Abstract
Sleep is a vital physiological process affecting physical, mental well-being, and cognitive performance, but it is not well studied in Vietnam medical students. Therefore, we aimed to assess the prevalence of poor sleep quality among Vietnamese medical students and identify associated factors. A cross-sectional study was conducted from April to May 2024 among all medical students at the University of Danang, Vietnam. Data were collected using a self-administered questionnaire about the Pittsburgh Sleep Quality Index (PSQI), demographic, mental health, and sleep-related characteristics. Poisson regression was used to identify factors associated with poor sleep quality (PSQI > 5). The study found that 45.8% of participants (264/577) (95% CI: 41.7–49.8%) had poor sleep quality, with a mean PSQI score of 5.4 ± 2.3. Clinical students had a higher prevalence of poor sleep quality (51.8%) than preclinical students (40.0%) (p = 0.005). In multivariable analysis, clinical training (PR = 1.37; 95%CI: 1.14–1.65), lower academic performance (PR = 1.43; 95%CI: 1.08–1.89), noise (PR = 1.49; 95%CI: 1.19–1.86), light disturbance during sleep (PR = 1.26; 95%CI: 1.04–1.53), stress (PR = 1.22; 95%CI: 1.00-1.48), anxiety (PR = 1.43; 95%CI: 1.15–1.78), and depression (PR = 1.29; 95%CI: 1.07–1.57) were significantly associated with poor sleep quality. In conclusion, poor sleep quality was highly prevalent, especially among clinical-year students.
Similar content being viewed by others
Introduction
Sleep is a vital physiological process essential for human survival and overall well-being1. Both the quantity and quality of sleep play a critical role in mental health, mood, cardiovascular, cerebrovascular, and metabolic health2. Moreover, sleep is essential for cognitive function, particularly in memory consolidation, which is fundamental to effective learning and performance3,4. Adequate and quality sleep enables individuals to retain information, improve concentration, and make effective decisions, which are key factors in academic success5,6. Conversely, poor sleep quality, characterized by insufficient sleep duration, low sleep efficiency, or irregular sleep, can impair these cognitive processes, leading to adverse effects on learning outcomes and overall academic performance7,8.
Medical students often stay up late and have irregular sleep schedules due to heavy academic load and clinical night shifts, which may further contribute to poor sleep quality9. A meta-analysis of 109 studies revealed that over 50% of medical students reported poor sleep quality10. In Vietnam, some studies have shown that poor sleep quality was prevalent among medical students, ranging from 44–60%11,12,13,14,15.
Several studies have identified academic stress, academic performance, family expectations, hospital night duties, social interactions, light and noise in the bedroom, use of mobile devices before sleeping, pathological pain, and mental health conditions such as anxiety and depression as significant contributors to poor sleep quality among medical students11,15,16,17. Lifestyle factors such as prolonged screen time, consumption of caffeinated beverages, irregular sleep schedules, and lack of physical activity have also been reported to exacerbate sleep disturbances17,18. In addition, sleep-related environment factors, including room temperature, humidity, air, light, and noise also affect sleep quality19,20. In Vietnam, a study of 200 students at a university in southern Vietnam in 2023 found that the sleep quality of third-year medical students was linked to physical activity level and caffeine consumption12. Another study on 1,502 students at the University of Medicine Pham Ngoc Thach, Vietnam in 2021 during the COVID-19 lockdown showed that higher level of stress experienced during the pandemic is associated with lower sleep quality13. However, critical sleep-related environmental factors such as light and noise disturbances during sleep have not been adequately explored in previous studies. Furthermore, existing studies in Vietnam have primarily targeted specific year groups, rather than the full cohort of medical students or explored differences in sleep quality between the clinical and preclinical phases of training. Therefore, we aimed to assess the prevalence of poor sleep quality among medical students, especially by clinical training status, and examine the association between sleep quality and potential risk factors, including clinical training status, mental health, and sleeping environment.
Methods
Study design and setting
A cross-sectional study was conducted from April to May 2024 among all medical students from first to sixth year at the School of Medicine and Pharmacy (SMP), University of Danang, Vietnam, outside of scheduled examination period.
