Abstract
Minimizing ionizing radiation is crucial in paediatric imaging due to children’s increased radiation sensitivity, especially at younger ages. To evaluate a CT attenuation-based Auto Prescription protocol for paediatric head imaging, testing whether it provides image quality and radiation dose comparable to age-based protocols. Auto Prescription was implemented on a 256-slice scanner for axial volumetric head CT, adjusting kV and mAs based on attenuation data from scout images. Radiation dose parameters (CTDIvol, SSDE, ED, DLP) and image quality metrics (SNR, CNR, subjective Likert scale from 1—unacceptable to 4—higher than needed) were assessed in 79 consecutive studies using Auto Prescription protocols. These were compared to 68 studies obtained with age-based protocols using non-parametric tests. A total of 147 patients (60 females, mean age 6.7 ± 5.1 years) were included. The auto prescription group included 29 patients aged 0–5, 20 aged 5–10, 25 aged 10–15, and 5 over 15 years; the age-based group included 36, 18, 9, and 5 patients respectively in the same age groups. The Auto Prescription protocol achieved a more balanced radiation dose distribution across age and water-equivalent diameter. The greatest dose reduction was observed in the 0–1 year (48.2%, p < 0.001) and 10–15 year (40.4%, p < 0.001) age groups. While diagnostic image quality was adequate in both settings, it was lower with the auto prescription protocols (mean image quality 3.0 ± 0.2 versus 2.8 ± 0.2; SNR 7.2 ± 1.5 vs. 5.1 ± 1.1; CNR 0.9 ± 0.5 vs. 0.7 ± 0.2; all p < 0.001). All subjective image quality parameters were statistically non-inferior to the age- based protocol (p < 0.05). Attenuation-based Auto Prescription resulted in a more homogeneous and head density adapted radiation dose across paediatric patients, with non-inferior image quality. Dose reduction was a secondary benefit of individualized scan settings based on patient attenuation rather than age alone.
Similar content being viewed by others
Introduction
Computed tomography (CT) is the largest contributor to patient doses worldwide1. Principles like “as low as reasonably achievable“ (ALARA)2 and campaigns such as “Image Gently”3 are leading toward a better understanding of radiation exposure. However, the radiation dose administered during CT examinations remains a significant public health concern1.
In paediatric healthcare, it is crucial to strike a balance between obtaining clear diagnostic images and minimizing radiation exposure, as younger patients are more vulnerable to its harmful effects4. Children are at greater risk because their longer life expectancy increases the potential for radiation-induced health issues. Anatomical differences, such as bones with more cartilage and less retroperitoneal fat, contribute to lower natural tissue contrast. Furthermore, the rapid cell division necessary for growth makes children’s cells more susceptible to radiation damage5,6,7,8,9. This highlights the critical importance of radiation protection in paediatric CT imaging.
CT of the head continues to be one of the most frequently performed CT examinations in children10. While magnetic resonance imaging (MRI) is the preferred imaging technique for the paediatric head in most cases, CT remains a valuable tool, particularly in emergency situations or when MRI is unavailable11. Recent review of the literature revealed that using low tube voltage in adult head CT scans is an effective approach for reducing radiation dose12. However, there is a lack of studies focusing on paediatric head CT examinations that involve reducing both tube voltage and tube current exposure-time product12,13.
In our tertiary centre, where MRI is available 24/7, head CT is primarily used in cases of trauma and acute neurological emergencies to rule out severe conditions such as intracranial bleeding, hydrocephalus, severe stroke, and midline shift.
The aim of this study was to implement and evaluate a new attenuation- and size-based protocol for paediatric head CT, focusing on dose and image quality, and compare it to the previously used conventional age-based protocol in order to optimise radiation dose in paediatric head CT scans.
Materials and methods
Study design
A retrospective analysis was conducted comparing consecutive patients scanned using a newly implemented attenuation-based Auto Prescription protocol with those previously scanned using an age-based protocol between December 2019 and November 2021. Inclusion criteria included age less than 18 years, without contrast media, age-based protocol or attenuation-based Auto Prescription protocol. All patients who met the inclusion criteria during this period were included, with no predefined target sample size. Exclusion criteria were other protocols such as contrast studies, angiography studies or bone studies.
