorporated age as a critical variable. This model demonstrated good discrimination and calibration in identifying TPE, emphasizing the role of age in the diagnostic process8. Our findings are similar to these previous studies.
The total protein concentration in pleural fluid is used to classify effusions as transudates or exudates, with exudates typically having higher protein levels. This classification is crucial because TPE is generally an exudative effusion, characterized by elevated protein levels due to increased capillary permeability and the presence of inflammatory cells and proteins in the pleural space. In the context of differentiating TPE from other types of exudative effusions, such as MPE or PPE, total protein levels alone may be insufficient but can be included as part of a broader diagnostic panel. Recent advances in diagnostic techniques, such as the use of machine learning algorithms, have further improved the ability to differentiate TPE from non-TPE. These algorithms can integrate multiple clinical and biochemical parameters, including total protein levels, to provide a more accurate diagnosis17.
Studies have shown that platelet parameters, including platelet count, can provide valuable insights into the underlying pathophysiology of PEs. One such study reported that PLT and plateletcrit were significantly higher in TPE patients than in those with MPE18. The immune regulation in TPE involves pathways such as the PD-1/PD-L1 axis, which can modulate immune cell activity, including that of platelets. The increase in soluble and membrane-bound PD-L1 in TPE has been reported to be associated with immune suppression and disease progression, which may impact platelet dynamics in the pleural fluid and peripheral blood19.
Limitations
First, because the study’s retrospective design may have led to biases in data collection and patient selection, future prospective studies are needed to verify the validity of this model in real-world clinical practice and to assess the impact on clinical decision-making and patient outcomes.
Second, while our model incorporates a comprehensive set of clinical and laboratory features, the inclusion of emerging biomarkers or advanced imaging techniques could further enhance its diagnostic accuracy.
Last, the performance of the model may vary in populations with different prevalence rates of TPE or in regions with varying healthcare resources. Future studies should validate the model in diverse populations and settings to confirm its generalizability.
Conclusion
Our study presents a predictive model for differentiating TPE from non-TPE in patients with high ADA levels. By leveraging multiple clinical and laboratory features, the model demonstrates high diagnostic accuracy and consistent performance across validation sets. This approach has the potential to improve diagnostic accuracy and guide the clinical management of patients with pleural effusion. Future research should focus on further validation and exploration of additional biomarkers to enhance the model’s applicability and accuracy.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Light, R. W. Pleural effusions. Med. Clin. N. Am. 95(6), 1055–1070. https://doi.org/10.1016/j.mcna.2011.08.005 (2011).
Gopi, A., Madhavan, S. M., Sharma, S. K. & Sahn, S. A. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest 131(3), 880–889. https://doi.org/10.1378/chest.06-2063 (2007).
Ruan, S. Y. et al. Revisiting tuberculous pleurisy: Pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 67(9), 822–827. https://doi.org/10.1136/thoraxjnl-2011-201363 (2012).
Kirsch, C. M. et al. The optimal number of pleural biopsy specimens for a diagnosis of tuberculous pleurisy. Chest 112(3), 702–706. https://doi.org/10.1378/chest.112.3.702 (1997).
Palma, R. M., Bielsa, S., Esquerda, A., Martínez-Alonso, M. & Porcel, J. M. Diagnostic accuracy of pleural fluid adenosine deaminase for diagnosing tuberculosis. Meta-analysis of Spanish studies. Arch. Bronconeumol. (Engl. Ed.) 55(1), 23–30. https://doi.org/10.1016/j.arbres.2018.05.007 (2019).
Aggarwal, A. N., Agarwal, R., Sehgal, I. S. & Dhooria, S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS ONE 14(3), e0213728. https://doi.org/10.1371/journal.pone.0213728 (2019).
Choe, J. et al. Features which discriminate between tuberculosis and haematologic malignancy as the cause of pleural effusions with high adenosine deaminase. Respir. Res. 25(1), 17. https://doi.org/10.1186/s12931-023-02645-6 (2024).