This study was conducted at the SMP, which is a key faculty within the University of Danang. The school is recognized as a leading institution for medical education in Central Vietnam, offering comprehensive training programs across five major disciplines: medicine, pharmacy, nursing, dentistry, and laboratory technology. The student population comprises over 1,300 individuals, with approximately 600 enrolled in medical programs from year 1 to year 621.
We excluded students with self-reported history of sleep disorders and/or chronic medical conditions known to affect sleep, such as depression, sleep apnea, or asthma (Fig. 1).
Data collection
Trained research assistants visited classrooms to introduce the study and invite students to participate. Those who agreed to participate signed the informed consent and filled out a paper-based questionnaire on-site. No personally identifiable information was collected to make it less sensitive for participants to share information related to their physical and mental health. Participants were informed that they could withdraw from the study at any time. Once the questionnaires were collected, the investigators entered and encrypted all data on a password-protected computer. As this was an anonymous survey, we were unable to create a list of students with potential mental health issues for follow-up or referral to psychological support services.
Questionnaire
The study utilized a self-administered questionnaire consisting of 52 items organized into five sections (see details in Table S4).
-
I.
General Characteristics includes questions about gender, school year, part-time employment, night duty at the hospital, stimulant use, electronic device use, academic performance (GPA), physical activity, and lunch napping habit.
-
II.
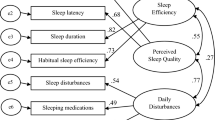
The Pittsburgh Sleep Quality Index (PSQI) assesses sleep quality over the past month using seven components: daytime dysfunction, sleep disturbances, sleep medication, sleep latency, sleep duration, habitual sleep efficiency, and subjective sleep quality. This scale was developed by Buysse et al. in 198922. The total score ranges from 0 to 21 points, with each component receiving a score from 0 to 3. A total PSQI score above 5 indicates poor sleep quality, with a sensitivity of 89.6% and specificity of 86.5%22. We used the Vietnamese version of the PSQI, which has been validated with a Cronbach’s alpha coefficient of 0.78923. The questionnaire has been widely used in various studies among Vietnamese medical students and the general population13,14,24,25. With a cut-off score of 5 points, it demonstrated a sensitivity of 89.66% and a specificity of 86.5% in the healthy population22. In this study, good sleep quality was defined as a total PSQI score of ≤ 5 points, while poor sleep quality was defined as a PSQI score of > 5 points22,23.
-
III.
Perceived Medical School Stress (PMSS) was developed by Vitaliano et al. in 1984. This scale includes 13 items rated on a 5-point Likert scale from 0 to 4 scores26. The study participant would choose 0- “Strongly disagree”, 1- “Disagree”, 2- “Neutral”, 3- “Agree”, or 4- “Strongly agree” for each question. These questions are designed to assess stress associated with academic, social, and financial pressures in medical schools. Total scores can range from 0 to 52 points, with higher scores indicating higher levels of perceived stress27. In this study, we classified participants with PMSS score higher than the median score of 27 as having stress.
-
IV.
Generalized Anxiety Disorder-7 (GAD-7) was developed by Spitzer et al. in 2006 to assess anxiety levels over the past two weeks with seven questions. Responses for each question are rated as 0- “Not at all”, 1- “Several days”, 2- “More than half the days,” or 3- “Nearly every day.” The GAD-7 has sensitivity and specificity of 89% and 82%, respectively28. The total GAD-7 score ranges from 0 to 21 points (< 5 points: no anxiety disorder; 5–9 points: mild anxiety disorder; 10–14 points: moderate anxiety disorder; > 14 points: severe anxiety disorder)28. In this study, having self-reported anxiety symptoms was identified as a total GAD-7 score of ≥ 10 points, whereas scores below 10 indicated no anxiety28.
-
V.
