Abstract
Human induced pluripotent stem cells (iPSCs) are gaining momentum as a powerful starting material in cell therapy. To fully harness their potential, CRISPR technology permits endogenous gene modifications as well as the introduction of advanced features, to increase the immune compatibility of the cells or insert suicide genes for enhancing therapeutic safety, for instance. However, genetic manipulation of iPSCs, in particular the generation of knock-in lines, remains relatively inefficient. Conventional mitigation strategies, such as enriching for positive cells using antibiotic selection or complex instrumentation, may, however, cause conflicts with good manufacturing practice (GMP) requirements. To address this challenge, we have systematically optimized a basic gene editing procedure using both Cas9 and Cas12a-based ribonucleoprotein (RNP) complexes. Based on the sequential delivery of RNPs and donor plasmids as a critical hallmark, this virus-free approach permits knock-ins of full-length transgenes at above 30% efficiency, while readily identifying positive clones through random screening at small scale. We exemplify these advances by creating and characterizing homozygous iPSC lines depleted of HLA class I and carrying an inducible caspase-9 suicide gene. Isolated clones from independent GMP iPSC lines retained genomic integrity, differentiation capability, and functionality of the safety switch in the differentiated state. This improved methodology will form a flexible platform for custom gene editing universally applicable both in basic iPSC research and therapy.
Similar content being viewed by others
Introduction
Due to their virtually unlimited differentiation potential, human induced pluripotent stem cells (iPSCs) have emerged as a promising starting material for various cell therapies1. These include, on the one hand, cell replacement therapies, for instance iPSC-derived cardiomyocytes for treatment after myocardial infarction2. On the other hand, the differentiation into cell types of the innate and adaptive immune system opens up allogeneic treatment options for different kinds of cancers. One key process for making iPSC-based therapies not only more effective and safe, but also more affordable, is the genetic manipulation of these cells using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR-Cas). For instance, the introduction of suicide genes may increase patient safety in case of adverse events3,4. The insertion of transgenes encoding chimeric antigen receptors (CARs) allows for directing therapeutic immune cells to the tumor5. And the manipulation of human leukocyte antigen (HLA) and other cell–cell recognition genes shall help to achieve universal immune compatibility in an allogeneic therapeutic setting6,7.
While knock-outs (KOs) of genes are well established in the field, the targeted integration of genes via knock-ins (KIs) still poses a more significant challenge. By experience, KIs are considered far less efficient in iPSCs compared to other cell types. While there are methods and strategies to improve KI efficiency, many of them are not compatible with GMP guidelines, which have to be met when producing cells for therapeutic applications. These guidelines are established to uphold the highest safety standards, encompassing rigorous donor screening, the use of high-quality, preferably non-animal-derived materials, comprehensive documentation, and stringent quality control measures8,9. Consequently, many strategies that enhance gene editing in research settings are not applicable within GMP-compliant workflows. For example, introducing antibiotic selection markers or fluorescent genes should be avoided in clinical processes, even though they simplify the editing process from a technical point of view3. Other ways for improving results may imply complex equipment like a cell sorting device, which is hard to operate under GMP conditions. Other studies demonstrate efficient and marker free KIs in iPSCs delivering their donor DNA via adeno-associated virus type 6 (AAV6) particles10. While this approach has demonstrated good results, the production of AAVs under GMP-compliant conditions is an additional step which requires more time, significant additional costs, and quality control.
In this study, we establish an efficient KI workflow which is GMP-compatible, virus-free, and does not require cell selection, enrichment, or use of any complex equipment. This workflow was developed using GMP iPSC lines described by Terheyden-Keighley et al., 202411. Applying this approach, we demonstrate the generation of homozygous iPSCs carrying a suicide switch based on an inducible Caspase 9 gene as recently introduced by Wunderlich et al. in 20223, together with a knock-out of Beta-2-Microglobulin (B2M), in a single step. The characterization of isolated clones from independent backgrounds revealed an intact geno- and phenotype, fully retained differentiation potential, as well as preserved transgene expression and activity after differentiation.
Results
Serial factor delivery is a key requirement for efficient knock-ins
To achieve the derivation of edited iPSCs carrying two copies of a desired transgene in a GMP-compatible manner, a prerequisite is a high fraction of cells carrying the desired knock-in. To do so, our goal was to develop a knock-in protocol, that was not only efficient, but also easy to apply by requiring simple handling steps and avoiding the use of complex equipment for sorting, as well as enrichment or selection. Three critical parameters were identified as key bottlenecks for efficient knock-ins, namely, efficient delivery of the required factors—gRNA, nuclease and donor DNA—the likelihood of cells to perform homology-directed repair (HDR) over non-homologous end joining (NHEJ), and cell survival or resilience of cells during the editing process.