Lei, X., Wang, J. & Yang, Z. Development and validation of a prediction model to assess the probability of tuberculous pleural effusion in patients with unexplained pleural effusion. Sci. Rep. 13(1), 10904. https://doi.org/10.1038/s41598-023-38048-2 (2023).
Wang, J. et al. The pleural fluid lactate dehydrogenase/adenosine deaminase ratio differentiates between tuberculous and parapneumonic pleural effusions. BMC Pulm. Med. 17(1), 168. https://doi.org/10.1186/s12890-017-0526-z (2017).
Blakiston, M., Chiu, W., Wong, C., Morpeth, S. & Taylor, S. Diagnostic performance of pleural fluid adenosine deaminase for tuberculous pleural effusion in a low-incidence setting. J. Clin. Microbiol. 56(8), e00258-e318. https://doi.org/10.1128/JCM.00258-18 (2018).
da Cunha, L. V. et al. Predominance of Th1 immune response in pleural effusion of patients with tuberculosis among other exudative etiologies. J. Clin. Microbiol. 58(1), e00927-e1019. https://doi.org/10.1128/JCM.00927-19 (2019).
Yi, F. S., Zhai, K. & Shi, H. Z. Helper T cells in malignant pleural effusion. Cancer Lett. 1(500), 21–28. https://doi.org/10.1016/j.canlet.2020.12.016 (2021).
Roofchayee, N. D. et al. T helper cell subsets in the pleural fluid of tuberculous patients differentiate patients with non-tuberculous pleural effusions. Front. Immunol. 2(12), 780453. https://doi.org/10.3389/fimmu.2021.780453 (2021).
Zhao, T., Zhang, J., Zhang, X. & Wang, C. Clinical significance of pleural fluid lactate dehydrogenase/adenosine deaminase ratio in the diagnosis of tuberculous pleural effusion. BMC Pulm. Med. 24(1), 241. https://doi.org/10.1186/s12890-024-03055-0 (2024).
Nyanti, L. E., Rahim, M. A. A. & Huan, N. C. Diagnostic accuracy of lactate dehydrogenase/adenosine deaminase ratio in differentiating tuberculous and parapneumonic effusions: A systematic review. Tuberc. Respir. Dis. 87(1), 91–99. https://doi.org/10.4046/trd.2023.0107 (2024).
Baumann, M. H. et al. Pleural tuberculosis in the United States: Incidence and drug resistance. Chest 131(4), 1125–1132. https://doi.org/10.1378/chest.06-2352 (2007).
Ren, Z., Hu, Y. & Xu, L. Identifying tuberculous pleural effusion using artificial intelligence machine learning algorithms. Respir Res. 20(1), 220. https://doi.org/10.1186/s12931-019-1197-5 (2019).
Ai, L., Li, J., Ye, T., Wang, W. & Li, Y. Use of platelet parameters in the differential diagnosis of lung adenocarcinoma-associated malignant pleural effusion and tuberculous pleural effusion. Dis. Markers. 27(2022), 5653033. https://doi.org/10.1155/2022/5653033 (2022).
Pan, X. et al. Increased soluble and membrane-bound PD-L1 contributes to immune regulation and disease progression in patients with tuberculous pleural effusion. Exp. Ther. Med. 12(4), 2161–2168. https://doi.org/10.3892/etm.2016.3611 (2016).
Acknowledgements
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Xuedan Lin conceived and designed the experiments, performed the data analysis, and wrote the manuscript. Yanchao Liu collected and organized the external validation data, performed the data analysis. Meiyun Chen and Anni Bao collected and organized the patients’ data, performed the data analysis. Tianxing Yang guided the determination of diagnostic criteria for clinical groups and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, X., Liu, Y., Chen, M. et al. Development and validation of a predictive model for tuberculous pleural effusion with high adenosine deaminase. Sci Rep 15, 31978 (2025). https://doi.org/10.1038/s41598-025-17864-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17864-8