Patient Health Questionnaire-9 (PHQ-9) was developed in 1999 by Spitzer et al. to screen depression symptoms over the past two weeks29. The tool has sensitivity and specificity values of 88.0% and 88.0%, respectively, for total scores of 10 and above30. The PHQ-9 consists of 9 questions with responses given on a scale of 0-“not at all”, 1-“several days”, 2-“more than half the days”, and 3-“nearly every day”. The total PHQ-9 score ranges from 0 to 27 points (< 5 points: no depression; 5–9 points: mild depression; 10–14 points: moderate depression; 15–19 points: moderately severe depression; and > 19 points: severe depression)30. In this study, participants with a total Patient Health Questionnaire-9 (PHQ-9) score of ≥ 10 points were classified as having self-reported depression symptoms, with scores below this threshold indicating the absence of a depressive disorder30.
In Viet Nam, the GAD-7 and PHQ-9 scale have been validated and used in various populations31,32,33. The PMSS scale was translated from English to Vietnamese and vice versa by two independent English-proficient researchers. The translation and backtranslation process followed the guidelines provided by the WHO34. All the questionnaires were modestly modified during this process to adapt to Vietnamese culture and language. The questionnaire was piloted on ten medical students to assess its readability and acceptability, allowing for more precise wording prior to data collection.
Data analysis
Categorical variables were reported as frequencies and percentages, while continuous variables were described using means and standard deviations (SD) or medians and interquartile ranges (IQR) when not normally distributed. Chi-square tests assessed differences between percentages, and Fisher’s exact test was used for tables with fewer than 20 samples or expected cell counts below 5. Differences between continuous variables were tested using the Independent T-test or Mann Whitney test.
The prevalence of poor sleep quality was 45.8% (> 10%) in this study, logistic regression is prone to overestimating associations between independent variables and binary outcomes35,36. In contrast, log-binomial regression models offer a more direct approach to estimating Prevalence Ratios (PRs). Prior studies by Zou et al. and Barros et al. have demonstrated that modified Poisson regression models, incorporating robust error variance, effectively calculate PRs or relative risks with binary outcome data35,36. Consequently, our analysis employed the modified Poisson regression model to estimate PRs and evaluate the associations between potential risk factors and sleep quality. Stata software version 17.0 was used to conduct the statistical analysis.
Ethical declaration
The study adhered to the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Scientific Council of the School of Medicine and Pharmacy, Danang University, and the Institutional Review Board of the University of Medicine and Pharmacy at Ho Chi Minh City, under Decision No. 576/HĐĐĐ-ĐHYD.
Results
Of the 610 students invited, nine chose not to participate, resulting in a response rate of 98.7%. Twenty-four participants who did not meet the inclusion criteria were excluded, leaving 577 participants for analysis.
Socio-demographic characteristics of participants
Among the 577 students, 295 (51.1%) were in the preclinical phase, and 282 (48.9%) were in the clinical phase (Table 1). Half the students in both the preclinical and clinical groups were male. Most students in both groups had good academic standing and above, a normal BMI, did not smoke or drink, and did some exercise, whereas caffeine consumption exceeded 65% in both groups. Nearly all students in both groups reported frequent use of electronic devices at about 4–5 h per day. Additionally, the prevalence of financial burden, anxiety symptoms (GAD-7), and depressive symptoms (PHQ-9) was similar across both groups.
Notably, preclinical students demonstrated a significantly higher grade point average (GPA) than their clinical counterparts (p = 0.001). Also, they were less likely to work part-time at night (p = 0.004), reported a higher frequency of gaming (p = 0.015), and higher levels of physical activity (p < 0.001).
Sleep characteristics and sleep quality components
Most students (> 80%) reported taking lunch naps more than 20 min per day (Table 2). The proportion of students whose sleep was affected by light and noise was similar between the two groups, at approximately 39.2% and 56.0%, respectively. Of the total 577 students, 45.8% of students had poor sleep quality (PSQI > 5 points). The median PSQI score was 5 points, with an interquartile range of 4 to 7 (Fig. 2). Compared to pre-clinical students, clinical students had shorter sleep duration (7.0 (6.0–7.0) vs. 6.0 (5.0–6.5) hours, p < 0.001, respectively), and poorer sleep quality scores (PSQI: 5.2 ± 2.1 vs. 5.6 ± 2.4, p = 0.014, respectively) (Table 2).
The prevalence of poor sleep quality (PSQI > 5) was 45.8% (95%CI: 41.7–49.8%). Students undergoing clinical training exhibited a significantly higher prevalence of poorer sleep quality (51.8%) compared to preclinical students (40.0%) (p = 0.005) (Table 2).