CT acquisition and image reconstruction
This head CT study included 147 paediatric patients and was conducted on a 256-row multidetector CT scanner (Revolution CT, GE Healthcare, Chicago, Illinois, United States of America).
The images were reconstructed using deep learning image reconstruction (DLIR, TrueFidelity, GE Healthcare, Chicago, Illinois, USA) using high strength denoising.
Patients and scan protocol
68 paediatric patients received head CT scans using an age-based protocol and 79 children were examined using an attenuation-based Auto Prescription protocol.
Age-based protocols were tailored to defined patient age ranges by setting the kV, noise index, and tube-current modulation (smart mA) limits (minimum and maximum mA) to achieve a specific dose and image quality. Age categories were 0 to 1 year, 1 to 5 years, 5 to 10 years, 10 to 16 years and 16 plus years. The appropriate age-based protocol for a given patient was manually selected by the radiographer/technologist based on the patient’s age and the corresponding protocol’s age range.
In a different approach, Auto Prescription calculates the patient size and water equivalent diameter from the attenuation information of the scout image over the prescribed scan range and then selects kV, mA, rotation time and noise index. To define the size ranges in the Auto Prescription profile, the age ranges from the age-based protocols were first converted to anterioposterior (AP) and lateral (LAT) measurement ranges using the conversion factors in Kleinman et al.14. These values were summed to create an AP + LAT measurement, which was then converted to a water equivalent diameter using the conversion factors from Burton et al.15. Thus, the size ranges defined in terms of water equivalent diameter for the Auto Prescription profile roughly correlated to the age ranges defined for the age-based protocols. The kV, noise index, rotation time, and Smart mA limits for each size range of the Auto Prescription profile were initially aligned with the corresponding age-based protocol values during protocol development. These were adjusted during a preliminary evaluation phase to optimize image quality and dose. Once finalized, the attenuation-based Auto Prescription protocol remained unchanged throughout the study period. For individual patients, the radiographer/technologist selects the Auto Prescription protocol, and the system calculates the optimal settings based on the water equivalent diameter derived from the scout image.
Both scan modes used tube current modulation (Smart mA) to fullfill the defined noise index. For the 16 + Auto Prescription category, the minimum mA was increased to 60 and the maximum mA was intentionally reduced to 270, based on internal dose and image quality evaluations. Table 1 lists selected scanner and protocol settings.
To evaluate the diagnostic accuracy of the attenuation-based Auto Prescription mode, we analyzed the follow-up of these patients. Either MRI follow-up or clinical follow-up was checked to determine if any severe diagnoses (e.g. intracranial bleeding, hydrocephalus, severe stroke, and midline shift) were missed.
DICOM anonymization
The first author anonymized and exported all studies from Workstation 21.2 (Sectra AB, Linköping, Sweden) to a local storage device. Randomly assigned ID numbers served as examination mixing procedure. We synchronized a standard head CT window (centre 50 Hounsfield units (HU), window width 95 HU) with 3 mm axial across all series. Each observer loaded the batch of anonymized examinations to rate them on calibrated 10-bit colour monitors Radiforce RX340 (EIZO Corporation, Hakusan, Ishikawa, Japan) in darkened reading rooms.
Objective image quality measurements
The first author manually placed circular regions of interest (ROI) in head window slices (3 mm) to retrieve the HU mean and standard deviation, the latter representing image noise. The minimum allowed ROI area was 5000 pixels. The average of three repeated measurements in the right thalamus and the right frontal white matter acted as basic objective image quality parameters. Based on these parameters, we calculated signal-to noise \(\:〈\:=SNR=\:\frac{MEAN\:tissue}{SD\:tissue}\:〉\)and contrast-to noise \(\:〈\:=CNR=\frac{(MEAN\:thalamus\:-\:MEAN\:white\:matter)}{\sqrt{\left(SD\:thalamu{s}^{2}+SD\:white\:matte{r}^{2}\right)}}\:〉\) ratios.
Subjective image quality ratings
Image quality of all head CT images were assessed by five radiologists (C.J.K 32-year experience in head CT, T.S. 30-year experience in head CT, R.K. 18-year experience in head CT, N.K. 8-year experience in head CT and M.Z. 10-year experience in head CT) using 5 image quality items on 4-point Likert scales.