As we were aiming for a universally applicable methodology, ribonucleoproteins (RNPs) were either based on Alt-R A.s. Cas12a Ultra or on Alt-R™ S.p. HiFi Cas9 Nuclease V3 (both from IDT). RNPs are complexes of a Cas protein and guide RNA (gRNA). Unlike delivery methods based on plasmids or mRNA, RNPs offer the highest editing efficiency with minimal off-target activity. They also exhibit reduced cytotoxicity and act immediately upon delivery, avoiding the delays associated with transcription or translation12,13,14. Donor templates were standard cloning plasmids. For optimization, a GFP KI into the AAVS1 locus was used as a model system (Supplementary Table S5). Initial work optimizing nucleofection conditions using the Lonza 4D Nucleofector and the pMaxGFP plasmid demonstrated a combination of the P4 Nucleofection Buffer with the program CA167 to yield the largest fractions of GFP + cells (Supplementary Figure S1 a). Moreover, a recovery step of 10 min in RPMI medium strongly increased cell survival after nucleofection as compared to recovery in iPSC medium (Supplementary Figure S1 b).
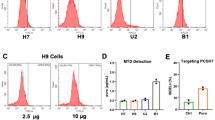
While plasmid delivery alone appeared highly efficient, and RNP delivery alone also resulted in high indel frequencies (Supplementary Figure S2), co-delivery of RNP and donor resulted in a low fraction of GFP + cells and poor cell survival (Supplementary Figure S1 c-d). Interestingly, when splitting delivery of RNP and donor into two serial nucleofections where on day one, cells received the plasmid, and on day two, the RNP was introduced- knock-in efficiency could be significantly increased (Fig. 1 a-b, Supplementary Figure S3 a-f). Notably, the recovery in RPMI medium after nucleofection was a prerequisite for the cells to survive two consecutive nucleofections. Next, scaling up the cell number per cuvette in the first nucleofection to 3*106, while keeping the amount of plasmid per cell the same, further increased KI efficiency and reduced variability (Fig. 1 a-b, Supplementary Figure S3 g-i. With the goal to further improve transgene integration into the genome, a “cold shock” step was introduced by incubating the cells at 32 °C after RNP nucleofection, as previously reported15. Indeed, this modification further improved KI efficiency (Fig. 1 a-b, Supplementary Figure S3 j-l). Moreover, replacing the standard culturing medium by a richer alternative two days prior to the first nucleofection resulted in slightly higher KI percentages (Fig. 1 a-b, Supplementary Figure S3 m-o). The aforementioned improvements collectively increased KI efficiencies from ~ 3% to up to 40%, as evidenced by flow cytometry and fluorescence microscopy in Fig. 1 c-d.
Optimized gene editing workflow enables efficient knock-ins in iPSCs. (a) Overview of optimized gene editing protocol. Critical optimizations are highlighted in blue. (b) Incremental increase of KI efficiency by implementation of each optimization indicated by flow cytometry quantification of GFP + cells 10 days after nucleofection. C = control, T = target. Controls received donor and RNP, but an irrelevant gRNA was used. (c-d) Example results of standard protocol (c) or fully optimized protocol (d) showing flow cytometry and fluorescence microscopy (with 5 × objective) of GFP. Scale bar = 200 µm. (e–g) Flow cytometry quantification of GFP integration comparing Cas12a against Cas9 (e), different target loci (f) or different GMP iPS cell lines (g). Replicate numbers are indicated by dots overlaying bar charts. Error bars denote standard deviation.
Interestingly, the serial delivery of donor plasmid followed by the RNP complexes appears to form a necessary requirement for enabling the other optimizations because omitting this critical step lead to a complete collapse in terms of KI efficiencies (Fig. 1 b, right; Supplementary Figure S5).
Further comparative investigation demonstrated that this protocol works well using either Cas12a or Cas9 nucleases at the same molecular concentration, as no significant difference in KI efficiency was observed between the two (Fig. 1 e). Moreover, targeting an independent genomic locus, the B2M gene, gave similar KI efficiencies as in AAVS1, suggesting that the method is target locus-independent (Fig. 1 f). Finally, comparable KI efficiencies were obtained using independent GMP iPS cell lines (Fig. 1 g). In sum, this optimized procedure appears to present a universal platform for manipulating therapeutically relevant PSCs regardless of the original donor, target locus, or nuclease used.
One-step iCaspase9 KI and B2M KO using optimized workflow
Genetic engineering of iPSCs is a promising strategy to enhance cell therapeutic strategies, in particular with regards to edits of broad utility. Hence, as a general safety measure in the context of creating immune compatible iPSCs, we sought to introduce an inducible Caspase 9 transgene into our iPSCs. We chose a CAG promoter due to its strong and stable expression properties. By placing a transcription terminator in front of iCASP9 and its promoter and by targeting the first intron of the B2M gene, a KI of the transgene would concomitantly result in a B2M KO (Supplementary Table S7). A lack of B2M prevents the transport of HLA-I to the cell surface avoiding T-Cell activation and thus immune rejection when transplanted into a patient (Fig. 2 a-b). While this strategy allows to perform two edits in one, it also offers a great opportunity to easily screen for cells with biallelic transgene integration in the correct locus: Under normal conditions, iPSCs only have low HLA-I expression on their surface, but after Interferon γ (IFNγ) stimulation, the marker becomes highly detectable via flow cytometry. A mono- or biallelic integration and, thereby, knock-out of the B2M gene would reduce, or completely ablate HLA-I surface expression, respectively (Fig. 2 c)—as exploited in our screening strategy. Hence, around one week after delivering the gene editing components, manipulated cells were seeded into 96 well plates via limiting dilution. These were later passaged into 2 plates, one remaining in culture for further expansion, the other used for screening via flow cytometry—to identify clones that show no HLA-I expression after INFγ stimulation (Fig. 2 c-d).