The radar plot compares the average scores of seven components of the PSQI between preclinical and clinical students (Fig. 3). The most notable difference is in sleep duration, with clinical students reported significantly poorer scores, indicating shorter sleep duration than preclinical students. Another notable difference was observed in daytime dysfunction which indicates that clinical students experienced higher levels of fatigue during the day. However, both groups showed modest differences in subjective sleep quality, sleep latency, habitual sleep efficiency, and sleep disturbances. The use of sleeping medication was very rare in both groups.
Among subgroups, the prevalence of poor sleep quality was highest in those with signs of anxiety (79.2%) and depressive disorder (71.1%) (Fig. 4 and Table S1). We also found a higher prevalence of poor sleep quality among students who reported caffeine consumption, sleep affected by light, or by noise (49.9%, 58.8%, and 56.3%, respectively) (Fig. 4).
Factors associated with the quality of sleep
In the multivariable Poisson regression model, clinical students had a 30% higher prevalence of poor sleep quality compared to preclinical students (PR = 1.37, 95% CI: 1.14–1.65) (Fig. 5). In terms of sleep - related environment, both light (PR = 1.26, 95% CI: 1.04–1.53) and noise disturbance (PR = 1.49, 95% CI: 1.19–1.86) had a significant associated with increased risk of poor sleep quality. Additionally, students with average or below-average academic performance (GPA < 2.5) were significantly more likely to have poor sleep quality compared to those with good performance (PR = 1.43, 95% CI: 1.08–1.89). Finally, mental health-related factors as having stress symptoms (PR = 1.22; 95% CI: 1.00–1.48), anxiety symptoms (PR = 1.46, 95% CI: 1.18–1.81) and depression symptoms (PR = 1.31, 95% CI: 1.09–1.58) were also significantly associated with poor sleep quality. The positive associations between these risk factors and poor sleep quality remained significant after adjusting for different sets of covariates (Table S2).
Discussion
This cross-sectional study assessed sleep quality and its associated factors among medical students from years 1 to 6 at the School of Medicine and Pharmacy, University of Danang, Vietnam. We found that 45.8% (95%CI: 41.7–49.8%) of medical students had poor sleep quality. Several factors were significantly associated with poor sleep quality, including clinical training status, light and noise disturbance during sleep, caffeine consumption, and having anxiety symptoms or depression symptoms.
Students in clinical training experienced poorer sleep quality compared to those in preclinical years. This finding was similar to prior studies conducted in Vietnam13,37 and other Asian countries38,39. Nevertheless, our results were lower than what had been reported in Saudi Arabia (63.2%), Kazakhstan (79.6%), China (74.33%), Brazil (80.95%), and Rwanda (80%)40,41,42,43,44. A comparison with other studies can be found in Table S3. These differences may be attributed to the fact that some of the earlier studies were conducted during the COVID-19 pandemic, which likely impacted students’ sleep quality. Additionally, variations in sample size, sampling techniques, research design, and study populations may also contribute to these discrepancies. On the other hand, a meta-analysis conducted among Chinese students found that medical students were more likely to experience sleep disturbances compared to students in non-medical fields45. This difference may be due to the fact that medical students are required to absorb a greater volume of knowledge, engage in more intense study, and experience higher levels of stress, all of which could contribute to poorer sleep quality. Our study also observed that clinical students had poorer sleep quality compared to preclinical students (PR = 1.37, 95% CI: 1.14–1.65), characterized by shorter sleep duration, poorer subjective sleep quality, and more frequent daytime dysfunction. This finding is consistent with previous studies conducted in other Asian countries, suggesting that the clinical phase of medical education, characterized by increased workload, longer shifts, and heightened responsibility, may contribute to greater stress and sleep disturbances46,47.