Overall image quality was rated as 1-unacceptable quality (images do not allow diagnostic interpretation), 2-limited quality (images are adequate only for limited clinical interpretation due to high noise), 3-adequate quality (images are adequate for diagnostic interpretation), 4-Higher than needed quality (images are much better than needed for interpretation: images with little or no noise). The grey-white matter differentiation was rated as 1-differences just depictable, 2-diagnostic, 3-very good differentiation, 4 perfect differentiation. The inner and outer cerebral fluid space was rated separately as 1-visualization just possible, 2-unsharp borders of pars centralis/frontalis but diagnostic, 3-very good visualization, 4-perfect delineation. Presence of artefacts was rated as 1-severe, 2-moderate, 3-mild, 4-none.
Dose estimates
We collected the computed tomography dose indices (CTDIvol, calibrated using a 16 cm head phantom, mGy) and dose length products (DLP = CTDIvol × scan length in cm, mGy*cm) from the DICOM dose reports. Tube current (mA) and tube current–time product (mAs, calculated as mA multiplied by rotation time) were extracted from the DICOM metadata for each scan. Water equivalent diameters were obtained from the scanner. These diameters, together with size-dependent correction factors and age-specific conversion factors derived from publications by Shrimpton et al. and the American Association of Physicists in Medicine (AAPM) report number 29316,17, formed the basis for calculating size-specific dose estimates (SSDE, mGy) and effective doses (ED, mSv). SSDE was calculated by multiplying CTDIvol by a size-specific factor (f) based on the water equivalent diameter, as outlined in the AAPM report. For ED, we used the formula ED = DLP x k, where k is an age-specific conversion factor (0–1 year: k = 0.0069; 1–5 years: k = 0.0044; >5 years: k = 0.0027), allowing for appropriate scaling of the DLP according to patient age.
Statistical analysis
We analysed the collected data in SPSS Statistics Version 26 (IBM Corp., Armonk, NY, USA) by computing descriptive statistics, as well as t-test mean value comparisons in cases of ascertained, and nonparametric Mann–Whitney U tests in missing normal distributions. Intraclass correlation coefficients (ICC) served as measures of inter-observer agreement in terms of ICC(3,k) two-way mixed, average measures. ICC values below 0.5 refer to poor, 0.5 to 0.75 moderate, 0.75 to 0.9 good, and higher than 0.9 to an excellent agreememt18. Inter-rater reliability of subjective image quality ratings (Likert scale) was assessed using the Intraclass Correlation Coefficient (ICC), in line with common practice in radiological research where Likert-type ratings are treated as quasi-continuous data18,19. Throughout this manuscript, p-values below 0.050 were regarded as statistically significant.
One-sided non-inferiority t-tests were calculated using Statgraphics Centurion 19 (Statgraphics Technologies Inc., The Plains, VA, USA), to assess our hypothesis that image quality was not significant inferior in attenuation based auto prescription protocol. Prior to the statistical analyses, the authors consensually agreed on the non-inferiority cut-off of 0.5 points as the clinically relevant margin. P-values below the significance level demonstrated the non-inferiority of specific attenuation based protocol parameters.
Results
Patients
Out of 147 patients (60 female) with a mean age of 6.7 ± 5.1 years (age range 1 day to 17.8 years), 68 patients (31 female, mean age 5.6 ± 5.1 years, age range 1 day to 16.6 years) were scanned using an age-based protocol and 79 patients (29 female, mean age 7.6 ± 5.1 years, range 0.2 to 17.6 years) were scanned with an attenuation-based Auto Prescription protocol.
Mean water equivalent diameter of the head was 16.2 ± 1.9 cm in age-based vs. 16.1 ± 1.5 cm in the attenuation-based protocols (Table 2 for each agegroup) with no significant difference (t test, p = 0.8). The mean water equivalent diameter was higher in the 10–15 year age-based group (17.91 ± 0.36) compared to the 15 years and above age-based group (17.79 ± 0.78) (Table 2).
Out of the 79 patients scanned with the attenuation- based Auto Prescription protocol, 24 patients (30%) received an MRI follow up study within 10 days and 55 patients (70%) were clinically evaluated and discharged in good health after observation. No severe diagnosis was missed.