Editing strategy and workflow. (a) Overview of functional consequence of B2M KO and iCasp9 KI. (b) Visualization of donor cassette integration into first B2M intron. E: Exon of B2M gene; CAG: Synthetic CAG promoter; iCASP9: iCASP9 coding sequence; SV40: SV40 polyA. (c) Illustration of HLA-I intensity based on hetero- or homozygous KI of iCASP9 construct. (d) Illustration of complete editing workflow from nucleofection to cryopreservation of candidate clones. (e) Fraction of HLA-I negative cells after editing and before subcloning in R26 and R36 indicating biallelic integration of iCASP9. gRNA-mediated cutting in the first B2M intron alone is not expected to diminish HLA-I expression. (f) Overview of screening results exemplified for one 96 well plate. Blue color refers to wells containing cells with HLA-I expression, white refers to empty wells. Clean HLA-I negative clones are indicated by yellow dots. Half yellow/half blue refers to mixed wells containing HLA-I negative and positive cells. (g) Flow cytometry results from clone screening providing examples for HLA-I+ cells (top), HLA-I- cells (middle) and mixed populations (bottom). Variability in the signals likely resulted from the use of a shortened high-throughput protocol for processing 96-well plates, which omitted washing steps to expedite sample handling.
The iCASP9 KIs were performed using an iPSC line derived from a European donor (R26) and one from a US donor (R36), both from our portfolio of GMP-compliant iPSC lines based on a validated reprogramming/banking process11. Figure 2 e illustrates the fractions of HLA-I negative cells, indicating biallelic editing – not necessarily biallelic knock-ins – at ~ 5% for R26 in this case and ~ 20% for R36. However, in this particular case, HLA-I negativity may roughly correlate with KI efficiency, as suggested by a control experiment in which GFP – not iCASP9 – was knocked into the B2M locus (Supplementary Table S6): Herein, analyzing GFP together with HLA-I expression demonstrated specific integration at the target site, as well as discrimination between heterozygous and homozygous transgene insertions (Supplementary Figure S4).
Based on these promising data, two 96 well plates were seeded for R36 and four for R26 to ensure derivation of enough homozygous clones for each line. Figure 2 f shows the flow cytometry results of one plate for R36 highlighting HLA-I fluorescence intensity and indicating three homozygous HLA-I knockout clones identified this way (marked yellow). Notably, six out of 39 wells with cells contained HLA-I negative clones (15.4%), although three of these were mixed with cells still expressing HLA-I – clearly a result of the imperfect single-cell seeding via limiting dilution. Figure 2 g shows representative flow cytometry results of the clone screening. Notably, due to variable HLA-I intensities, no clear discrimination between unedited and heterozygous clones was attempted, since only cells with bi-allelic editing were considered for further characterization.
Edited iPSCs lack HLA-I surface expression and carry functional suicide cassette in the correct genomic locus
Three clones per cell line were genotyped by PCR and investigated for iCASP9 expression. While gel electrophoresis indicated correct transgene insertion for 5 out of 6 clones, one clone generated from the R26 line did not show the expected PCR bands (Supplementary Figure S8). As expected from this result, this one clone also failed to die in the functional iCASP9 assay (data not shown). One clone per parental iPSC line—referred to as R26-iC and R36-iC—was then used for further characterization. First, we confirmed again that even after 3 days of IFNγ stimulation, HLA-I expression remained undetectable in the candidate clones (Fig. 3 a-c). PCR-based analysis confirmed integration of the transgene at the correct position (Fig. 3 d) and this was true for both alleles (Fig. 3 e). Moreover, RT-qPCR confirmed a complete lack of B2M mRNA as well as high levels of iCaspase9 (Fig. 3 f-g). Furthermore, flow cytometry confirmed expression of pluripotency markers (Fig. 3 h), which was supported by RT-qPCR data at RNA level (Supplementary Figure S6 a). Importantly, there was no sign of karyotypic aberrations in either of the candidate clones (Supplementary Figure S6 b-c).
Characterization of Edited iPSCs. (a-b) Lack of HLA-I in edited clones compared to parental line confirmed by flow cytometry. (c) Flow cytometry quantification of HLA-I expression. (d) PCR genotyping amplifying across upstream and downstream homology regions to confirm integration at correct position. Full gel images: Supplementary Figure S8. (e) PCR genotyping demonstrating absence of wild-type band. (f–h) RT-qPCR confirmation of successful B2M KO (f), iCASP9 expression (g) and retained pluripotency (h). (i) Overview of killing assay setup in T300 flasks. Spiking refers to the addition of a small fraction of wild type cells to the edited cells. Assuming all edited cells die, this demonstrates that from surviving single cells, full colonies can arise which can be detected. These conditions display positive controls for the pure edited conditions, where 100% suicide gene efficiency is claimed. (j) Microscopic view on edited iPSCs before start of killing assay and after recovery, including samples spiked with wild-type cells as controls. (k) Alkaline phosphatase staining of T300 flasks after recovery period comparing pure edited clones with spike-in controls. Scale bar = 200 µm.