In the current study, we also observed that students with generalized anxiety disorder or depression were more likely to have poor sleep quality, which is consistent with previous studies48,49,50,51. In Vietnam, a study by My Linh et al. similarly reported a higher prevalence of poor sleep quality among students with anxiety15. From a physiological perspective, depression leads to a reduction in serotonin neurotransmitters, which in turn impairs cognitive performance and disrupts normal sleep patterns50,51. Additionally, individuals with depression have been observed to experience alterations in brain waves, which inhibit rapid eye movement (REM) sleep, reduce non-rapid eye movement (NREM) sleep, and compromise sleep continuity52. On the other hand, an experimental study found that poor sleep quality was associated with greater difficulty in diverting attention from negative stimuli, which, in turn, predicted a rise in depressive symptoms53. Furthermore, sleep deprivation increases the levels of pro-inflammatory cytokines, which may promote the development of depression54,55. These results highlight the bidirectional relationship between sleep disturbances and psychological distress56, suggesting that interventions in mental health may also improve sleep quality.
Disruptive sleep environment is a significant factor influencing sleep quality. Particularly, noise and light were substantial contributors to poor sleep quality in this study, which is consistent with previous research11,39. Noise has been demonstrated to disrupt sleep, increase wakefulness during the night, reduce slow-wave sleep, and lead to shallower sleep. Additionally, it may induce metabolic disturbances, elevate heart rate, and increase blood pressure57,58,59. Light has also been shown to affect the circadian rhythm, making it more difficult to initiate and maintain sleep59. In the present findings, we also observed that light has a detrimental effect on the sleep quality of students. Addressing these modifiable factors, such as through noise and light reduction strategies or improved living conditions, may mitigate their impact.
Caffeine has a positive effect on alertness and concentration; however, high doses can negatively impact sleep14,60. Pharmacologically, caffeine acts as an adenosine receptor antagonist, primarily targeting A1 and A2A receptors, which disrupts sleep regulation and leads to reduced sleep duration and efficiency61,62. Therefore, reducing caffeine intake or consuming it at a modest level may help improve sleep quality among students.
The strength of our study was that it provides valuable insights into the sleep quality of medical students in Vietnam, a population for which limited data currently exist. Additionally, by including students from all academic years, both preclinical and clinical phases, we enhanced the representativeness of our sample, and obtained an overview of sleep quality across different stages of medical training. We used validated tools such as the PSQI, GAD-7, and PHQ-9, allowing for a reliable assessment of sleep quality and psychological factors. Moreover, our study identified an association between sleep-related environmental factors and financial burden with sleep quality, which has not been included in previous studies in Vietnam.
However, our study has several limitations. First, the cross-sectional design only allows us to identify associations rather than causal relationships. Second, self-reported data are subjected to bias. Third, as the study was conducted at a single institution, generalizability may still be limited despite structural similarities with other schools. Fourth, we were unable to assess other potential factors, such as dietary habits or air quality, which may also influence sleep quality. Moreover, translating the PMSS scale into Vietnamese may affect its validity. Lastly, although the tools used in this study can screen for symptoms of anxiety and depression, they do not allow for clinical diagnosis of these mental health conditions. Similarly, while the PSQI assesses subjective sleep quality, it does not provide objective measurements of sleep, such as accurately measuring sleep latency or sleep efficiency. Future studies should consider a longitudinal design and include objective sleep assessments to further validate these findings and inform mental health interventions for medical students in Vietnam.
Conclusion
In conclusion, this study underscores the high prevalence of poor sleep quality among medical students, particularly during clinical training. This study highlights the multifaceted factors contributing to this issue, including sleep environment disturbance, academic performance, clinical training status, having anxiety or depression symptoms. Addressing these factors through targeted sleep hygiene or mental health intervention strategies in medical education may potentially enhance medical students’ well-being and academic success.
Data availability
According to our application to the Institutional Review Board of the University of Medicine and Pharmacy at Ho Chi Minh City, the data cannot be shared publicly because of ethical restrictions to protect the confidentiality of the participants. A deidentified dataset is available for researchers who meet the criteria for access to confidential data. Requests for data should be submitted to the corresponding author, Dr. Linh Bui (contact via [linhbui.leo@gmail.com](mailto: linhbui.leo@gmail.com)).
References
Gross, M. The reasons of sleep. Curr. Biol. 29, R775–R777 (2019).
Watson, N. F. et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 38, 1161–1183 (2015).