Objective image quality
Mean CNR was slightly higher with 0.94 ± 0.46 in age-based protocols and 0.71 ± 0.24 in attenuation-based protocols (t-test, p < 0.001). Age-based protocols showed a higher mean SNR (white matter of 7.22 ± 1.50, thalamus of 7.87 ± 1.66) compared to attenuation-based protocols (white matter of 5.13 ± 1.06, thalamus of 5.62 ± 1.14, t-test all p < 0.001). Further details for each age group see Table 2.
Subjective image quality
Mean overall image quality was significantly higher with 3.05 ± 0.24 in age-based protocols and 2.84 ± 0.24 in attenuation-based protocols (u test, p < 0.001) showing diagnostic to near diagnostic image quality with similar results in all age groups. Among the scans, 14 out of 68 patients (20.6%) in the age-based group and 29 out of 79 patients (36.7%) in the attenuation-based group had a mean overall image quality score below 3, indicating near-diagnostic quality. No patients in either group received a score below 2. The age group 5–10 years had the least overall image quality score of 2.76 ± 0.27 using attenuation-based protocol (Table 2). All subjective image quality parameters in the attenuation-based Auto Prescription protocol were demonstrated to be statistically non-inferior compared to the age-based protocol, as confirmed by one-sided non-inferiority t-tests (p < 0.05).
Grey-white matter differentiation as well as differentiation between inner and outer cerebral fluid space was lower in attenuation-based protocol scans (grey-white matter differentiation 2.91 vs. 2.67, inner cerebral fluid space 3.32 vs. 3.07 and outer cerebral fluid space 3.07 vs. 2.90). Mild to no artefacts were observed in both protocols with less artefacts in the age-based protocols (mean 3.17 vs. 2.93). For p values and details see Table 2.
Interrater agreement in terms of ICC scores were 0.863 (95% confidence interval 0.812 to 0.906) in age-based protocol and 0.786 (95% confidence interval 0.712 to 0.849) in attenuation-based protocol, which is considered a good consensus.
Dose comparison of both protocols
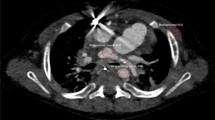
CTDIvol (age-based 22.20 vs. attenuation-based 16.88 mGy), SSDE (age-based 22.29 vs. attenuation-based 16.9 mGy), DLP (age-based 358.10 vs. attenuation-based 274.15 mGy cm), ED (age-based 1.32 vs. attenuation-based 0.88 mSv), mA (age-based 349.99 vs. attenuation-based 172.61 mA) and mAs (age-based 172.61 vs. attenuation-based 119.34 mAs) represent the higher radiation doses in age-based protocols throughout all patients, u test all p < 0.001. The greatest dose reduction was observed in the 0–1 year [CTDIvol of 15.13 age-based versus 7.84 mGy attenuation-based (48.2%, p < 0.001)] and 10–15 year [CTDIvol of 32.05 age-based versus 19.11 mGy attenuation-based (40.4%, p < 0.001) age groups (Table 3). Figure 1 illustrates the reduced dose and still diagnostic image quality between both protocols in patients of similar age.
(a, b) Axial head CT images illustrate the reduced dose with maintained sufficient image quality between both protocols in patients of similar age. (a) 16.5 year old female scanned with age-based protocol with an CTDIvol of 34.4 mGy, a DLP of 550.28 mGy cm, a SSDE of 33.02 mGy, an effective dose of 1.49 mSv and a mean overall image quality score by all readers of 3.2. (b) 16.8 year old male scanned with Auto Prescription protocol with an CTDIvol of 19.93 mGy, a DLP of 311.50 mGy cm, a SSDE of 19.27 mGy, an effective dose of 0.84 mSv and a mean overall image quality score by all readers of 3.0.
CTDIvol over water equivalent diameter shows a more homogenous distribution of radiation dose with a tighter confidence interval in attenuation-based protocol (Fig. 2). SSDE over age shows similar results with a tighter confidence interval and less dose in attenuation-based protocol (Fig. 3).
Discussion
This manuscript compares image quality and radiation doses in paediatric head CT scans with two different scanning protocols. Our implementation of an attenuation-based Auto Prescription protocol led to a significant reduction in radiation dose compared to the previous age-based approach while preserving sufficient, albeit slightly decreased image quality. Auto Prescription was designed to meet diagnostic image quality levels across a range of patient sizes at optimal dose by automatically adjusting kV and mA levels based on patient attenuation obtained from scout images.