Potential silencing events may question the safety of the suicide approach as a last resort in case of adverse events. Therefore, to demonstrate functionality of the transgene in all cells of a large population, both the selected R26 and the R36 clone were seeded into T300 flasks and grown until fully confluent. For each clone, a control flask was prepared, containing not only the edited cells, but also a low number of wild-type iPSCs (0.005%) spiked in as a control, to simulate putative rare cases of escaper cells. The cells from both scenarios were then treated with a chemical inducer of iCaspase 9 dimerization (CID, AP20187) for 48 h. Afterwards, cells were given time to recover for 7 days and an alkaline phosphatase staining was performed to label iPSC colonies (Fig. 3 i). While both R26-iC and R36-iC showed no viable cells after recovery, the conditions where edited cells were spiked with wild-type cells demonstrated multiple iPSC colonies (Fig. 3 j-k). Taken together, these data confirm the functionality of the inducible iCASP9 transgene and its activity in every cell without exception.
Edited cells are fully competent for differentiation and retain transgene expression and activity
As iPSCs are only a starting material for differentiated cells utilized in potential therapies, the KI clones were differentiated into three therapeutically relevant cell types, namely, cardiomyocytes, retinal pigment epithelium (RPE) cells, and natural killer (NK) – cells, using our previously developed protocols11. Cardiomyocyte differentiation (as illustrated in Fig. 4 a) gave rise to highly pure populations of cardiomyocytes as indicated by flow cytometry of cardiac troponin for both wild-type and edited iPSC lines (Fig. 4 b-c). To test the functionality of the suicide switch, cardiac aggregates were plated onto 6 well plates into monolayers. After 48 h of 1 nM CID stimulation and 7 days recovery, the plates were screened for surviving cells using brightfield microscopy and Calcein staining (Fig. 4 d-f). Indeed, the data demonstrated no effect of the CID on wild-type cardiomyocytes, while the edited cardiomyocytes were all eradicated as can be seen in Fig. 4 f.
Edited iPSCs give rise to highly pure cardiomyocyte cultures and retain a functional suicide switch. (a) Overview of cardiomyocyte differentiation protocol. (b) Representative image of cardiac aggregates generated from iPSCs. (c) Flow cytometry quantification of cardiac troponin in R26, R36 and edited cell lines. (d) Set up of killing assay, including re-plating, killing with 1 nM CID, recovery and Calcein staining. (e) Bright field images (scale bar = 200 µm) of cells after replating of spheres before start of killing assay. (f) Bright field images and Calcein staining after recovery period (scale bar = 50 µm).
Next, we investigated a representative cell type from the ectoderm lineage. Using the protocol of Fig. 5 a11, RPE cells were obtained from both R26-iC and R36-iC iPSCs at high purity as indicated by > 95% expression of RPE specific antigens premelanosome protein (PMEL17) and tyrosinase-associated protein (TYRP) (Fig. 5 b-c). Moreover, the engineered cells showed comparable expression of RPE-specific mRNAs as compared to their parental controls (Fig. 5 d). In addition, high levels of iCASP9 mRNA could still be detected in RPE cells from the edited clones (Fig. 5 e), whereas B2M mRNA remained undetectable (Fig. 5 f). Importantly, 48 h of 0.1 nM CID treatment was sufficient to kill all edited RPE cells, and no living cell was observed after 7 days of recovery (Fig. 5 g-h).
Edited clones successfully differentiate into RPE cells and retain functional kill switch. (a) Overview of differentiation protocol from iPSCs into RPE cells. (b-c) Flow cytometry quantification of of PMEL17 (b) and TYRP (c) expression. (d) RT-qPCR quantification of RPE-specific mRNAs. Expression of edited cells is normalized to expression of parental line (e–f) RT-qPCR quantification of iCASP9 expression (e) and B2M levels (f). Expression of edited cells is normalized to expression of parental line. (g-h) Brightfield images (5 × objective) of parental cell lines and edited cells before killing (g) and after recovery (h). Scale bars = 100 µm.
Finally, the edited cells were differentiated into NK cells as a representative cell type for cancer immunotherapy, using our GMP-compatible protocol based on feeder-free differentiation/expansion from iPSC-derived NK precursors as an intermediate stage (Fig. 6 a)11. Briefly, this differentiation procedure first generates a hemogenic endothelium (HE) harboring cell clusters on top of a more endothelial-like layer. In response to appropriate cues, these HE cells may give rise to hematopoietic progenitor cells transitioning into suspension and, subsequently, to NK cells (Fig. 6 b-c). Edited iPSCs gave rise to pure populations of NK cells indicated by flow cytometry as evidenced by ~ 99% of cells expressing the NK cell marker CD56 (Fig. 6 d). As predicted, in contrast to unedited NK cells, the iCASP9-positive lines did not show any trace of HLA-I surface expression (Fig. 6 e). To demonstrate cellular potency, the NK cells were used in a cytotoxicity assay against the K562 cancer cell line, at ratios 1:5, 1:1 and 5:1. The edited cells showed a slightly decreased efficacy at the two lower ratios but at 5:1, they were equal to their wild-type counterparts as the saturation point was reached (Fig. 6 f, Supplementary Figure S7). Finally, it was tested whether the edited NK cells could still be efficiently eliminated using the suicide switch. To quantify this, edited and wild-type NK cells were mixed 1:1, to then apply the iCASP9 activator (illustrated in Fig. 6 g). As predicted, following IFNγ stimulation, the two genotypes could readily be distinguished based on the presence or absence of HLA-I by flow cytometry (Fig. 6 h). After induction of the suicide switch, however, the HLA-I negative population representing edited cells completely disappeared (Fig. 6 i), showing that the killing is complete and confined to the edited iPSC-NK cells. Additionally, microscopic analysis of unmixed edited NK cells revealed no surviving edited cells after suicide gene activation, in harsh contrast to wild-type cells which were resistant to the drug (Fig. 6 j).