Diekelmann, S., Wilhelm, I. & Born, J. The whats and whens of sleep-dependent memory consolidation. Sleep. Med. Rev. 13, 309–321 (2009).
Fogel, S. M., Smith, C. T. & Cote, K. A. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav. Brain Res. 180, 48–61 (2007).
Alhola, P. & Polo-Kantola, P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis. Treat. 3, 553–567 (2007).
Zeek, M. L. et al. Sleep duration and academic performance among student pharmacists. Am. J. Pharm. Educ. 79, 63 (2015).
Alhusseini, N. K. et al. Effects of sleep quality on academic performance and psychological distress among medical students in Saudi Arabia. Health Scope 11, e123801 (2022).
Agu, A. U. et al. Impact of sleep patterns on the academic performance of medical students of college of medicine, university of Nigeria. Int. J. Med. Health Dev. 26, 31 (2021).
Azad, M. C. et al. Sleep disturbances among medical students: A global perspective. J. Clin. Sleep. Med. JCSM Off Publ Am. Acad. Sleep. Med. 11, 69–74 (2015).
Binjabr, M. A. et al. The worldwide prevalence of sleep problems among medical students by problem, country, and COVID-19 status: a systematic review, Meta-analysis, and Meta-regression of 109 studies involving 59427 participants. Curr. Sleep. Med. Rep. 9, 161–179 (2023).
Minh, L. H. & Phuong, P. T. V. Sleep quality and related factors among students of preventive medicine physician at university of medicine and pharmacy at Ho Chi Minh City in the context of the COVID-19 pandemic in 2022. Vietnam Med. J. 524, 253–257 (2023).
Tri, V. L. Q. et al. Sleep quality and associated factors among third-year medical students at can tho university of medicine and pharmacy. Can. Tho J. Med. Pharm. 9, 29–35 (2023).
Tran, D. S. et al. Stress and sleep quality in medical students: a cross-sectional study from Vietnam. Front. Psychiatry. 14, 1297605 (2023).
Duc Si, T. Thanh hiep, N. Sleep quality during COVID-19 social distancing in students at university of medicine pham Ngoc Thach. Vietnam Med. J. 509, 26–30 (2021).
Linh, T. M., Huong, D. T. & Ly, N. T. H. Sleep quality and associated factors of students in the faculty of nursing and medical technology, university of medicine and pharmacy at Ho Chi Minh City. Can. Tho J. Med. Pharm. 55, 87–94 (2022).
Wondie, T. et al. Magnitude and correlates of sleep quality among undergraduate medical students in ethiopia: cross –sectional study. Sleep. Sci. Pract. 5, 7 (2021).
Wang, F. & Bíró, É. Determinants of sleep quality in college students: A literature review. Explore N Y N. 17, 170–177 (2021).
Hershner, S. D. & Chervin, R. D. Causes and consequences of sleepiness among college students. Nat. Sci. Sleep. 6, 73–84 (2014).
Xu, X., Lian, Z., Shen, J., Lan, L. & Sun, Y. Environmental factors affecting sleep quality in summer: a field study in shanghai, China. J. Therm. Biol. 99, 102977 (2021).
Kang, M. et al. Associations between bedroom environment and sleep quality when sleeping less or more than 6 h: A cross sectional study during summer. Build. Environ. 257, 111531 (2024).
School of Medicine and Pharmacy. History of formation and development. https://smp.udn.vn/lich-su-hinh-thanh-va-phat-trien
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
To, M. N. Validity of the Vietnamese version of the Pittsburgh sleep quality index (V-PSQI). Vietnam J. Prev. Med. 27, 50–56 (2017).
Tham, L. T. H. & Thuy, L. T. Sleep quality and associated factors in older outpatients with hypertension in vietnam: A cross-sectional study. Nurs. Pract. Today. 11, 361–368 (2024).
Phan, T. et al. Sleep quality and poor Sleep-related factors among healthcare workers during the COVID-19 pandemic in Vietnam. J. Prev. Med. Public. Health Yebang Uihakhoe Chi. 56, 319–326 (2023).
Vitaliano, P. P., Russo, J., Carr, J. E. & Heerwagen, J. H. Medical school pressures and their relationship to anxiety. J. Nerv. Ment Dis. 172, 730–736 (1984).