CT scans with high radiation dose can contribute to the risk of developing brain cancers. Recent studies from Hauptmann et al. observed that over a median follow-up duration of 5.6 years, a total of 165 cases of brain cancer occurred20. They identified a notable linear correlation between radiation dose and the incidence of overall brain cancer; however, causality remains unproven. Their findings show a dose-response relationship between radiation exposure from CT scans and the occurrence of brain cancer20. European dose reference levels for paediatric patients suggest a DLP for head CT starting from 300 mGy*cm (0–3 month old), 385 mGy (3 month to 1 year old), 505 mGy*cm (1 to 6 year old) to 650 mGy*cm (6 year and older)21. The attenuation-based protocol used in this study achieved a mean DLP of 274 mGycm (range: 105.5 to 385.9 mGycm) across all patients, resulting in doses lower than the reference levels. In particular, in neonates and infants (age group 0 to 1 year) the dose was half to one-third of the recommended reference level (Table 3).
In Park et al.‘s study, reducing the tube voltage from 120kVp to 80kVp led to an increase in reference mA from 220 mA to 770 mA to balance the image noise. They observed a 4.7% decrease in CTDIvol and a 6.9% decrease in DLP in paediatric head CT22. Nakai et al. maintained the tube voltage at 120 kV but halved the mA, resulting in a 14.9% reduction in CTDIvol and a 17.2% reduction in DLP, using a specific low-dose technique for head CT in postoperative hydrocephalus cases and follow-up for craniosynostosis, rather than for routine head CT23. Priyanka’s latest study achieved the greatest reduction in radiation dose by lowering both tube voltage and tube current for different age groups in low dose protocols, emphasizing the importance of optimizing both parameters for efficient dose reduction13.
Although the overall image quality, CNR, and SNR in the attenuation-based Auto Prescription scans were reduced compared to the age-based approach, diagnostic image quality was still achieved. This is further supported by the results of one-sided non-inferiority t-tests, which confirmed that all subjective image quality parameters in the attenuation-based group were statistically non-inferior to those of the age-based group. This conclusion is also supported by patient follow-up data, where 30% of all patients received an MRI follow-up, and the remaining patients were discharged after unremarkable clinical course without any adverse events. Therefore, no severe diagnoses (e.g. intracranial hemorrhage, midline shift, severe stroke, hydrocephalus) were missed. While our study confirms diagnostic image quality for detecting critical findings, it is important to note that the protocol’s effectiveness in identifying subtler or lower-contrast abnormalities (such as early ischemia, encephalitis, or cortical dysplasia) was not directly assessed. Since head CT is predominantly used in our institution for acute emergencies, this was not a focus of the current evaluation. Our findings suggests that, although the image quality was lower, the attenuation-based method remains robust in identifying critical conditions. Furthermore, the attenuation-based Auto Prescription achieved a more homogenous distribution of dose across all age groups and head sizes (water equivalent diameters) compared to the age-based approach. The tighter confidence intervals observed throughout the study with the attenuation-based Auto Prescription highlight the more customised scan settings for each patient size, respectively for the true attenuation of the head (Figs. 2 and 3). Interestingly, the water equivalent diameter was higher in the 10–15 year age-based group compared to the 15 years and above age-based group, further highlighting the variability in head size within fixed age categories (Table 2).
Current guidelines, especially in Europe, which base radiation dose recommendations for paediatric patients on age and weight seem outdated21. Our study demonstrates that utilizing the water equivalent diameter of the head (size-based and attenuation-based approach) enables a balanced radiation dose, maintaining sufficient image quality while achieving lower doses compared to existing reference levels.
This suggests the potential for a more effective and tailored dose optimization strategy in paediatric head CT imaging. From a clinical perspective, attenuation-based Auto Prescription also reduces technologist dependency on manually selecting age-appropriate protocols, thereby minimizing operator variability. In addition, by basing acquisition parameters on attenuation rather than age, Auto Prescription holds the potential to standardize paediatric head CT dosing strategies across institutions.