Edited clones give rise to large numbers of active NK cells and retain functional kill switch. (a) Overview of differentiation protocol to generate and expand NK-cells from iPSCs. (b) Schematic overview of cluster formation in cell culture dispersing into suspension as hematopoietic progenitor cells. (c) Brightfield images showing the transition from endothelial monolayer into suspension cells. (d-e) Flow cytometry quantification of CD56 expression (d) and HLA-I levels (e). (f) Summary of cytotoxicity assay measuring killing of K562 cells by NK cells at three different ratios. (g) Schematic representation of suicide assay of wild-type and engineered cells using flow cytometry. (h-i) Flow cytometry quantification of HLA-I expression in wild-type NK cells and edited NK cells mixed 1:1 before iCASP9 activation (h) and after activation and recovery period (i).(j) Brightfield images of wells containing wild-type or edited NK cells after suicide gene activation and recovery period. HSPC = hematopoietic stem/progenitor cells; HE hemogenic endothelium. Scale bar = 100 µm.
Discussion
In this study we present an optimized protocol for KIs in iPSCs which not only proved to be highly efficient and applicable to different cell lines, loci and nucleases, but also comprises a very simple workflow free of complex equipment, selection or enrichment steps, complex plasmid modifications, or viral particles. Hence, this workflow can easily be used for GMP-compliant editing of clinical iPSCs. The engineering of two different iPSC lines with an inducible Caspase 9 knock-in into the first intron of B2M, resulting in the KO of this gene, demonstrates the practicability of this workflow.
Improvements to the generation of KIs in iPSCs have been reported in the past. However, most of these approaches tend to be rather complex and/or not universally applicable. For example, different publications showed high KI efficiencies of GFP into iPSCs using Cas916 or Cas12a10 but required the use of AAV6 particles to deliver the donor DNA into the target cell. By contrast, the procedure described here utilizes standard plasmids, which are easier to produce and to handle with regards to safety and quality control, especially in a GMP environment. Another effective approach targets essential housekeeping genes, with the intention to disrupt the c-terminus to create a loss of function, which can only be rescued by the correct insertion of the donor DNA by HDR17. This approach achieves high KI rates using both Cas9 and Cas12a in different cell types including iPSCs but it is limited to certain housekeeping genes, which is not always ideal, for instance, in the case of cell type-specific transgene expression. Tagging an immune cell-specific gene at the iPSC stage would be unlikely to work at high efficiency, as disrupting its C-terminus of such gene would not cause any selection pressure.
Our data demonstrates successful integration of the inducible Capase9 as a suicide switch, which is not only highly expressed in the pluripotent state, but also in various therapeutically relevant cell types, that is, after differentiation. Importantly, it facilitates efficient killing within 48 h in all tested cell types. As the cultures are given one week to recover before analysis, the chance that a few surviving cells remain unnoticed becomes highly unlikely. Combining the KI with a B2M KO in one step provides the cells with the ability to evade T-cell recognition without the need to target the cells with a second gRNA, reducing the risk of unwanted editing events and minimizing the workload. Of course, HLA-I surface depletion alone is not sufficient to achieve full immune evasion, as NK-cells would recognize its absence and become activated. Classical hypoimmune approaches combine HLA-I and -II depletion with the KI of a surface protein such as CD47 or HLA-E to prevent NK-cell activation6, but other strategies combining multiple surface modifications for immune cloaking have been developed as well18. For the cell lines presented here, a KI of CD47 might be sufficient to prevent immune activation in most cases, as HLA-II expression is usually restricted to a limited number of antigen-presenting cells. However, this needs to be confirmed experimentally. Interestingly, the edited iPSC-derived NK cells showed slightly decreased cytotoxicity against K562 cells. An interesting interpretation would be that this is due to the lack of HLA-I rather than to other gene editing-related effects. Indeed, studies in mouse models suggested that global downregulation of HLA-I may lead to hyporeactivity of NK cells in certain situations19.