Vitaliano, P. P., Maiuro, R. D., Mitchell, E. & Russo, J. Perceived stress in medical school: resistors, persistors, adaptors and maladaptors. Soc. Sci. Med. 1982 28, 1321–1329 (1989).
Spitzer, R. L., Kroenke, K., Williams, J. B. W. & Löwe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 166, 1092–1097 (2006).
Spitzer, R. L., Kroenke, K., Williams, J. B. W. & the Patient Health Questionnaire Primary Care Study Group. Validation and utility of a Self-report version of PRIME-MDThe PHQ primary care study. JAMA 282, 1737–1744 (1999).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Nguyen, M. X. et al. Validation of the combined patient health questionnaire anxiety and depression scale among people with HIV in Vietnam. Int. J. STD AIDS. 34, 832–840 (2023).
Nguyen, T. Q., Do, T. M. & Pham, T. A. Screening for psychological distress in Vietnamese cancer patients: an evaluation of the distress thermometer. Cancer Med. 10, 7793–7803 (2021).
Nguyen, D. T. et al. The prevalence of self-reported anxiety, depression, and associated factors among Hanoi medical university’s students during the first wave of COVID-19 pandemic. PloS One. 17, e0269740 (2022).
World Health Organization. Translation and linguistic evaluation protocol and supporting material. Man. WHO Disabil. Assess. Schedule WHODAS. 2, 1–8 (2010).
Barros, A. J. & Hirakata, V. N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 3, 21 (2003).
Zou, G. A modified Poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Pham, H. T., Chuang, H. L., Kuo, C. P., Yeh, T. P. & Liao, W. C. Electronic device use before bedtime and sleep quality among university students. Healthcare 9, 1091 (2021).
Janatmakan Amiri, A., Morovatdar, N., Soltanifar, A. & Rezaee, R. Prevalence of Sleep Disturbance and Potential Associated Factors among Medical Students from Mashhad, Iran. Sleep Disord. 1–4 (2020). (2020).
Zhou, Y. et al. Deteriorated sleep quality and influencing factors among undergraduates in Northern Guizhou. China PeerJ. 10, e13833 (2022).
Al-Khani, A. M., Sarhandi, M. I., Zaghloul, M. S. & Ewid, M. Saquib, N. A cross-sectional survey on sleep quality, mental health, and academic performance among medical students in Saudi Arabia. BMC Res. Notes. 12, 665 (2019).
Saygın, M. et al. Investigation of sleep quality and sleep disorders in students of medicine. Turk. Thorac. J. 17, 132–140 (2016).
Chen, J. et al. Association of depression symptoms and sleep quality with state-trait anxiety in medical university students in Anhui province, china: a mediation analysis. BMC Med. Educ. 22, 627 (2022).
Almeida, F. V. Q. et al. Influence of sleep quality on academic performance of medical students. Rev. Soc. Bras. Clínica Médica. 18, 6–10 (2020).
Nsengimana, A. et al. Sleep quality among undergraduate medical students in rwanda: a comparative study. Sci. Rep. 13, 265 (2023).
Li, L. et al. Prevalence of sleep disturbances in Chinese university students: a comprehensive meta-analysis. J. Sleep. Res. 27, e12648 (2018).
Khero, M., Fatima, M., Shah, M. A. A. & Tahir, A. Comparison of the Status of Sleep Quality in Basic and Clinical Medical Students. Cureus 11, e4326.
Kurdee, Z. S. et al. The association between sleep quality, stress, and academic performance in preclinical and clinical medical students. Int. J. Med. Dev. Ctries. 6, 1222–1226 (2022).
Cox, R. C. & Olatunji, B. O. A systematic review of sleep disturbance in anxiety and related disorders. J. Anxiety Disord. 37, 104–129 (2016).
Gregory, A. M. et al. Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. J. Psychosom. Res. 71, 250–255 (2011).
Sadock, B. J., Sadock, V. A. & Ruiz, P. Kaplan and Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 11th Ed. xvi, 1472Wolters Kluwer Health, US, (2015).