Limitations of our study have to be acknowledged. One limitation of our study was the significant higher average age of patients in the age-based protocol groups compared to the Auto Prescription protocol group. However, we believe that this limitation is mitigated by the consistent similarity in water equivalent diameter of the head across both protocols (16.2 ± 1.9 cm vs. 16.1 ± 1.5 cm) and the comparison made using age groups. Additionally, another notable limitation was the relatively small sample size (only 3 patients with attenuation-based Auto Prescription in age group 0 to 1 year old). Another limitation of our study is that the age-based protocols were designed with a lower noise index, which may create an imbalance in the comparison. We were unable to perform a comparison of age-based protocols using lower noise indices because the the noise index was adjusted in the process of developing and implementing the attenuation-based approach. However, within the actual study population, all scan parameters including the noise index remained unchanged once the final protocols (age based and attenuation-based auto prescription) were defined and applied. While the lower radiation dose achieved with the attenuation-based Auto Prescription protocol may be due to the lowered noise indices, the attenuation-based approach leads to a more homogeneous and balanced dose across age groups.
There were no updates to the scanner software or hardware between the studies conducted with the age-based and attenuation-based protocols.
Conclusions
The implementation of an attenuation-based Auto Prescription protocol for paediatric head CT led to a more homogeneous and size-adapted distribution of radiation dose across patient age and head size, with sufficient and non-inferior image quality compared to age-based protocols. The observed reduction in radiation dose was a favorable consequence of tailoring scan parameters to individual patient attenuation, rather than the primary aim of the protocol.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request. Additionally, the SPSS data file “Database 1.sav” has been included in the supplementary materials for reviewer access.
Abbreviations
- CT:
-
Computed tomography
- CTDIvol :
-
Computed tomography dose index volume
- CNR:
-
Contrast to noise ratio
- DLIR:
-
Deep learning image reconstruction
- DLP:
-
Dose length product
- Dw:
-
Water equivalent diameter
- ED:
-
Effective dose
- HU:
-
Hounsfield unit
- kV(p):
-
(peak) kilovoltage
- mA(s):
-
Milliampere (s)
- mGy:
-
Milligray
- mSv:
-
Millisievert
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SNR:
-
Signal to noise ratio
- SSDE:
-
Size specific dose index
References
Brenner, D. J. & Hall, E. J. Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med. 357, 2277–2284. https://doi.org/10.1056/NEJMra072149 (2007).
Oestreich, A. E. RSNA centennial article: ALARA 1912: as low a dose as possible a century ago. Radiographics. 34, 1457–1460. https://doi.org/10.1148/rg.345130136 (2014).
Goske, M. J. et al. Image Gently(SM): a National education and communication campaign in radiology using the science of social marketing. J. Am. Coll. Radiol. 5, 1200–1205. https://doi.org/10.1016/j.jacr.2008.06.007 (2008).
Kutanzi, K. R., Lumen, A., Koturbash, I. & Miousse, I. R. Pediatric exposures to ionizing radiation: carcinogenic considerations. Int. J. Environ. Res. Public. Health. 13, 1057. https://doi.org/10.3390/ijerph13111057 (2016).
Mese, I., Altintas Mese, C., Demirsoy, U. & Anik, Y. Innovative advances in pediatric radiology: computed tomography reconstruction techniques, photon-counting detector computed tomography, and beyond. Pediatr. Radiol. 54, 1–11. https://doi.org/10.1007/s00247-023-05823-2 (2024).
Nagy, E., Tschauner, S., Schramek, C. & Sorantin, E. Paediatric CT made easy. Pediatr. Radiol. 53, 581–588. https://doi.org/10.1007/s00247-022-05526-0 (2023).
Pearce, M. S. et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380, 499–505. https://doi.org/10.1016/S0140-6736(12)60815-0 (2012).
Mathews, J. D. et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346, f2360. https://doi.org/10.1136/bmj.f2360 (2013).
Bosch de Basea Gomez, M. et al. Risk of hematological malignancies from CT radiation exposure in children, adolescents and young adults. Nat. Med. 29, 3111–3119. https://doi.org/10.1038/s41591-023-02620-0 (2023).
Kalra, M. K., Sodickson, A. D. & Mayo-Smith, W. W. CT radiation: key concepts for gentle and wise use. RadioGraphics. 35, 1706–1721. https://doi.org/10.1148/rg.2015150118 (2015).