The data presented in this study suggest that, using the protocol described here, most genome editing events are specific, with a low frequency of unintended integrations or incomplete editing. Nevertheless, the potential for such events must be carefully considered when evaluating edited cell populations. Genotyping PCR, flow cytometry, and RT-qPCR analyses in this study support the bi-allelic integration of iCASP9. However, these methods cannot fully exclude the possibility that one allele may have undergone a complex rearrangement—such as a disruptive event leading to B2M loss without proper iCASP9 integration, resulting in a hemizygous cell line. Such aberrations may be difficult to detect without the use of more comprehensive genomic analyses20. Hence, whole genome sequencing may be a useful quality control assay for gene editing under GMP conditions.
Taken together, we have established a GMP-friendly and highly efficient KI platform which, due to its simple handling steps and flexibility, provides an attractive option for universal editing of iPSCs, applicable to all iPSC fields including cell therapy. The cell lines created in this study pose only one example of many useful edits of broad impact that could help to improve iPSC-based cell therapies in the near future.
Materials and methods
Generation and maintenance of iPSCs
R26 and R36 iPSC lines were derived under GMP conditions as described elsewhere11, by episomal reprogramming of CD34-positive cells from clincial-grade cord blood units from EU (R26) or US (R36) origin. They were manufactured by and are property of Catalent Duesseldorf GmbH. For the purpose of this study, under R&D conditions, iPSCs were cultured in Stem MACS iPS-Brew XF (iPS-Brew, Miltenyi # 130–104-368) medium on 0.15 µg/cm2 iMatrix-511 (Amsbio # 892,011) and replated 2 × per week using Accutase (Merck # A6964), usually Mondays and Thursdays (Mo 350,000 cells/6-well/Thu 120,000 cells/6-well). For gene editing, they were split 2 days before the first nucleofection (350,000 cells/6-well) [d-2 (Monday)] and seeded in StemMACS PSC-Brew XF (PSC-Brew # 130–127-865) + 10 µM Y-27632 (Y, Tocris # 1254).
Gene editing
Gene editing was performed via nucleofection of RNP complexes (4D Nucleofector, Lonza/program CA167/100 µl solution P4/cuvette format) and standard plasmids containing a transgene flanked by 600 bp homology arms in a kanamycin cloning backbone (synthesized by Genscript, Piscataway, NJ). The plasmids were amplified in E. Coli and purified using endotoxin-low midipreps and resuspended in EB buffer B (Qiagen # 19,086) at ~ 3 µg/µl. For RNPs, 2.42 µl of Cpf1 or 2.46 μl of Cas9 s (IDT # 1,081,061 or 10,001,273, respectively) were mixed with 4.5 µl Alt-R sgRNAs (IDT, 100 µM in IDTE pH = 7.5) targeting AAVS1 or B2M. 3 µg of donor vector was used per 106 cells. All nucleic acid sequences are given in Supplementary Table S4. In optimized protocol, 3*106 cells were nucleofected on day 1 with 9 µg donor vector. 24 h later, they were nucleofected again with RNP. Controls for unspecific integration were vector + RNP containing irrelevant gRNA. In brief, cells were seeded at 350,000 cells per 6-well in PSC-Brew two days prior to nucleofection. For nucleofections, cells were washed with 4 ml PBS-/- per well and incubated in Accutase for 20 min to ensure generation of single-cell suspension. After nucleofection, 400 µl RPMI medium was added and cuvettes were incubated at 37 °C for 10 min. Then they were transferred into 6 well plates with PSC-Brew supplemented with 1 × CloneR2 (Stemcell Technologies # 100–0691). After nucleofection, the cells were incubated at 32 °C for 24 h to improve HDR frequency. A patent application has been filed on the serial delivery aspect of this procedure (US provisional # 63/562,006). To assess editing efficiencies of different gRNAs, cells were nucleofected with RNPs, and genomic DNA was extracted after 24 h using the QIAamp DNA Mini Kit (Qiagen #51,304). Target regions were amplified with Q5 High-Fidelity 2 × Master Mix (NEB #M0491) using primers listed in Supplementary Table S3. PCR products were Sanger sequenced (Eurofins, TubeSeq Supreme), and indel frequencies were quantified using the ICE tool (EditCo), as shown in Supplementary Figure S2.
Single cell seeding
Single cell seeding was performed using limiting dilutions. Flat bottom 96 well plates were coated with 50 µl PSC-Brew supplemented with 1 × CloneR2 and iMatrix511. Cells were detached using Accutase for 20 min, centrifuged, resuspended, and filtered twice using a 20 µM cell strainer to achieve a real single cell solution. They were diluted to 33.3 cells/ml (equals 1 cell/30 µl) and 30 µl were seeded into each well using a multichannel pipette.
Cardiac differentiation
Differentiation was performed as described11 using medium based on Knockout DMEM supplemented with 0.1% (w/v) HSA, 250 µM 2-phospho-ascorbate, and L-glutamine, plus stage-specific factors/additives.
RPE differentiation
RPE differentiation was performed as described11. Undifferentiated cells were plated on 0.6 µg/cm2 iMatrix-511 and cultured in DMEMF12 supplemented with 15% (v/v) Knockout serum replacement with 1 × Glutamax. Differentiation was induced with 1 µM PD0325901, 5–10 µM SB431542, 0.25 µM Dorsomorphin, and 25 ng/mL Activin A for one week, followed by ActA alone for three weeks.