Adrien, J. Neurobiological bases for the relation between sleep and depression. Sleep. Med. Rev. 6, 341–351 (2002).
Steiger, A., Dresler, M., Kluge, M. & Schüssler, P. Pathology of sleep, hormones and depression. Pharmacopsychiatry 46 (Suppl 1), S30–35 (2013).
Vanderlind, W. M. et al. Sleep and sadness: exploring the relation among sleep, cognitive control, and depressive symptoms in young adults. Sleep. Med. 15, 144–149 (2014).
Fang, H., Tu, S., Sheng, J. & Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 23, 2324–2332 (2019).
Zhang, Y. M. et al. Sleep deprivation aggravates lipopolysaccharide-induced anxiety, depression and cognitive impairment: the role of pro-inflammatory cytokines and synaptic plasticity-associated proteins. J. Neuroimmunol. 386, 578252 (2024).
Yasugaki, S., Okamura, H., Kaneko, A. & Hayashi, Y. Bidirectional relationship between sleep and depression. Neurosci. Res. 211, 57–64 (2025).
Basner, M. & Samel, A. Effects of nocturnal aircraft noise on sleep structure. Somnologie 9, 84–95 (2005).
Maschke, C., Hecht, K. & Wolf, U. Nocturnal awakenings due to aircraft noise. Do wake-up reactions begin at sound level 60 dB(A)? Noise Health. 6, 21–33 (2004).
Selander, J. et al. Saliva cortisol and exposure to aircraft noise in six European countries. Environ. Health Perspect. 117, 1713–1717 (2009).
Alshumrani, R. et al. Consumption of energy drinks and their effects on sleep quality among medical students. J. Fam Med. Prim. Care. 12, 1609–1614 (2023).
Gardiner, C. et al. The effect of caffeine on subsequent sleep: A systematic review and meta-analysis. Sleep. Med. Rev. 69, 101764 (2023).
Reichert, C. F., Deboer, T. & Landolt, H. Adenosine, caffeine, and sleep–wake regulation: state of the science and perspectives. J. Sleep. Res. 31, e13597 (2022).
Acknowledgements
We would love to thank Dr. Hoang Thi Nam Giang for her valuable feedback on improving the questionnaire and study protocol, as well as for her support in connecting us with the students. We thank Dr. Hieu Le for his project leadership and coordination efforts. We appreciate Assoc. Prof. Thanh Huyen Vu for her helpful suggestions on improving the study protocol. We also thank Dao Thu Quynh for her language editing support. Also, we deeply thank our supervisors, collaborators, and study participants for their contributions and cooperation during the data collection process.
Funding
The study received funding (for study design and data acquisition) from the Research Advancement Consortium in Health (REACH) - a non-profit entity in Vietnam, of which Linh Bui and Tung Pham are co-managers. Linh Bui and Tung Pham did not receive any payment or compensation from this position at REACH. On behalf of REACH, they provided consultancy on study design, data collection and analysis, and manuscript preparation. However, Linh Bui and Tung Pham had no role in the decision to publish this study, and this final decision belongs to the funded research team.
The study received funding from the Partnership for Higher Education Reform (PHER) project, which is funded by the United States Agency for International Development (USAID) and Indiana University (USA), in collaboration with the University of Danang. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Nguyet Truong led conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing – original draft, and review & editing.Linh Bui contributed to conceptualization, formal analysis, methodology, project administration, writing – original draft, and review & editing.Hung Nguyen participated in data curation, funding acquisition, investigation, methodology review, and editing the manuscript.Tung Pham contributed to formal analysis, methodology, visualization, review, and editing the manuscript.Duyen Chu supported data curation, funding acquisition, investigation, and review manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study adhered to the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Scientific Council of the School of Medicine and Pharmacy, Danang University, and the Institutional Review Board of the University of Medicine and Pharmacy at Ho Chi Minh City, under Decision No. 576/HĐĐĐ-ĐHYD.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Truong, N., Nguyen, H., Pham, T. et al. Sleep quality by clinical training status among medical students and its associated factors: a cross-sectional study in Da Nang, Vietnam. Sci Rep 15, 33671 (2025). https://doi.org/10.1038/s41598-025-18256-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18256-8