Ferrazzano, P. A. et al. Use of magnetic resonance imaging in severe pediatric traumatic brain injury: assessment of current practice. J. Neurosurg. Pediatr. 23, 471–479. https://doi.org/10.3171/2018.10.PEDS18374 (2019).
Nakamura, K. et al. Computed tomography using a low tube voltage technique for acute ischemic stroke. Adv. Comput. Tomogr. 8, 24–35. https://doi.org/10.4236/act.2019.82003 (2019).
Priyanka, Kadavigere, R. & Sukumar, S. Low dose pediatric CT head protocol using iterative reconstruction techniques: A comparison with standard dose protocol. Clin. Neuroradiol. https://doi.org/10.1007/s00062-023-01361-4 (2023).
Kleinman, P. L. et al. Patient size measured on CT images as a function of age at a tertiary care children’s hospital. AJR Am. J. Roentgenol. 194, 1611–1619. https://doi.org/10.2214/AJR.09.3771 (2010).
Burton, C. S. & Szczykutowicz, T. P. Evaluation of AAPM reports 204 and 220: Estimation of effective diameter, water-equivalent diameter, and ellipticity ratios for chest, abdomen, pelvis, and head CT scans. J. Appl. Clin. Med. Phys. 19, 228–238. https://doi.org/10.1002/acm2.12223 (2018).
Shrimpton, P. C., Jansen, J. T. M. & Harrison, J. D. Updated estimates of typical effective doses for common CT examinations in the UK following the 2011 National review. Br. J. Radiol. 89, 20150346. https://doi.org/10.1259/bjr.20150346 (2016).
Boone, J. et al. Report No. 293 - Size Specific Dose Estimate (SSDE) for Head CT The Report of AAPM Task Group 293. (2019). https://doi.org/10.37206/185
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 (2016).
Shrout, P. E. & Fleiss, J. L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420–428. https://doi.org/10.1037/0033-2909.86.2.420 (1979).
Hauptmann, M. et al. Brain cancer after radiation exposure from CT examinations of children and young adults: results from the EPI-CT cohort study. Lancet Oncol. 24, 45–53. https://doi.org/10.1016/S1470-2045(22)00655-6 (2023).
Directorate-General for Energy (European Commission). European Guidelines on Diagnostic Reference Levels for Paediatric Imaging (Publications Office of the European Union, 2018).
Park, J. E. et al. Image quality and radiation dose of brain computed tomography in children: effects of decreasing tube voltage from 120 kVp to 80 kVp. Pediatr. Radiol. 47, 710–717. https://doi.org/10.1007/s00247-017-3799-8 (2017).
Nakai, Y. et al. Evaluation of radiation dose reduction in head CT using the half-dose method. Jpn. J. Radiol. 41, 872–881. https://doi.org/10.1007/s11604-023-01410-5 (2023).
Acknowledgements
I would like to express my gratitude to Beate Schmidt and Paul Deak at GE Healthcare for their invaluable assistance in addressing technical questions related to this paper. Special thanks to Drazen Udovicic for his dedicated efforts in conducting the scans, contributing significantly to the success of this research.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
M.Z., F.R., and C.K. contributed to the study concept. Data acquisition was performed by M.Z., and C.K. Data analyses and visualization were conducted by S.S., N.K., T.S. and R.K. M.Z. prepared the manuscript draft. All authors contributed to reviewing and editing of the manuscript. C.K. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and informed consent
Approval for this study was obtained from the Ethics Committee of Zurich, Switzerland (BASEC No. 2020 -01644). All patients and/or their legal guardians had previously provided general informed consent for the use of anonymized clinical and imaging data for research purposes. Only patients with documented consent were included in this retrospective analysis. All study procedures were conducted in accordance with institutional guidelines and the principles outlined in the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zellner, M., Sirin, S., Kocher, N. et al. Radiation dose optimisation in paediatric head CT using attenuation-based auto prescription. Sci Rep 15, 33276 (2025). https://doi.org/10.1038/s41598-025-18097-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18097-5
This article is cited by
-
Attenuation-based ultra-low-dose lung computed tomography at 0.1 mSv to 0.3 mSv effective dose in children
Pediatric Radiology (2026)