NK-cell differentiation
Differentiation was performed as described, with slight adjustments11. iPSCs were reseeded at 2*105 per laminin-511-coated 12-well and treated with 25 ng/mL BMP4 and 8 µM CHIR99021 in StemPro-34 for 72 h and, from days 3–6, with 200 ng/mL VEGFA and 10 µM SB431542. An EHT was then induced in APEL2 medium with 20 ng/mL SCF and 20 ng/mL IL-7. Emerging suspension cells were typically harvested at day 21 and were transferred to new 12-wells at 2*105/well in APEL2 medium with SCF, FLT3L, IL-7, and IL-15 at 20, 10, 20, and 10 ng/mL, respectively. Cultures were fed every 3–4 days applying 50% medium changes (2 mL total) until day 28 to then be repeatedly split weekly in DF12 with 10% hPL, 1 × Glutamax, and 250 µM 2-phospho-L-ascorbate, plus IL-15 for three weeks.
PCR-based methods
Primer sequences are given in Supplementary Table S2. SYBR Green-based RT-qPCRs were carried out as described and results were expressed as fold changes relative to an indicated reference21. RPL37A was used as a housekeeping gene. cDNA was prepared using 1–2 µg total RNA and M-MLV reverse transcriptase (Promega # M1701) with 0.5 µg oligo-sT15 priming in 25 µl reactions for 1 h at 42 °C. Conventional PCRs followed standard procedures and were performed using the Q5 High-Fidelity 2 × Master Mix (NEB # M0491). Annealing temperatures and elongation times are indicated in Supplementary Table S3. Genomic DNA was isolated using the QIAamp DNA Mini Kit (Qiagen #51,304). PCR amplicons were visualized using gelelectrophoresis performed for 1 h at 120 V. Gels consisted of 1.5% agarose in TAE buffer.
Immunofluorescence-based methods
Flow cytometry and immunofluorescence microscopy were carried out according to standard procedures. Microscopic pictures were obtained with the help of Axiovert 200 fluorescence microscope. Microscopy was performed only for GFP, so no staining procedure was necessary except for Calcein labelling. Cardiomyocytes were incubated in their standard culturing medium containing 1 µg/mL Calcein (Thermo Fisher # C3100MP) for 60 min. Afterwards, wells were washed twice with 4 ml PBS-/- and fresh medium was applied to the cells before imaging. For flow cytometry, a MACSQuant® Analyzer 10 Flow Cytometer was used. When flow cytometry was applied for clone screenings, staining and measurements were performed using 96 well plates. For flow cytometry, cells were incubated with antibodies for 10 min at 4 °C and washed before and after with PBS-/- supplemented with Y. Optionally, dead cells were additionally discriminated by staining with propidium iodide (1 µg/ml) included in PBS solution. FACS antibodies and controls are given in Supplementary Table S1. For HLA-I staining, cells were stimulated for 48 h with 100 ng/ml Interferon γ (Peprotech # 300–02).
Statistical methods
Quantitative data were processed in MS Excel or GraphPad Prism. Replicates were biological, not technical throughout. Error bars in charts highlighting individual replicates indicate SDs. Statistical testing was done using 2-sided unpaired t-tests against appropriate control samples. When comparing more than two groups, ANOVAs were performed followed by Bonferroni’s multiple comparison test. P values of < 0.05 (*), < 0.01 (**), < 0.001 (***) or < 0.0001 (****) statistically significant differences. Normality was assessed via QQ-plots (Supplementary Figure S9).
Killing Assay
Killing assay was performed as described in Terheyden-Keighley et al., 2024. In brief, K562 cells (strain AAC10) were used as target cells and labelled with Cell Trace Violet (CTV, ThermoFisher # C34564). Unlabeled iPSC-NK were used for killing at 1:5, 1:1, and 5:1 ratios of NK:K562. CellEvent Caspase-3/7 Green (ThermoFisher # C10423) and SYTOX 7AAD (ThermoFisher # S10274) was used to label apoptotic cells or dead cells respectively.
Alkaline Phosphatase staining
Alkaline phosphatase labeling was performed using BCIP®/NBT-Blue (Thermo Fisher # B3679). Cells were washed with PBS-/- once. Afterwards they were fixed with 4% formaldehyde (pH 7.4) for 5 min. Then they were washed twice with PBS-/- and incubated with BCIP®/NBT-Blue so that the whole plate surface was covered. After 30 min incubation in the dark at RT, cells were washed twice with PBS-/- before pictures were taken.
Data availability
All relevant data are available in the main article or supplementary information.
References
Yamanaka, S. Pluripotent stem cell-based cell therapy—Promise and challenges. Cell Stem Cell 27, 523–531. https://doi.org/10.1016/j.stem.2020.09.014 (2020).
Morita, Y., Kishino, Y., Fukuda, K. & Tohyama, S. Scalable manufacturing of clinical-grade differentiated cardiomyocytes derived from human-induced pluripotent stem cells for regenerative therapy. Cell Prolif. 55, e13248. https://doi.org/10.1111/cpr.13248 (2022).
Wunderlich, S. et al. Targeted biallelic integration of an inducible Caspase 9 suicide gene in iPSCs for safer therapies. Mol. Ther. - Methods Clin. Dev. 26, 84–94. https://doi.org/10.1016/j.omtm.2022.05.011 (2022).
Lipus, A. et al. Targeted integration of inducible caspase-9 in human iPSCs allows efficient in vitro clearance of iPSCs and iPSC-macrophages. Int. J. Mol. Sci. 21, 2481. https://doi.org/10.3390/ijms21072481 (2020).
Zhou, Y. et al. Engineering induced pluripotent stem cells for cancer immunotherapy. Cancers 14, 2266. https://doi.org/10.3390/cancers14092266 (2022).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258. https://doi.org/10.1038/s41587-019-0016-3 (2019).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 42, 413–423. https://doi.org/10.1038/s41587-023-01784-x (2024).
Rivera, T., Zhao, Y., Ni, Y. & Wang, J. Human-induced pluripotent stem cell culture methods under cGMP conditions. Curr. Protoc. Stem Cell Biol 54, e117. https://doi.org/10.1002/cpsc.117 (2020).
Baghbaderani, B. A. et al. cGMP-Manufactured human induced pluripotent stem cells are available for pre-clinical and clinical applications. Stem Cell Rep. 5, 647–659. https://doi.org/10.1016/j.stemcr.2015.08.015 (2015).
Hammad, R. et al. CRISPR-Cas12a for Highly efficient and marker-free targeted integration in human pluripotent stem cells. Int. J. Mol. Sci. 25, 985. https://doi.org/10.3390/ijms25020985 (2024).
Terheyden-Keighley, D. et al. GMP-compliant iPS cell lines show widespread plasticity in a new set of differentiation workflows for cell replacement and cancer immunotherapy. Stem Cells Transl. Med. 13, 898–911. https://doi.org/10.1093/stcltm/szae047 (2024).
Huang, J., Zhou, Y., Li, J., Lu, A. & Liang, C. CRISPR/Cas systems: Delivery and application in gene therapy. Front. Bioeng. Biotechnol. 10, 942325. https://doi.org/10.3389/fbioe.2022.942325 (2022).
Guo, C., Ma, X., Gao, F. & Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 11, 1143157. https://doi.org/10.3389/fbioe.2023.1143157 (2023).
Bloomer, H., Khirallah, J., Li, Y. & Xu, Q. CRISPR/Cas9 ribonucleoprotein-mediated genome and epigenome editing in mammalian cells. Adv. Drug Deliv. Rev. 181, 114087. https://doi.org/10.1016/j.addr.2021.114087 (2022).
Guo, Q. et al. ‘Cold shock’ increases the frequency of homology directed repair gene editing in induced pluripotent stem cells. Sci. Rep. 8, 2080. https://doi.org/10.1038/s41598-018-20358-5 (2018).
Martin, R. M. et al. Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated homologous recombination. Cell Stem Cell 24, 821-828.e5. https://doi.org/10.1016/j.stem.2019.04.001 (2019).
Allen, A. G. et al. A highly efficient transgene knock-in technology in clinically relevant cell types. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01779-8 (2023).
Lanza, R., Russell, D. W. & Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev Immunol. 19, 723–733. https://doi.org/10.1038/s41577-019-0200-1 (2019).
Bern, M. D. et al. Inducible down-regulation of MHC class I results in natural killer cell tolerance. J. Exp. Med. 216, 99–116. https://doi.org/10.1084/jem.20181076 (2019).
Schmid B, Prehn KR, Nimsanor N, Garcia BIA, Poulsen U, Jørring I, et al. Corrigendum to “Generation of a set of isogenic, gene-edited iPSC lines homozygous for all main APOE variants and an APOE knock-out line” [Stem Cell Res. 34/1873–5061 (2019) 101349–55]. Stem Cell Res 48:102005. https://doi.org/10.1016/j.scr.2020.102005. (2020)
Rao, J. et al. Stepwise clearance of repressive roadblocks drives cardiac induction in human ESCs. Cell Stem Cell 18, 341–353. https://doi.org/10.1016/j.stem.2015.11.019 (2016).
Acknowledgements
We acknowledge Prof. Ulrich Martin, Hannover Medial School, for discussions and sharing data on the iCaspase-based safety switch. Parts of this work have been supported by the EU-funded HEAL consortium (HORIZON-HLTH-2021-TOOL-06-02; # 101056712).
Author information
Authors and Affiliations
Contributions
Conception and design: Thomas Berger and Boris Greber; Collection and/or assembly of data: Thomas Berger, Elitsa Borisova and Daniel Terheyden-Keighley; Data analysis and interpretation:, Thomas Berger, Elitsa Borisova and Daniel Terheyden-Keighley; Development of differentiation methods: Daniel Terheyden-Keighley, Soraia Martins, Anna Gamerschlag and Boris Greber; Manuscript writing: Thomas Berger; Final approval of manuscript: Thomas Berger, Elitsa Borisova and Boris Greber.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Berger, T., Borisova, E., Gamerschlag, A. et al. Sequential factor delivery enables efficient workflow for universal gene editing in clinical grade iPS cells. Sci Rep 15, 32514 (2025). https://doi.org/10.1038/s41598-025-17876-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17876-4